Abstract
The hippo pathway and its downstream mediator yes-associated protein 1 (YAP1) regulate mammalian organ size in part through modulating progenitor cell numbers. YAP1 has also been implicated as an oncogene in multiple human cancers. Currently, little is known about the expression of YAP1 either in normal human brain tissue or in central nervous system neoplasms. We used immunohistochemistry to evaluate nuclear YAP1 expression in the fetal and normal adult human brains and in 264 brain tumors. YAP1 was expressed in fetal and adult brain regions known to harbor neural progenitor cells but there was little YAP1 immunoreactivity in the adult cerebral cortex. YAP1 protein was also readily detected in the nuclei of human brain tumors. In medulloblastoma, expression varied between histologic subtypes and was most prominent in nodular/desmoplastic tumors. In gliomas it was frequently expressed in infiltrating astrocytomas and oligodendrogliomas, but rarely in pilocytic astrocytomas. Using a loss of function approach we show that YAP1 promoted growth of glioblastoma cell lines in vitro. High levels of YAP1 mRNA expression were associated with aggressive molecular subsets of glioblastoma and with a non-significant trend toward reduced mean survival in human astrocytoma patients. These findings suggest that YAP1 may play an important role in normal human brain development and that it could represent a new target in human brain tumors.
Keywords: Brain tumor, Glioma, Hippo pathway, Medulloblastoma, YAP1
Introduction
The control of progenitor cell fate specification and differentiation during development are highly conserved processes. Reactivation or dysregulation of the cell signaling pathways that mediate these programs have been identified in many human disease states, including cancer. Recent work in Drosophila and mammalian systems has implicated the hippo pathway in the regulation of progenitor cell fate during development (1-3). The core components of the hippo pathway in Drosophila include the serine/threonine kinases hippo (in humans MST1 and MST2) and warts (in humans LATS1 and LATS2) (4). The hippo pathway is regulated proximally by several proteins, including the protein product of the NF2 tumor suppressor gene merlin (5). The hippo pathway distally regulates the nuclear translocation of yes-associated protein-1 (YAP1) (2). YAP1 in the nucleus acts to co-activate transcription factors including TEAD family members (6, 7). Because YAP1 works through the activity of its transcriptional binding partners, it appears to have the ability to promote proliferation and apoptosis, depending on the cellular context (8-10).
Patients with neurofibromatosis type II (NFII) represent a specific patient population with a defined genetic defect in an upstream mediator of the hippo pathway. However, alterations in the hippo pathway or upregulation of YAP1 have been identified in many cancers that are not classically associated with NFII. For example, amplicons, including the YAP1 locus, have been reported in hepatocellular carcinoma, medulloblastoma, squamous cell carcinoma and mesothelioma (11-14). Upregulation of YAP1 has been shown to promote tumorigenesis in most but not all tumor types evaluated (15). YAP protein overexpression has been shown to be an independent poor prognostic factor in hepatocellular carcinoma (16).
Apart from the classic NFII-associated lesions, little is known about the role of the hippo pathway and YAP1 in nervous system tumors. Upregulation of YAP1 mRNA has been reported in medulloblastoma and ependymoma, both through gene amplification and an increase in transcription (12, 17), and in animal models of medulloblastoma it acts to support tumor growth. However, the expression and potential function of YAP1 in the majority of primary human brain tumors is unknown.
In the current study, we used immunohistochemistry to evaluate YAP1 in normal human brain tissue and found that it is highly expressed in regions of adult and fetal brain known to harbor a high concentration of progenitor cells, but at very low levels in adult cerebral cortex. We also examined the expression of YAP1 in a large panel of primary brain tumors and found that YAP1 upregulation is a common feature of many human brain tumors including infiltrating gliomas. Finally, we found that YAP1 overexpression promotes growth of glioblastoma cell lines in vitro.
Materials and Methods
Construction of Tissue Microarrays and Immunohistochemistry
Human brain tumor samples and normal control tissue from autopsy specimens were obtained from the archives of the Johns Hopkins Hospital Department of Pathology following appropriate institutional review board approval. Classification of each tumor by subtype was performed according to World Health Organization guidelines (18). No information was obtained with respect to NF2 mutation status for tumor samples and no patients were known to have NFII. Formalin-fixed, paraffin-embedded tissue was utilized to construct tissue microarrays according to standard procedures at the Johns Hopkins tissue microarray core facility (19). Immunohistochemistry for YAP1 was performed using a polyclonal antibody specific for human YAP1 (Epitomics, Burlingame, CA; catalog #2209-1; 1:200 dilution), as previously described (20). Four cores of each tumor were used per array. Tumors containing less than 2 cores in the array were excluded from analysis. Each tumor was scored with respect to the percentage of positive tumor nuclei. After obtaining a value of percentage positive nuclei for each tumor, a 3-tiered scoring system was constructed as follows: “no staining” corresponded to no nuclear staining in tumor cells; “low staining” corresponded to 1% to 10% of tumor nuclei positive; and “high staining” corresponded to greater than 10% of the tumor nuclei positive for YAP1, similar to that previously described (20). Nuclear positivity for YAP1 was used as a surrogate of the active fraction of the protein. Formalin-fixed brain sections from an 18-week female fetus and 8 different adult brains were immunostained for YAP1 as a comparison group
Cell Lines and Cell Culture
The human glioblastoma neurosphere line, HSR-GBM1 was cultured as described (21). The adherent glioblastoma cell lines U87MG, U373, and A172 were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, http://www.atcc.org) and maintained in ATCC's recommended growth medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA).
Viral Vectors and Transduction
Control lentiviral vector pKO.1 was obtained from Addgene (Cambridge, MA). The lentiviral vector pKO.1-YAP1, which expresses an shRNA specific for human YAP1, was a kind gift from Dr. Anirban Maitra (Johns Hopkins University). Viral production and transduction were performed as described (22). Following transduction, cell lines were maintained in 2 μg/ml of puromycin (Sigma-Aldrich, St. Louis, MO).
RNA Extraction and Q-PCR
RNA was extracted from cell lines using the Qiagen RNeasy RNA extraction kit (Qiagen, Valencia, CA). RNA levels were analyzed by real-time PCR performed in triplicate with SYBR Green reagents (Applied Biosystems, Foster City, CA), using an I-Cycler IQ5 real-time detection system (Bio-Rad, Hercules, CA), according to the manufacturer's instructions. Expression levels were determined using the standard curve method with all expression levels being normalized to actin. Primers for YAP1 reverse transcription polymerase chain reaction were obtained from SABiosciences (Frederick, MD). Primers for PTCH1B, GLI1, and GLI2 have been previously described (23).
Cell Growth Assay
Cell number and viability were assessed using the Guava PCA and Viacount reagent according to instructions (Guava Technologies, Hayward, CA). Viable cells were plated at equal density in a 96-well plate containing appropriate growth media. Cell growth was measured using CellTiter-96 Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI). Fold increase in cell mass was calculated as the percentage increase over starting A490 nm.
Patient Datasets
Microarray and clinical data were acquired from the Repository for Molecular BRAin Neoplasia DaTa (REMBRANDT) database (https://caintegrator.nci.nih.gov/rembrant/) on September 15, 2010. A total of 343 glioma samples were evaluated. Analysis within tumor grade was performed by evaluating the survival in 181 glioblastomas, 50 grade II astrocytomas, and 44 grade III astrocytomas. Astrocytomas in which the clinical grade was not known were excluded from the analysis. For mRNA expression analysis, expression values derived from Affimetrix U133 Plus 2.0 arrays were obtained from the REMBRANDT dataset. Mean reporter values were evaluated within each tumor type. Tumors with no defined tumor grade were excluded. To evaluate the expression of YAP1 in molecular subsets of glioblastoma, normalized YAP1 expression values from Verhaak et al (24) were evaluated within the 180 glioblastoma samples most representing the 4 distinct subsets of glioblastoma. The mean YAP1 expression was calculated for each subset.
Statistical Analysis
Statistical analyses were performed using the GraphPad Prism 4 software unless otherwise specified (GraphPad software). Evaluation of immunohistochemistry from tissue microarrays utilized either chi-square or the Fisher exact test to evaluate the comparison of negative YAP1 staining vs. positive YAP1 staining (low YAP1 + high YAP1) or low YAP1 vs. high YAP1 staining (negative and low YAP1 vs. high YAP1). In analysis of MTS assays an unpaired Student t test was performed at each time point. Graphed data represent the mean of 3 to 6 replicated samples collected. Error bars represent the standard error of the mean. For mRNA expression analysis, Student t test was performed. In survival analysis the percent survival was calculated using the product limit (Kaplan-Meier) method. Curve comparisons were evaluated using the log rank test (Mantel-Haenszel). For all analyses, differences were considered significant at p ≤ 0.05.
Results
YAP1 Protein Expression in Non-neoplastic Human Brain
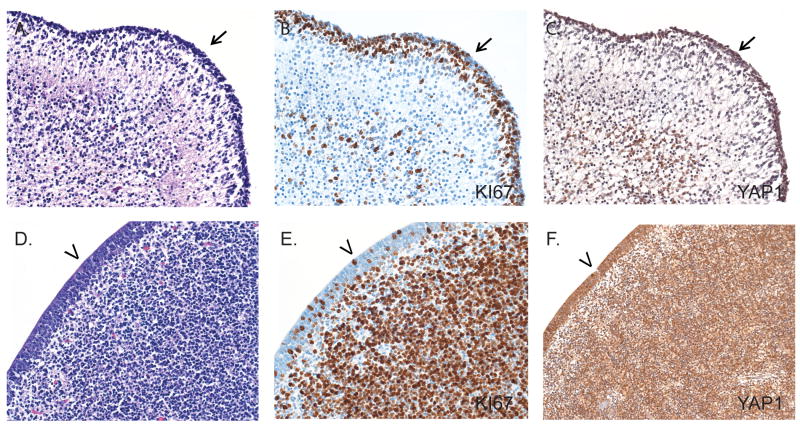
We first examined the expression of YAP1 in non-neoplastic adult and fetal autopsy specimens by immunohistochemistry. We hypothesized that, similar to other developmental pathways such as wnt, notch, and hedgehog, the downstream hippo pathway mediator YAP1 would be expressed during development in the specialized niches known to harbor multipotent neural progenitor cells. In the fetal brain, nuclear YAP1 immunoreactivity was found to be enriched in 2 regions known to contain stem and progenitor cells: the forebrain subventricular zone and the external granular cell layer of the cerebellum (Fig. 1). YAP1 staining was mostly observed in cell nuclei, where it is seen when it is active (25, 26). To evaluate whether YAP1 was enriched in proliferating cells, we stained the fetal tissue for the proliferation marker, Ki67. There was substantial overlap between the immunohistochemical staining for YAP1 and Ki67, suggesting that YAP1 is enriched in proliferating progenitor cell populations.
Figure 1.
Immunohistochemical analysis of YAP1 in normal fetal brain. (A-F) Sections from the external granular cell layer of 18-week-old fetal cerebellum (black arrows, A-C) and the ventricular (arrows) and subventricular zones (D-F) show high levels of YAP1 immunoreactivity (C, F). Areas of YAP1 expression overlap with cells showing high proliferation identified using Ki67 (B, E). Panels A and D are stained with hematoxylin and eosin. Original magnification for all: 100×.
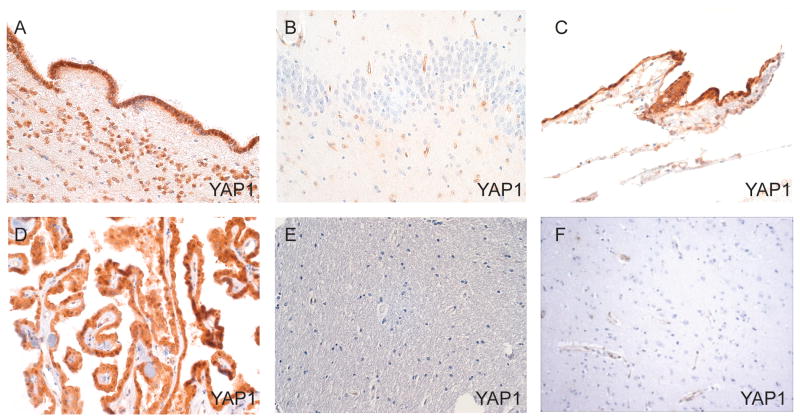
In the adult, YAP1 immunoreactivity was more restricted and showed the greatest staining in the ependyma, choroid plexus, and leptomeninges (Fig. 2). Of note, we did see some weak staining of reactive astrocytes in 1 specimen. Consistent with the high levels of expression observed in the fetal progenitor niche, YAP1 showed high expression in the adult subventricular zone of the lateral ventricle (Fig. 2A). Focal YAP1 positivity was identified in the adult subgranular zone of the dentate gyrus (Fig. 2B), but this staining was not identified in all samples. Very little YAP1 immunoreactivity was identified in the adult cerebral cortex; when present, it was confined to weak vascular staining (Fig. 2E, F). Taken together, these findings support a role for YAP1 in neural development in humans, particularly in brain areas in which there is expansion of neural progenitor cells.
Figure 2.
Immunohistochemical analysis of YAP1 expression in normal adult brain. (A-F) YAP1 expression is identified in the ependymal subventricular zone (A) and focally in the subgranular zone of the dentate gyrus (B). Constitutive YAP1 immunoreactivity is also seen in arachnoid meningothelial cells (C) and choroid plexus (D). In contrast, the cerebral cortex shows little YAP1 expression. The deep white matter of the frontal cortex (E) and the gray matter of the temporal lobe (F) show only weak vascular staining. Original magnification for all: 100×.
YAP1 Protein Expression in Embryonal Brain Tumors
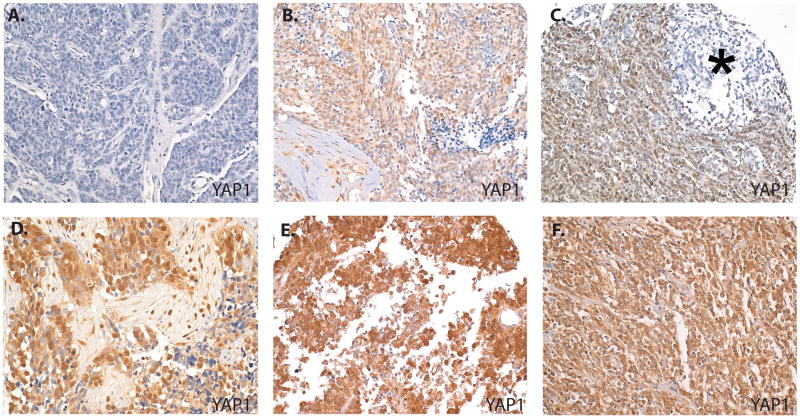
To evaluate the expression of YAP1 protein in human embryonal brain tumors we employed a tissue microarray containing 4 central nervous system primitive neuroectodermal tumors (CNS PNET), 2 medulloepitheliomas, 3 atypical teratoid rhabdoid tumors (ATRTs), and 57 medulloblastomas of various subtypes (Fig. 3; Table 1). Of the 66 embryonal tumors examined, 30 (45%) showed some nuclear YAP1 expression, with no nuclear YAP1 expression in the control brain tissue (0/7). Among medulloblastomas, 22 out of 57 (39%) showed at least some staining for nuclear YAP, with 33% showing high level staining. The nodular desmoplastic subtype had the highest percentage of positive tumors (67%), whereas 32% of anaplastic medulloblastoma and 20% of classic medulloblastoma showed YAP1 immunoreactivity. This enrichment of nuclear YAP1 in the nodular desmoplastic subtype was statistically significant when compared to both classical (p = 0.007) and anaplastic medulloblastomas (p = 0.05). Interestingly, the nodular desmoplastic subtype had a distinct distribution of immunostaining such that the highly proliferative internodular areas showed intense positive staining for YAP1, whereas the intranodular areas had weak or negative immunoreactivity (Fig. 3C). This staining pattern is identical to those we have observed previously for hedgehog mediators including GLI1 (27), suggesting that activity of the 2 pathways may be connected in medulloblastoma.
Figure 3.
Variable YAP1 expression in embryonal tumors. (A) A classical medulloblastoma shows no staining for YAP1. (B) A classical medulloblastoma shows high level YAP1 staining. (C) A nodular desmoplastic medulloblastoma shows high level staining in internodular areas but weak or negative staining in the nodule (*). High YAP1 expression is also shown in a medulloepithelioma (D), an atypical teratoid rhabdoid tumor (E), and a central primitive neuroectodermal tumor (F). Original magnifications: A, B, D-F, 100×; C, 64×.
Table 1. Summary of Nuclear YAP1 Immunohistochemistry in Brain Tumors.
| Tumor | WHO grade | Total (N) |
Extent of staining (% nuclei positive) * |
Negative staining (#) |
Negative (%) |
Low YAP1 (#) |
Low YAP1 (%) |
High YAP1 (#) |
High YAP1 (%) |
|---|---|---|---|---|---|---|---|---|---|
| Embryonal Tumors | |||||||||
| Medullo. (Classic) | IV | 20 | 73 | 16 | 80 | 0 | 0 | 4 | 20 |
| Medullo. with Anaplasia | IV | 19 | 20 | 13 | 68 | 3 | 16 | 3 | 16 |
| Medullo. (Nodular) | IV | 18 | 65 | 7 | 33 | 0 | 0 | 12 | 67 |
| ATRT | IV | 3 | 80 | 0 | 0 | 0 | 0 | 3 | 100 |
| CNS PNET | IV | 4 | 90 | 1 | 25 | 0 | 0 | 3 | 75 |
| Medulloepithelioma | IV | 2 | 78 | 0 | 0 | 0 | 0 | 2 | 100 |
| Astrocytic Tumors | |||||||||
| Pilocytic Astrocytoma | I | 72 | 24 | 50 | 69 | 13 | 18 | 9 | 13 |
| Diffuse Astrocytoma | II | 9 | 14 | 0 | 0 | 7 | 78 | 2 | 22 |
| Anaplastic Astrocytoma | III | 15 | 39 | 0 | 0 | 3 | 20 | 12 | 80 |
| Glioblastoma | IV | 91 | 40 | 18 | 20 | 22 | 24 | 51 | 56 |
| Pediatric GBM | IV | 35 | 33 | 9 | 26 | 12 | 34 | 14 | 40 |
| Adult GBM | IV | 56 | 44 | 9 | 16 | 10 | 18 | 37 | 66 |
| Oligodendroglial Tumors | |||||||||
| Oligodendroglioma | II | 5 | 25 | 0 | 0 | 1 | 20 | 4 | 80 |
| Anaplastic Oligodendroglioma | III | 3 | 13 | 1 | 33 | 1 | 33 | 1 | 33 |
| Oligoastrocytic Tumors | |||||||||
| Oligoastrocytoma | II/III | 3 | 33 | 0 | 0 | 1 | 33 | 2 | 67 |
| Total | 264 | 105 | 40 | 51 | 19 | 108 | 41 |
Mean percent nuclei positive in YAP1-positive tumors within each tumor subtype. “negative staining” = no nuclear staining in tumor cells; “low” = 1-10% of tumor nuclei positive; “high” = > 10 percent of tumor nuclei positive for YAP1; ATRT=atypical teratoid/rhabdoid tumor; CNS PNET=Central nervous system primitive neuroectodermal tumor, GBM=Glioblastoma; Medullo. = medulloblastoma; WHO = World Health Organization.
Among the other embryonal tumors examined, YAP1 immunoreactivity was generally diffuse and intense. High level nuclear YAP1 immunoreactivity was observed in 2 of 2 medulloepitheliomas (Fig. 3D) and 3 of 3 ATRTs (Fig. 3E); 3 out of 4 CNS PNET also showed high level nuclear immunoreactivity (Fig. 3F; Table 1).
YAP1 Protein Expression in Glial Brain Tumors
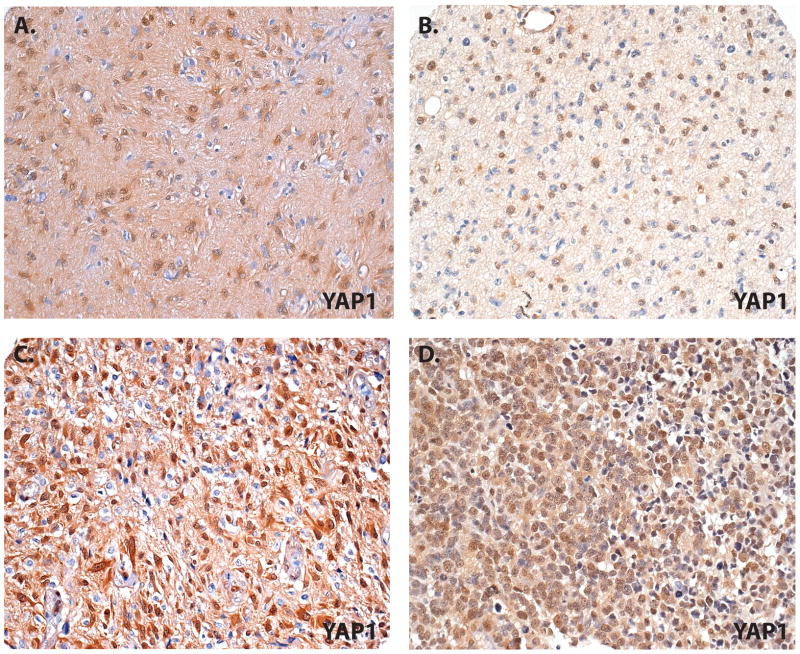
We next evaluated YAP1 expression and cellular localization in 198 astrocytic and oligodendroglial tumors of various World Health Organization (WHO) grades. In each glioma type there was at least 1 case that expressed some nuclear YAP1. Pilocytic astrocytomas (WHO grade I) showed the lowest proportion of YAP1 positivity, with 22/72 tumors (30%) showing some immunoreactivity and only 9/72 (13%) showed high level nuclear staining. In contrast, very high proportions of WHO grade II diffuse astrocytomas (100%), WHO grade III anaplastic astrocytomas (100%), and WHO grade IV glioblastomas (73/91 or 80%) showed at least some nuclear reactivity (Fig. 4). The YAP1 expression (low or high) in infiltrating astrocytomas of all grades was significantly higher vs. that in pilocytic astrocytoma (p <0.001 for all comparisons). Within the group of infiltrating astrocytoma, high-grade tumors, including anaplastic astrocytoma and glioblastoma, showed greater high level staining (80% and 56%, respectively) than diffuse astrocytoma (22%). Both anaplastic astrocytoma and adult glioblastoma had significantly greater high-level nuclear YAP1 expression compared to diffuse astrocytoma (p = 0.01 and 0.02, respectively).
Figure 4.
YAP1 expression in glial neoplasms. (A-D) A pilocytic astrocytoma shows high levels of nuclear YAP1 immunoreactivity (A). High levels of nuclear YAP1 immunoreactivity are more common in examples of diffuse astrocytoma (B), anaplastic astrocytoma (C), and glioblastoma (D). Original magnification for all: 100×.
A significantly greater proportion of glioblastomas resected from adults showed high level staining (66%) vs. pediatric glioblastomas (40%; p = 0.01). Consistent with this observation is that in a recent study by Paugh et al that identified YAP1 among the genes differentially expressed in adult glioblastoma compared to pediatric glioblastoma, higher levels were found in the adult tumors (28).
Nuclear YAP1 was detected in 7/8 oligodendrogliomas including 5/5 grade II oligodendrogliomas and 2/3 grade III oligodendrogliomas. Similarly, 3/3 oligoastrocytomas showed some nuclear YAP1 immunoreactivity (Table 1).
Functional Significance of YAP1 in Glioblastoma
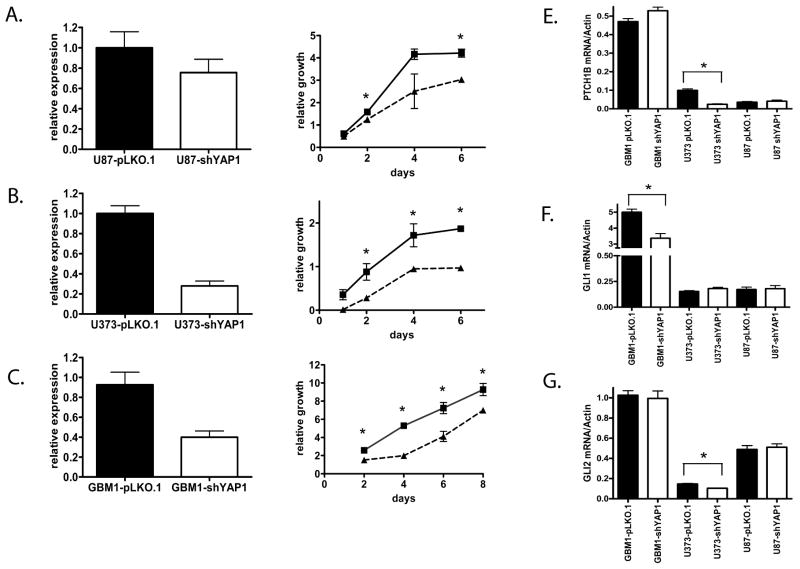
Because YAP1 can have opposite effects on cellular growth depending on the nuclear binding partner utilized (8), we sought to determine whether YAP1 would promote or inhibit glioblastoma growth in vitro using both gain and loss of function approaches. To evaluate the effects of knockdown, we used a lentivirus vector to express an shRNA specific for YAP1. The adherent glioblastoma lines U87 and U373 and the neurosphere line HSR-GBM1 were transduced with virus containing either pLKO.1-shYAP1 or empty pLKO.1 vector. Using quantitative PCR, we documented 25% to 72% reductions in YAP1 mRNA levels following 1 week of selection with puromycin (Fig. 5A-C). Cultures with decreased YAP1 levels were evaluated in a MTS assay; in all 3 glioma lines reductions in YAP1 mRNA were associated with significantly reduced growth. In the U87 cell line, significantly reduced growth was observed at 2 and 6 days (p< 0.001 each) (Fig. 5A), whereas in the U373 cell line significantly reduced growth was observed at 2 days (p < 0.05), 4 days (p < 0.05) and 6 days (p < 0.001) (Fig. 5B). The glioblastoma neurosphere cell line HSR-GBM1 (Fig. 5C) showed significantly reduced growth at 2, 4, 6, and 8 days (all p < 0.05) (Fig. 5C).
Figure 5.
Knockdown of YAP1 decreases cell growth in glioblastoma cell lines. (A-C) YAP1 mRNA expression was knocked down in glioblastoma cell lines U87 (A), U373 (B), and GBM1 (C) using the lentiviral vector pLKO.1 expressing a shYAP1. The decrease in YAP1 mRNA was monitored by Q-PCR (left). Cellular growth was evaluated using the MTS assay (right). The growth relative to starting value was graphed for each cell line transduced with either empty pLKO.1 vector (solid line) or pLKO.1-shYAP1 (hatched lines). Statistical analysis was performed using the Student's t test at each time point. (D-F) The ability of YAP1 knockdown to modulate the hedgehog targets PTCH1B (D), GLI1 (E), and GLI2 (F) in glioblastoma cell lines GBM1, U373, and U87 was assessed by Q-PCR. Statistical analysis was performed using the Student t-test. Asterisks (*) in all graphs represent significance below the p = 0.05 level. Error bars represent the standard error of the mean for 3 to 6 replicates.
Because YAP1 interacts with the hedgehog pathway (12), we next evaluated effects of YAP1 knockdown on mRNA levels of hedgehog targets in glioblastoma (Fig. 5D-F). By quantitative PCR there was a significant downregulation of hedgehog target GLI1 in HSR-GBM1 (p = 0.009) following YAP1 knockdown, whereas downregulation of PTCH1B and GLI2 (p = 0.0008 and 0.004, respectively) were demonstrated following knockdown of YAP1 in U373. U87 showed no difference in expression of hedgehog targets following YAP1 knockdown. Although these findings did not show consistent modulation of individual hedgehog targets, they suggest that (as in medulloblastoma) there may be an interaction between the hedgehog pathway and YAP1 in glioblastomas.
Survival Analysis of YAP1 Expression in Glioblastoma
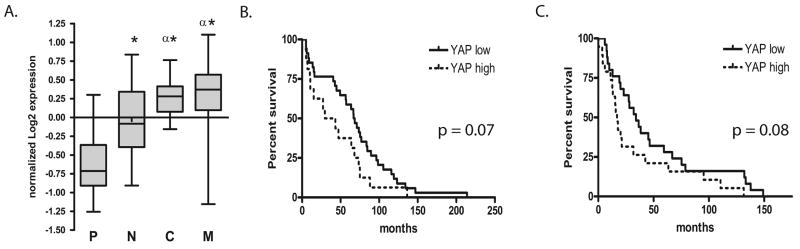
We next evaluated whether YAP1 expression was prognostic of survival. The publically available REMBRANDT database was used to compare glioma patients that overexpress YAP1 by over 2-fold (high YAP1) to the remaining patient samples (low YAP1). Three different cohorts of patients were examined, including those with grade II astrocytomas (Fig. 6B), grade III astrocytomas (Fig. 6C), and glioblastomas (data not shown). Survivals were evaluated using the log rank test. In each comparison group there were significantly worse outcomes in the high YAP1 group vs. the low YAP1 group with respect to median survival (36 vs. 68 months in grade II astrocytomas; 17 vs. 34 months in grade III astrocytoma; and 13 vs. 17 months in glioblastoma). These trends towards differences in survival were not statistically significant (p = 0.07, p = 0.08 and p = 0.10, respectively; Table 2).
Figure 6.
YAP1 Expression and survival in glioma patients. (A) Normalized YAP1 expression values from Verhaak et al (24) were graphed for each subtype of glioblastoma. Significant difference was analyzed by the Student t-test. Asterisks represent p < 0.0001 for the neural (N), classic (C), and mesenchymal (M) subtypes compared to the proneural subgroup (P). A significant increase was also observed in the mesenchymal and classical subtypes compared to the neural subtype (“α”, p < 0.001). (B, C) Patients with either grade II astrocytomas (B) or grade III astrocytomas (C) were stratified with respect to YAP1 either greater than 2-fold overexpression (“high YAP1”, hatched curve) or less than 2-fold overexpression (“low YAP1”, solid curve). Using the log rank test, significance between groups was not met for grade II astrocytoma (p = 0.07) or grade III astrocytoma (p = 0.08).
Table 2. Relationship between Survival and YAP1 Expression in Glioma Patients.
| YAP1 Expression | |||||
|---|---|---|---|---|---|
| Tumor | <2 (N) |
>2 (N) |
<2.0 Median Survival (months) |
>2.0 Median Survival (months) |
p value |
| All gliomas | 198 | 145 | 28.3 | 15.0 | <0.0001 |
| Grade II astrocytomas | 34 | 16 | 67.5 | 36.3 | 0.07 |
| Grade III astrocytomas | 25 | 19 | 34.1 | 17.0 | 0.08 |
| Glioblastomas | 84 | 97 | 17.0 | 13.4 | 0.10 |
Survival data in glioma patients showing high YAP1 expression (>2-fold expression) compared to low YAP1 expression (<2.0-fold expression). p values were generated using the log rank test; p < 0.05 is considered significant.
Discussion
We show that YAP1 is highly expressed in the developing human fetus and adult brain in regions known to harbor populations of stem and neural progenitor cells but at relatively low levels in the adult cerebral cortex. This is consistent with studies in other organisms such as the chick neural tube (29). Similarly, overexpression of the YAP1 homologue yorkie in Drosophila neuroepithelia prevents differentiation and promotes neural progenitor cell expansion (30). Fernandez et al demonstrated YAP1 expression in the external granular cell layer of the developing murine cerebellum (12) and our data suggest that this also occurs in humans. We found virtually no YAP1 immunoreactivity in neurons in the adult cerebral cortex, consistent with recent in vitro studies showing that YAP1 expression in embryonic stem cells is downregulated following neural differentiation (3).
Previous studies have shown that YAP1 is regulated downstream of the hippo pathway and can be modulated by loss of NF2 in mammals (5). Interestingly, we found YAP1 expressed at high levels in arachnoid meningothelial cells and in ependymal cells lining the ventricles. Because patients with NF2 have a predisposition to meningiomas and ependymomas (18), our findings suggest that high constitutive YAP1 expression might confer a special vulnerability to these tissues for neoplastic transformation. This expression pattern in normal tissue also suggests that YAP1 is not merely a surrogate of proliferating cells, as it is present in some highly proliferative cells (i.e. fetal neural progenitor populations) and some relatively quiescent populations such as adult ependymal cells.
An interaction between the hedgehog pathway and YAP1 was recently identified in medulloblastoma by Fernandez et al and validated in murine models (12). Our findings of enrichment of nuclear YAP1 expression in the nodular/desmoplastic subtype of medulloblastoma, which is associated with high hedgehog signaling (31), support this relationship. Furthermore, the distribution of YAP1 staining (high in internodular areas and weak or absent within nodules) is identical to the pattern we previously reported for mediators of the hedgehog pathway including GLI1 (27). The finding of high YAP1 expression in the nodular/desmoplastic subtype of medulloblastoma was also recently reported by others (32). We also found high levels of nuclear YAP1 protein in ATRT and PNET, suggesting a potential role in embryonal tumors other than medulloblastoma.
In glial tumors we identified expression of YAP1 protein in tissue derived from multiple types of astrocytoma and oligodendroglioma. Nuclear YAP1 protein expression was a common feature in infiltrating astrocytic tumors including glioblastoma, but was less common in pilocytic astrocytomas. Consistent with our immunohistochemical studies of YAP1 protein, analysis of the REMBRANDT database revealed that YAP1 mRNA was elevated in infiltrating astrocytomas compared to control brain tissue (Figure, Supplemental Digital Content 1, http://links.lww.com/NEN/A244). High levels of nuclear YAP1 protein were more common in adult glioblastomas on our arrays as compared to those in pediatric patients, which is consistent with a recent report that YAP1 mRNA expression is significantly higher in adult than in pediatric glioblastomas (28). Little else has been reported to date regarding the expression or role of YAP1 in gliomas, although its mRNA has been noted in ependymomas (17).
Although in many tumors YAP1 functions as an oncogene (4), previous studies have demonstrated that YAP1 can act to promote or inhibit tumor growth depending on cellular context (10). Thus, defining the functional role of YAP1 expression in individual tumor types is critical. Using a loss of function approach we found that YAP1 promotes glioblastoma growth in vitro. Moreover, we observed modulation of hedgehog targets GLI1, GLI2, and PTCH1B in some, but the results were not consistent among the 3 glioblastoma cell lines examined.
Evaluation of the REMBRANDT database revealed a trend toward YAP1 overexpression and poor prognosis in glioma patients. Among those with astrocytic tumors grades II, III, and IV, median survival was consistently shorter in the high YAP1 group, although the differences were not statistically significant. Finally, when we examined the 4 molecular glioblastoma subtypes recently reported by Verhaak et al (24), our analysis of their dataset revealed significantly higher YAP1 levels in the “classical” and “mesenchymal” groups that are associated with the worst median survival. It is unclear why high YAP1 expression associates with more clinically aggressive glioblastoma subtypes (classical and mesenchymal) but more favorable medulloblastoma subtypes (WNT- and SHH-tumors) (24, 32). Whether this represents a complex interaction of YAP1 with different treatment modalities or whether YAP1 acts as a surrogate for other factors that segregate within different tumor types remains to be determined. The latter appears to be at least partially true in medulloblastoma as few of the YAP-positive tumors have c-myc amplification (32).
The mechanism(s) by which YAP1 expression and activity are regulated in gliomas are unclear. NF2 is known to affect the hippo pathway, but while Lau et al previously demonstrated that NF2 is decreased in glioblastoma but not anaplastic astrocytoma (33), we found high nuclear expression of YAP1 in both grade III and grade IV astrocytomas, suggesting that NF2 may not be the key regulator. It is possible that the hippo pathway may be modulated by other factors upstream of YAP1. For example, Xu et al found that overexpression of CD44 in glioblastoma inhibited the hippo pathway and allowed YAP1 to remain in the active, hypophosphorylated state in U87MB and U251 glioblastoma cell lines (34). Furthermore, Jiang et al evaluated 88 human astrocytoma specimens and found frequent loss of LATS1 and LATS2 expression due to promoter hypermethylation in infiltrating astrocytomas (35).
In summary, this study demonstrates that YAP1 protein is expressed in both human neural stem and progenitor cells and a number of human brain tumor types. Elevated nuclear immunoreactivity was prominent in high-grade gliomas, suggesting a potential role in the pathobiology of the most common malignant brain tumors. Consistent with this concept, YAP1 promoted glioblastoma growth in vitro and high-level expression was associated with clinically aggressive glioblastoma subtypes. Taken together, these findings suggest that the hippo pathway and YAP1 represent a potential new therapeutic target in malignant gliomas.
Supplementary Material
Acknowledgments
We thank Norman Barker in the Johns Hopkins Pathology Photography, Digital Imaging, and Graphic Arts Laboratory for expert assistance preparing photomicrographs.
This work was supported by R01 NS055089 (C.G.E.), R01 DK081417 (R.A.A.), and a resident research grant from The Johns Hopkins Department of Pathology (B.A.O.)
References
- 1.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lian I, Kim J, Okazawa H, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–18. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang N, Bai H, David KK, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu S, Liu Y, Zheng Y, et al. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–98. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Ren F, Zhang Q, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–87. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapi E, Di Agostino S, Donzelli S, et al. PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol Cell. 2008;32:803–14. doi: 10.1016/j.molcel.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Matallanas D, Romano D, Yee K, et al. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell. 2007;27:962–75. doi: 10.1016/j.molcel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan M, Tomlinson V, Lara R, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–9. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 11.Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-L A, Northcott PA, Dalton J, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–41. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taniguchi T, Karnan S, Fukui T, et al. Genomic profiling of malignant pleural mesothelioma with array-based comparative genomic hybridization shows frequent non-random chromosomal alteration regions including JUN amplification on 1p32. Cancer Sci. 2007;98:438–46. doi: 10.1111/j.1349-7006.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snijders AM, Schmidt BL, Fridlyand J, et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene. 2005;24:4232–42. doi: 10.1038/sj.onc.1208601. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-L A, Kenney AM. The Hippo in the room: A new look at a key pathway in cell growth and transformation. Cell Cycle. 2010 Jun 30;9(12) doi: 10.4161/cc.9.12.11919. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Xu MZ, Yao TJ, Lee NP, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–85. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modena P, Lualdi E, Facchinetti F, et al. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol. 2006;24:5223–33. doi: 10.1200/JCO.2006.06.3701. [DOI] [PubMed] [Google Scholar]
- 18.Louis DN International Agency for Research on Cancer. WHO classification of tumours of the central nervous system. Lyon: International Agency for Research on Cancer; 2007. [Google Scholar]
- 19.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 20.Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–9. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vescovi AL, Parati EA, Gritti A, et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol. 1999;156:71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- 22.Bar EE, Lin A, Mahairaki V, et al. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. 2010;177:1491–1502. doi: 10.2353/ajpath.2010.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–33. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yagi R, Chen LF, Shigesada K, et al. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999;18:2551–62. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu S, Totty NF, Irwin MS, et al. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 27.Bar EE, Chaudhry A, Farah MH, et al. Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am J Pathol. 2007;170:347–55. doi: 10.2353/ajpath.2007.060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28:3061–8. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–34. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy BV, Rauskolb C, Irvine KD. Influence of fat-hippo and notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. Development. 2010;137:2397–2408. doi: 10.1242/dev.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–31. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 32.Ellison DW, Dalton J, Kocak M, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381–96. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau YK, Murray LB, Houshmandi SS, et al. Merlin is a potent inhibitor of glioma growth. Cancer Res. 2008;68:5733–42. doi: 10.1158/0008-5472.CAN-08-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Stamenkovic I, Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010;70:2455–64. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Z, Li X, Hu J, et al. Promoter hypermethylation-mediated down-regulation of LATS1 and LATS2 in human astrocytoma. Neurosci Res. 2006;56:450–8. doi: 10.1016/j.neures.2006.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.