Abstract
The pace of research on the potential therapeutic uses of liver stem cells or “oval cells” has accelerated significantly in recent years. Concurrent advancements in techniques for the isolation and characterization of these cells have helped fuel this research. Several models now exist for the induction of oval cell proliferation in rodents. Protocols for the isolation and culture of these cells have evolved to the point that they may be set up in any laboratory equipped for cell culture. The advent of magnetic cell sorting has eliminated reliance on expensive flow cytometric sorting equipment to generate highly enriched populations of oval cells. Our laboratory has had much success in using the oval cell surface marker Thy-1 in combination with magnetic sorting to produce material suitable for testing the influence of a myriad of chemical signaling molecules on the oval cell phenotype. This chapter will describe our basic strategy for oval cell induction and isolation. Additionally, two in vitro procedures are described which the reader may find useful in the early stages of developing an oval cell research project.
Keywords: Liver stem cells, oval cells, 2-acetylaminofluorene (2AAF), partial hepatectomy (PH), collagenase perfusion, simple gravity enrichment, immunomagnetic sorting (IMS), flow cytometric sorting (FACS), proliferation assay, migration assay
1. Introduction
The past decade has shown great strides in our understanding of the potential therapeutic use of adult, tissue-committed stem cells. The extensively characterized rat hepatic stem/progenitor cell, or oval cell, has been demonstrated by our laboratory, as well as several others, to show particular promise as a tool for the treatment of genetic liver disorders and as a bridging therapy or alternative to liver transplant (1–4).
The term “oval cells” (OC) is currently used to define the population of small proliferating cells with oval-shaped nuclei which arise within the liver following certain types of liver injury (1–7). OC proliferation is activated when the proliferative capacity of hepatocytes is impaired, as well as in certain models of carcinogenesis. In OC-mediated liver regeneration, OC first appear in the portal tract periphery, and eventually migrate deep into the lobular parenchyma. Due to their bipotency and high clonogenicity, OC have been considered putative liver stem cells. Ongoing research is beginning to elucidate the signaling molecules involved in controlling the activation, proliferation. and differentiation of this unique cell population (5–7).
2. Materials
2AAF (2-acetylaminofluorene). 70 mg 2AAF pellets, 28 day time release (Innovative Research of America; Sarasota, FL).
Isoflurane (Webster Veterinary; Sterling, MA).
Iodine solution.
70% Ethanol (EtOH).
2–0 silk suture (Ethicon Inc.; Somerville, NJ).
S&M solution (solution A): 500 mg KCl, 8.3 g NaCl, 2.4 g HEPES, 190 mg NaOH. Bring to 1 L with dH2O. Adjust pH to 7.4 (see Note 6).
CaCl2 solution (solution B): 636 mg CaCl2·2H2O. Bring to 1 L with S&M solution (see Note 1).
Collagenase H (Roche; Germany), at a working concentration of 320 μg/ml (see Note 2).
20-gauge catheter, 1–1/4″ (Medex; Carlsbad, CA).
Nylon mesh (100–150 μm pore diameter).
MACS® cell separator, columns, and anti-FITC microbeads (Miltenyi Biotec Inc., Auburn, CA).
FITC-conjugated Thy-1/mouse CD90.1 (Thy-1.1) monoclonal antibody. (BD Biosciences, Pharmingen; San Diego, CA).
FITC-mouse IgG1 isotype control (Vector, Burlingame, CA).
PBS buffer (pH 7.4).
IMDM supplemented with 10% FBS, 1% insulin, and antibiotics (GIBCO; Grand Island, NY).
Bovine serum albumin, BSA, 0.5% (Roche; Germany).
Transwell culture dishes with 5 μm pore filters, 6.5 mm diameter, 24-well cell clusters (Coring Inc.; NY, USA).
3. Methods
3.1. Oval Cell Activation: 2AAF/PH Protocol
While several methods are available for the activation of oval cells, they all involve the same basic principal: inhibition of the proliferative potential of mature hepatocytes followed by liver injury (1–7). Chemical inhibition of hepatocyte proliferation is made possible by the unique expression of mixed function oxidases (P450 s) within the parenchymal cells of the liver. Popular OC induction models in the rat include choline-deficient diet followed by ethionine exposure, galactosamine, 2-acetylaminofluorene (2AAF)/CCl4, 2AAF/partial hepatectomy (PH), and allyl alcohol. In our experience, the 2AAF/PH protocol has generated the most reproducible OC response in rats and has become the gold standard method within this field (8, 9).
3.1.1. 2AAF Administration
The 2AAF/PH protocol is a well-established model of OC activation in rats (8, 9). 2AAF is metabolized by hepatocytes to an N-hydroxyl derivative, which interferes with the cyclin D1 pathway. Biliary epithelial cells and oval cells lack the ability to convert the 2AAF to its toxic metabolite. Therefore, the administration of 2AAF prior to PH inhibits hepatocyte proliferation and force oval cell recruitment to mediate liver regeneration. This procedure results in a robust oval cell response following PH (peaking between day 9 and 11 post-surgery) and within 14 days, OC start to differentiate into hepatocytes (10).
Rats must be continuously exposed to 2AAF for 7 days prior to liver injury. Two strategies for treatment with 2AAF have been successfully employed by our laboratory. The first is administration by gastric gavage. 2AAF is dissolved in corn oil and administered daily by gavage at a dosage of 10 mg/kg for 14 days, with PH being performed after 1 week (11) (see Note 3). Our laboratory currently commissions the production of time release 2AAF pellets (Innovative Research of America; Sarasota, FL). These pellets can be custom made for different release rates and durations. In our experience, 70 mg 2AAF, 28-day pellets result in almost complete inhibition of hepatocyte proliferation and little toxicity in F344 rats implanted subsequent to 10 weeks of age (see Note 4). We insert these pellets intraperitoneally through a small (~5 mm) incision in the right lower quadrant of the abdomen.
3.1.2. Partial Hepatectomy
Following 7 days exposure to 2AAF, a partial hepatectomy is performed. The surgical technique we employ is a modification, optimized by Piscaglia, of the method previously described by Higgins and Anderson (12).
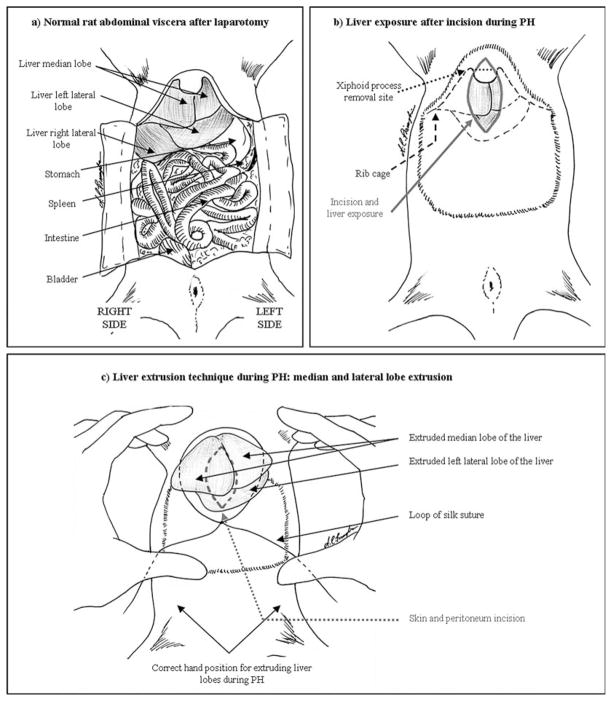
The rat is maintained under anesthesia with isoflurane, and standard aseptic technique should be used. After shaving the abdomen, disinfect with iodine solution, followed by 70% EtOH. A 2.5 –3.0 cm median-line longitudinal incision is made through the skin, above the xiphoid process of the sternum. The incision is continued through the abdominal muscles (<1 cm), just to the right side of the linea alba (see Note 5). The upper edge of the incision should approach the diaphragm and extreme care must be used so as to not puncture the thoracic cavity, because a pneumothorax is rapidly fatal to the animal (seeFig. 24.1a for a diagram of the rat abdominal viscera). At this point the xiphoid process may be removed with dissecting scissors. The laparotomy may be enlarged (up to 1.5–2.0 cm), until the median lobe and the left lateral lobe are clearly visible (Fig. 24.1b) (see Note 6). A loop of 2–0 silk suture is placed loosely over the incision. The medial and left lobes of the liver are gently extruded through the incision, using the index fingers of both hands to brace the lower rib cage while using the thumbs to push in and up below the liver. The right central and left central portions of the medial lobe will come out first, followed by the left lateral lobe (Fig. 24.1c). This will result in 65–75% of the entire liver mass protruding through the suture loop. The suture is carefully constricted around the base of the exposed liver lobes and tightened. Care must be given not to capture skin or muscle while tightening the loop. The exposed lobes may now be cut off distal to the ligature. The remaining liver stump should be examined for active bleeding before suturing the muscle and skin. No special post-operative care is needed, complications are extremely rare, and post-operative mortality is uncommon (<3%).
Fig. 24.1.
Partial hepatectomy: (a) Normal anatomy of the rat abdominal viscera. (b) Following incision, three lobes should be clearly visible. (c) The median and left lateral lobes of the liver should be externalized, through the loop of silk suture, by pressure from the thumbs. The index fingers brace the lower portion of the rib cage.
3.2. Two-Step Collagenase Perfusion
The two-step collagenase perfusion is a common technique, employed to obtain viable cells from a solid organ. This procedure uses two liver perfusates. The first consists of an isotonic buffer to flush blood cells from the vasculature. The second perfusate contains collagenase which digests the extracellular matrix, allowing for the collection of a single cell suspension suitable for isolation of discrete cell populations (13).
Regarding OC isolation from liver perfusion following 2AAF/PH in rats, the peak of the OC response will occur between days 9 and 11 following partial hepatectomy (8–10). It is during this time frame that the following procedures will yield the greatest number of OC (see Note 7).
For each rat to be perfused, aliquot 250 ml of solutions A and B. Each of these should be pre-warmed to 40°C in a circulating water bath. Ideally, the solution should cool to 37°C as it is pumped from the water bath to the animal. The containers should be weighted, so they do not float and tip over during the perfusion. An amount of collagenase appropriate for 250 ml should be aliquoted and stored on ice.
Set up a peristaltic pump directly next to the water bath. A section of appropriate diameter tubing should be used to transfer solutions from the water bath, through the pump, to the animal. Use a sufficient length to allow free manipulation of the tubing during catheterization, but be aware that the solution should not cool below 37°C before it enters the animal.
Anesthetize the rat for a terminal procedure (see Note 8).
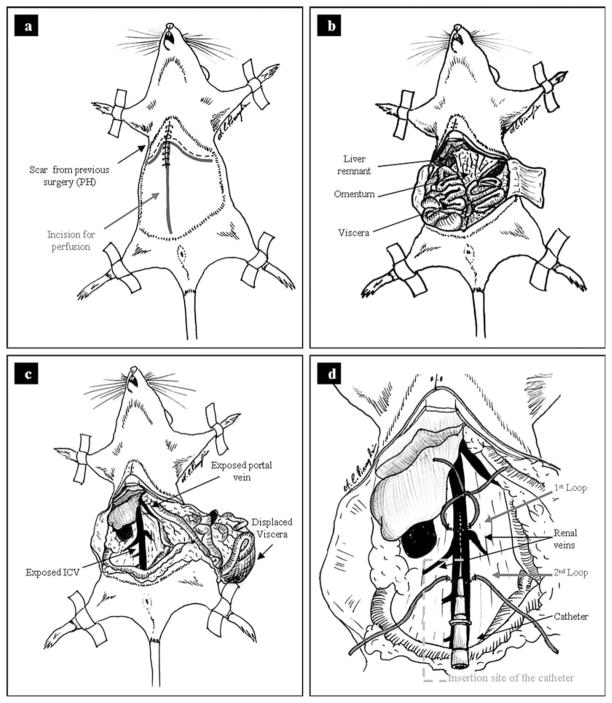
Secure the rat to a dissection board by taping or pinning the extremities. The abdomen may be shaved prior to disinfecting if sterility of the product is of concern. Rinse the abdomen and lower thorax with 70% EtOH. Cut a large mid-line incision through the skin and peritoneum. Make a subsequent cut perpendicular to the laparotomy incision on both sides below the subcostal margin (Fig. 24.2a). Retract the wound edges and expose the abdominal viscera (Fig. 24.2b).
Displace the intestines toward the lower left quadrant and expose the IVC. Use a gauze pad in each hand to tear the fascia covering the IVC. Identify the renal veins which branch from the IVC. Also, identify the portal vein which will be the large vessel entering the liver within the lesser omentum (Fig. 24.2c).
Run a 2–0 silk suture under the IVC immediately above to the renal vein branches. Tie but do not tighten the suture leaving a 1 cm diameter loop above the IVC. Run a second suture under the IVC distal to the renal branches. Do not tie this suture (Fig. 24.2d).
Turn on the pump and recirculate solution A through the tubing back into the flask containing the S&M solution. Gently tap the tubing to dislodge as many air bubbles as possible. Once the tubing is completely full of solution with no air bubbles, turn off the pump.
Cannulate the IVC using a 20-gauge catheter, between the two sutures that were put in place in step 6. Insert the catheter very carefully, keeping it just a few degrees from parallel to the IVC (see Note 9). Gently slide the catheter into the IVC until the end is past the most proximal loop of suture (Fig. 2d). Blood should begin to ooze back into the catheter. If this does not happen, use a small syringe to apply negative pressure to the catheter.
Without moving the catheter, carefully tighten the suture loop around the IVC and catheter. Tie a second knot on top of the first to secure. Bring the ends of the second suture over the top of the portion of catheter which is external to the IVC and secure with a double knot. At this point the catheter should be locked securely within the IVC with no blood flow through the IVC due to constriction of the vessel by the sutures. (It is helpful to have a second set of hands while performing this step.)
Attach the outflow end of the tubing to the catheter and turn on the pump with a flow rate of approximately 15 ml/min. Attach the tube to the catheter and secure the tube with tape so that the catheter is not pulled out of position. Blanching of the liver indicates successful perfusion. Immediately cut the portal vein between the liver and the stomach with dissecting scissors. A large volume of blood followed by solution A will flow from the severed vein.
Open the chest cavity, cutting through the diaphragm, above the liver. Insert a curved hemostat clamp through this hole and clamp off the superior vena cava.
The liver may have patches that do not perfuse well. Gently massage the liver lobes with a cotton swab, to assist in moving the blood from the sinusoids, until the effluent is clear. At this point, add the collagenase to solution B, mix well and keep the solution with collagenase at 40°C. Allow solution A to run until no blood is visible within the liver and the effluent is clear (up to 15 min, see Note 10).
Once the liver is completely blanched, turn off the pump and move the in-flow end of the tubing to the solution B with collagenase. Turn the pump on and confirm liver perfusion as described in step 12. Decrease the flow rate to about 12 ml/min and let it run for 10–15 min (the duration of this step is largely dependent on the activity of the collagenase used, see Note 11).
When adequate digestion of the liver has been achieved, shut off the pump and disconnect the tubing from the catheter. The liver must now be carefully dissected away from tissues which connect to it. Be aware that the liver is extremely fragile following digestion.
Transfer the liver into a beaker containing about 50 ml of ice-cold solution A. Keep the top of the beaker covered at all times to limit contamination.
From this point onwards, every step must be performed under a laminar flow hood if cells are to be cultured. Grasp the tissue with sterile pliers and shake/agitate so that it breaks apart, dispersing cells in the buffer, which will turn brown (the darker the color, the better the perfusion).
Carefully pass the cell suspension through a nylon mesh (100–150 μm pore diameter) placed over the top of a 250 ml beaker. Wash the mesh with an adequate volume of S&M buffer to collect all of the cells. Aliquot the supernatant into 50 ml conical tubes.
Proceed to simple gravity enrichment, to separate hepatocytes from non-parenchymal cells.
Fig. 24.2.
Two step collagenase perfusion: (a) Mid-line incision from just below the diaphragm to the groin. (b) Make cuts perpendicular to the main incision to expose viscera. (c) Identify the IVC and portal vein. (d) Diagram showing the correct placement of the silk sutures and catheter.
3.3. Simple Gravity Enrichment
A crude separation of hepatocytes and non-parenchymal cells (NPC) may be accomplished by centrifugation alone. This procedure will result in a very pure NPC population. Analysis of this population by immunostaining for P450 (CYP3A2) demonstrates less than 2% of the fractionated NPC express this hepatocyte marker. Oval cells will partition to the NPC fraction. Purity of the parenchymal cell fraction will vary depending on the number of washing steps, though it is difficult to achieve greater than 75% enrichment by this method.
Centrifuge the cell suspension at 55 g for 5 min at 4°C. Transfer the supernatant (enriched for NPC) to another container and save it on ice. Resuspend the pellet (enriched for parenchymal cells) in an additional 50 ml ice-cold S&M buffer and repeat centrifugation (see Note 12). Discard the final pellet, unless you need to collect the hepatocyte fraction.
Centrifuge the supernatants at 220 g for 5 min at 4°C. Collect the final pellets, which represent the enriched non-parenchymal cell fraction. Resuspend and pool the NPC, using an adequate volume of filtered phosphate-buffered saline (PBS; pH 7.4).
Wash cells twice in PBS (220 g for 5 min at 4°C).
Check cell viability by trypan blue exclusion and proceed to OC isolation.
3.4. Oval Cell Isolation: Immunomagnetic and Flow Cytometric Cell Sorting
Several methods have been employed for the isolation of OC. These techniques range in complexity and include Nicodenze gradient separation, and sorting by flow cytometric (FACS) or immunomagnetic sorting (IMS). FACS had been the gold standard for isolating OC prior to the introduction of IMS (13). IMS has become a widely used method for separating different cell types. It has numerous advantages compared to other methods. It is a convenient, easy to use system which can yield enrichment of up to 99% depending on the specificity of the cell surface marker used. Stem cell biology has benefited significantly from the introduction of magnetic cell sorting (14, 15).
3.4.1. Thy-1+ Cell Sorting
Thy-1 (CD90) is a GPI-anchored membrane glycoprotein of the Ig superfamily which is involved in signal transduction (16–18), and it is expressed by a wide spectrum of hematopoietic stem and progenitor cells (16–19), as well as non-hematopoietic cells, including neurons (16), edothelium at inflammatory sites (20), and hepatic OC (21, 22). High purity OC enrichment is achieved through IMS using the OC surface marker Thy-1. We demonstrated that, using Thy-1 in conjunction with FACS sorting, a 95–97% enriched population of OC may be obtained (21).
Prior to sorting, the NPC population must be isolated by the simple gravity enrichment method described above. Because the collagenase perfusion protocol eliminates virtually all blood cells from the liver, the Thy 1+ cell population is comprised almost entirely of oval cells. Thy-1+ cells from rat liver can be isolated using indirect labeling strategies. We currently employ fluorescein isothiocyanate (FITC)-conjugated mouse anti-rat CD90 (Thy-1.1) to positively select OC.
3.4.2. Immuno magnetic Sorting
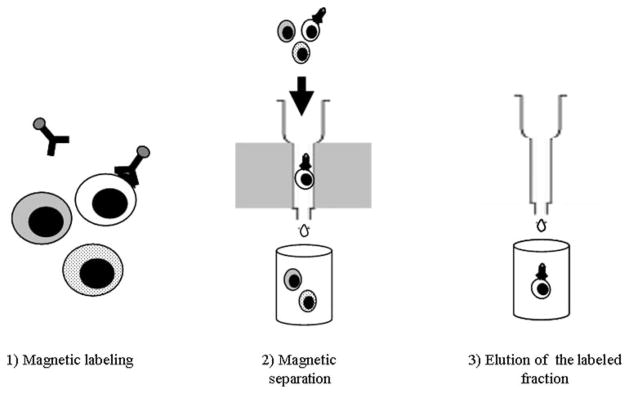
IMS technology is based on the use of microbeads, columns, and a magnetic field. The microbeads are super-paramagnetic particles (approximately 50 nm in size, biodegradable, and non-toxic to cells), which are coupled, directly or indirectly, to specific monoclonal antibodies. These beads magnetically label the target cell population. The patented column technology (MACS®, Miltenyi Biotec Inc., Auburn, CA) is specifically designed to generate the high-strength magnetic field that is required to retain the labeled cells, while maintaining optimal cell viability and function. By placing the column in a permanent magnet (called the “separator”), the magnetic force is sufficient to retain the target cells. By simply rinsing the column with buffer, all the unlabeled cells are washed away. Once the column is detached from the magnet, the labeled fraction can be eluted (Fig. 24.3). See Note 13.
Fig. 24.3.
Immunomagnetic sorting.
Separate the NPC fraction through simple gravity enrichment and wash it three times, as described above.
Resuspend the pellet in 10 ml of ice-cold filtered PBS and transfer the cell suspension to a 15 ml tube.
Add 100 μl FITC-conjugated mouse anti-rat CD90 and incubate for 1 h at 4°C. Cover the tube with sterile aluminum foil, and leave on ice in a shaker (gentle agitation, ~1 oscillation/sec).
Centrifuge the cell suspension at 220 g for 5 min at 4°C, discard the supernatant and resuspend the pellet in PBS. Repeat the washing step.
Resuspend the pellet in 900 μl of PBS and add anti-rat FITC microbeads (100 μl), mix well and incubate for 30 min at 4°C. Cover the tube with sterile aluminum foil, and leave on ice in a shaker as described above.
Add PBS (up to 15 ml) and centrifuge at 220 g for 5 min at 4°C. Remove the supernatant, and resuspend the pellet in 3 ml of PBS. At this point, a small sample may be removed for flow cytometric analysis.
Place the column (combined with the appropriate column adapter) in the magnetic field of the MACS Separator. Place an empty tube below the column. Equilibrate the column by washing with 1 ml of ice-cold PBS.
When the buffer has passed through, apply the cell suspension to the top of the column. Let the unlabeled cells pass through. Rinse twice with 3 ml of PBS.
Remove the column from the separator, and place it over a suitable collection tube. Pipette 1 ml of PBS onto the column and flush out magnetically labeled fraction using the plunger supplied with the column. Repeat two more times, using 1 ml PBS each time (final volume 3 ml). Push hard and fast, in order to get complete cell elution.
To achieve higher purity, apply the magnetically labeled fraction onto a new, freshly prepared column. Let the unlabeled cells pass through. Rinse with 2 × 3 ml of PBS. Elute the magnetically labeled fraction as described above.
Check cell viability by trypan blue exclusion, and count the cells on a hemocytometer. From one rat, 5 × 107 Thy-1+ cells with 90–95% viability can be achieved on a regular basis.
3.4.3. Flow Cytometric Sorting
Separate the NPC fraction through simple gravity enrichment and wash it three times, as described above.
Resuspend the pellet and incubate cell suspension with FITC-conjugated mouse anti-rat CD90 (1 μl/106 cells), for 20 min at 4°C. Cover the tube with sterile aluminum foil, and leave on ice in a shaker (gentle agitation, ~1 oscillation/sec).
Wash the cells twice in PBS supplemented with 1% fetal bovine serum. Store at 4°C in the dark until sorting.
Sort cells into Thy-1.1+ and Thy-1.1− fractions, using the FACS flow cytometer.
Check cell viability by trypan blue exclusion, and count the cells on a hemocytometer. From one rat, 5 × 107 Thy-1+ cells with 90–95% viability can be achieved on a regular basis.
3.5. Oval Cell Characterization: In Vitro Assays
Several laboratory techniques may be used to characterize OC. Cytospins from OC suspensions can be employed for immunohistochemistry or immunofluorescence (Fig. 24.4). RNA, DNA, or protein can be extracted and processed for molecular biology. Additionally, OC may be cultured for in vitro assays. Several media have been proposed to culture OC, with varying degrees of success. It is very difficult to maintain and expand OC in culture without spontaneous differentiation. We have been able to culture OC for up to 5 months without signs of senescence or spontaneous differentiation (Piscaglia et al., unpublished data) using a relatively minimal medium (see Note 14).
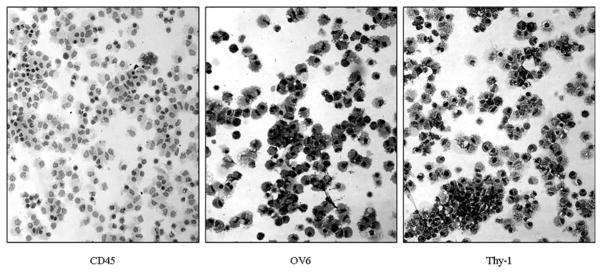
Fig. 24.4.
Oval cell cytospin: immunomagnetic sorting of Thy-1+ oval cells yields a cell population, which is almost entirely Thy-1+/OV-6+ (both are oval cell markers) while almost completely devoid of CD45+ leucocytes.
In vitro assays are very useful in analyzing the properties of OC and their response to exogenous factors. In particular, two properties are critical to the OC phenotype: proliferation following certain types of liver injury, and migration from the periportal space to the liver parenchyma (1–7). Trafficking, mobilization, and homing of OC are multifactorial processes that are regulated by several factors, including adhesion molecules, cytokines, and chemotactic molecules (6). Therefore, proliferation and migration assays may be useful in clarifying the molecular mechanisms underlying OC activation. In our hands, in vitro assays on OC isolated through the previously described techniques have contributed to the identification of several factors, such as SDF-1, SST, and G-CSF, which are involved in OC activation (23–25).
3.5.1. Proliferation Assay
Proliferation assays may be used to assess the effects of a specific factor on oval cell proliferation. Briefly, the factor is added at different dosages to the basic OC medium (IMDM). Growth kinetics of the OC are evaluated at different time points (usually at 1, 3, 5, and 7 days). A positive control (FBS 10%) and a negative control (bovine serum albumin, BSA, 0.5%) are required. Every determination should be performed at least in triplicate.
Seed cells in six-well plates (105 cells/well) in IMDM supplemented with 10% FBS, 1% insulin, and antibiotics, and incubate at 37°C, 5% CO2 overnight.
The following day, prepare proliferation buffers for each experimental group (see Note 15). Count the attached cells in three wells to establish initial cell number.
Begin the proliferation assay by replacing medium with the various experimental buffers.
Count cells at the pre-determined time points (see Note 16).
3.5.2. Migration Assay
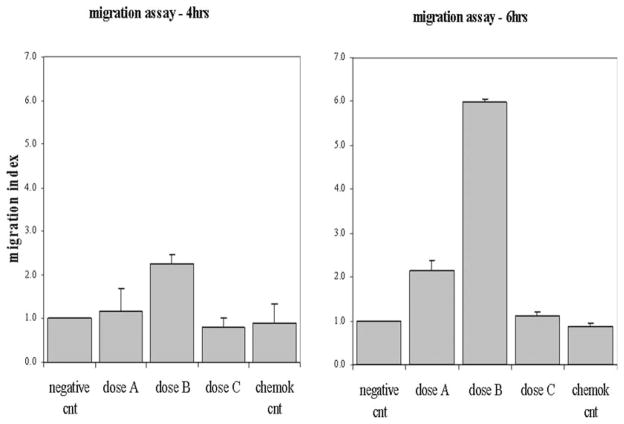
Migration assays may be used to assess the effects of a specific factor on oval cell motility. Briefly, OC are seeded on the microporous membrane of a transwell (Fig. 24.5). The factor is added at different dosages to the basic OC medium (see Note 17) in the lower chamber. This will result in the migration of OC through the membrane, following the chemoattractive factor gradient. OC motility can be assessed at different time points (see Note 18). A negative control and a chemokinetic control are required (see Note 19). Fig. 24.6 shows an example of migration assay.
Prepare a 24-well plate by pre-coating wells with 0.006% RTC (rat tail collagen in 0.1% acetic acid) for 3 h at 37°C, 5% CO2 (store the plates in a clean bag at RT for up to several hours until used). Add 0.6 ml of migration buffer (IMDM + insulin 1% + FBS 10% + antibiotics)/well. Insert a transwell into each well. Incubate for a few minutes at 37°C, 5% CO2.
Seed cell suspension on the top of each transwell (105 cells in 100 μl of migration buffer).
Allow the cells to attach overnight at 37°C, 5% CO2.
Before beginning the test, prepare the various migration buffers by adding the proper dose of the experimental factor. Add each migration buffer to a new cluster plate.
Carefully remove non-adherent cells prior to the assay with a 100 μl pipette. Begin the migration assay by transferring the transwell chambers to the cluster plate containing the various buffers.
Incubate at 37°C, 5% CO2 for either 4 or 6 h (time 1 and 2, respectively).
At the end of the test, fix and stain the cells as described by Stolz et al. (26) (see Note 20). Remove the collagen and stationary cells from the top of the transwell filter by rubbing with a cotton-tipped applicator. Transfer the transswells to a new, clean 24-well plate, and dry overnight.
Enumerate the cells that have migrated to the lower chamber, by counting each transwell chamber at ×10 magnification.
3.6. Applications of Isolated Liver Stem Cells
During the last 20 years, the development of new biological technologies has set the stage for a plethora of novel therapies. In particular, the field of stem cell biology has demonstrated enormous potential in the prevention and treatment of several human diseases (27, 28). Stem cells play a fundamental role in the maintenance of tissue homeostasis. Improving our knowledge of stem cell biology is the conditio sine qua non to regenerative medicine. Advantages of the use of adult stem cells are many, and include the availability of cells and the lack of any ethical concerns (29).
Acute or chronic liver diseases may benefit greatly from stem cell therapies (30). Inborn errors of metabolism, such as Crigler-Najjar syndrome, LDL-receptor defect, hereditary hemochromatosis, Wilson’s disease, etc., may, someday, be treated with genetically modified, Thy-1-sorted cells (31). Finally, identifying the complex mechanisms involved in maintenance of the hepatic stem cell phenotype may lead to the development of novel therapies for the treatment of liver cancers (32, 33).
Continued improvement of techniques for the identification, isolation, and in vitro expansion of the hepatic oval cells will contribute to the development of stem cell-based approaches for treating genetic liver disorders, preventing hepatic cancers, and providing bridging therapies or alternatives to liver transplant (1–4).
Fig. 24.5.
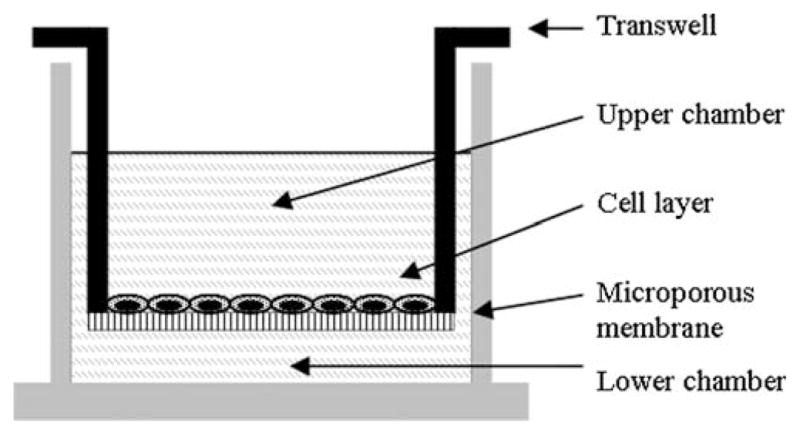
Schematic representation of transwell in chamber for migration assay.
Fig. 24.6.
Example of migration assay, where three different doses of a specific factor are tested.
Footnotes
If cells are to be used for culture or transplantation, the solutions S&M (solution A) and CaCl2 (solution B) should be filtered through a 0.2 μm filter, under a laminar flow hood. Antibiotics must be added to each buffer.
Many collagenase preparations are commercially available. The activity of collagenase varies among different preparations, and it is important to determine an appropriate concentration for the particular collagenase which will be used. Too little activity will result in incomplete disassociation of cells within the liver, whereas too much activity will quickly compromise the integrity of the vasculature resulting in nonuniform perfusion. Also, high collagenase activity can be toxic, decreasing the viability of harvested cells. We have had good success with collagenase H from Roche, Germany, at a working concentration of about 320 μg/ml. The following protocol has been optimized for this particular collagenase preparation.
Gastric gavage can be fraught with complications and is time consuming. Most often, a person with limited experience in gavage will occasionally insert the gavage needle into the trachea, resulting in almost certain death of the animal. Time released pellets are expensive, but well worth the price.
Younger animals seem to be more susceptible to the toxicity of 2AAF (most notably necrosis within the liver and intestines). A lower dosage may be required for younger animals.
Compared to the classical mid-line incision (12), our technique is more effective in exposing the liver, but it also increases the risk of cutting the right epigastric vessels. Therefore, attention must be paid in identifying and sparing these vascular structures.
Avoid making the incision longer than necessary, as this can make it difficult to extrude the liver without also externalizing portions of intestine.
With slight modification, this protocol may be adapted to mice. A smaller catheter must be used for cannulation. Also, we suggest using collagenase A (Boehringer Mannheim) at a working concentration of 1 mg/ml.
It is critical that the heart does not stop beating before the inferior vena cava (IVC) is cannulated. The resulting drop in blood pressure will make insertion of the catheter very difficult.
The best way to insert the catheter is to carefully “catch” the surface of the vessel on the bevel of the catheter needle, and lift it up slightly (less than 1 mm) before pushing it in all the way. This will eliminate the possibility of puncturing the back wall of the vessel.
Closely monitor the surface of the liver during perfusion. A moist shiny appearance indicates continued perfusion of the organ. If the surface of the liver begins to dry, this may indicate that the perfusion has been interrupted. Also, a gentle heaving of the liver in synchrony with the rollers of the peristaltic pump should be apparent. It is often difficult to determine whether or not the clear perfusate is flowing from the severed portal vein. Use a cotton swab to soak up some blood and squeeze it out above the portal vein. The blood should allow you to visualize flow from the severed vein. If perfusion is interrupted, gently reposition the catheter until flow from the portal vein is re-established.
Pay attention to the texture of the liver surface, as this will be the main indicator of the degree of collagenase digestion. Towards the end of the perfusion, channels will begin to form under the hepatic capsule. Trabeculation and fragmentation indicate a breakdown of the extracellular matrix of the liver. These channels will progressively enlarge and connect up with each other, giving the organ a “brain-like” appearance when digestion is sufficient.
This step may be repeated several times to increase purity of the parenchymal cell fraction; we suggest at least three centrifugations before proceeding to the next step.
For cell culture, all procedures must be performed in a laminar flow hood under aseptic conditions, and all buffers and solutions must be filtered and supplemented with antibiotics.
Iscove’s modified Dulbecco’s medium (IMDM) supplemented with insulin 1%, fetal bovine serum (FBS) 10%, and antibiotics, at 37°C, 5% CO2.
Negative control: IMDM + 0.5% BSA; positive control: IMDM + 10% FBS, treatment groups: IMDM + BSA 0.5% + experimental factor at various doses.
Usually 1, 3, 5, and 7 days.
OC basic medium: IMDM + insulin 1% + FBS 10%.
Usually at 4 and 6 h.
Negative control: add the experimental factor to neither the lower chamber nor the upper chamber. Chemokinetic control: add the factor to both the chambers. Every determination should be performed at least in triplicate.
Briefly, remove the medium from the upper chamber of each transwell and transfer the transwells to a new 24-well plate containing 0.6 ml of PBS/well. Add 0.5 ml PBS to the upper chamber of each transwell to wash the membrane. Transfer the transwells into a new 24-well plate containing 0.6 ml of 4% paraformaldehyde (PFA) in PBS/well; add 0.5 ml of PFA to the upper chamber of each transwell. Incubate for 30 min at RT. Remove PFA from the chamber of each transwell by transferring the transwells to new 24-well plates containing 0.6 ml PBS/well with 0.1% Coomassie blue (CB), 10% MeOH, and 10% acetic acid. Add 0.5 ml of this same solution to the upper chamber of each transwell. Incubate for 1–2 h at RT. Wash with PBS at least twice by transferring the transwells into new 24-well plates, containing 0.6 ml of PBS/well.
References
- 1.Newsome PN, Hussain MA, Theise ND. Hepatic oval cells: helping redefine a paradigm in stem cell biology. Curr Top Dev Biol. 2004;61:1–28. doi: 10.1016/S0070-2153(04)61001-5. [DOI] [PubMed] [Google Scholar]
- 2.Oh SH, Hatch HM, Petersen BE. Hepatic oval ‘stem’ cell in liver regeneration. Semin Cell Dev Biol. 2002;13:405–409. doi: 10.1016/s1084952102001271. [DOI] [PubMed] [Google Scholar]
- 3.Petersen BE. Hepatic “stem” cells: coming full circle. Blood Cells Mol Dis. 2001;27:590–600. doi: 10.1006/bcmd.2001.0422. [DOI] [PubMed] [Google Scholar]
- 4.Piscaglia AC, Di Campli C, Gasbarrini G, Gasbarrini A. Stem cells: new tools in gastroenterology and hepatology. Dig Liver Dis. 2003;35:507–514. doi: 10.1016/s1590-8658(03)00226-3. [DOI] [PubMed] [Google Scholar]
- 5.Lowes KN, Croager EJ, Olynyk JK, Abraham LJ, Yeoh GC. Oval cell-mediated liver regeneration: Role of cytokines and growth factors. J Gastroenterol Hepatol. 2003;18:4–12. doi: 10.1046/j.1440-1746.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- 6.Libbrecht L, Desmet V, Van Damme B, Roskams T. Deep intralobular extension of human hepatic ‘progenitor cells ‘correlates with parenchymal inflammation in chronic viral hepatitis: can ‘progenitor cells’ migrate? J Pathol. 2000;192:373–378. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH700>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Mohle R, Bautz F, Denzlinger C, Kanz L. Transendothelial migration of hematopoietic progenitor cells. Role of chemotactic factors. Ann N Y Acad Sci. 2001;938:26–34. doi: 10.1111/j.1749-6632.2001.tb03571.x. discussion 34–35. [DOI] [PubMed] [Google Scholar]
- 8.Petersen BE, Zajac VF, Michalopoulos GK. Bile ductular damage induced by methylene dianiline inhibits oval cell activation. Am J Pathol. 1997;151:905–909. [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen BE, Zajac VF, Michalopoulos GK. Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology. 1998;27:1030–1038. doi: 10.1002/hep.510270419. [DOI] [PubMed] [Google Scholar]
- 10.Alison MR, Golding M, Sarraf CE, Edwards RJ, Lalani EN. Liver damage in the rat induces hepatocyte stem cells from biliary epithelial cells. Gastroenterology. 1996;110:1182–1190. doi: 10.1053/gast.1996.v110.pm8613008. [DOI] [PubMed] [Google Scholar]
- 11.Evarts RP, Hu Z, Omori N, Omori M, Marsden ER, Thorgeirsson SS. Precursor-product relationship between oval cells and hepatocytes: comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis. 1996;17:2143–2151. doi: 10.1093/carcin/17.10.2143. [DOI] [PubMed] [Google Scholar]
- 12.Higgins GM, Anderson RM. Experimental pathology of the liver. Arch Pathol. 1931;12:186–201. [Google Scholar]
- 13.Petersen BE, Hatch HM. Methods of Tissue Engineering. Academic Press; 2002. Stem Cell Culture: Liver Stem Cells; pp. 429–437. [Google Scholar]
- 14.Schmitz B, et al. Magnetic activated cell sorting (MACS)–a new immunomagnetic method for megakaryocytic cell isolation: comparison of different separation techniques. Eur J Haematol. 1994;52:267–275. doi: 10.1111/j.1600-0609.1994.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 15.Von Schönfeldt V, Krishnamurthy H, Foppiani L, Schlatt S. Magnetic Cell Sorting Is a Fast and Effective Method of Enriching Viable Spermatogonia from Djungarian Hamster, Mouse, and Marmoset Monkey Testes. Biology Reprod. 1999;61:582–589. doi: 10.1095/biolreprod61.3.582. [DOI] [PubMed] [Google Scholar]
- 16.Campbell DG, Gagnon J, Reid KBM, Williams AF. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981;195:15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermans MHA, Opstelten D. In situ visualization of hemotopoietic cell subsets and stromal elements in rat and mouse bone marrow by immunostaining of frozen sections. J Histochem Cytochem. 1991;39:1627–1634. doi: 10.1177/39.12.1940317. [DOI] [PubMed] [Google Scholar]
- 18.Garnett D, Barclay AN, Carmo AM, Beyers AD. The association of the protein tyrosine kinases p56lck and p60fyn with the glycosyl phosphatidylinositol anchored proteins Thy-1 and CD48 in rat thymocytes is dependent on the state of cellular activation. Eur J Immunol. 1993;23:2540–2544. doi: 10.1002/eji.1830231024. [DOI] [PubMed] [Google Scholar]
- 19.Crook K, Hunt SV. Enrichment of early fetal-liver hemopoietic stem cells of the rat using monoclonal antibodies against the transferrin receptor, Thy-1, and MRC-OX82. Dev Immunol. 1996;4:235–246. doi: 10.1155/1995/85036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishizu A, et al. Thy-1 induced on rat endothelium regulates vascular permeability at sites of inflammation. Int Immunol. 1995;7:1939–1947. doi: 10.1093/intimm/7.12.1939. [DOI] [PubMed] [Google Scholar]
- 21.Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- 22.Mason DW, Williams AM. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980;187:1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–51. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- 24.Jung Y, Oh SH, Zheng D, Shupe TD, Witek RP, Petersen BE. A potential role of somatostatin and its receptor SSTR4 in the migration of hepatic oval cells. Lab Invest. 2006;86:477–489. doi: 10.1038/labinvest.3700410. [DOI] [PubMed] [Google Scholar]
- 25.Piscaglia AC, Shupe TD, Oh S, Gasbasrini A, Petersen BE. Granulocyte-Colony Stimulating Factor Promotes Liver Repair and Induces Oval Cell Migration and Proliferation in Rats. Gastroenterology. 2007;133:619–631. doi: 10.1053/j.gastro.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stolz DB, Michalopoulos GK. Synergistic enhancement of EGF, but not HGF, stimulated hepatocyte motility by TGF-beta 1 in vitro. J Cell Physiol. 1997;170:57–68. doi: 10.1002/(SICI)1097-4652(199701)170:1<57::AID-JCP7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Weissman IL. Stem cells - scientific, medical, and political issues. New Eng J Med. 2002;346:1576–1579. doi: 10.1056/NEJMsb020693. [DOI] [PubMed] [Google Scholar]
- 28.Marshall E. The business of stem cells. Science. 2000;287:1419. doi: 10.1126/science.287.5457.1419. [DOI] [PubMed] [Google Scholar]
- 29.Winstor R. Embryonic stem cell research. The case for …. Nat Med. 2001;7:396–397. doi: 10.1038/86442. [DOI] [PubMed] [Google Scholar]
- 30.Ferber S. Can we create new organs from our own tissues? Isr Med Assoc J. 2000;2 (suppl 2):32–36. [PubMed] [Google Scholar]
- 31.Grompe M. Liver repopulation for the treatment of metabolic diseases. J Inherit Metab Dis. 2001;24:231–244. doi: 10.1023/a:1010375203539. [DOI] [PubMed] [Google Scholar]
- 32.Paul S, Regulier E. Molecular basis of oncogenesis. Ann Biol Clin. 2000;59:393–402. [PubMed] [Google Scholar]
- 33.Bach SP, Renehan AG, Potten CS. Stem cells: the intestinal cell as a paradigm. Carcinogenesis. 2000;21:469–476. doi: 10.1093/carcin/21.3.469. [DOI] [PubMed] [Google Scholar]