Abstract
The Fragile Histidine Triad gene or FHIT functions as tumor suppressor in many epithelial cell types. Although its tumor suppressive mechanism is the subject of intense study, less is known about how FHIT gene expression itself is regulated. Here we show that PI3 kinase and its downstream target AKT suppress FHIT gene expression in response to growth factor stimulation in actively cycling cells. Upon removal of mitogens from the culture environment, FHIT mRNA and protein levels are observed to increase as a result of derepression from these protooncogenic kinases. AKT signaling through the FOXO transcription factors appears to be the basis for FHIT gene regulation. Increases in FHIT gene expression are directly dependent on endogenous FOXO3a in MCF7 breast carcinoma cells as evidenced by experiments with RNAi targeting FOXO transcription factor family members. Thus, this is the first report demonstrating that FHIT gene expression is normally repressed in actively cycling cells through the PI3K/AKT/ FOXO3a axis.
Keywords: FHIT, mitogen signaling, PI3K, AKT, FOXO
Introduction
Fragile Histidine Triad or FHIT is a tumor suppressor gene vital to the maintenance of proper cell growth and division. Although the exact mechanism of tumor suppression by FHIT remains unclear, it is apparent that in its absence, cell cycle homeostasis is often perturbed resulting in the development of soft tissue tumors. First identified in digestive tract cancers, FHIT gene expression was discovered to be absent as a consequence of deletions in the coding region of the gene [1, 2]. Analysis of human lung cancer samples provided evidence for a link between chronic exposure to tobacco smoke and loss of FHIT expression [3]. It was later revealed that the FHIT locus was indeed particularly sensitive to the genomic damage inflicted by recurring exposure to carcinogenic compounds present in tobacco products [2, 4-6]. Subsequently, it has been determined that the FHIT locus, 3p14.2, encompasses one of the most common chromosomal fragile sites in the human genome—the FRA3B locus [1]. Recurrent carcinogen exposure is suspected to result in fragile site expression and consequently increased susceptibility to allelic loss of function and as such, loss of protection from tumor development normally afforded by FHIT [7]. Moreover, loss of FHIT gene expression by these mechanisms is now known to be one of the earliest events in lung cancer carcinogene-sis—an observation that provides a rationale for monitoring FHIT as a biomarker for high throughput detection of early stage lung carcinoma [8].
In addition to links between FHIT expression and lungcarcinogenesis, FHIT expression levels are reduced in certain types of breast cancer [9-20]. FHIT has been suggested to protect against HER2-driven breast tumor development. Additionally, loss of heterozygosity in breast tumors is associated with decreased patient survival and increased tumor burden [9]. Further, reduced expression of FHIT has been shown to correlate with the expression of other fragile site -susceptible tumor suppressors such as WWOX in high grade ductal carcinoma in situ (DCIS) breast cancers [9].
It is clearly evident that our understanding of how FHIT functions as a tumor suppressor is advancing; however less is known regarding how FHIT gene expression itself is regulated. Guo and Vishwanathan reported that FHIT gene expression was constitutive throughout the cell cycle of 293FT cells [21]. Somewhat paradoxically for a putative tumor suppressor, the overexpression of E2F1 has been reported to result in the elevation of FHIT [22]. Counter to these findings we present evidence in the present study that the regulation of FHIT mRNA and protein expression is determined by cell cycle status. In particular, FHIT gene expression increases as cells are arrested in the G1 phase of the cell cycle, where E2F1 is normally inactive. We provide evidence that FHIT gene expression is dependent on the modulation of the PI3K/ AKT/FOXO pathway downstream of cell cycle status.
Materials and methods
FOXO expression vectors FLAG-FOXO3a WT (Addgene plasmid 8360) and FLAG-FOXO3a TM (Addgene plasmid 8361) were kind gifts from Michael Greenburg through the Addgene repository (www.addgene.org) [23, 24]. FOXO expression vectors FLAG-FOXO3a WT (Addgene plasmid 8360) and FLAG-FOXO3a TM (Addgene plasmid 8361) were kind gifts from Michael Greenburg through the Addgene repository (www.addgene.org) [23, 24]. FLAG FKHR AAA mutant was a generous gift from Kunliang Guan [25]. FLAG FKHR AAA mutant (FOXO1 TM, Addgene plasmid 13508) was a generous gifts from Kunliang Guan [25]. pcDNA3.1-(constitutively active)-caAKT and pcDNA3.1-(kinase dead)-kdAKT were kind gifts from Dr. Lindsay Mayo (Indiana University). A validated lenti-shFOXO3a Mission construct was purchased from Sigma Aldrich.
RT-PCR
Total RNA was isolated using the e.Z.N.A. Total RNA kit (Omega Bio-Tek) according to the manufacturer's instructions. RNA quality and quantity was assessed on a RNA nanochip (Agilent Technologies). One microgram of total RNA was used as a template for cDNA synthesis using the reverse transcriptase core reagent kit (Applied Biosystems). Taqman based PCR was performed in triplicate using Assay on Demand probe sets (Applied Biosystems) and an Applied Biosystems 7900 Sequence Detection System. GAPDH was used as an endogenous control.
Cell lines
All cell lines were maintained at 37 degrees Celsius in a 5% carbon dioxide environment. All cell lines with the exception of MCF10A immortalized mammary epithelial cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with either 10% fetal bovine serum (FBS) or 10% neonatal calf serum (NCS) and 50 μg/mL Gentamycin sulfate. MCF10A cells were grown in Mammary Epithelial Growth Medium (Lonza) supplemented with a Clonetics MEGM BulletKit containing the following supplements: recombinant epidermal growth factor (rEGF), insulin, 50 μg/mL Gentamycin, bovine pituitary extract (BPE), hydrocortisone and 100 ng/ml Cholera toxin. Each BulletKit contained pre-aliquoted amounts of each supplement that would establish 1X concentrations after dilution in 500 mL of MEGM base media. All of these tumor lines were purchased from the American Type Culture Collection.
Immunoblotting
Cells were scraped into 1X PBS on ice and pelleted by centrifugation. Whole-cell extracts were obtained by lysing cells in single lysis buffer [50 mmol/L Tris (pH 8.0), 150 mmol/L NaCI, 1% NP40] containing a protease inhibitor cocktail (Sigma) on ice for 30 minutes followed by soni-cation in ice water with a tapped-horn dismem-brator (Fisher Scientific) for 2 minutes at 40% amplitude. Sixty micrograms of total protein was assayed by SDS-PAGE and transferred to an Immobilon transfer membrane (Millipore) at one constant amp for one hour. Membranes were blocked in 5% bovine serum albumin and 0.1% Tween20 in 1X TBS for one hour at room temperature with gentle agitation. Primary antibodies were applied to blots overnight at 4 degrees C, followed by five eight minute washes with IX TBS containing 0.1% Tween20. Primary antibody dilutions in blocking buffer are as follows: polyclonal anti-FHIT 1:1000 (Zymed), Anti rabbit -phospho S473 AKT 1:1000 (Santa Cruz) polyclonal anti-AKT 1:1000 (Cell Signaling), monoclonal anti-FLAG 1:5000 (Invitrogen), monoclonal anti-actin 1:10,000 (Santa Cruz), polyclonal anti-FHIT 1:1000 (Zymed) and polyclonal anti-GADD45A 1:2500 (Santa Cruz). Blots were incubated with either anti-rabbit or anti-mouse HRP-conjugated secondary antibodies 1:5000 (Promega) for one hour at room temperature. Blots were then washed as mentioned above and exposed to SuperSignal West Pico Chemilu-minescent Substrate and imaged by LAS-4000 imaging system (Fujifilm).
Lentiviral induction
Lentiviral vectors were produced by cotransfection of 293FT cells with either plenti-shFOXO3a or pLenti-shControl and lentiviral packaging mix (Invitrogen) according to the manufacturer's instructions. Lentivirus-containing supernatant was harvested at 48 hours after transfection, purified by filtration through a 0.45μm filter and stored at -80°C. Infections were carried overnight in the presence of 10 μg/mL Polybrene (Sigma). Infections were followed with 10 μg/mL puromycin for 48h prior to the initiation of wortmannin treatment.
Results
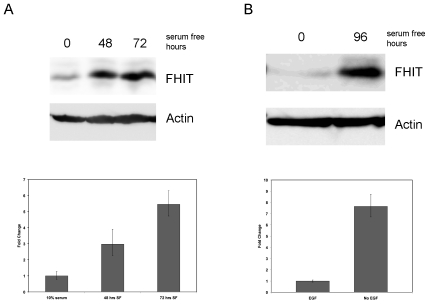
To determine the effect of cellular entry into quiescence on FHIT gene expression, MCF7 breast carcinoma cells were deprived of serum for forty-eight and seventy-two hours to elicit a G1 cell cycle arrest followed by examination of FHIT mRNA and protein levels. A time-dependent increase in FHIT expression at both the transcript and protein levels was evident when compared to cells permitted to propagate in 10% serum (Figure 1A). A similar increase in FHIT protein expression was also seen in HCT116 colon carcinoma cells 48h after serum removal (data not shown). To ensure that the increase in FHIT expression seen in serum starved tumor cell lines was not dependent on genetic deregulation inherent to transformed cells, immortalized MCF10A mammary epithelial cells were deprived of epidermal growth factor (EGF) and assessed for FHIT expression by qPCR and Western blot (Figure 1B). An increase in both FHIT mRNA and protein levels was evident compared to actively dividing control cells exposed to EGF. From these experiments it was concluded that an upregulation of FHIT gene expression occurs as mitogen-deprived cells arrested in the G1 phase of the cell cycle.
Figure 1.
FHIT expression increases in response to mitogen deprivation. (A) MCF7 breast carcinoma cells were treated with serum containing media (0) or serum starved for 48 and 72 hours. FHIT protein and mRNA levels were determined by Western blotting (upper panel) or RT-PCR (lower panel). The Western blot was reprobed for beta-actin to demonstrate equivalent loading. Error bars represent 95% confidence intervals in the fold change of FHIT expression. (B) MCF10A immortalized mammary epithelial cells were grown with (0) or without (96) EGF for 96 hours. Western (top panel) and RT-PCR (bottom panel) were determined as described in (A).
PI3 kinase functions downstream of many growth factor receptor families as a second messenger kinase. Its involvement in transmitting progrowth/proliferation signals to the nucleus from growth factor receptors imbedded in the cell membrane has been studied extensively [26]. We hypothesized that growth factor receptor stimulation may act to repress FHIT expression in actively dividing cells. It was thought that when cell division is arrested by mitogen depletion, the consequential inhibition of second messenger signaling transmission via PI3 kinase may lead to the release of this repression on FHIT gene expression. To address this possibility, PI3 kinase was investigated using small molecule inhibitors to determine if FHIT gene expression could be modulated in a similar fashion to that observed during mitogen deprivation.
MCF7 cells were treated with the PI3 kinase inhibitor Wortmannin (Figure 2) or LY294002 (Figure 3), both of which have been shown to inhibit PI3K activity in vivo [27-29]. As anticipated, an increase in FHIT mRNA expression was observed 20 hours following Wortmannin treatment (Figure 2) and 7 hours following LY-294002 (Figure 3). The increase in phosphorylation of AKT by PI3 kinase at serine 273 demonstrates that the Wortmannin treatment inhibited PI3 kinase activity (Figure 2, inset). Also increases in p27 (Figure 3) and Gadd45 (data not shown), genes known to be inhibited by PI3K [24], demonstrate that LY293004 inhibited PI3K.
Figure 2.
Inhibition of PIP3 kinase activity with Wortmannin leads to upregulation FHIT gene expression. MCF7 cells were treated for increasing periods of time (hours) with 1 μM Wortmannin, a specific inhibitor of PIP3 kinase activity and then analyzed for FHIT mRNA expression by RT-PCR. (Inset) Western blot demonstrating a decrease in AKT phos-phorylation relative to total AKT following exposure of MCF7 cells to Wortmannin.
Figure 3.
Inhibition of PIP3 kinase with LY-294002 leads to an upregulation FHIT gene expression. MCF7 cells were treated with 50 μM LY-294002, a specific PIP3K inhibitor for increasing amounts of time and then analyzed for FHIT (black bars) and p27 (grey bars). Gadd45a mRNA expression was also shown to increase with LY-294002 treatment (data not shown). Error bars represent 95% confidence intervals.
The serine/threonine protein kinase, AKT is activated downstream of PI3 kinase in response to cellular quiescence mediated by mitogen depletion. Also known as protein kinase B, AKT functions as a critical mediator of growth factor signal transduction by phosphorylating target proteins involved in perpetuation or inhibition of cell growth and division [30]. To explore whether FHIT gene expression was regulated through AKT signaling, transient transfections were performed where constitutively active AKT (AKT) or kinase-dead AKT (kd-AKT) were transfected into MCF7 cells that were the subsequently serum starved for 72 hours and treated with Wortmannin. Analysis of FHIT mRNA levels by qPCR revealed that transfection of AKT was able to partially block the increase in FHIT mRNA normally seen in response to serum deprivation/Wortmannin treatment while the kinase dead AKT mutant showed no inhibition of FHIT expression (Figure 4).
Figure 4.
Overexpression of a constitutively active AKT impedes the serum deprivation-dependent increase in FHIT expression. MCF7 cells were trans-fected with expression plasmids encoding no protein (CMV), constitutively active AKT (AKT) or kinase dead AKT (KD-AKT) prior to being serum deprived or remaining in complete media. Cells were serum starved for 72 hours and treated with 1 μM Wortmannin for 24 hour prior to analysis of FHIT gene expression by RT-PCR. Error bars represent 95% confidence intervals.
The results from these experiments where encouraging in that they provided evidence that FHIT expression could be increased by inhibiting PI3K/AKT activation downstream of growth factor stimulation. However, determining the identity of other intermediates involved in this regulation proved challenging due to the extensive number of growth regulating proteins that are downstream of AKT signaling. In order to narrow this search, we focused first on transcription factors that were themselves negatively regulated by PI3 kinase/AKT activation. The Fork-head or FOXO transcription factors were selected as candidates that could be potentially implicated in the regulation of FHIT gene expression in that their transcriptional activity is impeded by phosphorylated (active) AKT. As homo-logues of the Daf-16 gene in C. elegans, FOXOs have been associated with the molecular regulatory mechanisms of aging and insulin signaling [31]. Additionally, they have been found to regulate genes that are involved in tumor suppression [32-35]. In cells that are actively dividing, FOXOs are phosphorylated at three key alanines by AKT and as a result, sequestered from the nucleus and effectively prevented from driving target gene expression [31]. With the onset of growth arrest, such as during the absence of mitogenic stimulus, AKT is no longer active. FOXOs in turn are no longer phosphorylated and therefore permitted to enter the nucleus to transactivate gene targets. Important FOXO target genes include p27, p21, GADD45, BIM and FasL, which all have roles in tumor suppression either by facilitating cell cycle regulation, apoptosis or DNA repair [31].
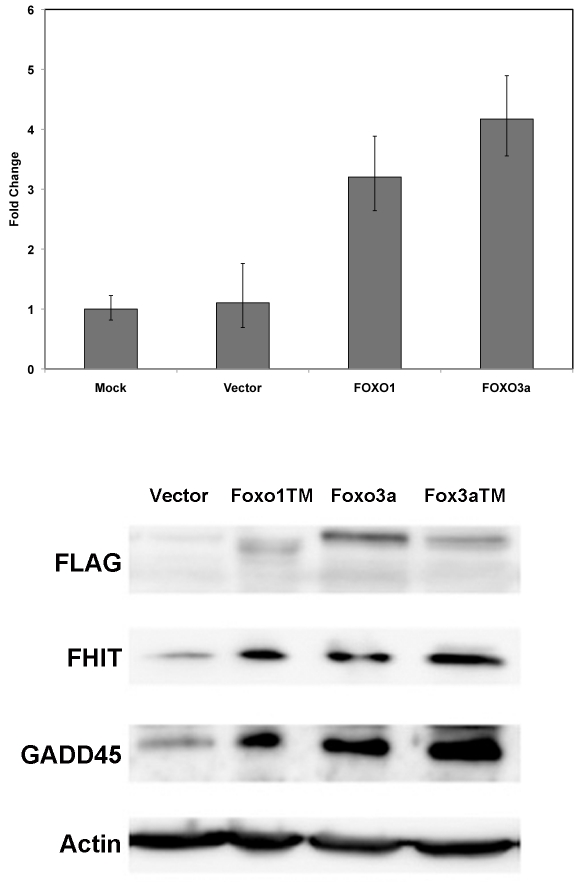
To explore the possibility that FOXO transcription factors may play a role in FHIT regulation, expression vectors of FOXO1 TM, FOXO3a, FOXO3a TM where transiently transfected into MCF7 cells. The “TM” denotes a triple mutation of all three serines targeted for phosphorylation by AKT to alanines [23]. These TM mutants are not susceptible to cytoplasmic sequestration in actively dividing cells and as such facilitate more efficient FOXO transactivation of FOXO transcriptional target genes. Exogenous expression of various FOXOs resulted in increases in FHIT gene expression (Figure 5, top panel) and FHIT protein (Figure 5, lower level). GADD45, a known FOXO regulated gene also showed increases at the protein level following overexpression of the various FOXOs.
Figure 5.
FHIT protein expression increases in response exogenous expression of the FOXO1 or FOXO3a transcription factors. Top panel: MCF7 cells were transfected with no DNA (mock), CMV plasmid (Vector), FLAG -FOXO1 TM plasmid or FLAG-FOXO3a plasmid. Forty-eight hours post-transfection RNA was isolated and used in RT-PCR to determine FHIT gene expression in the various cell transfections. Error bars represent 95% confidence intervals. Bottom panel: MCF7 cells were transfected with the indicated plasmids. Forty-eight hours later, whole cell extracts were analyzed by SDS-PAGE and probed with the indicated antibodies.
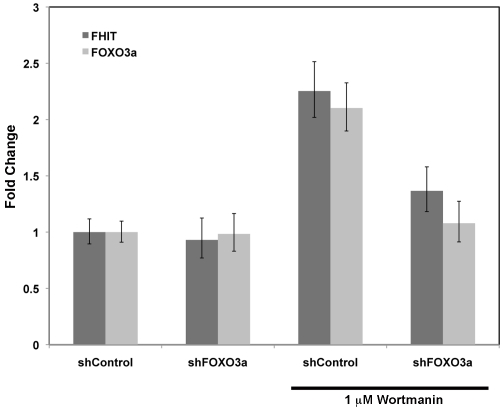
Finally we examined whether the loss of FOXO3a expression impacted Wortmannin-induced FHIT expression. FOXO3a was chosen because this was the FOXO family member most abundantly expressed in MCF7 cells (data not shown). Utilizing a shRNA designed to target FOXO3a expression, MCF7 cells were infected with lenti-shFOXO3a and after 48h of selection with puromycin, treated with 1μM Wortmannin for twenty-four hours. As anticipated, compared to cells infected and selected with control shRNA lentivirus, infection with shFOXO3a blocked the increase in FHIT transcript levels seen in response to PI3K inhibition with Wortmannin (Figure 6). This result demonstrates that modulation of FHIT gene expression down-stream of Wortmannin-dependent inhibition of PI3K is through FOXO3a.
Figure 6.
PIP3K-dependent FHIT gene repression requires FOXO3a. MCF7 cells were infected with lenti-shRNA expression constructs targeting no human gene (shControl) or FOXO3a (shFOXO3a). Seventy-two hours post-infection, 1 μM Wortmannin was applied to cells for twenty-four hours. RNA was isolated and FHIT (dark gray bars) and FOXO3a (light gray bars) gene expression analyzed by RT-PCR. Error bars represent 95% confidence intervals.
Discussion
The goal of this study was to determine how FHIT gene expression may be regulated with the anticipation that the mechanism and timing of this regulation may influence its biological function with regard to its tumor suppressive potential. Others have shown prior to this study for example that exogenous E2F1 can activate FHIT transcription [22]. This transcription factor is normally released from repression by Rb to drive expression of genes involved in cellular progression through S phase. Thus we might expect to see FHIT expression levels modulate similarly to that of S-phase cyclins, in a more cyclical pattern of expression during the cell cycle. In contrast to this expectation, Guo and collogues have reported in 293T cells that FHIT expression does not appear to be under any type of cell cycle control [21]. With these studies in mind, it is likely that there are several competing factors involved in the regulation FHIT gene expression and that each may influence FHIT transcription and transcript stability to different degrees based on cell type and line-age as well as microenvironment and growth factor conditions.
Our results provide evidence that FHIT expression is increased as cells, deprived of growth factors, enter a quiescent state, as opposed to those that are actively cycling. Further we provide a potential mechanism whereby the AKT/ PI3K/FOXO3a axis is likely to be contributing to these changes in gene expression. The data support the model whereby FHIT gene expression is normally kept at low levels in asynchronously growing cells by growth factor receptor stimulation and concomitant activation of PI3K and AKT. These oncogenic kinases indirectly suppress FHIT's expression by preventing FOXO3a from entering the nucleus. Regarding what is known about FHIT as a growth suppressive tumor suppressor protein, it would make biological sense that FHIT's basal expression in the actively cycling cell would be maintained at repressed levels, and that upon exit from the cell cycle, FHIT expression is seen to increase. Drawing from these observations and what is known about FHIT's biological activity, FHIT may contribute to facilitating the balance between cellular quiescence and cell division. It is anticipated that these findings will assist in pinpointing the mechanism of this enigmatic protein's tumor suppressive function.
Acknowledgments
We would like to thank the members of the Berberich Laboratory for their insightful comments. These studies were supported by the National Cancer Institute (CA66430 to SJB).
References
- 1.Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, Croce CM, Huebner K. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 2.Smith DI, McAvoy S, Zhu Y, Perez DS. Large common fragile site genes and cancer. Semin Cancer Biol. 2007;17:31–41. doi: 10.1016/j.semcancer.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Yanagisawa K, Kondo M, Osada H, Uchida K, Takagi K, Masuda A, Takahashi T. Molecular analysis of the FHIT gene at 3p14.2 in lung cancer cell lines. Cancer Res. 1996;56:5579–5582. [PubMed] [Google Scholar]
- 4.D'Agostini F, Izzotti A, Balansky R, Zanesi N, Croce CM, De Flora S. Early loss of Fhit in the respiratory tract of rodents exposed to environmental cigarette smoke. Cancer Res. 2006;66:3936–3941. doi: 10.1158/0008-5472.CAN-05-3666. [DOI] [PubMed] [Google Scholar]
- 5.Pang Y, Li W, Ma R, Ji W, Wang Q, Li D, Xiao Y, Wei Q, Lai Y, Yang P, Chen L, Tang S, Lin Y, Zhuang Z, Zheng Y, Chen W. Development of human cell models for assessing the carcinogenic potential of chemicals. Toxicol Appl Pharmacol. 2008;232:478–486. doi: 10.1016/j.taap.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Pichiorri F, Palumbo T, Suh SS, Okamura H, Trapasso F, Ishii H, Huebner K, Croce CM. Fhit tumor suppressor: guardian of the preneo-plastic genome. Future Oncol. 2008;4:815–824. doi: 10.2217/14796694.4.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huebner K, Croce CM. FRA3B and other common fragile sites: the weakest links. Nat Rev Cancer. 2001;1:214–221. doi: 10.1038/35106058. [DOI] [PubMed] [Google Scholar]
- 8.Li R, Todd NW, Qiu Q, Fan T, Zhao RY, Rodgers WH, Fang HB, Katz RL, Stass SA, Jiang F. Genetic deletions in sputum as diagnostic markers for early detection of stage I non-small cell lung cancer. Clin Cancer Res. 2007;13:482–487. doi: 10.1158/1078-0432.CCR-06-1593. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi F, Tagliabue E, Menard S, Campiglio M. Fhit expression protects against HER2-driven breast tumor development: unraveling the molecular interconnections. Cell Cycle. 2007;6:643–646. doi: 10.4161/cc.6.6.4033. [DOI] [PubMed] [Google Scholar]
- 10.Gatalica Z, Lele SM, Rampy BA, Norris BA. The expression of Fhit protein is related inversely to disease progression in patients with breast carcinoma. Cancer. 2000;88:1378–1383. [PubMed] [Google Scholar]
- 11.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, Hauck WW, McCue P, Huebner K. The fragile genes FHIT and WWOX are inactivated coor-dinately in invasive breast carcinoma. Cancer. 2004;100:1605–1614. doi: 10.1002/cncr.20137. [DOI] [PubMed] [Google Scholar]
- 12.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, McCue P, Huebner K. Concordant loss of fragile gene expression early in breast cancer development. Pathol lnt. 2005;55:471–478. doi: 10.1111/j.1440-1827.2005.01855.x. [DOI] [PubMed] [Google Scholar]
- 13.Huiping C, Jonasson JG, Agnarsson BA, Sigbjornsdottir BI, Huebner K, Ingvarsson S. Analysis of the fragile histidine triad (FHIT) gene in lobular breast cancer. Eur J Cancer. 2000;36:1552–1557. doi: 10.1016/s0959-8049(00)00143-x. [DOI] [PubMed] [Google Scholar]
- 14.Iliopoulos D, Guler G, Han SY, Johnston D, Druck T, McCorkell KA, Palazzo J, McCue PA, Baffa R, Huebner K. Fragile genes as bio-markers: epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene. 2005;24:1625–1633. doi: 10.1038/sj.onc.1208398. [DOI] [PubMed] [Google Scholar]
- 15.Ingvarsson S, Agnarsson BA, Sigbjornsdottir BI, Kononen J, Kallioniemi OP, Barkardottir RB, Kovatich AJ, Schwarting R, Hauck WW, Huebner K, McCue PA. Reduced Fhit expression in sporadic and BRCA2-linked breast carcinomas. Cancer Res. 1999;59:2682–2689. [PubMed] [Google Scholar]
- 16.Ingvarsson S, Sigbjornsdottir BI, Huiping C, Jonasson JG, Agnarsson BA. Alterations of the FHIT gene in breast cancer: association with tumor progression and patient survival. Cancer Detect Prev. 2001;25:292–298. [PubMed] [Google Scholar]
- 17.Tang XL, Yao GY, Chen LR, Yang ZR, Li SL. [Loss of heterozygosity on chromosome 3p in breast cancer and precancerous lesion] Zhonghua Wai Ke Za Zhi. 2006;44:1314–1317. [PubMed] [Google Scholar]
- 18.Wang ZB, Zhao P, Liu M, Li XH. [Expression of the genes FHIT, Bcl-2 and Bax in breast infiltrating ductal carcinoma and clinicopathological significance thereof] Zhonghua Yi Xue Za Zhi. 2006;86:2171–2176. [PubMed] [Google Scholar]
- 19.Yang Q, Nakamura M, Nakamura Y, Yoshimura G, Suzuma T, Umemura T, Shimizu Y, Mori I, Sakurai T, Kakudo K. Two-hit inactivation of FHIT by loss of heterozygosity and hypermethylation in breast cancer. Clin Cancer Res. 2002;8:2890–2893. [PubMed] [Google Scholar]
- 20.Zochbauer-Muller S, Fong KM, Maitra A, Lam S, Geradts J, Ashfaq R, Virmani AK, Milchgrub S, Gazdar AF, Minna JD. 5' CpG island methylation of the FHIT gene is correlated with loss of gene expression in lung and breast cancer. Cancer Res. 2001;61:3581–3585. [PubMed] [Google Scholar]
- 21.Guo Z, Vishwanatha JK. Effect of regulated expression of the fragile histidine triad gene on cell cycle and proliferation. Mol Cell Biochem. 2000;204:83–88. doi: 10.1023/a:1007068823848. [DOI] [PubMed] [Google Scholar]
- 22.Ishii H, Mimori K, Vecchione A, Sutheesophon K, Fujiwara T, Mori M, Furukawa Y. Effect of exogenous E2F-1 on the expression of common chromosome fragile site genes, FHIT and WWOX. Biochem Biophys Res Commun. 2004;316:1088–1093. doi: 10.1016/j.bbrc.2004.02.159. [DOI] [PubMed] [Google Scholar]
- 23.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 24.Tran H, Brunet A, GrenierJM, Datta SR Fornace AJ Jr., DiStefano PS Chiang LW., Greenberg ME. DNA repair pathway stimulated by the fork-head transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 25.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 26.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foukas LC, Daniele N, Ktori C, Anderson KE, Jensen J, Shepherd PR. Direct effects of caffeine and theophylline on pllO delta and other phosphoinositide 3-kinases. Differential effects on lipid kinase and protein kinase activities. J Biol Chem. 2002;277:37124–37130. doi: 10.1074/jbc.M202101200. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto T, He Z, Ma WY, Schmid PC, Bode AM, Yang CS, Dong Z. Caffeine inhibits cell proliferation by G0/G1 phase arrest in JB6 cells. Cancer Res. 2004;64:3344–3349. doi: 10.1158/0008-5472.can-03-3453. [DOI] [PubMed] [Google Scholar]
- 29.Kong D, Yamori T. Phosphatidylinositol 3-kinase inhibitors: promising drug candidates for cancer therapy. Cancer Sci. 2008;99:1734–1740. doi: 10.1111/j.1349-7006.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu M, Wang B, Fei J, Santanam N, Blough ER. Important roles of Akt/PKB signaling in the aging process. Front Biosci (Schol Ed) 2010;2:1169–1188. doi: 10.2741/s125. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 32.Arden KC. FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene. 2008;27:2345–2350. doi: 10.1038/onc.2008.27. [DOI] [PubMed] [Google Scholar]
- 33.Dansen TB, Burgering BM. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008;18:421–429. doi: 10.1016/j.tcb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009;29:395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weidinger C, Krause K, Klagge A, Karger S, Fuhrer D. Forkhead box-O transcription factor: critical conductors of cancer's fate. Endocr Relat Cancer. 2008;15:917–929. doi: 10.1677/ERC-08-0153. [DOI] [PubMed] [Google Scholar]