Abstract
The present study investigated the development of audiovisual speech perception skills in children who are prelingually deaf and received cochlear implants. We analyzed results from the Pediatric Speech Intelligibility (Jerger, Lewis, Hawkins, & Jerger, 1980) test of audiovisual spoken word and sentence recognition skills obtained from a large group of young children with cochlear implants enrolled in a longitudinal study, from pre-implantation to 3 years post-implantation. The results revealed better performance under the audiovisual presentation condition compared with auditory-alone and visual-alone conditions. Performance in all three conditions improved over time following implantation. The results also revealed differential effects of early sensory and linguistic experience. Children from oral communication (OC) education backgrounds performed better overall than children from total communication (TC backgrounds. Finally, children in the early-implanted group performed better than children in the late-implanted group in the auditory-alone presentation condition after 2 years of cochlear implant use, whereas children in the late-implanted group performed better than children in the early-implanted group in the visual-alone condition. The results of the present study suggest that measures of audiovisual speech perception may provide new methods to assess hearing, speech, and language development in young children with cochlear implants.
Introduction
Although most researchers working in speech and hearing science have focused their efforts on listeners’ auditory skills, it is now firmly established that visual information from lipreading can enhance speech perception in adults with normal hearing (Erber, 1969; Sumby & Pollack, 1954), adults with hearing loss (Erber, 1975), and adults with hearing loss who use cochlear implants (Grant, Walden, & Seitz, 1998; Kaiser, Kirk, Lachs, & Pisoni, 2003; Tyler, Parkinson, et al., 1997). Audiovisual (AV) enhancement in speech perception has also been found in children with normal hearing (Arnold & Kopsel, 1996; Desjardins, Rogers, & Werker, 1997; Erber, 1972) and those with hearing loss (Arnold & Kopsel, 1996; Erber, 1972, 1975). A few studies have also reported AV enhancement in children with hearing loss who use cochlear implants (Geers & Brenner, 1994; Geers, Brenner, & Davidson, 2003; Lachs, Pisoni, & Kirk, 2001; Staller, Dowell, Beiter, & Brimacombe, 1991; Tyler, Fryauf-Bertschy, et al., 1997). The primary purpose of the present study was to investigate the effects of early linguistic experience on the development of AV speech perception skills in children who are prelingually deaf after cochlear implantation. A secondary goal was to determine the role of age at implantation on the development of AV speech perception skills following implantation.
In one of the first studies of AV speech perception in children with cochlear implants, Staller et al. (1991) administered the Word Intelligibility by Picture Identification (Lerman, Ross, & McLauchlin, 1965) closed-set test of spoken word perception to 8-year-old children, and the Central Institute for the Deaf (Davis & Silverman, 1978) open-set test of sentence perception to 12-year-old children. The children received cochlear implants at a mean age of 9.2 years and all children had used their cochlear implants for at least 1 year. Most, but not all, of the children were prelingually deafened. Children in both age groups performed better in the AV condition compared with a lipreading-alone (visual-alone, or V-alone) condition, revealing that they benefited from the additional auditory information provided by their implant. However, because the investigators did not administer these tests in an auditory-alone (A-alone) condition, it is possible these children would have performed equally well in the AV condition and an A-alone condition.
More recent studies of AV speech perception in children with prelingual hearing loss who received cochlear implants at younger ages than children in the Staller et al. (1991) study have administered speech perception tests under three presentation conditions: A-alone, V-alone, and AV (Geers et al., 2003; Lachs et al., 2001; Tyler, Fryauf-Bertschy, et al., 1997). In the Tyler, Fryauf-Bertschy, et al. (1997) study, two separate groups of children completed the Audiovisual Feature Test (Tyler, Fryauf-Bertschy, & Kelsay, 1991), a closed-set test of consonant feature recognition, at 2 and 4 years post-implantation. The results showed that performance was better in the AV presentation condition compared with the A-alone and V-alone conditions, regardless of the consonant feature.
More recently, Lachs et al. (2001) found similar AV enhancement results using the Common Phrases Test (Robbins, Renshaw, & Osberger, 1995), an open-set test of spoken sentence comprehension, in 4- to 8-year-old children at 2 years post-implantation. Finally, Geers et al. (2003) administered the Children’s Audio-Visual Enhancement Test (Tye-Murray & Geers, 2001) to assess spoken word recognition skills in a group of 181 8- and 9-year-old children who had used their implants for 5 years. They found the best performance in the AV condition, followed by A-alone and V-alone conditions. Taken together, these studies confirm that children with hearing loss who use cochlear implants display AV enhancement over simply lipreading (V-alone) or listening (A-alone) to consonants, words, and sentences.
Does the pattern of AV enhancement that is observed after implantation change over time from pre-implantation to several years post-implantation? Although Tyler, Fryauf-Bertschy, et al. (1997) found that consonant feature recognition performance increased in all presentation conditions in children with 4 years of implant use as compared to children with 2 years of implant use, the overall pattern of performance among the consonant features in each presentation condition was similar for both post-implantation intervals. For example, when tested on consonant place, children performed best in the AV condition, followed closely by V-alone, but they performed very poorly in the A-alone condition regardless of the post-implantation interval.
In a longitudinal study of AV enhancement in 13 children with cochlear implants between the ages of 2 and 12 years, Geers and Brenner (1994) assessed AV and V-alone word and sentence perception before receiving a cochlear implant, and again at 12, 24, and 36 months post-implantation. Their results revealed that performance was similar for both the AV and V-alone conditions prior to implantation, but by 3 years post-implantation, performance in the AV condition surpassed that in the V-alone condition. This suggests that AV enhancement or auditory gain changes over time following implantation. However, like the earlier study by Staller et al. (1991), Geers and Brenner (1994) did not measure performance in an A-alone condition. Also, their results were based on a composite score that reflected performance obtained in a variety of closed-set and open-set word and sentence perception tests. For example, at the pre-implantation interval, 5 children completed closed-set tests of word recognition, 1 child completed a closed-set test of sentence recognition, and 4 children completed open-set tests of sentence comprehension varying in complexity. The results reported for this interval for these different tests were then compiled into a single lipreading enhancement score representing performance in the AV and V-alone conditions. One of the primary goals of the present study was to investigate the development of AV enhancement for spoken word recognition as well as spoken sentence recognition, using similar testing procedures and materials, in A-alone, V-alone, and AV presentation conditions. How does performance under these three presentation conditions change over time after implantation?
Another source of variability in performance that has largely been overlooked in previous studies on AV enhancement in children with cochlear implants is the effect of the early sensory and linguistic environment on perceptual development. Although a variety of communication methods are available to families of children with hearing loss, most children are grouped into one of two communication modes: oral communication (OC), in which children are educated using auditory/oral skills, and total communication (TC), in which children are educated using simultaneous signed and spoken English (Geers et al., 1999). Typical OC methods can range from Auditory-Verbal therapy (AVT), in which auditory information is heavily emphasized and lipreading is discouraged (Ling, 1993; Rhoades, 1982), to Cued Speech, in which specific hand cues are used to supplement auditory information (Cornett & Daisey, 2000). In contrast, TC methods can range from an emphasis on spoken English, an equal emphasis on signed and spoken English (e.g., Signing Exact English [Gustason & Zawolkow, 1993]), to an emphasis on manual signs (Geers et al., 1999). Note that even the latter extreme is still not a completely manual sign language, such as American Sign Language (ASL).
Although previous research has shown significant speech perception and production advantages for using OC over TC with children (Cullington et al., 2000; Hodges et al., 1999; Miyamoto, Kirk, Svirsky, & Sehgal, 1999; Svirsky et al., 2000), only one study to date has investigated the effects of communication mode on AV enhancement. In a recent cross-sectional study, Lachs et al. (2001) used the CP test to measure comprehension of everyday sentences under A-alone, V-alone, and AV conditions in children who had cochlear implants for 2 years. The authors found much stronger correlations between auditory and visual gain scores and outcome measures of spoken word recognition and speech intelligibility for OC children than TC children. To assess the effects of communication mode on AV speech perception, we recently re-analyzed the Lachs et al. (2001) data from the scores provided in their published report (Bergeson & Pisoni, in press). OC children performed better than TC children on the CP test not only in the A-alone and AV presentation conditions, but also in the V-alone condition.
These results demonstrate effects of early sensory experience and language-processing activities in the form of OC and TC on the ability to use multimodal sources of speech information. However, Lachs et al. (2001) only assessed the performance of 4- to 8-year-old children after 2 years of implant use. They did not investigate the development of AV speech perception skills in OC and TC children over time following implantation. Moreover, the CP sentence comprehension test that Lachs et al. (2001) used is appropriate for children with hearing loss over the age of 6 years. Thus, another goal of the present study was to assess the effects of early experience (i.e., OC vs. TC education) on the development of AV speech perception skills in younger children with cochlear implants.
Age at implantation also affects a wide range of outcome measures in children with cochlear implants. Large effects of age at implantation are consistently reported in both the adult and pediatric CI literature (Fryauf-Bertschy et al., 1997; Kirk et al., 2002; Kirk, Pisoni, & Miyamoto, 2000; Staller et al., 1991; Waltzman et al, 1994; Waltzman et al., 1997). Duration of deafness prior to implantation may differentially affect the development of lipreading skills in children with cochlear implants. Therefore, the secondary goal of this study was to assess the effects of age at implantation on the development of AV speech perception skills in the same group of children.
To investigate the development of AV speech perception skills in children with hearing loss who use cochlear implants, we analyzed a dataset that was obtained using the Pediatric Sentence Intelligibility (Jerger et al., 1980) test. The test scores were collected from a large group of 3- to 6-year-old children enrolled in a longitudinal study of speech perception and language development from pre-implantation to 3 years post-implantation. The PSI test was developed to measure spoken word recognition and sentence perception (Jerger & Jerger, 1982; Jerger, Jerger, & Lewis, 1981; Jerger et al., 1980). In our center, the PSI test is routinely administered live-voice under three presentation conditions: A-alone, V-alone, and AV. Testing in all three conditions allowed us to assess differences in speech perception in both the developing auditory and visual modalities, as well as measure enhancement of speech perception in the combined AV condition.
Several features of the PSI test are beneficial for testing very young children. First, the test words are tailored to young children’s vocabulary level. Also, the PSI is a closed-set test that requires the children to listen to a word or sentence and then to point to one of six pictures that corresponds with the word or sentence. Thus, both spoken word and sentence recognition ability can be compared directly using two subtests that have the same task demands. Therefore, the PSI is unlike previous studies that compared word recognition and sentence recognition using different tests (Staller et al., 1991) or consonant feature perception (Tyler, Fryauf-Bertschy et al., 1997), or by simply combining the results of word recognition and sentence recognition tests for a global composite speech perception score (Geers & Brenner, 1994). In addition, the same group of children was followed over time in a longitudinal design.
Method
Participants
Participants in this study consisted of 80 children who experienced a profound hearing loss before the age of 36 months, received a cochlear implant before 9 years of age, and used either OC or TC communication methods. Classification of communication method is based primarily on parental report and confirmed by the child’s educational setting. Two age-at-implantation categories (i.e., early-implanted and late-implanted) were determined by a median split. Children in the early implanted group received a cochlear implant before the age of 53 months and children in the late implanted group received cochlear implants after 53 months. Table I provides a summary of the demographics of these children. Children were tested once every 6 months to 1 year for 3 years. Not all children were tested at each interval for several reasons. Many of the participants lived or moved a great distance from the Indianapolis area, some participants were judged to be too tired to complete testing, and others did not finish testing because of time constraints. Finally, by 2 and 3 years post-implantation, many of the children had graduated from the baby test battery to the pre-school test battery because they were performing at or near ceiling or because they were too old for the baby test battery. Table II shows the number of children tested at each interval in the Words and Sentences subtests of the PSI. Most of the children used a Nucleus® 22 or 24 implant model; only one child had a Clarion 1.2 implant. The majority of the children used Spectra or MSP processors with MPEAK or SPEAK strategies at the time of testing. Other processors used were WSP, Esprit, and Sprint, and other strategies used were F0F1F2, F0F1F2F5, CIS, and ACE. Thirty of the children changed processors and/or strategies over the 3-year testing period.
Table I.
Demographic Characteristics of PSI Participants
| Communication Mode | Age Group | Age at Implantation | Unaided PTA | Aided PTA (CI) | Number of Electrodes | |
|---|---|---|---|---|---|---|
| Oral Communication (n = 38) | Early (n = 22) | Mean | 36 months | 111 dB HL | 34 dB HL | 19.32 |
| Range | 17–53 | 98–120 | 25–40 | 8–22 | ||
| Late (n = 16) | Mean | 73 months | 110 dB HL | 32 dB HL | 19.69 | |
| Range | 57–106 | 97–120 | 22–39 | 9–22 | ||
| Total Communication (n = 42) | Early (n = 20) | Mean | 37 months | 113 dB HL | 37 dB HL | 20.72 |
| Range | 22–53 | 103–120 | 25–58 | 8–22 | ||
| Late (n = 22) | Mean | 72 months | 114 dB HL | 35 dB HL | 20.22 | |
| Range | 55–104 | 100–121 | 21–58 | 11–22 | ||
Table II.
Number of Participants for PSI at Each Interval
|
Implant Use (Yrs.) |
||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Words | A | OC | 25 | 25 | 23 | 9 |
| TC | 36 | 38 | 28 | 17 | ||
| V | OC | 24 | 21 | 22 | 10 | |
| TC | 35 | 37 | 28 | 17 | ||
| AV | OC | 25 | 24 | 22 | 9 | |
| TC | 36 | 36 | 26 | 15 | ||
| Sentences | A | OC | 17 | 26 | 23 | 10 |
| TC | 29 | 35 | 27 | 15 | ||
| V | OC | 16 | 24 | 21 | 10 | |
| TC | 30 | 35 | 25 | 14 | ||
| AV | OC | 16 | 26 | 21 | 9 | |
| TC | 30 | 33 | 26 | 14 | ||
Note. A = auditory-alone, V = visual-alone, AV = audiovisual; OC = oral communication, TC = total communication.
Procedures
All test measures were administered by a licensed speech-language pathologist or audiologist at the Indiana University Medical Center. The PSI test (Jerger et al., 1980) was administered live-voice under three presentation conditions in the following order: auditory-alone (A), visual-alone (V), and audiovisual (AV). To eliminate visual cues in the A-alone condition, the clinician covered her face with a black mesh-cloth screen that did not mask the auditory signal.
The PSI test is a six-alternative, closed-set test originally developed by Jerger and colleagues to assess the speech perception skills of children as young as 3 years of age, using familiar words and short sentences based on studies of typically developing children’s first vocabularies (Jerger et al., 1980). There was no language criterion for administering the test. The PSI test consists of two conditions: a word recognition subtest and a sentence recognition subtest. The word recognition condition uses four plates, each containing six pictures of concrete nouns. Prior to testing, the child is familiarized with each of the pictures and the corresponding test words for a particular plate. The child then hears one of five words and is asked to point at the picture on the plate that corresponds to the word. This procedure is repeated for each of the four plates. A correct response is awarded 1 point; the child’s final score is calculated based on the percent correct out of a possible 20 trials.
The sentence recognition condition consists of two plates, each containing six pictures that portray animals engaged in a specific action. Prior to testing, the child is familiarized with each of the pictures and the corresponding test sentences for a particular plate. The child then hears one of five sentences and is asked to point to the picture that portrays the sentence read to them. The procedure is repeated for each of the two plates. Each correct response is awarded 1 point, and a final percent correct score is calculated out of a possible 10 trials. Because the test stimuli remained the same in all testing intervals, it is possible that children in this study might have become familiar with the words, sentences, and pictures over time.
In addition to the PSI test, scores were examined from several other clinical tests of speech and language development that are routinely used to measure outcome and assess benefit of cochlear implantation (Kirk, 2000). The Phonetically Balanced-Kindergarten (PBK) test (Haskins, 1949) is a live-voice, open-set test used to assess A-alone speech perception. The child hears a spoken word and is asked to repeat the word aloud. Children’s responses are scored as the percentage of words and/or phonemes repeated correctly. The items on the PBK test are phonetically balanced, monosyllabic words.
The Peabody Picture Vocabulary Test (Dunn & Dunn, 1997) is a closed-set test used to assess receptive vocabulary knowledge. The clinician presents a spoken word to the child, and the child is asked to point to the target word depicted by one of four pictures. In our center, this test is administered using the child’s preferred mode of communication, either spoken English or Signing Exact English (SEE), which is simultaneously signed and spoken English. That is, the test words were presented only in the auditory modality for OC children, but they were simultaneously spoken and signed for TC children. An age equivalence (AE) score was calculated by comparing the raw score to normative data obtained from children with normal hearing and determining the age of most children who receive a similar score.
The Reynell Developmental Language Scales–3rd Edition (Edwards et al., 1997; Reynell & Huntley, 1985) is a test used to assess children’s language skills. The receptive language scale consists of 10 subtests that assess skills ranging from word recognition and sentence comprehension to verbal comprehension of ideational content. The expressive language scale consists of three subtests that assess such skills as children’s spontaneous expression of speech and their ability to describe a novel picture. In the present study both the receptive and expressive language scores were also obtained using the child’s preferred mode of communication. The children received credit for signed and/or spoken correct responses. Raw scores on the RDLS–III scales were converted into AE scores based on normative data obtained from children with normal hearing and determining the age of most children who receive a similar score.
The Beginner’s Intelligibility Test (Osberger et al., 1994) was administered to obtain an objective measure of the child’s speech intelligibility. The child is asked to repeat aloud 10 sentences presented by the clinician. Audio recordings of children’s speech productions are then presented to three naïve adult listeners who are asked to transcribe what the child said. An intelligibility score is computed based on the average number of words transcribed correctly by the three listeners.
Results
The data set used in this study was obtained from a clinical population enrolled in a larger longitudinal research project, and therefore not all children could be tested at each interval. To deal with the problem of missing data, the SAS Mixed Procedure (Wolfinger & Chang, 1995) was used to analyze the fixed effects in this study. The traditional repeated measures ANOVA, commonly used to analyze variance in longitudinal designs, eliminates participants with missing data. However, systematically eliminating participants with missing data can often lead to skewed or biased results, as well as an underestimation of variability (Schafer & Graham, 2002). Because the data used for the present study consisted of repeated measures from the same participants, a maximum-likelihood estimation method (e.g., Mixed Procedure) can use all available data to create a model without eliminating any participants (Schafer & Graham, 2002). Table II shows the number of participants tested in the PSI at each interval in the study.
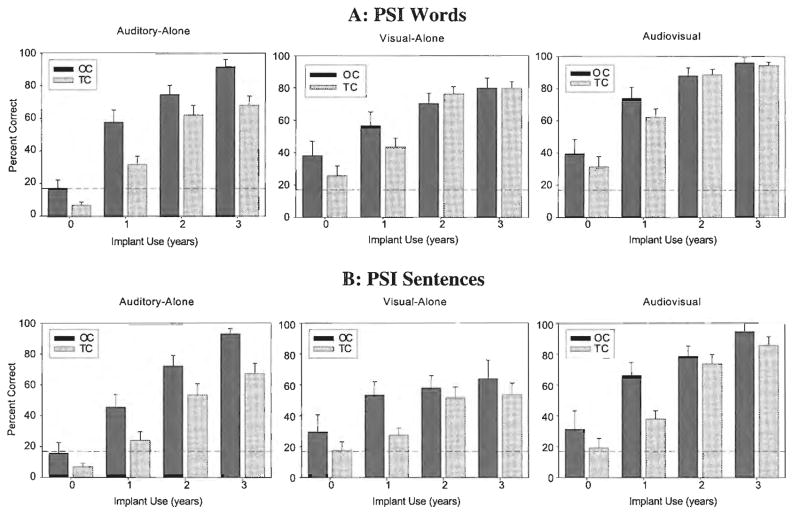
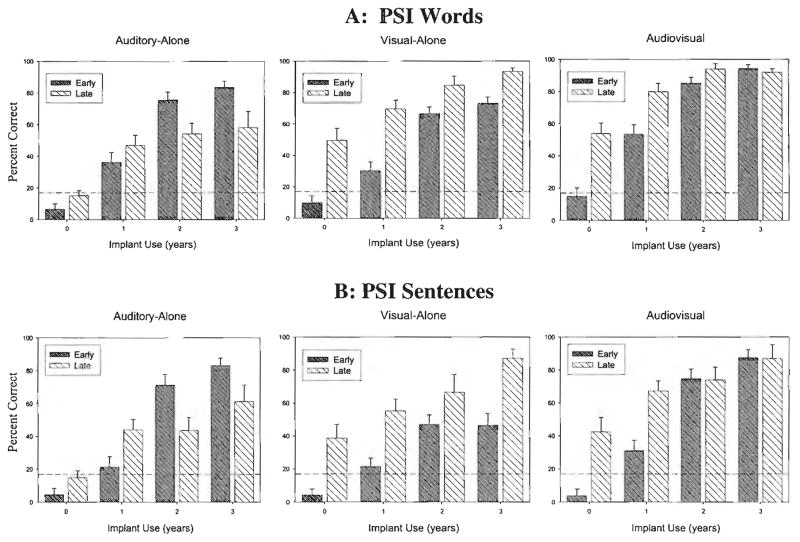
Figure 1 shows the longitudinal results obtained in the three presentation formats (A-alone, V-alone, AV) for OC and TC children in the Words (top panel) and Sentences (bottom panel) subtests of the PSI. Figure 2 shows the longitudinal results obtained in the three presentation formats (A-alone, V-alone, AV) for children in the early-implanted and late-implanted groups in the Words (top panel) and Sentences (bottom panel) subtests. Accuracy scores averaged over all groups of children in the three presentation conditions of the Words subtest were strongly correlated with the scores obtained from the Sentences subtest at Year 2 post-implantation (A-alone: r =.83, p <.001; V-alone: r =.77, p <.001; AV: r =.81, p <.001). In fact, inspection of both figures shows that the patterns are strikingly similar across the PSI Words and Sentences subtests. These findings reveal that both subtests of the PSI measure the same underlying processes used in AV speech perception. However, overall performance was significantly better on the Words subtest than the Sentences subtest (F(1, 1026) = 69.29, p <.0001), most likely due to substantial ceiling effects in the Words subtest. In light of these results, as well as space constraints, we will focus the remainder of the results section on the Sentences subtest.
Figure 1.
Mean percent correct word recognition (top panel) and sentence recognition (bottom panel) over time on the Pediatric Speech Intelligibility (PSI) test under auditory-alone, visual-alone, and audiovisual conditions for oral communication (OC) and total communication (TC) children. Error bars represent standard error; dotted lines represent chance levels.
Figure 2.
Mean percent correct word recognition (top panel) and sentence recognition (bottom panel) over time on the Pediatric Speech Intelligibility (PSI) test under auditory-alone, visual-alone, and audiovisual conditions for early-implanted (Early) and late-implanted (Late) children. Error bars represent standard error; dotted lines represent chance levels.
PSI Accuracy Scores: Sentences
The effects of presentation format (A-alone, V-alone, AV), duration of implant use, communication mode (OC vs. TC), and age at implantation (early [implanted before 53 months of age] vs. late [implanted after 53 months of age]) were the main effects included in the analyses. The bottom panels of Figures 1 and 2 show the longitudinal results obtained for the Sentences subtest in the three presentation formats (A-alone, V-alone, AV) for OC and TC (see Figure 1), and children in the early-implanted and late-implanted groups (see Figure 2). We found significant main effects of presentation format (F(2, 429) = 26.52, p < 0.0001), duration of implant use (F(3, 476) = 101.16, p < 0.0001), communication mode (F(1, 113) = 10.02, p < 0.01), and age at implantation (F(1, 77.6) = 12.81, p < 0.001). These results indicate that, overall, performance was consistently better in the AV condition compared with the A-alone and V-alone conditions. Also, performance of all children, regardless of communication mode and presentation format, improved consistently over time from pre-implantation to 3 years post-implantation. Finally, OC children consistently performed better than TC children, and children in the late-implanted group performed better than children in the early-implanted group.
Several two-way interactions among the main effects were also statistically significant: duration of implant use and communication mode (F(3, 476) = 3.71, p < 0.05), duration of implant use and age at implantation (F(3, 476) = 12.99, p < 0.0001), and presentation condition and age at implantation (F(2, 429) = 34.73, p < 0.0001). OC children showed larger improvements over time than TC children. Also, OC children performed somewhat better than TC children in the V-alone and AV conditions even before they received cochlear implants. Children in the early-implanted group showed larger improvements over time than children in the late-implanted group.
Finally, two three-way interactions were significant: presentation condition by age at implantation by duration of implant use (F(12, 429) = 3.72, p < 0.0001), and communication mode by age at implantation by duration of implant use (F(4, 286) = 5.12, p < 0.001). Although children in the late-implanted group performed better than the children in the early-implanted group in the pre-implantation and 1-year post-implantation intervals in the A-alone and AV conditions, by 2 years post-implantation, performance for both groups of children was quite similar in the AV condition and children in the early-implanted group performed better than children in the late-implanted group in the A-alone condition. In contrast, the children in the early-implanted group’s performance in the V-alone condition increased over time but never reached the performance levels of the children in the late-implanted group in the pre- and post-implantation testing intervals.
When the data shown in Figures 1 and 2 are broken down into four groups (early-implanted OC, late-implanted OC, early-implanted TC, and late-implanted TC), they reveal the second three-way interaction. The OC children in the late-implanted group performed much better than all other groups of children in the V-alone and AV conditions, particularly from pre-implantation to 2 years post-implantation. In the pre-implantation A-alone condition, both late-implanted groups performed better than the children in the early-implanted group. Whereas the children in the early-implanted OC and TC groups did eventually reach the performance level of the children in the late-implanted OC group by 2 years post-implantation, the children in the late-implanted TC group improved very little over time.
Correlations Between PSI and Outcome Measures of Speech and Language
To assess the relation between the Words and Sentences subtests and the other clinical outcome measures of speech and language skills, correlation analyses on these measures were performed for all children after 2 years of cochlear implant use (see Lachs et al., 2001). In order to obtain an adequate sample size for this analysis, the correlations were carried out by combining the scores for children in the OC, TC, early-implanted, and late-implanted groups.
Table III presents a summary of the correlations between accuracy scores on the Words and Sentences subtests and several clinical outcome measures of speech perception, speech intelligibility, and language skills at 2 years post-implantation. Both A-alone Words and Sentences were strongly correlated with measures of open-set speech perception (PBK words and phonemes) and speech intelligibility (BIT). These results suggest that children who had higher scores in the A-alone conditions tended to have better speech perception and speech production skills.
Table III.
Correlations for PSI and Outcome Measures 2 Years Post-implantation
| A | V | AV | |||
|---|---|---|---|---|---|
| Words | PBK–Words | r | .668** | .037 | .189 |
| N | 23 | 21 | 19 | ||
| PBK–Phonemes | r | .872** | −.042 | .029 | |
| N | 22 | 21 | 19 | ||
| PPVT | r | −.213 | .562** | .367* | |
| N | 50 | 50 | 47 | ||
| RDLS–III Expr. | r | .048 | .700** | .509* | |
| N | 40 | 42 | 40 | ||
| RDLS Rec. | r | .008 | .663** | .364* | |
| N | 41 | 43 | 41 | ||
| BIT | r | .480** | .388* | .375* | |
| N | 42 | 43 | 41 | ||
| Sentences | PBK–Words | r | .560** | .065 | .300 |
| N | 23 | 20 | 21 | ||
| PBK–Phonemes | r | .773** | .100 | .430 | |
| N | 23 | 20 | 21 | ||
| PPVT | r | −.058 | .426** | .227 | |
| N | 48 | 45 | 46 | ||
| RDLS Expr. | r | .287 | .646** | .562** | |
| N | 38 | 38 | 38 | ||
| RDLS Rec. | r | .191 | .589** | .408** | |
| N | 39 | 39 | 39 | ||
| BIT | r | .459** | .321* | .406* | |
| N | 42 | 41 | 41 | ||
p < .05,
p < .01.
Interestingly, both V-alone Words and Sentences were significantly correlated with vocabulary (PPVT), language measures (RDLS–III, Expressive and Receptive), and speech intelligibility (BIT). These results suggest that children who performed well in the V-alone presentation condition of the PSI test displayed better vocabulary, expressive and receptive language, and speech intelligibility skills. However, no significant relationship was found between V-alone performance and the PBK open-set word recognition scores.
Finally, both AV Words and Sentences were strongly correlated with language skills (RDLS–III, Expressive and Receptive) and speech intelligibility (BIT). These results suggest that children who performed well in the AV conditions had better expressive and receptive language skills, and better speech intelligibility scores than the children who performed poorly on the PSI test. Also, only the AV Words subtest was significantly correlated with vocabulary level (PPVT). This result suggests that children who perform well on a test of spoken word perception also have more advanced vocabulary and language skills compared with children who have poorer spoken word perception skills.
Correlations Between Pre-implantation PSI and Post-implantation Measures of Speech and Language
Further correlation analyses were also conducted to determine whether children’s pre-implantation scores on the PSI test could predict their skills on speech and language outcome measures at 2 years post-implantation. To obtain an adequate sample size for this analysis, the correlations were also carried out combining the scores for children in the OC, TC, early-implanted, and late-implanted groups.
Table IV shows a summary of the correlations between the pre-implantation Words and Sentences and several clinical outcome measures of speech perception, speech intelligibility, and language skills 2 years post-implantation. The analyses revealed strong positive correlations between the A-alone, pre-implantation Words scores and post-implantation outcome measures of Expressive and Receptive language (RDLS–III) and speech intelligibility (BIT). Also, both V-alone and AV scores on the pre-implantation Words subtest were significantly correlated with post-implantation measures of vocabulary (PPVT), Expressive and Receptive language (RDLS–III), and speech production (BIT). Thus, children’s pre-implantation performance on the Words subtest may provide a behavioral measure that can be used to predict their vocabulary, language, and speech intelligibility performance after 2 years of implant use.
Table IV.
Correlations for Pre-implantation PSI and Outcome Measures 2 Years Post-implantation
| A | V | AV | |||
|---|---|---|---|---|---|
| Words | PBK–Words | r | .351 | .334 | .361 |
| N | 29 | 27 | 29 | ||
| PBK–Phonemes | r | .373 | .219 | .297 | |
| N | 28 | 26 | 28 | ||
| PPVT | r | .239 | .486** | .357** | |
| N | 54 | 52 | 55 | ||
| RDLS–III Expr. | r | .661** | .633** | .625** | |
| N | 42 | 40 | 43 | ||
| RDLS Rec. | r | .470** | .603** | .561** | |
| N | 43 | 41 | 44 | ||
| BIT | r | .322* | .426** | .409* | |
| N | 44 | 42 | 45 | ||
| Sentences | PBK–Words | r | .451* | .314 | .354 |
| N | 21 | 21 | 22 | ||
| PBK–Phonemes | r | .235 | .188 | .236 | |
| N | 20 | 20 | 21 | ||
| PPVT | r | .432** | .489** | .487** | |
| N | 40 | 40 | 41 | ||
| RDLS Expr. | r | .575* | .520** | .663** | |
| N | 29 | 29 | 30 | ||
| RDLS Rec. | r | .697** | .524** | .617** | |
| N | 30 | 30 | 31 | ||
| BIT | r | .303 | .331 | .445** | |
| N | 32 | 32 | 33 | ||
p < .05,
p < .01.
The analyses also revealed strong positive correlations between the A-alone pre-implantation Sentences scores and post-implantation measures of open-set word recognition (PBK), vocabulary (PPVT), and Expressive and Receptive language (RDLS–III). Post-implantation measures of vocabulary (PPVT) and Expressive and Receptive language (RDLS–III) were also significantly correlated with V-alone and audiovisual scores on the pre-implantation Sentences subtest. In addition, the pre-implantation AV Sentences subtest was significantly correlated with post-implantation speech intelligibility (BIT) scores. Thus, children’s pre-implantation performance on the Sentences subtest also predicts their subsequent word recognition, vocabulary, language, and speech intelligibility performance at 2 years post-implantation.
Discussion
Several consistent patterns of performance emerged from the results of the present analysis of the longitudinal data obtained from a large group of children who are prelingually deaf over a period of 3 years following cochlear implantation. Performance was consistently best in the AV conditions. When data from all groups were pooled, overall performance was similar in the A-alone and V-alone conditions. This pattern of performance was not surprising and was expected based on results from earlier studies of children with hearing loss who have cochlear implants (Geers et al., 2003; Lachs et al., 2001; Staller et al., 1991; Tyler, Fryauf-Bertschy, et al., 1997). Also, performance in all three presentation conditions improved over the 3-year post-implantation period. Although performance was better overall in the Words subtest than the Sentences subtest, the patterns of performance were remarkably similar in both conditions. This finding is theoretically important because these two subtests measure two qualitatively different types of processing operations. The Words subtest is simply a measure of pattern matching, whereas the Sentences subtest requires the construction of a more detailed linguistic representation. For the sentences, children must encode and represent not only the name of the animal, but also parse the input and encode the action that the animal takes (e.g., “A bear is brushing his teeth.” vs. “A bear is combing his hair.”).
The results of the present study also revealed strong differential effects of early sensory and linguistic experience on spoken word and sentence recognition performance that reflected differences in the children’s communication mode and educational environment. Children who were immersed in OC educational environments displayed consistently higher scores than children in TC environments. In general, OC children performed better than TC children in the V-alone and AV presentation conditions both prior to implantation and across the 3 years post-implantation, although it appeared that the TC children’s performance begins to reach the performance levels of OC children by 2 years post-implantation. On the other hand, TC and OC children displayed chance scores in the A-alone presentation condition prior to implantation, but OC children performed better than TC children across the 3 years post-implantation. Finally, the A-alone scores of the children from TC environments were not only consistently lower overall than those of OC children, but their performance improved more slowly over time after implantation.
Taken together, these results suggest that the children in OC environments are becoming sensitive to the correlations and coupling between visual and/or auditory speech cues even prior to cochlear implantation, and that this sensitivity improves their attention to auditory and visual cues to speech as they gain additional experience with sound after implantation. Several factors may be responsible for the large and consistent differences observed in performance between these two groups of children in the early time intervals. Because of the heavy emphasis on aural-oral skills and explicit activities in perceiving and producing spoken language, OC children may have more experience focusing their attention on combined auditory and visual articulation information, even before they receive cochlear implants, compared with TC children. Children in typical TC environments are instead required to divide their limited visual attention between the talker’s face and hands, optical and acoustic signs that are uncorrelated and arbitrarily related to each other only by simple association. In contrast, lipreading and speech are causally related, reflecting common underlying articulatory events.
It is important to emphasize that a very large proportion of children with hearing loss who receive cochlear implants have parents with normal hearing who do not have a good working knowledge of sign language or manual communication methods. Thus, the language samples that children with hearing loss in TC environments are exposed to, are not only produced more slowly, but are very likely to be impoverished models of language (Moeller & Luetke-Stahlman, 1990; Spencer, 1993; Swisher & Thompson, 1985). TC children may receive degraded or incomplete sign sequences because their parents speak fluently, but present impoverished manual signs. In addition, parents’ manual signs may be delayed in time rather than produced simultaneously relative to the spoken words. As a consequence, TC children are exposed to poor instances of manual signs as well as degraded auditory signals via the cochlear implant. Inputs from either the visual or the auditory modality are therefore unlikely to be sufficient to promote optimal language acquisition.
Another finding that emerged from our analysis of the PSI Sentences results was an effect of age at implantation. When data from all conditions were pooled, children who received cochlear implants later in life (i.e., had a longer period of deafness before implantation) performed better overall than children who received cochlear implants at a younger age (i.e., had a shorter period of deafness before implantation; see Figure 2). The direction of this finding was surprising given that large effects of age at implantation have been consistently reported in both the adult and pediatric CI literature (e.g., Kirk et al., 2000; Staller et al., 1991; Waltzman et al., 1994; Waltzman et al., 1997). It is important to note that the children in the late-implanted group in this study were more than 3 years older than the children in the early-implanted group across all testing intervals (see Table I). Also, the main effect of age at implantation in this study is qualified by several complex interactions with other factors included in this study.
Of particular interest was the finding that children in the late-implanted group initially displayed superior performance over children in the early-implanted group in the A-alone presentation condition, but by 2 years post-implantation children in the early-implanted group performed better than children in the late-implanted group in the A-alone condition. In contrast, children in the late-implanted group consistently performed better than children in the early-implanted group in the V-alone presentation condition both prior to implantation and all 3 years post-implantation. Thus, children who were profoundly deaf for longer periods actually turned out to be better lipreaders than children who were profoundly deaf for shorter periods. This finding with children who are prelingually deaf replicates previous research findings in which adults with early-onset hearing loss display better lipreading performance than adults with late-onset hearing loss (Bergeson & Pisoni, in press; Tillberg, Ronnberg, Svard, & Ahlner, 1996). Interestingly, although children in the late-implanted group performed better than children in the early-implanted group in the first two intervals (i.e., pre-implantation and 1 year post-implantation), both groups of children performed at similar levels by 2 years post-implantation. Thus, it appears that children in the early-implanted group primarily use auditory information and children in the late-implanted group primarily use visual information to reach similar performance levels in the AV presentation condition.
The present results of the children in the early-implanted group vs. children in the late-implanted group in the A-alone condition are also consistent with the recent suggestion that it might never be possible for spoken language to develop fully or for the underlying sensory, perceptual, and cognitive processes to reach their optimal states without sufficient auditory stimulation to the central nervous system during critical periods of speech and language development (e.g., Bruer, 2001; Neville & Bruer, 2001). Some cortical reorganization may take place after implantation, but the process of development proceeds based on information obtained by the intact sensory modalities. In fact, a recent study of cortical response latencies to speech in children who are born deaf and adults with cochlear implants revealed maximal plasticity in a sensitive period up to about 3.5 years of age, with plasticity remaining in some children up to 7 years of age (Sharma, Dorman, & Spahr, 2002).
The effects of age at implantation observed in this study were also influenced by communication mode over the 3-year period of implant use. The children in the late-implanted OC group performed best in the V-alone and AV presentation conditions; all other children (early-implanted OC, early-implanted TC, and late-implanted TC) displayed similar, though somewhat lower scores. On the other hand, in the A-alone condition, children in the late-implanted OC group performed best in the pre-implantation and 1-year post-implantation intervals, but by 2 years post-implantation, children in the early-implanted OC group displayed the best scores in both PSI Words and Sentences. Thus, very early experience, in terms of both communication method (OC vs. TC) and onset of auditory experience (early-implanted vs. late-implanted), markedly influences the development of AV speech perception in young children with cochlear implants.
The correlational analyses revealed that performance on both the Words and Sentences subtests was strongly associated with clinical outcome measures of speech perception, speech intelligibility, and language at the 2-year post-implantation interval. These two sets of correlations obtained with words and sentences suggest that both measures of AV speech perception obtained using the PSI test share a common underlying source of variance with other behavioral tests that are routinely used to measure speech and language skills in this clinical population. This pattern of correlations is revealing and of interest theoretically because it indicates that the basic underlying sensory, cognitive, and linguistic processes used to carry out AV speech perception are also accessed and used in other language-processing tasks. Thus, scores on the PSI test are not measuring isolated and independent perceptual skills that are task- and modality-specific and do not generalize beyond the specific experimental paradigm. Instead, the results of our analyses of the PSI test suggest that central auditory, cognitive, and linguistic factors are responsible for the enormous variation and individual differences observed in traditional clinical outcome measures (see also Bergeson & Pisoni, in press).
In addition to the intercorrelations with these outcome measures after 2 years of implant use, we also carried out a set of correlations between the pre-implantation PSI scores and several speech and language outcome measures after 2 years of implant use to identify early predictors of performance and benefit. Despite small sample sizes, pre-implantation PSI scores were strongly correlated with vocabulary, Receptive and Expressive language, and speech intelligibility scores obtained at 2 years post-implantation. The present findings suggest that pre-implantation lipreading and AV speech perception scores can be used to predict speech and language skills after several years of implant use. Measures of AV speech perception may provide reliable behavioral markers that can be used to predict and identify the children who will obtain the most benefit from their cochlear implants at an early point following implantation. Although the pre-implantation correlations are based on small sample sizes obtained by combining OC and TC children together, the results suggest that measures of AV speech perception—even simple lipreading measures obtained prior to implantation—might reveal fundamental processes that are used to recover phonetic information about speech articulation and the linguistically significant gestures of the speaker that are used to encode and represent distinctive phonological contrasts in the sound system of the target language in environment.
Finally, it should be noted that the PSI test has several methodological limitations. First, the PSI test is a closed-set, forced-choice test with a restricted response set of six pictures. The child is asked merely to point to the picture that represents what he or she heard (or saw) the clinician say. Second, in the clinical version of the PSI test that is used in our center, the same target words and sentences are used repeatedly in all three presentation conditions, and these conditions are always presented in the same order. Finally, the PSI test was originally designed by Jerger and colleagues (Jerger & Jerger, 1982; Jerger et al., 1980; Jerger et al., 1981) to measure word and sentence recognition only in very young children between the ages of 3 and 7 years. Thus, we only have PSI data for 3 years of cochlear implant use.
All of these limitations may conspire to make the PSI test extremely easy even for children who are prelingually deaf and use cochlear implants, perhaps especially for those children who received cochlear implants later in life and thus are chronologically older and have better overall language skills than their peers who are early-implanted, hearing age-matched, resulting in ceiling effects. Not only do ceiling effects make it harder to detect absolute differences among conditions, but they also systematically influence AV gain scores. For example, children who performed near or at ceiling on the A-alone presentation condition of the PSI test would have no possibility of receiving additional benefits and enhancement under the visual information in the AV presentation condition. To deal with these problems, it is important to obtain additional measures of AV speech perception performance using tests that provide a better range of scores that are above the floor but below ceiling (see Bergeson & Pisoni, in press; Bergeson, Pisoni, & Davis, 2003).
Considering that the pre-implantation PSI test scores predict later speech and language outcome, and that infants as young as 12 months of age are now routinely receiving cochlear implants, it is also important to develop new AV speech perception tests and even V-alone non-speech perception tests that can be administered to infants and children younger than 3 years of age. Recent studies have made use of existing preferential paradigms to assess speech perception and word-learning skills in infants with cochlear implants (e.g., Barker & Tomblin, 2003; Houston, Pisoni, Kirk, Ying, & Miyamoto, 2003; Houston, Ying, Pisoni, & Kirk, 2003). We are currently using these experimental paradigms to explore the development of infants with cochlear implants’ AV speech perception skills. Interestingly, some researchers have proposed that multimodal perception in infants guides their development of language skills (Gogate, Walker-Andrews, & Bahrick, 2001). However, it remains to be seen whether the measures obtained from AV tests appropriate for infants and toddlers with cochlear implants will predict later speech and language performance post-implantation using traditional clinical measures of outcome and benefit.
Acknowledgments
This work was supported by NIH–NIDCD Training Grant T32DC00012 to Indiana University, and NIH–NIDCD Research Grant R01DC00064 to the Indiana University School of Medicine. We thank Amy Teoh, Elizabeth Collison, and Cindy Hiltgen for their help in testing the children, and Sujuan Gao and Amy Rong Qi for their statistical assistance. We also thank Lorin Lachs and Karen Iler Kirk for their insightful comments on this project.
Contributor Information
Tonya R. Bergeson, NIH Postdoctoral Research Fellow at the Indiana University School of Medicine in Indianapolis, IN
David B. Pisoni, Chancellors’ Professor of psychology and cognitive science at Indiana University in Bloomington, IN and an adjunct professor of otolaryngology-head and neck surgery at the Indiana University School of Medicine in Indianapolis, IN
Rebecca A. O. Davis, Research associate at the Indiana University School of Medicine in Indianapolis, IN
References
- Arnold P, Kopsel A. Lipreading, reading and memory of hearing and hearing-impaired children. Scandinavian Audiology. 1996;25:13–20. doi: 10.3109/01050399609047550. [DOI] [PubMed] [Google Scholar]
- Barker BA, Tomblin JB. Comparing bimodal perception skills in infant hearing-aid and cochlear-implant users. Paper presented at the 9th Symposium on Cochlear Implants in Children; Washington, DC. 2003. [Google Scholar]
- Bergeson TR, Pisoni DB. Audiovisual speech perception in deaf adults and children following cochlear implantation. In: Calvert G, Spence C, Stein BE, editors. Handbook of multisensory integration. Cambridge, MA: MIT Press; in press. [Google Scholar]
- Bergeson TR, Pisoni DB, Davis RAO. Development of audiovisual speech perception skills in prelingually deaf children with cochlear implants. 2003. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruer JT. A critical and sensitive period primer. In: Lichtman JW, editor. Critical thinking about critical periods. Baltimore: Paul H. Brookes Publishing Company; 2001. pp. 3–26. [Google Scholar]
- Cornett RO, Daisey ME. The Cued Speech resource book for parents of deaf children. Cleveland, OH: The National Cued Speech Association, Inc; 2000. [Google Scholar]
- Cullington H, Hodges AV, Butts SL, Dolan-Ash S, Balkany TJ. Comparison of language ability in children with cochlear implants placed in oral and total communication education settings. Annals of Otology, Rhinology, & Laryngology. 2000;185:121–123. doi: 10.1177/0003489400109s1253. [DOI] [PubMed] [Google Scholar]
- Davis H, Silverman S. Hearing and deafness. 4. New York: Holt, Rinehart & Winston; 1978. [Google Scholar]
- Desjardins RN, Rogers J, Werker JF. An exploration of why preschoolers perform differently than do adults in audiovisual speech perception tasks. Journal of Experimental Child Psychology. 1997;66:85–110. doi: 10.1006/jecp.1997.2379. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Edwards S, Fletcher P, Garman M, Hughes A, Letts C, Sinka I. Reynell Developmental Language Scales. 3. Windsor, England: NFER-Nelson Publishing Company, Ltd; 1997. [Google Scholar]
- Erber NP. Interaction of audition and vision in the recognition of oral speech stimuli. Journal of Speech and Hearing Research. 1969;12:423–425. doi: 10.1044/jshr.1202.423. [DOI] [PubMed] [Google Scholar]
- Erber NP. Auditory, visual, and auditory-visual recognition of consonants by children with normal and impaired hearing. Journal of Speech and Hearing Research. 1972;15:413–422. doi: 10.1044/jshr.1502.413. [DOI] [PubMed] [Google Scholar]
- Erber NP. Auditory-visual perception of speech. Journal of Speech and Hearing Disorders. 1975;40:481–492. doi: 10.1044/jshd.4004.481. [DOI] [PubMed] [Google Scholar]
- Fryauf-Bertschy H, Tyler RS, Kelsay D, Gantz B, Woodworth G. Cochlear implant use by prelingually deafened children: The influences of age at implant and length of device use. Journal of Speech, Language, and Hearing Research. 1997;40:183–199. doi: 10.1044/jslhr.4001.183. [DOI] [PubMed] [Google Scholar]
- Geers A, Brenner C. Speech perception results: Audition and lipreading enhancement. The Volta Review. 1994;96(5):97–108. [Google Scholar]
- Geers A, Brenner C, Davidson L. Factors associated with development of speech perception skills in children implanted by age five. Ear and Hearing. 2003;24:24S–35S. doi: 10.1097/01.AUD.0000051687.99218.0F. [DOI] [PubMed] [Google Scholar]
- Geers A, Nicholas J, Tye-Murray N, Uchanski R, Brenner C, Crosson J, et al. Periodic Progress Report No. 35, Central Institute for the Deaf. 1999. Cochlear implants and education of the deaf child: Second-year results; pp. 5–16. [Google Scholar]
- Gogate LJ, Walker-Andrews AS, Bahrick LE. The intersensory origins of word comprehension: An ecological-dynamic systems view. Developmental Science. 2001;4:1–37. [Google Scholar]
- Grant KW, Walden BE, Seitz PF. Auditory-visual speech recognition by hearing-impaired subjects: Consonant recognition, sentence recognition, and auditory-visual integration. Journal of the Acoustical Society of America. 1998;103(5):2677–2450. doi: 10.1121/1.422788. [DOI] [PubMed] [Google Scholar]
- Gustason G, Zawolkow E. Signing Exact English. Los Alamitos, CA: Modern Signs Press, Inc; 1993. [Google Scholar]
- Haskins HA. Unpublished master’s thesis. Northwestern University; Evanston, IL: 1949. A phonetically balanced test of speech discrimination for children. [Google Scholar]
- Hodges AV, Dolan-Ash S, Balkany TJ, Schloffman JJ, Butts SL. Speech perception results in children with cochlear implants: Contributing factors. Otolaryngology-Head and Neck Surgery. 1999;12:31–34. doi: 10.1016/S0194-5998(99)70119-1. [DOI] [PubMed] [Google Scholar]
- Houston DM, Pisoni DB, Kirk KI, Ying EA, Miyamoto RT. Speech perception skills of deaf infants following cochlear implantation: A first report. International Journal of Pediatric Otorhinolaryngology. 2003;67:479–495. doi: 10.1016/s0165-5876(03)00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DM, Ying EA, Pisoni DB, Kirk KI. Pre word-learning skills in normal-hearing infants and deaf infants who use cochlear implants. Paper presented at the biennial meeting of the Society for Research in Child Development; Tampa, Florida. 2003. [Google Scholar]
- Jerger S, Jerger J. Pediatric speech intelligibility test: Performance-intensity characteristics. Ear and Hearing. 1982;3(6):325–334. doi: 10.1097/00003446-198211000-00007. [DOI] [PubMed] [Google Scholar]
- Jerger S, Jerger J, Lewis S. Pediatric speech intelligibility test. II. Effect of receptive language age and chronological age. International Journal of Pediatric Otorhinolaryngology. 1981;3(2):101–118. doi: 10.1016/0165-5876(81)90026-4. [DOI] [PubMed] [Google Scholar]
- Jerger S, Lewis S, Hawkins J, Jerger J. Pediatric Speech Intelligibility Test. I. Generation of test materials. International Journal of Pediatric Otorhinolaryngology. 1980;2 doi: 10.1016/0165-5876(80)90047-6. [DOI] [PubMed] [Google Scholar]
- Kaiser AR, Kirk KI, Lachs L, Pisoni DB. Talker and lexical effects on audiovisual word recognition by adults with cochlear implants. Journal of Speech, Language, and Hearing Research. 2003;46(2) doi: 10.1044/1092-4388(2003/032). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KI. Challenges in the clinical investigation of cochlear implant outcomes. In: Niparko JK, Kirk KI, Mellon NK, Robbins AM, Tucci DL, Wilson BS, editors. Cochlear implants: Principals and practices. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 225–259. [Google Scholar]
- Kirk KI, Miyamoto RT, Ying EA, Perdew AE, Zuganelis H. Cochlear implantation in young children: Effects of age at implantation and communication mode. The Volta Review. 2002;102(4):127–144. monograph. [Google Scholar]
- Kirk KI, Pisoni DB, Miyamoto RT. Lexical discrimination by children with cochlear implants: Effects of age at implantation and communication mode. In: Cohen N, editor. Proceedings of the Vth International Cochlear Implant Conference; New York: Thieme Medical Publishers; 2000. [Google Scholar]
- Lachs L, Pisoni DB, Kirk KI. Use of audiovisual information in speech perception by prelingually deaf children with cochlear implants: A first report. Ear and Hearing. 2001;22:236–251. doi: 10.1097/00003446-200106000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman J, Ross M, McLauchlin R. A picture-identification test for hearing-impaired children. Journal of Auditory Research. 1965;5:273–278. doi: 10.1044/jshr.1301.44. [DOI] [PubMed] [Google Scholar]
- Ling D. Auditory-verbal options for children with hearing impairment: Helping to pioneer an applied science. The Volta Review. 1993;95:187–196. [Google Scholar]
- Miyamoto RT, Kirk KI, Svirsky MA, Sehgal ST. Communication skills in pediatric cochlear implant recipients. Acta Otolaryngol. 1999;119:219–224. doi: 10.1080/00016489950181701. [DOI] [PubMed] [Google Scholar]
- Moeller MP, Luetke-Stahlman B. Parents’ use of Signing Exact English: A descriptive analysis. Journal of Speech and Hearing Disorders. 1990;55:327–338. doi: 10.1044/jshd.5502.327. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Bruer JT. Language processing: How experience affects brain organization. In: Lichtman JW, editor. Critical thinking about critical periods. Baltimore: Paul H. Brookes Publishing Company; 2001. pp. 151–172. [Google Scholar]
- Osberger MJ, Robbins AM, Todd SL, Riley AI, Miyamoto RT. Speech intelligibility of children with cochlear implants. The Volta Review. 1994;96:169–180. [Google Scholar]
- Reynell JK, Huntley M. Reynell Developmental Language Scales–Revised. 2. Windsor, England: NFER-Nelson Publishing Company, Ltd; 1985. [Google Scholar]
- Rhoades EA. Early intervention and development of communication skills for deaf children using an auditory-verbal approach. Topics in Language Disorders. 1982;2:8–16. [Google Scholar]
- Robbins AM, Renshaw JJ, Osberger MJ. Common Phrases Test. Indianapolis: Indiana University School of Medicine; 1995. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear and Hearing. 2002;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Spencer PE. The expressive communication of hearing mothers and deaf infants. American Annals of the Deaf. 1993;138(3):275–283. doi: 10.1353/aad.2012.0414. [DOI] [PubMed] [Google Scholar]
- Staller SJ, Dowell RC, Beiter AL, Brimacombe JA. Perceptual abilities of children with the Nucleus 22-Channel cochlear implant. Ear and Hearing. 1991;12(4, Supplemental):34S–47S. doi: 10.1097/00003446-199108001-00006. [DOI] [PubMed] [Google Scholar]
- Sumby WH, Pollack I. Visual contribution to speech intelligibility in noise. The Journal of the Acoustical Society of America. 1954;26(2):212–215. [Google Scholar]
- Svirsky MA, Robbins AM, Kirk KI, Pisoni DB, Miyamoto RT. Language development in profoundly deaf children with cochlear implants. Psychological Science. 2000;11(2):153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher MV, Thompson M. Mothers learning simultaneous communication: The dimensions of the task. American Annals of the Deaf. 1985;130(3):212–217. [PubMed] [Google Scholar]
- Tillberg I, Ronnberg J, Svard I, Ahlner B. Audio-visual speechreading in a group of hearing aid users: The effects of onset age, handicap age, and degree of hearing loss. Scandinavian Audiology. 1996;25:267–272. doi: 10.3109/01050399609074966. [DOI] [PubMed] [Google Scholar]
- Tye-Murray N, Geers A. Children’s Audio-Visual Enhancement Test. St. Louis, MO: Central Institute for the Deaf; 2001. [Google Scholar]
- Tyler RS, Fryauf-Bertschy H, Kelsay D. Audiovisual feature test for young children. Iowa City, Iowa: University of Iowa; 1991. [Google Scholar]
- Tyler RS, Fryauf-Bertschy H, Kelsay DMR, Gantz BJ, Woodworth GP, Parkinson A. Speech perception by prelingually deaf children using cochlear implants. Otolaryngology-Head and Neck Surgery. 1997;117(3):180–187. doi: 10.1016/s0194-5998(97)70172-4. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Parkinson AJ, Woodworth GG, Lowder MW, Gantz BJ. Performance over time of adult patients using the Ineraid or Nucleus cochlear implant. Journal of the Acoustical Society of America. 1997;102:508–522. doi: 10.1121/1.419724. [DOI] [PubMed] [Google Scholar]
- Waltzman SB, Cohen NL, Gomolin RH, Shapiro WH, Ozdamar SR, Hoffman RA. Long-term results of early cochlear implantation in congenitally and prelingually deafened children. American Journal of Otology. 1994;15:9–13. [PubMed] [Google Scholar]
- Waltzman SB, Cohen NL, Gomolin RH, Green JE, Shapiro WH, Hoffman RA, et al. Open-set speech perception in congenitally deaf children using cochlear implants. American Journal of Otology. 1997;18:342–349. [PubMed] [Google Scholar]
- Wolfinger R, Chang M. Comparing the SAS GLM and MIXED procedures for repeated measures. Paper presented at the Proceedings of the Twentieth Annual SAS Users Group International Conference; Orlando, Florida. 1995. [Google Scholar]