Abstract
Background
Thrombocytopenia is frequent among neonates, and 20-25% of affected infants are treated with platelet transfusions. These are frequently given for mild thrombocytopenia (platelets 50-100×109/L), largely due to the known hyporeactivity of neonatal platelets. In tests of primary hemostasis, however, neonates have shorter bleeding and closure times (CTs) than adults. This has been attributed to their higher hematocrits, higher von Willebrand factor (VWF) concentrations, and predominance of longer VWF polymers.
Objective
To determine whether the “transfusion” of adult (relatively hyper-reactive) platelets into neonatal blood results in a hypercoagulable profile.
Methods
Cord blood (CB) and adult peripheral blood (PB) were separated (using a modified buffy-coat method) to generate miniaturized platelet concentrates (PCs) and thrombocytopenic blood. PB- and CB-derived PCs (n=7 per group) were then “transfused” in-vitro into thrombocytopenic CB and PB. The effects of autologous vs. allogeneic (developmentally mismatched) “transfusions” were evaluated using whole blood aggregometry, platelet function analyzer (PFA-100), and thromboelastography (TEG).
Results
Adult platelets aggregated significantly better than neonatal platelets in response to TRAP, ADP and collagen, regardless of the blood into which they were transfused. The “transfusion” of adult platelets into thrombocytopenic CB resulted in shorter CTs-Epi (PFA-100) and higher clot strength and firmness (TEG), compared to “transfusion” of neonatal autologous platelets.
Conclusions
In vitro “transfusion” of adult platelets into neonatal blood results in shorter CTs than “transfusion” with neonatal platelets. Our findings should raise awareness of the differences between the neonatal and adult hemostatic system and the potential “developmental mismatch” associated with platelet transfusions on neonatal hemostasis.
Introduction
Thrombocytopenia (platelet count <150×109/L) affects 20-35% of patients admitted to neonatal intensive care units (NICUs) [1]. Its incidence is inversely proportional to the gestational age of the infants, and reaches approximately 70% among the most premature infants (birth weight <1000g) [1, 2]. Most affected neonates have transient and/or mild thrombocytopenia, which does not prompt intervention. However, as many as 20-25% of them (5-9% of NICU admissions, or 20,000-36,000 neonates per year in the USA) receive platelet transfusions [2, 3], and approximately 50% of them receive two or more [4]. The majority of these transfusions, up to 98% in some reports, are given to non-bleeding patients whose platelet counts have fallen below an arbitrary trigger [4]. The precise platelet count at which a neonatal transfusion is ordered is very variable, but generally substantially higher than in older children or adults [5]. Indeed, a large proportion of platelet transfusions are given to non-bleeding neonates whose platelet counts are in the 50 to 150×109/L range [2, 3]. To a large extent, this practice is based on the fact that neonates have the highest risk of intracranial hemorrhage of any patient group. Specifically, 25-31% of preterm infants born with a birth weight <1500g experience intraventricular hemorrhage (IVH) [6], which almost always presages adverse neurodevelopmental consequences. However, the etiology of IVH in preterm infants is most likely multifactorial, and the specific contribution of thrombocytopenia has been difficult to establish. In fact, it has been previously demonstrated that platelet transfusions in the setting of mild to moderate thrombocytopenia (platelet counts 50-150 × 109/L) do not decrease the incidence or severity of IVH in preterm infants [7].
A second factor leading to the liberal use of platelet transfusions in neonatology is the well known hyporeactivity of neonatal platelets. Over the last three decades, multiple studies using aggregometry and flow cytometry consistently demonstrated that neonatal platelets are hyporeactive in response to most agonists, leading to the widely accepted belief that neonatal platelets are dysfunctional [8-10]. In contrast to these findings, however, studies of primary hemostasis revealed significantly shorter bleeding times in neonates compared to adults [11]. Similarly, in-vitro studies of primary hemostasis using the Platelet Function Analyzer-100 (PFA-100®) demonstrated that closure times (CTs) were shorter in term cord blood (CB) than in adult peripheral blood (PB) samples [12]. More recently, whole blood global coagulation assays such as the thromboelastogram also demonstrated that coagulation in full term neonates is equal to or somewhat more robust than in adults [13, 14].
This paradoxical finding of enhanced primary hemostasis in the face of platelet hyporeactivity has been attributed to the higher hematocrits, higher mean corpuscular volumes (MCV) of erythrocytes, higher von Willebrand factor (VWF) concentrations, and predominance of longer VWF polymers in the blood of neonates [15]. In fact, the VWF concentrations and polymer composition in neonates resemble those of patients with ADAMTS-13 deficiency [16]. These factors, which increase blood viscosity and clot formation, functionally counterbalance the relative platelet hyporeactivity of neonates. Based on these findings, the relative hyporeactivity of neonatal platelets is currently viewed as part of a delicate neonatal hemostatic balance, rather than as a developmental deficiency.
Given that all clinical transfusions are derived from adult donors, this perspective led us to question whether transfusing adult (comparatively hyperreactive) platelets into neonatal blood would result in a hemostatic “developmental mismatch”, possibly leading to a hypercoagulable profile. To test this hypothesis, we carried out experiments mixing neonatal or adult platelets with neonatal vs. adult thrombocytopenic blood. Since every attempt was made to mimic the plasma and cellular composition of clinical platelet concentrates and thrombocytopenic blood, throughout the manuscript we refer to these mixing studies as in-vitro transfusions or simply “transfusions”. We then evaluated the effects of these in-vitro transfusions on hemostasis using a combination of whole blood platelet aggregometry, in-vitro primary hemostasis (PFA-100®), and thromboelastography (TEG).
Material and methods
Sources of blood
Two sources of blood were used. First, CB was collected from healthy full term infants born by elective cesarian section following an uncomplicated pregnancy. PB was collected from the antecubital vein of healthy adults who had not taken any medications during the ten days prior to the study. These studies were approved by the Institutional Review Board.
All samples (CB and PB) were gently drawn through a 21-gauge needle into plastic syringes containing 3.2% sodium citrate (9:1 blood/citrate). The first 2 ml of each draw were used for cell count in an Acl-10 Analyzer (Beckman Coulter, Miami, FL) and for ABO typing (Immucor, Norcross, GA). Five ml of blood were stored at room temperature as an un-manipulated control for each sample.
Preparation of platelet concentrates and “thrombocytopenic blood”
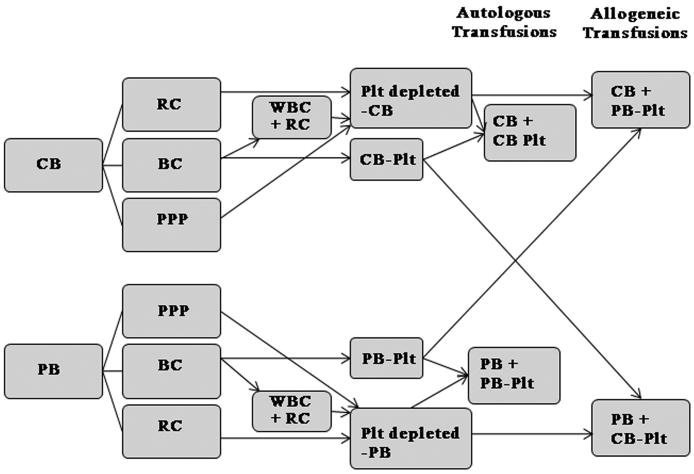
Platelet concentrates (PCs) were generated using a modified buffy-coat (BC) method (Fig. 1). Briefly, a hard spin (1200g × 12 min) was used initially to separate whole blood into platelet poor plasma (PPP), red blood cells (RBCs), and a BC layer. After removing the PPP, the BC was transferred to a new tube and was allowed to “rest” for 30 min. Following this, a soft spin (200g ×10 min) was used to separate the platelets (in the supernatant) and generate a small PC with a mean platelet concentration of 1,232×109/L (714-1,600×109/L), similar to that of a typical PC prepared for clinical use. The pellet resulting from this spin (containing RBCs, leukocytes, and some contaminating platelets) was then diluted 1:2 with autologous PPP and subjected to a new soft spin to obtain a platelet-free pellet containing mostly leukocytes. This pellet and the PPP from the same sample were then added to the RBC concentrate obtained during the first centrifugation, to reconstitute thrombocytopenic whole blood. The final product had a hematocrit similar to that of the initial whole blood and a platelet count <50×109/L, thus mimicking common transfusion thresholds in neonatology [5].
Figure 1.
‘In vitro’ transfusion model. Whole blood obtained from adult subjects (peripheral blood [PB]) or cord blood (CB) was separated into platelet-poor plasma (PPP), red blood cells (RBCs), and buffy coat (BC). The BC was allowed to rest and, after a soft spin, a platelet concentrate was generated from the supernatant. The pellet resulting from this spin was mixed with the PPP and RBCs from the first centrifugation to reconstitute platelet-depleted blood. PB-derived and CB-derived platelet concentrates were mixed with platelet-depleted CB or PB to mimic autologous and allogeneic transfusions (n = 7 per group). Plt, platelet; WBC, white blood cell; RC, red cells.
In-vitro platelet “transfusions”
As shown in Fig. 1, ABO-matched adult PB-PCs, generated as described, were then gently mixed with thrombocytopenic CB, to mimic a standard platelet transfusion in a thrombocytopenic neonate (CB+PB platelets). The number of platelets added was calculated to generate a final platelet count of approximately 150×109/L. Given the platelet concentrations in our PCs, this approach resulted in a PC to thrombocytopenic blood ratio of 1:5 to 1:9 (mean of 1:7.8), which closely mimicked a standard platelet transfusion of 10-15 ml/kg into a neonate with a blood volume of 80 ml/kg. Thus, the plasma in our post-“transfusion” whole blood samples was predominantly recipient-derived.
PCs obtained from the same CB sample and prepared using the same procedure, were also “transfused” into thrombocytopenic CB, to serve as age-matched autologous controls (CB+CB platelets). A platelet count was obtained before and after each in-vitro transfusion.
The reverse experiments were carried out using thrombocytopenic adult PB samples. Specifically, thrombocytopenic PB samples were gently mixed with ABO-matched CB-derived platelets (PB+CB platelets). Autologous in-vitro platelet transfusions were also performed to serve as age-matched controls (PB+PB platelets).
Hemostasis tests
Platelet aggregation
Whole blood platelet aggregation was evaluated following in-vitro transfusions using an impedance aggregometer (Multiplate®, Dynabyte Medical). This system measures increases in impedance between electrodes due to the adhesion and aggregation of platelets. Briefly, 1:1 mixtures of pre-heated 0.9% NaCl and citrated reconstituted whole blood were stirred for 3 minutes at 37°C in cuvettes specifically designed for low-volume samples (Multiplate® Mini test cells). Immediately thereafter, adenosine 5′-diphosphate (ADP, 6.5 μM), collagen (3.2 μg/ml), thrombin receptor–activating peptide (TRAP, 32 μM), or ristocetin (0.77 mg/ml) were added, and the increase in electrical impedance was continuously recorded over 6 minutes. The mean values of two determinations were expressed as the area under the curve (AUC), a measure of the aggregation response over time.
PFA-100
Whole blood primary hemostasis was evaluated using the PFA-100® (Siemens, Inc.). Briefly, 800 μl of “transfused” whole blood were placed in a test cartridge and aspirated through a capillary under constant negative pressure (high shear rate) toward a membrane with a small aperture coated with collagen plus ADP or collagen plus epinephrine (Epi), to enhance platelet aggregation. The reported values, closure time-Epi (CT-Epi) or closure time-ADP (CT-ADP), represent the time until the aperture was occluded by the platelet plug.
TEG coagulation analysis
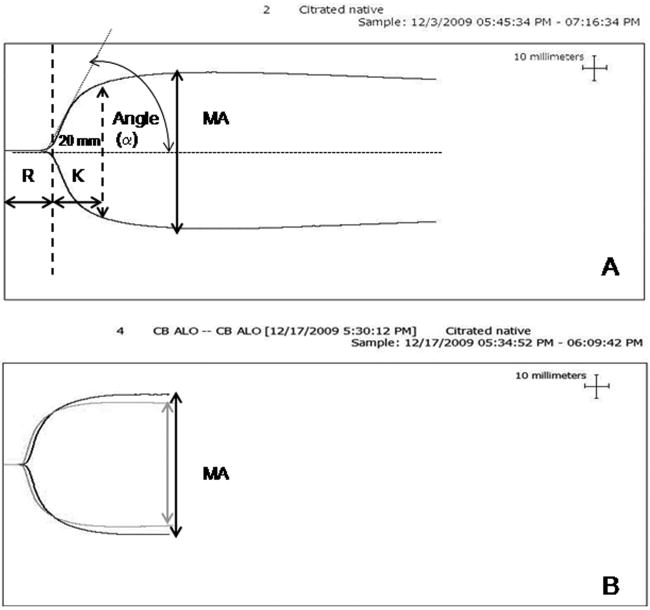
Whole blood clotting was evaluated using the TEG® (5000 Series Analyzer, Haemoscope, Skokie, IL). Citrated “transfused” whole blood (340 μl) was recalcified with 0.2 M calcium chloride inside a preheated plastic cup, and clot formation was recorded following manufacturer's instructions. The variables measured, described in Fig.2A, included: Reaction time (R); Clot formation time (K); α, angle; and Maximal Amplitude (MA). The elastic shear (G) is related to MA [by 5000 × MA/(100 – MA)], and it indicates clot firmness expressed in dyne per cm2.
Figure 2.
Thromboelastogram profile parameters and representative profiles of post-transfusion CB samples. (A) Thromboelastogram profile. Reaction time (R) is the time from the start of tracing until the curve is 2 mm wide, and represents the time to start of clot formation. Clot formation time (K) is the time measured from R to the point where the amplitude reaches 20 mm. Maximum amplitude (MA) is the widest part of the graph, and reflects clot strength; Angle (α) is the size (in degrees) of the angle formed by a line tangent to the thromboelastogram tracing, and reflects the speed of clot formation. (B) Overlapping thromboelastogram graphs showing the results of a cord blood (CB) sample transfused with neonatal platelets (gray line) vs. adult platelets (black line). Transfusion with neonatal platelets resulted in a shorter R and a lower MA
Statistical analysis
All results are reported as mean ± SD. The statistical significance of the differences between un-manipulated CB and adult samples was assessed using unpaired two-tailed T-tests. Differences between post-“transfusion” samples (CB+CB platelets vs. CB+PB platelets and PB+PB platelets vs. PB+CB platelets) and between un-manipulated samples and samples reconstituted with autologous platelets were analyzed by using paired two-tailed T tests. Multiple group comparisons were carried out by ANOVA. The influence of hematocrit and WBC counts on aggregation parameters was evaluated using linear regression analysis (Pearson's correlation coefficients). Inter-individual variability was expressed as % coefficient of variation (CV). Statistical significance was set at a p<0.05. All statistical tests were carried out using SSPS for Windows version 15.0 (Chicago, IL).
Results
Cell Counts
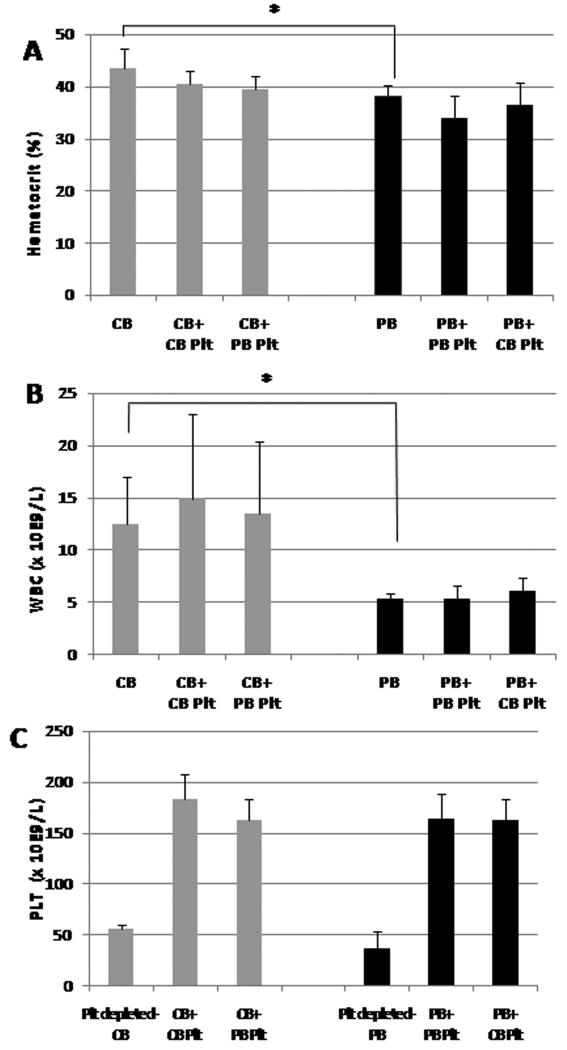
Platelet counts were similar in un-manipulated CB and PB samples (n=7 per group). As expected, however, WBC counts and hematocrit were significantly higher in neonates (CB) than in adults (p=0.006 and p=0.02 respectively; Table 1). During the reconstitution process, attention was placed to generate blood that maintained the characteristics of the original sample, with the exception of the platelet count. As a result, following reconstitution and in-vitro transfusion, both WBC and hematocrit were very similar to those in the original un-manipulated whole blood samples (Fig. 3 A and B). All platelet counts were close to the target values of 50×109/L for the thrombocytopenic blood (range: 20-60×109/L), and 150 × 109/L for the post-“transfusion” blood (range: 140-207×109/L) (Fig. 3 C).
Table 1.
Cell counts in CB vs. PB (adult) samples.
| CB | PB | p-value | |
|---|---|---|---|
| Platelets (× 109/L) | 246 ± 44 | 221 ± 45 | 0.4 |
| Leukocytes (× 109/L) | 12.5 ± 4.6 | 5.3 ± 0.5 | 0.006 |
| Hematocrit (%) | 43.4 ± 3.8 | 38.2 ± 2.0 | 0.02 |
Values represent the mean ± SD of 7 independent samples per group.
Figure 3.
Hematocrit, white blood cell (WBC) counts and platelet concentrations in cord blood (CB) (gray bars) and peripheral blood (PB) (black bars). (A) Unmanipulated CB samples had significantly higher hematocrits than unmanipulated PB samples (*P < 0.05). Post-transfusion samples had similar hematocrits as their original whole blood sources. (B) Similarly, unmanipulated CB samples had significantly higher WBC counts than unmanipulated PB samples. Post-transfusion samples had similar WBC counts as their original whole blood sources. (C) Platelet counts in platelet-depleted whole blood and in post-transfusion CB (gray bars) and PB (black bars) samples. Plt, platelets
Platelet Aggregation Testing
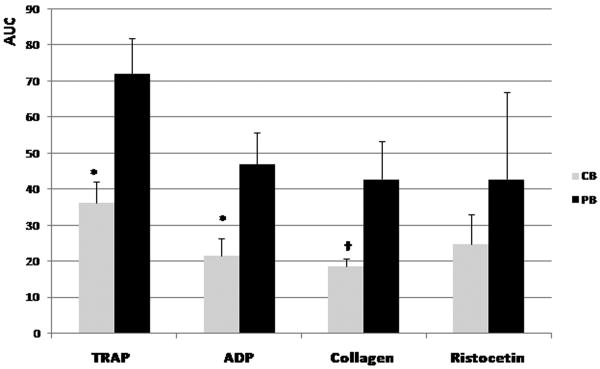
Since no prior studies had used the whole blood Multiplate® system to evaluate platelet aggregation in newborn infants, and given the significant developmental differences in hematocrit and WBC counts that we observed, platelet aggregation was evaluated in CB and PB non-manipulated whole blood samples in addition to our post-“transfusion” samples. Consistent with prior observations in standard platelet-rich plasma assays, non-manipulated CB whole blood samples exhibited significantly less platelet aggregation (AUC) than non-manipulated PB samples in response to TRAP (36±6 vs. 72±10, p<0.001), ADP (22±5 vs. 47±9, p<0.001) and collagen (18±2 vs. 43±10, p<0.01) (Fig. 4). No statistically significant difference was found between CB and PB in response to ristocetin (25±8 vs. 43±24, p=0.15), as a result of the high inter-individual variability in response to this agonist (CVs: 32% and 56% in CB and PB, respectively) and/or the fact that ristocetin-induced aggregation (more properly referred to as agglutination) is not dependent on glycoprotein IIb-IIIa.
Figure 4.
Impedance platelet aggregometry results obtained with the Multiplate system, following stimulation with thrombin receptor-activating peptide (TRAP), ADP, collagen, and ristocetin. Gray bars represent non-manipulated cord blood (CB) samples and black bars represent non-manipulated adult peripheral blood (PB) samples (mean ± standard deviation of seven independent samples per group). AUC, area under the curve. *P < 0.001; †P < 0.01.
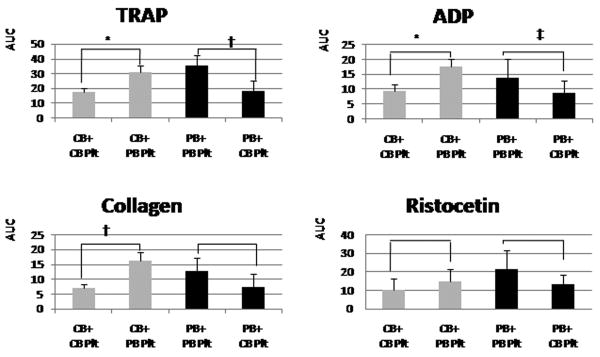
Overall, platelet aggregation was lower in all post-“transfusion” samples compared to non-manipulated samples, even when autologous platelets were used for the in-vitro transfusion. Since all samples were run at the same time, these differences did not reflect time-dependent changes, but likely some degree of platelet activation and de-sensitization during the preparation of PCs. Nevertheless, all post-“transfusion” samples responded to the different agonists. Furthermore, following in-vitro transfusion into thrombocytopenic CB, adult platelets aggregated (AUC) better than neonatal platelets in response to TRAP (32±5 vs. 18±4, p<0.001), ADP (18±2 vs. 9±2, p<0.001), and collagen (16±3 vs. 7±1, p<0.01). No significant developmental differences were observed in response to Ristocetin (15±6 vs. 10±6, p=0.15) (Fig. 5).
Figure 5.
Mean platelet aggregation (± standard deviation) results from thrombocytopenic cord blood (CB) samples (gray bars) and adult peripheral blood (PB) samples (black bars) transfused in vitro with neonatal (CB) or adult (PB) platelets. Adult platelets aggregated better than neonatal platelets, regardless of the blood into which they were transfused, in response to thrombin receptor-activating peptide (TRAP), ADP, and collagen. The same trend was observed in response to ristocetin, but the differences were not significant. *P < 0.001, †P < 0.01, ‡P < 0.05. AUC, area under the curve; Plt, platelets.
Similarly, following in-vitro transfusion into thrombocytopenic adult (PB) blood, adult platelets aggregated better than neonatal platelets in response to TRAP (35±7 vs. 18±7, p<0.01), and ADP (14±6 vs. 9±4, p<0.05). The same trend was observed in response to collagen and ristocetin, although these differences were not statistically significant (Fig. 5).
Overall, we observed that CB platelets exhibited similar degrees of agonist-induced aggregation, irrespective of the blood into which they were “transfused” (PB or CB). The same was true for PB-derived platelets, thus suggesting that differences in hematocrit and WBC did not significantly influence the degree of platelet aggregation in the whole blood Multiplate® system. Confirming this hypothesis, a linear regression analysis showed no association between the impedance platelet aggregation and either the WBC count or the hematocrit (data not shown).
Primary Hemostasis
Next, we evaluated the effects of transfusing adult vs. neonatal (autologous) platelets into thrombocytopenic CB on primary hemostasis, using the PFA-100. Consistent with prior reports [12], our non-manipulated CB samples had shorter CTs than non-manipulated adult PB samples (76.8±6.8 vs. 115.8±31.0 sec. for CT-Epi and 50.6±4.3 vs. 76.4±14.9 sec. for CT-ADP, respectively). In general, CTs were longer in blood reconstituted with autologous platelets than in non-manipulated samples, likely reflecting some degree of platelet desensitization associated with sample manipulation.
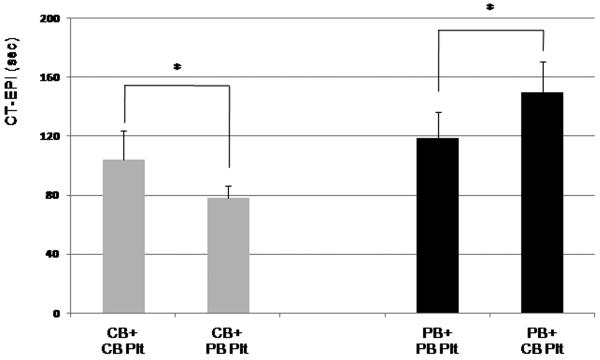
When comparing “post-transfusion” samples, however, we found that thrombocytopenic CB samples transfused in-vitro with adult platelets yielded significantly shorter CT-Epi than samples “transfused” with autologous CB platelets (78±8 vs. 104±19 sec., respectively; p=0.03) (Fig. 6). Conversely, in-vitro transfusion of newborn platelets into thrombocytopenic adult blood resulted in prolonged CT-Epi, compared to autologous “transfusion” with adult platelets (150±21 sec. for PB+CB platelets vs. 118±18 sec for PB+PB platelets, p=0.02). In general, CT-Epi were significantly shorter in neonatal compared to adult blood. Within each different whole blood milieu, however, “transfusion” with adult platelets consistently resulted in a CT-Epi shortening of approximately 25%, compared to “transfusion” with neonatal platelets (Fig. 6). CT-ADP followed similar trends, but the differences were not statistically significant (data not shown).
Figure 6.
PFA-100 closure times following stimulation with collagen and epinephrine (CTs-EPI). Cord blood (CB) samples (gray bars) generated shorter CTs-EPI than peripheral blood (PB) samples (black bars). Within each whole blood source, transfusion with adult platelets resulted in significantly shorter CTs-EPI than with neonatal platelets. Plt, platelets. *P < 0.05.
Whole blood coagulation by TEG
In concordance with prior studies, un-manipulated CB samples exhibited faster initiation of clot formation (R) and more rapid clot accumulation (K and α) compared to un-manipulated adult samples (Table 1-S) [13, 14]. No statistically significant differences were found when un-manipulated samples and age-matched autologous “post-transfusion” samples were compared (Table 1S and 2). In contrast, when blood samples reconstituted in vitro with autologous vs. allogeneic platelets were compared, small but significant differences were observed in three TEG parameters: MA, G and R (Table 2). When “transfused” into thrombocytopenic CB, adult platelets yielded significantly increased clot strength (MA) and clot firmness (G) values, compared with “transfused” neonatal platelets (Table 2 and Fig. 2). Contrariwise, neonatal platelets tended to reduce these parameters when transfused into adult thrombocytopenic whole blood, although these differences were not statistically significant (Table 2). The R also changed in response to platelet “transfusions”. Overall, the R was shorter in reconstituted CB compared to reconstituted PB samples. In both environments, however, it was shorter following in vitro transfusion with neonatal platelets and longer following “transfusion” with adult platelets.
Table 2.
Results of TEG studies in the four different in vitro transfusion conditions.
| Thromboctopenic CB + | Thromboctopenic PB + | |||
|---|---|---|---|---|
| CB Platelets | PB Platelets | PB Platelets | CB Platelets | |
| R (min) | 4.5 ± 0.9 | 5.1 ± 0.9* | 7.5 ± 1.9 | 5.5 ± 1.0 † |
| K (min) | 1.7 ± 0.3 | 1.6 ± 0.2 | 2.1 ± 0.6 | 1.9 ± 0.6 |
| α(deg) | 55.9 ± 7.0 | 61.0 ± 3.6 | 55.7 ± 8.1 | 53.5 ± 11.4 |
| MA (mm) | 55.4 ± 1.8 | 59.4 ± 1.5* | 58.5 ± 4.8 | 55.4 ± 2.9 |
| G (dynes/cm2) | 6.2 ± 0.5 | 7.4 ± 0.5* | 7.2 ± 1.4 | 6.3 ± 0.8 |
Data are mean ± SD (n=7).
p < 0.05 between CB+CB platelets and CB+PB platelets
p<0.05 between PB+PB platelets and PB+CB platelets.
Discussion
As a first step, our studies validated the use of Multiple Electrode Aggregometry (Multiplate®) to study neonatal samples. The Multiplate® is a new generation impedance aggregometer, which includes single-use test cells containing duplicate impedance sensors. This test offers many advantages for the study of platelet aggregation in neonates, since it avoids the need to separate platelets from blood and can be performed using very small blood volumes (175 μl for the mini-test cells). However, the Multiplate® had not been previously used with neonatal blood, characterized by high hematocrits and WBC counts. Seyfert et al. previously demonstrated that the WBC count does not influence platelet aggregometry in the Multiplate® [17], but the potential effects of variable hematocrits had not been previously investigated. In that regard, we found no correlation between impedance platelet aggregation and hematocrit or WBC count, within the ranges of our study. Furthermore, our results were in agreement with the previously described hyporeactivity of neonatal platelets [10], thus validating the use of the Multiplate® to evaluate our samples. Most importantly, however, our “transfusion” studies demonstrated that the developmental differences in platelet aggregation between neonates and adults persisted despite changing the milieu of the platelets, thus strongly suggesting that these differences are intrinsic to the platelets.
In these studies, platelet aggregation was lower in all “post-transfusion” samples compared to non-manipulated samples. These findings likely reflected some degree of platelet activation and de-sensitization occurring during the preparation of PCs. This de-sensitization was also evident on the global tests of hemostasis used: PFA-100 closure times were significantly longer in reconstituted compared to un-manipulated samples, and the MA and G were slightly lower in the TEG of samples reconstituted with autologous platelets than in non-manipulated samples, although this difference was not statistically significant. Nevertheless, our platelet aggregation studies demonstrated that adult and neonatal platelets were equally affected by sample manipulation and that manipulated platelets retained their developmental functional differences, thus allowing for direct comparisons between samples transfused with platelets of different developmental origins.
As a first step, we evaluated the effects of “developmentally mismatched” platelet “transfusions” on primary hemostasis, using the PFA-100. As expected, we observed that CB-CTs were shorter than PB-CTs (regardless of the source of the platelets “transfused”), a finding likely due to the high concentrations of VWF, the predominance of ultralong VWF polymers, the high hematocrits, and the high MCVs in neonatal blood [15]. When adult platelets were “transfused” into thrombocytopenic CB, however, the resulting CTs-Epi were approximately 25% shorter than when neonatal autologous platelets were used, presumably due to the interaction of the relatively hyperreactive adult platelets with the somewhat pro-thrombotic characteristics of neonatal whole blood. In fact, the combination of thrombocytopenic neonatal blood and adult platelets consistently yielded the shortest CTs-Epi among the four whole blood/platelets combination, although all values were within the normal range for the test. Similar trends were observed when CTs-ADP were measured, although the differences were not significant. In that regard, CT-Epi has been previously reported to be a more sensitive test than CT-ADP for platelet function disorders [18]. Furthermore, the developmental differences between neonatal and adult platelet aggregation are particularly pronounced in response to epinephrine [19], because of the lower number of α-adrenergic receptors in neonatal platelets[8].
Although in clinical practice the PFA-100 is mainly used as a screening test for VWD [18], there is also a growing body of evidence supporting the association of short PFA-100 CTs and increased risk of thrombosis. In the adult cardiology literature, shorter CTs have been reported in patients with unstable angina, compared with sex- and age-matched subjects with chronic stable angina [20]. Similarly, patients with a recent myocardial infarction were found to have shorter CT-Epi than patients with coronary artery disease but no infarction [21], and in a prospective study involving more than 200 subjects with acute coronary syndrome, shortened CTs predicted recurrent acute coronary syndrome [22].
Our studies also evaluated the effects of developmentally mismatched transfusions on global hemostasis, using the TEG. This test has been used clinically to evaluate both bleeding and thrombotic disorders, including hypercoagulable states during pregnancy and postpartum [23], cancer [24], and surgery [25]. Two TEG parameters reflect platelet function [14]: the clot strength (MA) and the clot firmness (G). In a prior study, G has been shown to be lower in neonatal compared to adult samples, presumably reflecting decreased platelet function [26]. In our un-manipulated samples, G values followed a similar trend, but the difference was not statistically significant. As predicted, however, the in vitro transfusion studies revealed a statistically significant increase in both maximal clot firmness (G) and clot strength (MA) in thrombocytopenic CB samples transfused in-vitro with adult platelets, compared to neonatal platelets. While the magnitude of these differences was small, and their clinical significance is difficult to ascertain (particularly in the context of some platelet desensitization), elevated G values have been associated with thromboembolic events in critically ill postoperative surgical patients [27]. In that study, the odds of a thromboembolic event increased by 25% for every 1 dyne/cm2 increase in G, although the specific G values could not be compared to those in our study due to the use of activators to trigger the clotting reaction (kaolin and tissue factor) in their study, but not in ours [27]. Similarly, a recent review [25] concluded that the MA was the most useful TEG indicator of a post-operative hypercoagulable state. However, there is no consensus regarding the cut-off MA value associated with a higher incidence of thromboembolism [25].
In concordance with several prior studies, we also found a shorter R in neonatal compared to adult samples [13, 14]. This apparently paradoxical finding (considering the low levels of most coagulation factors found in neonates [28]) has been attributed to the significantly faster rate of thrombin generation in newborn blood (a consequence of their low levels of antithrombin III, protein C, and protein S) and to their physiologically low levels of tissue factor pathway inhibitor [29, 30]. In our study, CB samples “transfused” with neonatal platelets had shorter R times than those “transfused” with adult platelets. Similarly, thrombocytopenic PB samples “transfused” with neonatal platelets had shorter R times than those “transfused” with adult platelets. Since R times are not thought to be influenced by platelets and are rather determined by coagulation factor levels [14, 27], we believe these intriguing observations reflect the effects of the plasma present in our PCs, albeit in very small amounts.
We recognize limitations in our study. First, our experiments were carried out using CB samples, and not peripheral blood from neonates. While this would have been ideal, the amount of neonatal blood required precluded this approach. Second, we recognize that the unavoidable platelet activation and desensitization secondary to the sample manipulation is a weakness of our study, as desensitized adult platelets somewhat resembled the normal hyporeactivity of un-manipulated neonatal platelets. Partly because of this, findings from our in-vitro transfusion model cannot be directly extrapolated to clinical scenarios. The platelet reactivity of blood bank PCs could be different to that of our miniaturized fresh PCs, and this would significantly influence the effects of transfusions on neonatal whole blood hemostasis. Nevertheless, we demonstrated that all our samples were similarly affected by the manipulation, and that platelets retained their developmental differences in the manipulated samples. This, coupled with the bidirectional design of our mixing studies, allowed us to conclude that the developmental stage of the platelet per se significantly influences the effects of a transfusion on hemostasis.
While the clinical significance of our findings remains to be determined, our observations should raise awareness of the substantial differences between the neonatal and adult hemostatic system and of the potential “developmental hemostatic mismatch” associated with platelet transfusions. Thrombocytopenia is common in the NICU, and adult-derived platelets are frequently transfused to non-bleeding neonates with mild/moderate thrombocytopenia [2, 3]. Interestingly, neonates also have the highest incidence of thrombosis within pediatrics, a propensity attributed to the fragility of the neonatal hemostatic balance [31]. Symptomatic thromboses occur in 1% of neonates with vascular catheters [32], and in up to 7% of coagulopathic neonates [33]. Furthermore, most of the risk factors for neonatal thrombosis (prematurity, sepsis, extracorporeal membrane oxygenation, necrotizing enterocolitis, intrauterine growth retardation, and maternal diabetes) [34], are also causes of thrombocytopenia [2, 4]. Additional studies will be necessary to determine whether and how platelet transfusions administered to neonates in clinical practice affect their hemostatic profile and thrombotic risk.
Supplementary Material
Acknowledgments
The authors thank C. Brugnara and J. Rivera for their technical support and helpful discussions. This work was partially supported by NIH grant HL69990 (MSV) and Grant BAE-90058 from the Health Research Fund, Ministry of Health, Spain, and a Health Service grant from the Comunidad Autonoma of Murcia, Spain (both to FFM).
Footnotes
Disclosure of Conflict of Interests: The authors declare that they have no conflicts of interest.
References
- 1.Castle V, Andrew M, Kelton J, Giron D, Johnston M, Carter C. Frequency and mechanism of neonatal thrombocytopenia. J Pediatr. 1986;108:749–55. doi: 10.1016/s0022-3476(86)81059-9. [DOI] [PubMed] [Google Scholar]
- 2.Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Sola-Visner MC, Lambert DK, Kiehn TI, Ainsworth S. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol. 2006;26:348–53. doi: 10.1038/sj.jp.7211509. [DOI] [PubMed] [Google Scholar]
- 3.Del Vecchio A, Sola MC, Theriaque DW, Hutson AD, Kao KJ, Wright D, Garcia MG, Pollock BH, Christensen RD. Platelet transfusions in the neonatal intensive care unit:factors predicting which patients will require multiple transfusions. Transfusion. 2001;41:803–8. doi: 10.1046/j.1537-2995.2001.41060803.x. [DOI] [PubMed] [Google Scholar]
- 4.Dohner ML, Wiedmeier SE, Stoddard RA, Null D, Jr, Lambert DK, Burnett J, Baer VL, Hunt JC, Henry E, Christensen RD. Very high users of platelet transfusions in the neonatal intensive care unit. Transfusion. 2009;49:869–72. doi: 10.1111/j.1537-2995.2008.02074.x. [DOI] [PubMed] [Google Scholar]
- 5.Josephson CD, Su LL, Christensen RD, Hillyer CD, Castillejo MI, Emory MR, Lin Y, Hume H, Easley K, Poterjoy B, Sola-Visner M. Platelet transfusion practices among neonatologists in the United States and Canada: results of a survey. Pediatrics. 2009;123:278–85. doi: 10.1542/peds.2007-2850. [DOI] [PubMed] [Google Scholar]
- 6.Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, Phibbs R, Soll RF. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics. 2002;110:143–51. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 7.Andrew M, Vegh P, Caco C, Kirpalani H, Jefferies A, Ohlsson A, Watts J, Saigal S, Milner R, Wang E. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr. 1993;123:285–91. doi: 10.1016/s0022-3476(05)81705-6. [DOI] [PubMed] [Google Scholar]
- 8.Corby DG, O'Barr TP. Decreased alpha-adrenergic receptors in newborn platelets: cause of abnormal response to epinephrine. Dev Pharmacol Ther. 1981;2:215–25. [PubMed] [Google Scholar]
- 9.Israels SJ, Daniels M, McMillan EM. Deficient collagen-induced activation in the newborn platelet. Pediatr Res. 1990;27:337–43. doi: 10.1203/00006450-199004000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Rajasekhar D, Kestin AS, Bednarek FJ, Ellis PA, Barnard MR, Michelson AD. Neonatal platelets are less reactive than adult platelets to physiological agonists in whole blood. Thromb Haemost. 1994;72:957–63. [PubMed] [Google Scholar]
- 11.Andrew M, Paes B, Bowker J, Vegh P. Evaluation of an automated bleeding time device in the newborn. Am J Hematol. 1990;35:275–7. doi: 10.1002/ajh.2830350411. [DOI] [PubMed] [Google Scholar]
- 12.Israels SJ, Cheang T, McMillan-Ward EM, Cheang M. Evaluation of primary hemostasis in neonates with a new in vitro platelet function analyzer. J Pediatr. 2001;138:116–9. doi: 10.1067/mpd.2001.109794. [DOI] [PubMed] [Google Scholar]
- 13.Kettner SC, Pollak A, Zimpfer M, Seybold T, Prusa AR, Herkner K, Kuhle S. Heparinase-modified thrombelastography in term and preterm neonates. Anesth Analg. 2004;98:1650–2. doi: 10.1213/01.ANE.0000115149.25496.DD. [DOI] [PubMed] [Google Scholar]
- 14.Miller BE, Bailey JM, Mancuso TJ, Weinstein MS, Holbrook GW, Silvey EM, Tosone SR, Levy JH. Functional maturity of the coagulation system in children: an evaluation using thrombelastography. Anesth Analg. 1997;84:745–8. doi: 10.1097/00000539-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Saxonhouse MA, Sola MC. Platelet function in term and preterm neonates. Clin Perinatol. 2004;31:15–28. doi: 10.1016/j.clp.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Schmugge M, Dunn MS, Amankwah KS, Blanchette VS, Freedman J, Rand ML. The activity of the von Willebrand factor cleaving protease ADAMTS-13 in newborn infants. J Thromb Haemost. 2004;2:228–33. doi: 10.1046/j.1538-7933.2003.00575.x. [DOI] [PubMed] [Google Scholar]
- 17.Seyfert UT, Haubelt H, Vogt A, Hellstern P. Variables influencing Multiplate(TM) whole blood impedance platelet aggregometry and turbidimetric platelet aggregation in healthy individuals. Platelets. 2007;18:199–206. doi: 10.1080/09537100600944277. [DOI] [PubMed] [Google Scholar]
- 18.Podda GM, Bucciarelli P, Lussana F, Lecchi A, Cattaneo M. Usefulness of PFA-100 testing in the diagnostic screening of patients with suspected abnormalities of hemostasis: comparison with the bleeding time. J Thromb Haemost. 2007;5:2393–8. doi: 10.1111/j.1538-7836.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 19.Monagle P, Andrew M. Developmental Hemostasis: Relevance to Newborns and Infants. In: Nathan DO, Ginsburg D, editors. Hematology of Infancy and Chilhood. Philadelphia: WB Saunders Co; 2003. pp. 121–68. [Google Scholar]
- 20.Sestito A, Sgueglia GA, Spinelli A, Navarese EP, Infusino F, Crea F, Lanza GA. Increased platelet reactivity in unstable angina patients is not related to C-reactive protein levels. Platelets. 2006;17:336–9. doi: 10.1080/09537100600759329. [DOI] [PubMed] [Google Scholar]
- 21.Linden MD, Furman MI, Frelinger AL, 3rd, Fox ML, Barnard MR, Li Y, Przyklenk K, Michelson AD. Indices of platelet activation and the stability of coronary artery disease. J Thromb Haemost. 2007;5:761–5. doi: 10.1111/j.1538-7836.2007.02462.x. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs I, Frossard M, Spiel A, Riedmuller E, Laggner AN, Jilma B. Platelet function in patients with acute coronary syndrome (ACS) predicts recurrent ACS. J Thromb Haemost. 2006;4:2547–52. doi: 10.1111/j.1538-7836.2006.02239.x. [DOI] [PubMed] [Google Scholar]
- 23.Sharma SK, Philip J, Wiley J. Thromboelastographic changes in healthy parturients and postpartum women. Anesth Analg. 1997;85:94–8. doi: 10.1097/00000539-199707000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Francis JL, Francis DA, Gunathilagan GJ. Assessment of hypercoagulability in patients with cancer using the Sonoclot Analyzer and thromboelastography. Thromb Res. 1994;74:335–46. doi: 10.1016/0049-3848(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 25.Dai Y, Lee A, Critchley LA, White PF. Does thromboelastography predict postoperative thromboembolic events? A systematic review of the literature. Anesth Analg. 2009;108:734–42. doi: 10.1213/ane.0b013e31818f8907. [DOI] [PubMed] [Google Scholar]
- 26.Edwards RM, Naik-Mathuria BJ, Gay AN, Olutoye OO, Teruya J. Parameters of thromboelastography in healthy newborns. Am J Clin Pathol. 2008;130:99–102. doi: 10.1309/LABNMY41RUD099J2. [DOI] [PubMed] [Google Scholar]
- 27.Kashuk JL, Moore EE, Sabel A, Barnett C, Haenel J, Le T, Pezold M, Lawrence J, Biffl WL, Cothren CC, Johnson JL. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146:764–72. doi: 10.1016/j.surg.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 28.Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Powers P. Development of the human coagulation system in the full-term infant. Blood. 1987;70:165–72. [PubMed] [Google Scholar]
- 29.Cvirn G, Gallistl S, Rehak T, Jurgens G, Muntean W. Elevated thrombin-forming capacity of tissue factor-activated cord compared with adult plasma. J Thromb Haemost. 2003;1:1785–90. doi: 10.1046/j.1538-7836.2003.00320.x. [DOI] [PubMed] [Google Scholar]
- 30.Cvirn G, Gallistl S, Leschnik B, Muntean W. Low tissue factor pathway inhibitor (TFPI) together with low antithrombin allows sufficient thrombin generation in neonates. J Thromb Haemost. 2003;1:263–8. doi: 10.1046/j.1538-7836.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 31.Andrew M. Developmental hemostasis: relevance to hemostatic problems during childhood. Semin Thromb Hemost. 1995;21:341–56. doi: 10.1055/s-2007-1000655. [DOI] [PubMed] [Google Scholar]
- 32.Edstrom CS, Christensen RD. Evaluation and treatment of thrombosis in the neonatal intensive care unit. Clin Perinatol. 2000;27:623–41. doi: 10.1016/s0095-5108(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 33.Puetz J, Darling G, Brabec P, Blatny J, Mathew P. Thrombotic events in neonates receiving recombinant factor VIIa or fresh frozen plasma. Pediatr Blood Cancer. 2009;53:1074–8. doi: 10.1002/pbc.22160. [DOI] [PubMed] [Google Scholar]
- 34.Saxonhouse MA, Manco-Johnson MJ. The evaluation and management of neonatal coagulation disorders. Semin Perinatol. 2009;33:52–65. doi: 10.1053/j.semperi.2008.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.