SUMMARY
In flies and mammals extracellular Hedgehog (Hh) molecules alter cell fates and proliferation by regulating the levels and activities of Ci/Gli family transcription factors. How Hh-induced activation of transmembrane Smoothened (Smo) proteins reverses Ci/Gli inhibition by Suppressor of Fused (SuFu) and kinesin-family protein (Cos2/Kif7) binding partners is a major unanswered question. Here we show that the Fused (Fu) protein kinase is activated by Smo and Cos2 via Fu- and CK1-dependent phosphorylation. Activated Fu can recapitulate a full Hh response, stabilizing full-length Ci via Cos2 phosphorylation and activating full-length Ci by antagonizing Su(fu) and by other mechanisms. We propose that Smo/Cos2 interactions stimulate Fu autoactivation by concentrating Fu at the membrane. Autoactivation primes Fu for additional CK1-dependent phosphorylation, which further enhances kinase activity. In this model, Smo acts like many transmembrane receptors associated with cytoplasmic kinases, such that pathway activation is mediated by kinase oligomerization and trans-phosphorylation.
INTRODUCTION
Changes in cell fate and proliferation instructed by extracellular Hedgehog (Hh) signaling molecules are critical in development, tissue maintenance and cancer (Ingham and McMahon, 2001; Jiang and Hui, 2008). Understanding exactly how Hh signals are transduced is therefore essential to appreciate how signaling is integrated into the organized development and regulation of a complex organism, and in order to diagnose and devise therapies for a variety of human genetic conditions. Hh signaling was studied first and with the greatest resolution in a physiological setting in Drosophila, revealing the pivotal role of a single transcription factor, Cubitus interruptus (Ci) (Hooper and Scott, 2005). When Hh is absent, its receptor Patched (Ptc) prevents the accumulation and activity of the seven trans-membrane protein Smoothened (Smo), full-length Ci-155 is retained in the cytoplasm and slowly processed to a shorter product, Ci-75, which accumulates in the nucleus, binds DNA and represses Hh target genes. When Hh binds Ptc, Smo becomes active, Ci-155 processing is blocked and Ci-155 activates Hh target genes. This basic scheme was found also to apply to vertebrates, in which Ci has multiple paralogs called Gli proteins (Huangfu and Anderson, 2006; Wilson and Chuang, 2010).
Precisely how Smo regulates Ci is unresolved. In Drosophila this involves a kinesin-family protein, Costal 2 (Cos2), three protein kinases that phosphorylate Ci-155 to promote its processing to Ci-75, a repressive binding partner of Ci-155 and a fourth protein kinase, Fused (Fu). In mice the same three protein kinases, Protein Kinase A (PKA), Glycogen Synthase Kinase 3 (GSK3) and Casein Kinase 1 (CK1), act analogously on Gli proteins together with a Cos2 ortholog, Kif7 to direct Gli ubiquitination and proteasome-mediated proteolysis (Huangfu and Anderson, 2006; Wilson and Chuang, 2010). The repressive binding partner, Suppressor of Fused (designated Su(fu) in flies and SUFU in mammals) is also conserved. However, mouse Fu appears to play no role, even though Fu has been implicated in Hh signaling in zebrafish (Wilson and Chuang, 2010). Conversely, many additional proteins associated with the regulation of the primary cilium are essential for normal Hh signaling in mice, but not in flies (Goetz and Anderson, 2010).
In mice, loss of SUFU produces strong ectopic Hh target gene induction, provoking the suggestion that Hh signaling requires Smo to antagonize the silencing of Gli activators by SUFU (Wilson and Chuang, 2010). However, there is no known direct contact between Smo and SUFU and no mechanistic model that connects the two. In Drosophila, Smo contacts Cos2 directly and is thought to inhibit Ci-155 processing by reducing the association of Cos2 with Ci-155 or with PKA, GSK3 and CK1 (Ruel et al., 2003; Zhang et al., 2005). Smo also activates Fu, which can bind Smo, Cos2 and Su(fu), leading to increased activity of stabilized Ci-155 (Hooper and Scott, 2005). Fu is thought only to antagonize Su(fu) because loss of Su(fu) substantially restores Hh signaling impaired by genetic inactivation of Fu kinase (Preat, 1992). On the basis of correlated biochemical changes it has been speculated that Fu modifies Su(fu) function by direct phosphorylation and that Fu is activated by a process involving Fu phosphorylation (Hooper and Scott, 2005).
Here we define specific Fu phosphorylation sites that suffice to activate Fu fully by participating in a positive feedback loop of intermolecular auto-phosphorylation reactions. We find that the productive interaction of Fu molecules stimulated by Hh is mediated by both Smo and Cos2. Contrary to previous ideas, we also show that Fu can stabilize Ci-155 via phosphorylation of Cos2 on S572, that regulation of Ci-155 silencing by Su(fu) involves Casein Kinase 1, that Fu activates Ci-155 by mechanisms additional to Su(fu) antagonism, and that silencing of Ci-155 by mouse SUFU can be regulated by Hh in flies. These observations show Fu kinase to be at a fulcrum of Drosophila Hh signaling and strongly suggest the participation of an analogous protein kinase in mammalian Hh signaling.
RESULTS
Casein Kinase 1 is required downstream of Smo to activate Fu and antagonize Su(fu)
In Drosophila wing discs, Hh is expressed in posterior compartment cells and moves into a narrow stripe of anterior cells, known as AP border cells, to activate several target genes, including decapentaplegic (dpp) and ptc, conveniently reported by a ptc-lacZ transgene (Ingham and McMahon, 2001). The highest levels of Hh also induce Engralied (En), which is distinguished from Hh-independent posterior En expression by its spatial overlap with strictly anterior ptc-lacZ expression (Figure 1A).
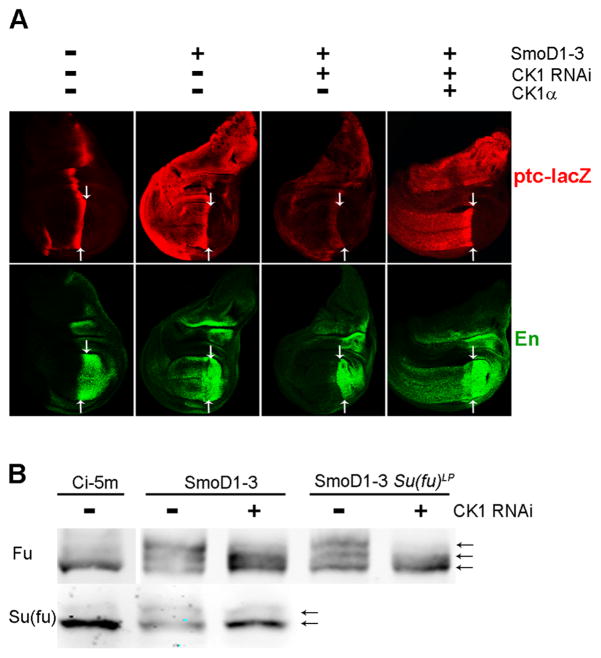
Figure 1. CK1α acts downstream of Smo.
(A) The indicated transgenes were expressed evenly throughout wing discs using C765-GAL4 at 25C, followed by staining for expression of the Hh target genes ptc-lacZ (red) and En (green). The posterior (right) edge of ptc-lacZ expression (arrows) marks the boundary between posterior Hh-producing cells and anterior (left) Hh-responsive cells. Constitutively active SmoD1–3 induced strong ectopic anterior ptc-lacZ and En expression, which was largely prevented by an RNAi transgene directed towards CK1α and restored by co-expression of excess CK1α. (B) Extracts of wild-type or Su(fu)LP mutant wing discs expressing the indicated transgenes under the control of C765-GAL4 at 29C were subjected to Western Blot analysis using antibodies to Fu and Su(fu). Ci-5m induces Hh target genes but does not activate Fu ectopically (Price and Kalderon, 1999). SmoD1–3-induced upward shifts, indicative of phosphorylation of Fu and Su(fu), which were reduced by inhibition of CK1α.
We began by re-examining the requirements for CK1 in Hh signaling. Smo activation by Hh was previously shown to require a specific cluster of PKA-primed CK1 sites (Apionishev et al., 2005; Jia et al., 2004; Zhang et al., 2004); accordingly, replacing all known significant PKA and CK1 sites with acidic residues generated a constitutively active Smo variant, SmoD1–3 (Jia et al., 2004) and expression of a Casein Kinase 1α (CK1α) RNAi transgene reduced ptc-lacZ expression and eliminated En expression at the AP border of wing discs (Figure S1A). Surprisingly, CK1α kinase activity was also required for SmoD1–3 to induce ectopic anterior expression of En and ptc-lacZ (Figure 1A). Removal of Su(fu) restored strong anterior En expression to wing discs expressing SmoD1–3 and CK1 RNAi (Figure S1C). Similarly, CK1 RNAi did not inhibit strong En induction by a Ci variant lacking a known binding site for Su(fu) in smo pka mutant clones (Figure S1D). Thus, CK1, like Fu, is required downstream of activated Smo to overcome inhibition of activated Ci by Su(fu).
SmoD1–3 expression, like Hh stimulation, increases both Fu and Su(fu) phosphorylation, manifest as gel mobility shifts on Western blots (Ho et al., 2005; Lum et al., 2003; Therond et al., 1996). Both responses to SmoD1–3 were reduced by co-expressing CK1 RNAi (Figure 1B). Fu kinase activity can be assayed in situ by using a phospho-epitope antibody specific to a known direct target, S572 of Cos2 (Raisin et al., 2010). Phospho-S572 staining was strongly induced in anterior cells by SmoD1–3 but substantially reduced by co-expression of CK1 RNAi (Figure S1B). We conclude that CK1 is required for normal Fu phosphorylation and activation, and for phosphorylation and antagonism of Su(fu) in response to activated Smo.
To test whether the critical CK1 target might be in Cos2 or in Smo phosphorylation sites that are not altered in SmoD1–3, we used a constitutively active Fu variant, GAP-Fu, which was constructed by adding a palmitoylation signal from GAP-43 to the N-terminus of Fu in order to promote constitutive membrane localization (Claret et al., 2007). CK1 RNAi blocked the strong induction of En by GAP-Fu in both smo pka and smo cos2 mutant clones (Figures S1E and F) and in each case, loss of Su(fu) restored strong En induction. We therefore conclude that CK1 has a critical target in the Hh pathway other than Smo or Cos2.
Fu activation involves phosphorylation of its activation loop
We investigated whether Fu might be a critical target for CK1 by looking for potential consensus CK1 motifs (S/T three or four residues C-terminal to a potentially phosphorylated S/T) (Smelkinson et al., 2007). Protein kinases are frequently regulated by activation loop phosphorylation (Nolen et al., 2004) and the Fu activation loop includes two potential CK1 sites at threonines 154 and 158, with a potential priming site at threonine 151 (Figure 2B). We investigated the importance of potential activation loop phosphorylation sites in a complementation assay.
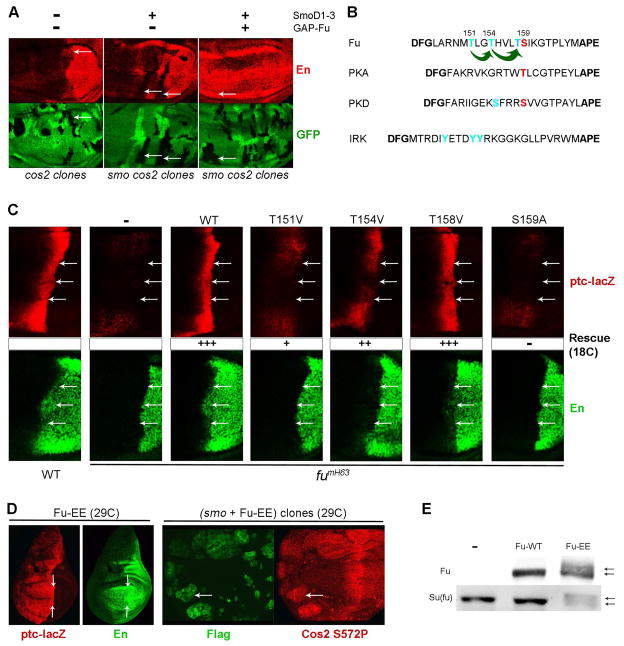
Figure 2. Fu kinase activation requires Cos2 and Fu activation loop phosphorylation.
(A) Clones lacking Cos2 activity (arrows, no green GFP) failed to induce En expression (red) in response to Hh at the AP border (left panel) or in response to SmoD1–3 (middle panel) unless GAP-Fu was expressed together with SmoD1–3 (right panel) throughout the wing disc using C765-GAL4 at 29C (B) Sequence alignment of kinase activation loops bordered by conserved (bold) DFG and APE motifs. Potential Fu phosphorylation sites are colored, with green arrows representing consensus primed CK1 sites. For the mammalian kinases shown, phosphorylation sites contributing to activation are colored, with red highlighting residues equidistant from the conserved APE motif. (C) Wild-type (WT) or single residue variants of Flag-tagged Fu were expressed under the control of C765-GAL4 at 18C in fumH63 wing discs and stained for both ptc-lacZ (red) and En (green) expression. Arrows indicate the posterior borders of ptc-lacZ expression, providing a landmark for assessing anterior, Hh-dependent, En expression (to the left of the arrows). Full (+++), strong (++), poor (+) and zero (-) rescue of ptc-lacZ and En induction by Hh are indicated. (D) Flag-tagged Fu with T151E and T154E substitutions (Fu-EE) expressed under the control of C765-GAL4 at 29C induced (left panels) strong ectopic ptc-lacZ (red) and En (green) in anterior cells (left panels; arrows indicate the AP boundary) and increased Cos2 S572P phospho-epitope staining (red) in anterior smo mutant clones (marked by green Flag staining and arrows; right panels). (E) Western blot of extracts of wing discs expressing Fu transgenes using C765-GAL4 at 29C, showing gel mobility shifts (arrows) of tagged Fu-EE and endogenous Su(fu), indicative of increased phosphorylation promoted by Fu-EE but not by wild-type Fu.
In fumH63 wing discs, which lack Fu kinase activity, there is no anterior En expression, ptc is induced only weakly at the AP border and, as a consequence of reduced Ptc protein levels, Hh spreads unusually far to induce a broadened stripe of very weak ptc-lacZ expression (Ohlmeyer and Kalderon, 1998) (Figure 2C). A wild-type Fu transgene (UASGAL4-Fu-WT) expressed ubiquitously and evenly in wing discs using C765-GAL4 at 18C fully rescued normal AP border expression of En and ptc-lacZ (Figure 2C). Fu-T158V fully rescued all phenotypes at 18C, whereas Fu-S159A showed no rescue of AP border ptc-lacZ or En expression at any temperature (Figure 2C and S2A). These data contradict previous findings in tissue culture using T158A and S159A variants (Fukumoto et al., 2001) and show that S159, rather than T158 phosphorylation is essential for Fu activity. S159 is in the precise position characteristic of single activating phosphorylation sites in other protein kinases, such as PKA (Figure 2B).
Fu-T151V provided modest ptc-lacZ rescue and no rescue of AP border En expression at 18C (Figure 2C) but complete ptc-lacZ rescue and partial En rescue at 25C, where higher, temperature-dependent GAL4 activity drives stronger transgene expression (Figure S2A, B). Fu-T154V was more active than Fu-T151V but still failed to rescue AP border En at 18C (Figure 2C). Fu-T151E was more active than Fu-T151V at all temperatures (Figure S2A) but, like Fu-T154V, did not rescue En at 18C (data not shown). Acidic residues mimic some aspects of phosphorylated residues, but they do not prime CK1 phosphorylation efficiently (Jia et al., 2004; Smelkinson et al., 2007; Zhang et al., 2004). Thus, the similar phenotypes of Fu-T151E and Fu-T154V, coupled to the stronger defect of Fu-T151V, fits well with the assertions that T151 primes T154 phosphorylation by CK1, and that both phosphorylated residues (T151 and T154) contribute independently to Fu activity.
When both T151 and T154 were altered to acid residues, in Fu-EE, Fu acquired constitutive activity, inducing strong ectopic anterior expression of ptc-lacZ at 25C and also strong En expression at 29C (Figure 2D). High-level Fu-EE expression in wing discs also induced Cos2 phosphorylation at S572 in smo mutant clones (Figure 2D) and phosphorylation of both Fu-EE itself and Su(fu), judged by gel mobility shifts in Western blots (Figure 2E). Neither Fu-T151E nor Fu-T154E (or wild-type Fu) exhibited analogous Hh-independent activity at any temperature. Thus, from both loss of function and gain of function analyses, we infer that both T151 and T154 phosphorylation contribute to Fu activation.
The constitutive activity of Fu-EE is much stronger than that of GAP-Fu, as judged by Hh target gene expression (Figure S5D, E). Furthermore, the extent of Su(fu) phosphorylation induced by GAP-Fu in Kc cells, gauged by gel mobility shift, was markedly increased by introducing T151E /T154E substitutions and was eliminated by T151V and T154V substitutions (Supplemental Figure S2C). Thus, the constitutive activity of GAP-Fu, like the Hh-dependent activity of wild-type Fu, depends on phosphorylation of the activation loop.
CK1 sites in the non-catalytic C-terminal half of Fu
We tested whether T154 accounts for the effects of CK1 inhibition downstream of Smo by using Fu-EE. CK1 RNAi did not strongly reduce ectopic Cos2 S572P phospho-epitope staining induced by Fu-EE, indicating that Fu-EE retained substantial protein kinase activity (Figure S3A). However, CK1 RNAi still inhibited ectopic En induction by SmoD1–3 in wing discs expressing Fu-EE (Figure 3A). CK1 RNAi also inhibited En induction by Fu-EE in both smo pka and smo cos2 mutant clones, where other potential CK1 targets in Smo and Cos2 are simultaneously absent (Figure S3C). CK1 RNAi also reduced the extent of Fu-EE phosphorylation in wing discs expressing SmoD1–3 and Fu-EE (Figure S3B).
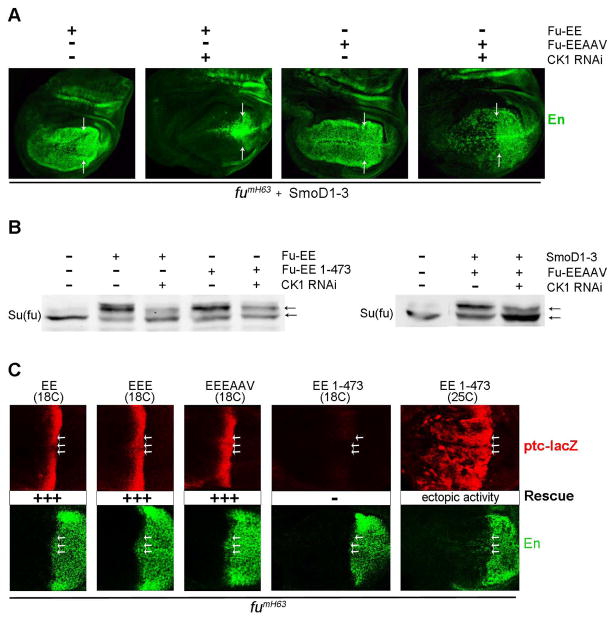
Figure 3. Regulation of Fu activity by CK1 and Hh.
(A) Co-expressing SmoD1–3 with Fu-EE or Fu-EEAAV using C765-GAL4 at 29C in fumH63 wing discs induced strong ectopic anterior En (green) expression (left of arrows), which was largely prevented by CK1 RNAi transgene expression. (B) Extracts of wing discs expressing the indicated transgenes under the control of C765-GAL4 at 29C were subjected to Western Blot analysis using antibodies against Su(fu). CK1 RNAi reduced upward shifts, indicative of phosphorylation, induced by SmoD1–3, Fu-EE, Fu-EE 1–473 and Fu-EEAAV. (C) Flag-tagged Fu variants were expressed using C765-GAL4 at the indicated temperatures in fumH63 wing discs and stained for both ptc-lacZ (red) and En (green) expression to reveal zero (-) or full (+++) rescue, or full rescue accompanied by ectopic pathway activity (right panels). Arrows indicate the posterior borders of ptc-lacZ expression.
We therefore searched for additional CK1 sites on Fu, which might be important for Fu activity. Through systematic mutagenesis studies, we found that the mobility shift of Fu-EE resulting from high level expression in Kc cells was lost in derivatives with S482A, S485A/T486V (”AV”), or all three (S482A/S485A/T486A: “AAV”) substitutions (Figure S3D) or in the absence of Fu residues beyond 473. S485 and T486 are consensus CK1 sites once S482 is phosphorylated (Figure S1E).
A Fu transgene lacking these three potential phosphorylation sites combined with acidic substitutions at T151 and T154 (Fu-EEAAV) induced ectopic Hh target gene expression very much like Fu-EE (Figure S3F). Fu-EEAAV also underwent a clear gel mobility shift when expressed in wing discs alone or together with SmoD1–3, but, in contrast to Fu-EE, the shift was not reduced by co-expression of CK1 RNAi (Figure S3B). Thus, S485 and T486 do appear to be CK1 sites primed by S482 phosphorylation in vivo and collectively, the sites described here appear to account for all CK1-dependent phosphorylation of Fu.
We tested the importance of S485 and T486 for Fu activity in the context of GAP-Fu. GAP-Fu induced a gel mobility shift for Su(fu) in Kc cells but not when S485 and T486 were altered (Figure S3H). Similarly, in wing discs, GAP-Fu induction of the (highly sensitive) Hh target gene reporter dpp-lacZ was lost when S485 and T486 were substituted to create GAP-Fu-AV (Supplemental Figure S3G). Thus, phosphorylation sites in the C-terminal half of Fu (S485, T486), can contribute to Fu activity, at least under conditions of weak activation by membrane tethering.
However, CK1 RNAi still inhibited En induction by SmoD1–3 in the presence of Fu-EEAAV, just as for Fu-EE (Figure 3A) and the strong mobility shift for Su(fu) induced in wing discs by Fu-EE, Fu-EEAAV, and a truncated activated Fu (Fu-EE 1–473) were all strongly reduced by co-expression of CK1 RNAi (Figure 3B). Thus, CK1 appears to phosphorylate Fu at residues T154, S485 and T486, all of which can contribute to Fu activation, but CK1 also clearly has at least one additional role in promoting Su(fu) phosphorylation and antagonizing Su(fu) activity. Su(fu) itself is an obvious potential CK1 target.
Role of T151 and T154 in activation of Fu by Hh
We tested whether Hh regulates Fu solely by stimulating phosphorylation of T151 and T154. First, we saw that Fu-EE expressed at lower levels (using the same C765-GAL4 driver, but at 18C) restored normal Hh signaling at the AP border in fumH63 discs without inducing ectopic ptc-lacZ expression (Figure 3C). We also found that Fu-EE with an additional T158E substitution (Fu-EEE) had no constitutive activity even at 29C, presumably because an acidic residue does not mimic T158 phosphorylation well (Figure S3J). Even in anterior smo pka mutant clones, where Ci-155 is stabilized, Fu-EEE did not enhance the low levels of En induced (Figure S3J). Fu-EEE was nevertheless able to complement fumH63 function fully at the AP border, even at 18C (Figure 3C), indicating activation by Hh. We also found that Fu-AV, Fu-AAV and Fu-EEEAAV all rescued AP border signaling in fumH63 discs without exhibiting any constitutive activity (Figure 3C and S3F).
We conclude that T151 and T154 phosphorylation normally plays a central role in activation of Fu by Hh, while S482, S485 and T486 phosphorylation contribute to Fu activation in a more subtle manner. Nevertheless, additional mechanisms allow robust regulation of Fu by Hh, most likely centering on phosphorylation of S159, which is essential for Fu activity.
Fu kinase activity promotes Fu phosphorylation
To test whether the robust gel mobility shift of Fu-EE expressed at high levels in Kc cells (Figures 4A and 4E) results from its own kinase activity we introduced the kinase-inactivating S159A and G13V substitutions (Liu et al., 2007). In each case no shift was observed (Figure 4A). Both Flag-tagged Fu-EE S159A and Fu-S159A did, however, undergo a clear gel mobility shift when co-expressed with HA-tagged Fu-EE (but not kinase-dead HA-FuEE), indicating that an active Fu kinase molecule can promote the activation of another Fu molecule in trans (Figure 4B). The shift was much reduced when using Fu-EEAAV S159A as substrate, indicating that S482 is a major site of auto-phosphorylation detected in this assay (likely followed by CK1 phosphorylation of S485 and T486).
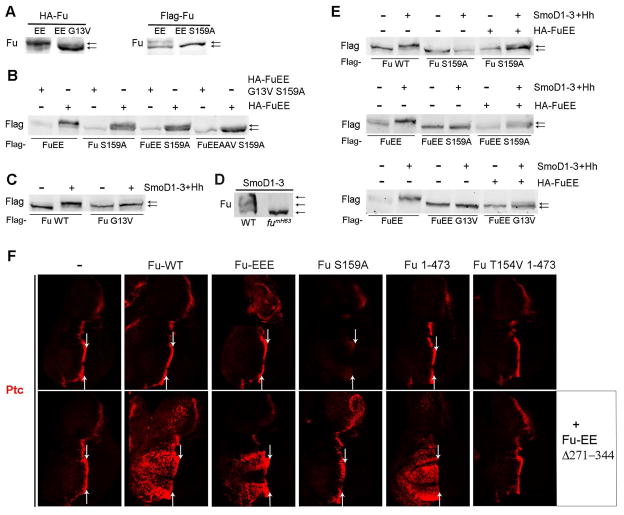
Figure 4. Fu-stimulated Fu phosphorylation activates Fu.
(A) Western blots with HA or Flag antibody of extracts of Kc cells expressing high levels of tagged Fu-EE variants and Cos2. G13V and S159A substitutions prevented a robust gel mobility shift. (B) Western blot with Flag antibody of extracts of Kc cells co-expressing high levels of Cos2 together with the indicated Flag-tagged Fu and HA-tagged Fu variants (using a 3:1 ratio of HA-Fu to Flag-Fu). HA-FuEE, but not kinase-defective HA-FuEE G13V S159A, induced upward shifts of Flag-Fu variants, indicating Fu intermolecular phosphorylation. (C) Western blot with Flag antibody of extracts of Kc cells co-expressing low levels of Cos2 and the indicated Flag-tagged Fu variants, with or without SmoD1–3 and Hh, showing a Hh-stimulated mobility shift for wild-type Fu but not for kinase-defective Fu G13V. (D) Western blot with Fu antibody of wild-type (WT) or fumH63 wing disc extracts expressing SmoD1–3, showing Fu mobility shift depends on Fu kinase activity. (E) Western blot with Flag antibody of extracts of Kc cells co-expressing low levels of Cos2 and the indicated Flag-tagged Fu variants. Kinase-defective Flag-Fu proteins (due to S159A or G13V substitutions) showed mobility shifts only when co-expressed with SmoD1–3, Hh and low levels of HA-FuEE. (F) Fu variants were expressed under the control of C765-GAL4 at 29C in wing discs either alone (top row) or together with Fu-EE Δ271–344 (bottom row). Ptc (red) was ectopically induced in anterior cells only when Fu-WT, Fu-EEE or Fu1–473 were co-expressed with Fu-EE Δ271–344. Arrows indicate the AP boundary.
To test whether Hh could stimulate Fu kinase dependent phosphorylation of Fu we expressed wild-type Fu or Fu-EE at lower levels in Kc cells together with Cos2. Under these conditions we observed a strong Fu gel mobility shift only when SmoD1–3 and Hh were co-expressed (Figures 4C and 4E). The Hh-induced shift of wild-type Fu was reduced substantially by a T151V substitution, eliminated by S159A and G13V substitutions, and enhanced by T151E/T154E substitutions (Figure 4E and S4A), implying an essential requirement for Fu kinase activity. A robust Hh-induced shift was still observed for Fu-EEE, Fu-EEAAV and (to a lesser degree) Fu-EEEAAV, implying that the substrate residues involved encompassed more than T151, T154, T158, S482, S485 and T486, and most likely include S159. All phosphorylation of Fu induced by SmoD1–3 in wing discs, detected by a gel mobility shift, was also dependent on Fu kinase activity (Figure 4D), consistent with Fu kinase dependent phosphorylation of Fu at multiple sites during normal Hh pathway activation, and suggesting that the primary means by which Hh signaling impinges on Fu activation is initiated by Fu autoactivation.
When Flag-tagged kinase-dead variants of Fu (Fu-S159A, Fu-EE S159A or Fu-EE G13V) were expressed together with low levels of HA-tagged Fu-EE in Kc cells we saw a clear gel mobility shift for each Flag-tagged inactive Fu molecule only in the presence of SmoD1–3 and Hh (Figure 4E). We conclude that an active Fu molecule can promote phosphorylation of another Fu molecule in trans either when Fu is expressed at very high levels or when Hh activates lower levels of Fu. In this regard, it is intriguing to note that known physical interactions among Smo, Cos2 and Fu would be expected to promote Fu localization to the plasma membrane and thus increase the local Fu concentration (Hooper and Scott, 2005), particularly when Smo levels and Smo/Cos2 interactions are stimulated by Hh-induced activation of the pathway (see below).
Fu is activated by Fu-dependent phosphorylation
To test whether Fu kinase dependent phosphorylation of Fu suffices to activate Fu we used a transgene encoding a derivative of Fu-EE lacking residues 271–344. This activated Fu variant promotes its own phosphorylation and a Su(fu) mobility shift when expressed at high levels in Kc cells (Figure S4C). However, in wing discs Fu-EE Δ271–344 was expressed at relatively low levels using C765-GAL4 at 29C and did not induce any ectopic activation of Hh target genes or a Su(fu) mobility shift (Figure 4F and S4B-D). Co-expressing Fu-WT, which did not have any constitutive activity alone, induced a number of changes indicative of constitutive pathway activity, including ectopic anterior dpp-lacZ expression (data not shown) and most clearly, extensive ectopic Ptc expression (Figure 4F). Co-expression of Fu-S159A with Fu-EE Δ271–344 showed no evidence of ectopic Hh pathway activity. Indeed, Fu-S159A alone inhibited Hh signaling at the AP border (Figure 4F). The apparent trans-activation of kinase-competent Fu and the dominant-negative activity of Fu-S159A support the idea that Fu is activated by Fu kinase dependent phosphorylation. We also observed trans-activation of Fu-EEE by Fu-EE Δ271–344 (Figure 4F). Since T151 is absent from Fu-EEE, S159 must be a key target for trans-activation.
Fu activation depends on Cos2 and Fu-Fu interactions
It is not known how Hh promotes Fu activation via Smo. Fu contacts Smo directly and also indirectly via Cos2 (Malpel et al., 2007), suggesting two possible routes for Smo to influence Fu activation. To test whether the known requirement for Cos2 to allow En induction by Hh in AP border cells (Wang and Holmgren, 2000; Zhou et al., 2006)(Figure 2A) reflects a direct function in Fu activation, rather than just the known roles of Cos2 in promoting Smo activation (Lum et al., 2003) and Fu stabilization (Ruel et al., 2003) we used constitutively active Smo. We found that anterior En expression induced by SmoD1–3 was blocked in smo cos2 mutant clones (Figure 2A). Since Ci-155 processing is blocked in smo cos2 mutant clones (Methot and Basler, 2000) we infer that loss of Cos2 impairs Ci-155 activation, which requires Fu kinase activity. Indeed, GAP-Fu restored En induction to smo cos2 mutant clones expressing SmoD1–3 (Figure 2A), whereas wild-type Fu did not (data not shown). We conclude that Cos2 is normally required for activated Smo to activate Fu, and that concentrating Fu at the membrane can substitute for this function of Cos2.
We then tested whether Cos2-Fu association was important for Fu activation by Hh. Fu binds to Cos2 through a C-terminal domain (mapping from residue 437–805) that is disrupted in class II fu alleles (Monnier et al., 2002; Preat et al., 1993; Robbins et al., 1997). These loss-of-function alleles encode truncated proteins with intact kinase domains, but it is not known if they are defective for activation or for recognizing downstream substrates. We found that Fu-EE 1–473 induced strong ectopic ptc-lacZ expression when expressed at high levels in fumH63 wing discs, but it was inactive at lower levels, with no evidence of activation by Hh at the AP border (Figure 3C and S5G, H). Similarly, even high levels of Fu 1–473 (without activating mutations) showed little or no ability to rescue Hh target gene expression at the AP border of fumH63 wing discs (Figure S3F). We deduce that activation of Fu by Hh requires binding of Fu to Cos2. This further suggests that Smo promotes autoactivation by using Cos2 to increase the local concentration of Fu.
The constitutive activity of Fu-EE 1–473 expressed at high levels (Fig. 3C) and the ability of Fu 1–473 to synergize with Fu-EE Δ271–344 to induce strong ectopic Ptc expression (Figure 4F), show that even molecules which are refractory to Hh-stimulated, Cos2-mediated activation can be activated synthetically simply by Fu-stimulated phosphorylation. To ask whether that synthetic activation by Fu required any interactions beyond the phosphorylation of one catalytic domain by another we tested the properties of the Fu catalytic domain alone. We found that Fu-EE 1–305, containing little more than the kinase domain, did not induce ectopic Hh target gene expression in wing discs even when expressed at high levels and did not promote Su(fu) phosphorylation in Kc cells (Supplemental Figures S4C and S4E). We infer that Fu-EE 1–305 lacks a critical non-catalytic domain (present in Fu 1–473) that is essential for productive Fu-Fu interactions. Supporting that deduction, the Fu kinase domain and non-catalytic region have in fact previously been shown to bind to each other in vitro and to complement to some degree in wing discs (Ascano et al., 2002).
Even though Fu-EE 1–305 did not exhibit constitutive activity, high levels of Fu-EE 1–305 allowed full rescue of Fu function at the AP border of fumH63 wing discs (Supplemental Figure S4F). By contrast, Fu-EE 1–305 provided no rescue in a fuM1 background, where the predicted fuM1 gene product contains only residues 1–80 (Supplemental Figure S4E). We infer that the fumH63 gene product, which is full-length but has an essential catalytic residue substitution, complements Fu-EE 1–305 by providing a binding interface for Fu-EE 1–305 and a binding site for Cos2, both of which are required for Hh to stimulate the association and cross-phosphorylation of Fu-EE 1–305 catalytic domains.
Fu stabilizes Ci-155 via Cos2 S572 phosphorylation
Normal Hh signaling involves activating Ci-155 and blocking the PKA-dependent processing of Ci-155 to Ci-75, with Fu kinase activity implicated only in Ci-155 activation by opposing the actions of Su(fu) (Hooper and Scott, 2005; Ohlmeyer and Kalderon, 1998). It was therefore surprising to find that Fu-EE increased Ci-155 levels, detected by 2A1 antibody, in anterior wing disc cells (Figure 5A). Elevated Ci-155 levels and ectopic ptc-lacZ expression induced by Fu-EE were both opposed by expression of constitutively active PKA (Figure 5A), supporting the idea that stabilization of Ci-155 by Fu-EE results from inhibition of PKA-dependent Ci-155 processing, as in all other situations where the Hh pathway is activated.
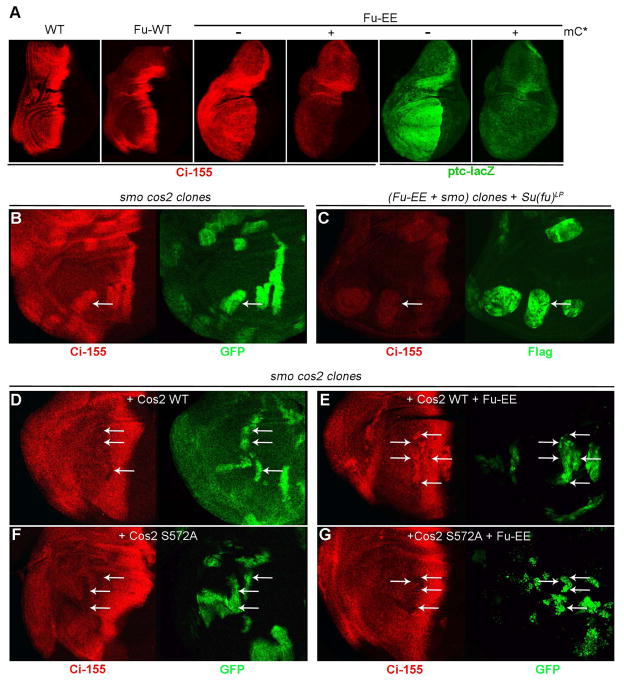
Figure 5. Fu stabilizes Ci-155 via Cos2 S572 phosphorylation.
(A) Strongly elevated anterior full-length Ci-155 (red; 2A1 antibody) and ptc-lacZ (green) were induced by expressing Fu-EE but not wild-type Fu using C765-GAL4 at 29C, and were substantially reduced by co-expressing activated mouse PKA catalytic subunit (mC*). (B) smo cos2 double mutant clones, marked by GFP (green, arrows) showed elevated full length Ci-155 (red). (C) smo clones expressing Flag-Fu-EE, marked by Flag staining (green, arrows), in Su(fu)LP discs at 29C, showed elevated Ci-155 (red) levels. (D-G) smo cos2 clones expressing Cos2 WT or Cos2 S572A, marked by GFP (green, arrows), showed normal or reduced anterior Ci-155 (red) staining (D, F), while co-expressing Fu-EE increased Ci-155 levels in clones expressing Cos2 WT (E) but not in clones expressing Cos2 S572A (F). Transgene expression in (C-F) was limited to smo cos2 clones using MARCM and C765-GAL4 at 29C.
Even low levels of GAP-Fu stabilized Ci-155, with little or no accompanying ptc-lacZ and En induction (Figure S5D, E), indicating that Ci-155 stabilization is a particularly sensitive response to Fu activation. Ci-155 stabilization by GAP-Fu was previously attributed to an intermediate activation of Smo by Fu (Claret et al., 2007). However, we found that both Fu-EE and GAP-Fu induced high levels of Ci-155 whether functional Smo was present or absent (Figures S5A–C, F). Ci-155 stabilization by Fu-EE was also observed in smo mutant clones within discs lacking Su(fu) protein (Figure 5C). Thus, in contrast to previous widely-held expectations for the actions of Fu kinase, activated Fu appears to inhibit Ci-155 processing through a mechanism that does not involve Smo or Su(fu).
We therefore tested if stabilization of Ci-155 by Fu-EE depends on the known Fu phosphorylation site on Cos2 at S572 (Raisin et al., 2010). We did this by replacing endogenous Cos2 with the variant, Cos2-S572A. Ci-155 levels are high in smo cos2 mutant clones but were restored to normal anterior levels (or lower) by expressing either Cos2-WT or Cos2-S572A in the mutant clones (Figures 5B, D and F). Fu-EE expression in smo cos2 clones increased Ci-155 levels when Cos2-WT was co-expressed, but did not increase Ci-155 levels when Cos2-S572A was co-expressed (Figures 5E and G). We conclude that Fu-dependent phosphorylation of Cos2 on S572 is essential for activated Fu to stabilize Ci-155.
It was previously shown that Hh strongly increases Ci-155 levels at the AP border of fumH63 wing discs (Ohlmeyer and Kalderon, 1998), implying that Fu kinase is not required for Hh to inhibit Ci-155 processing. Residual Ci-155 processing can be detected more sensitively by assaying for repression of hh-lacZ by Ci-75 than by staining for Ci-155 (Smelkinson et al., 2007). We therefore examined hh-lacZ expression in posterior fumH63 clones in discs expressing wild-type Ci but we found no reduction in hh-lacZ expression (Figure S5I). Thus, even though Hh can stabilize Ci-155 via Fu activation, high levels of Hh can still block Ci-155 processing completely in the absence of Fu kinase activity.
Su(fu) phosphorylation sites and substitution by mouse SUFU
Since Hh has been postulated to regulate Ci-155 activity via Fu-dependent phosphorylation of Su(fu) (Ho et al., 2005; Lum et al., 2003), we sought to identify the phosphorylated residues on Su(fu) by expressing Fu-EE together with Su(fu) variants in Kc cells. S321 and S324 proved to be the only phosphorylation sites essential for a robust Su(fu) gel mobility shift in Kc cells and in wing imaginal discs (Figure S6A–C). S324 is a predicted CK1 site and, consistent with this prediction, the Su(fu) gel mobility shift was reduced by CK1 RNAi in wing discs expressing activated Smo and Fu variants lacking CK1 sites (Figure 3B). Su(fu) variants with alanine or acidic substitutions at S321, S324 and nearby residues were nonetheless indistinguishable from wild-type Su(fu) in diverse assays of Hh signaling in wild-type, fumH63, and Fu-EE or CK1 RNAi expressing discs (Figure 6A, B and S6A, E-H). Even when other potential targets of Fu were eliminated by studying smo cos2 mutant clones, GAP-Fu induced ectopic En equally well in discs expressing Su(fu)-WT or Su(fu)-5A in place of endogenous Su(fu) (Figure S6D). Thus, Hh-stimulated, Fu-dependent phosphorylation of Su(fu) on residues S321 and S324 leads to a clear gel mobility shift but does not play a major role in the regulation of Ci-155 activity by Hh.
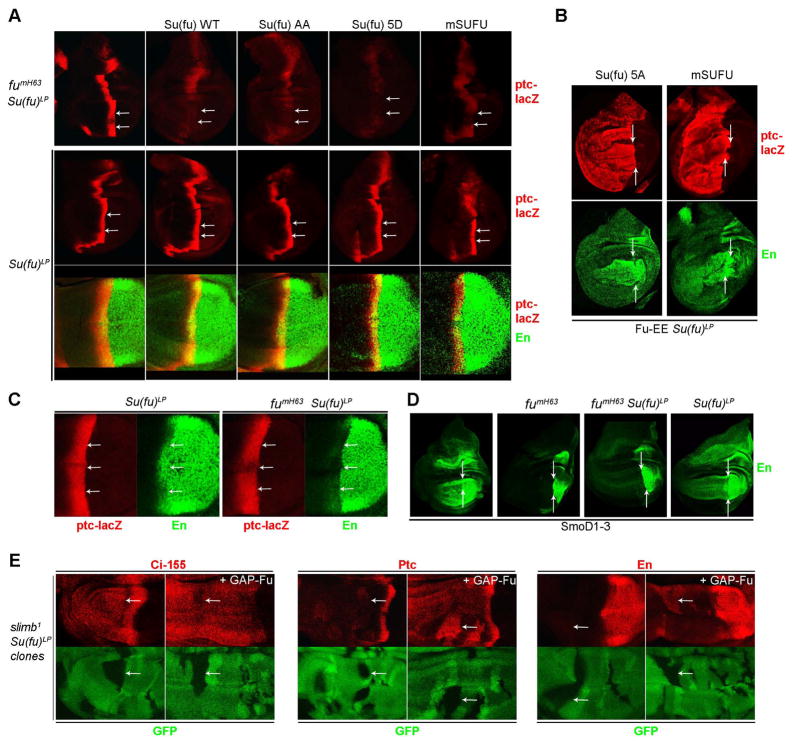
Figure 6. Fu antagonizes mouse SUFU and activates the Hh pathway independent of Su(fu).
(A) Normal AP border ptc-lacZ (red) expression in fumH63 Su(fu)LP wing discs (top left) was inhibited by expression of the indicated fly Su(fu) variants or mouse Su(fu) (mSUFU) using C765-GAL4 at 29C (top row). Arrows indicate the posterior edge of ptc-lacZ expression in the wing pouch region, where responses to Hh are clearest. The same Su(fu) variants permitted normal induction of ptc-lacZ (red) and En (green) by Hh in the wing pouch region of the AP border in Su(fu)LP wing discs (middle and bottom row). (B) Fu-EE co-expressed with Su(fu)-5A or mSUFU using C765-GAL4 at 29C induced strong ectopic ptc-lacZ (red) and En (green) in Su(fu)LP mutant wing discs. (C) AP border En (green, arrows) staining was reduced in fumH63 Su(fu)LP wing discs (right panels) compared to normal (not shown) or Su(fu)LP wing discs (left panels), while ptc-lacZ (red) expression was normal in each case. (D) Wing discs expressing SmoD1–3 using C765-GAL4 at 29C showed strong ectopic anterior En (green) expression for wild-type (left) and Su(fu)LP discs (right), no ectopic En in fumH63 discs and only very little ectopic En in fumH63 Su(fu)LP discs (middle panels). Arrows indicate the posterior border of ptc-lacZ in (A-D). (E) Expressing GAP-Fu using C765-GAL4 at 29C (right panels of each pair) in slimb1 Su(fu)LP clones (lacking green GFP, arrows) did not increase Ci-155 levels (red, left) but increased ectopic expression of both Ptc (red, middle) and En (red, right) in the mutant clones.
To see if pathways regulating Su(fu) actions might be conserved, we expressed mouse SUFU (mSUFU) in Drosophila wing discs. Both fly and mammalian Suppressor of fused showed similar abilities to stabilize Ci-155 (Figure S6F), inhibit AP border ptc-lacZ in fumH63 wing discs (Figure 6A) and allow induction of ptc-lacZ and En by Hh (Figure 6A), SmoD1–3 and Fu-EE in a manner susceptible to inhibition by CK1 RNAi (Figure 6B and S6G). It therefore seems likely that the underlying mechanisms regulating Su(fu) action in the Hh pathway may in fact be conserved between flies and mammals.
Fu activates Ci-155 by mechanisms additional to Ci stabilization and Su(fu) antagonism
Finally, we re-examined the evidence for the widely-held belief that Su(fu) inhibition is the sole key target for activation of Ci-155 by Fu kinase. That assertion derives principally from the finding that the spacing of adult wing veins 3 and 4, which depends on induction of collier by relatively high levels of Hh signaling (Vervoort, 2000), is fully restored in Fu-kinase deficient animals by eliminating Su(fu) (Preat, 1992). However, while wing vein spacing and AP border ptc-lacZ staining of fumH63 wing discs was indeed rescued by loss of Su(fu), we found that AP border En expression was only very weakly restored (Figure 6C). Similarly, we found that ectopic anterior En expression induced by SmoD1–3 was much reduced in fumH63 discs lacking Su(fu) (Figure 6D), despite strong ectopic ptc-lacZ expression (data not shown). Comparable deficits in En induction were not seen in wing discs lacking Su(fu) alone (Figure 6C, D) and must therefore be attributed to a Su(fu)-independent function of Fu rather than to a hypothetical positive function of Su(fu) contributing to Hh target gene induction.
We have already shown that Fu can stabilize Ci-155 via Cos2 S572 phosphorylation but we thought that was unlikely to be a critical Fu activity because Hh can fully block Ci-155 processing in the absence of Fu kinase activity. We therefore tested whether Fu can activate Ci-155 in the absence of both Su(fu) and Ci-155 processing. In anterior slimb1 Su(fu)LP mutant clones Ci-155 processing is blocked but only low levels of Ptc and no ectopic En are induced, as shown previously (Wang et al., 1999) (Figure 6E). Expression of GAP-Fu in these clones greatly increased Ptc expression and even induced strong En expression (Figure 6E), providing direct evidence that Fu kinase can indeed enhance Hh pathway activity by a mechanism independent of both Su(fu) and Ci-155 processing.
DISCUSSION
Here we have found that Fu is activated by phosphorylation in a Hh-initiated positive feedback loop and that Fu kinase activity alone can provoke the two key outcomes of Hh signaling in Drosophila, namely Ci-155 stabilization and Ci-155 activation. This previously unrecognized central thread of the Drosophila Hh pathway is strikingly similar to receptor tyrosine kinase (RTK) pathways or cytokine pathways, where the trans-membrane receptor itself or an associated cytoplasmic tyrosine kinase initiates signal transduction via intermolecular phosphorylation (Hubbard and Till, 2000). In Hh signaling, engagement of the Ptc receptor leads indirectly to changes in Smo conformation, and perhaps oligomerization (Kalderon, 2008; Zhao et al., 2007) that are relayed to Fu via a mutual binding partner, Cos2.
Fu activation mechanism
We identified three activation loop residues as critical for normal Fu activity. Fu with acidic residues at T151 and T154 (Fu-EE) was not active at physiological levels in the absence of Hh but could initiate Fu activation in three different ways. First, increasing Fu-EE levels induced the full spectrum of Hh target genes and responses in wing discs and was accompanied by extensive phosphorylation, undoubtedly including S159, indicating that phosphorylation can fully activate Fu. Second, low levels of a Fu-EE derivative could synergize with an excess of wild-type Fu, provided the latter molecule had an intact activation loop and was kinase-competent, indicating that a feedback phosphorylation loop could initate Fu activation even from a ground state containing no phosphorylated residues or their mimics. Third, Hh could activate Fu-EE or wild-type Fu, but this, unlike the above mechanisms, required Cos2 and the Cos2-binding region of Fu. Activation by Hh alters Smo conformation and increase the plasma membrane concentration of Smo-Cos2 complexes (Kalderon, 2008; Zhao et al., 2007), suggesting that the role of activated Smo-Cos2 complexes may simply be to aggregate Fu molecules.
In all of the above situations there is likely an important contribution of binding between the catalytic and regulatory regions of pairs of Fu molecules (Ascano et al., 2002) to allow cross-phosphorylation, as suggested by the impotence of the Fu-EE 1–305 kinase domain alone. The sites of inferred cross-phosphorylation, T151, S159 and S482 might most simply be direct Fu auto-phosphorylation sites but they may involve the participation of an intermediate kinase. Importantly, since Fu is the key activating stimulus and Fu is the key target for activation, there is no need to postulate additional upstream regulatory inputs into a hypothetical intermediary protein kinase.
Phosphorylated residues in positions analogous to Fu S159 generally stabilize the active form of the protein kinase, while unphosphorylated residues at other positions, closer to the DFG motif may also, or exclusively, stabilize specific inactive conformations (Favelyukis et al., 2001; Kornev et al., 2006). By analogy, phosphorylated T151, T154 and S159 are likely to serve independent, additive functions, all of which are required to generate fully active Fu kinase. There are clearly additional phosphorylated residues on Fu, including the cluster at S482, S485 and T486. These residues are not essential for Hh or Fu-EE to generate fully active Fu when Fu is expressed at high levels. However, S485A/T486A substitutions did suppress activation of GAP-Fu in wing discs and in Kc cells, suggesting that stimulation of physiological levels of Fu, perhaps by lower levels of Hh uses S482, S485 and T486 phosphorylation to favor an active conformation of Fu or productive engagement of Fu molecules. Since the S482 region may be recognized directly as a substrate by the Fu catalytic site, this region may initially mask the catalytic site (in cis or in trans) and then reduce its affinity for the catalytic site once it is phosphorylated, permitting further phosphorylation of Fu in its activation loop.
Fu activities
For a long time it was thought that Fu kinase acts only to prevent inhibition of Ci-155 by Su(fu), and Fu was postulated to accomplish this by phosphorylating Su(fu). Here we mapped the sites responsible for the previously observed Hh- and Fu-stimulated phosphorylation of Su(fu) and showed that they were not important for regulating Hh pathway activity. We found that CK1, like Fu, was required for Hh to oppose Su(fu) inhibition of Ci-155 and because each of the Fu-dependent phosphorylation sites in Fu and Su(fu) that we mapped in this study prime CK1 sites we suspect that the critical unidentified Fu and CK1 sites for antagonizing Su(fu) will be found in the same molecule, with Ci-155 itself being a prime candidate.
Here we have found that Fu does considerably more than just antagonize Su(fu). We found unexpectedly that Fu kinase can also stabilize Ci-155 via phosphorylation of Cos2 on S572, which likely leads to reduced association of Cos2 with Ci-155 (Ruel et al., 2007). We also found that Fu promoted Ci-155 activation independently of Su(fu), even when Ci-155 processing was blocked by other means.
We also gained some insight into the key regulatory role that Fu plays in Hh signaling. The truncated partially activated Fu derivative, Fu-EE 1–473, exhibited constitutive activity when expressed at high levels but, unlike full-length Fu-EE, it was not activated by Hh. Importantly, we could not find a level of Fu-EE 1–473 expression in fumH63 mutant wing discs where Hh target genes were induced at the AP border but not ectopically. Hence, Hh regulation of Fu activity appears to be essential for normal Hh signaling. This contrasts with the normal Hh signaling observed in animals lacking Su(fu) and emphasizes that Fu is a key regulatory component that has essential actions beyond antagonizing Su(fu).
Conservation of Hh signaling pathways
In mice, SUFU increases Gli protein levels and inhibits Gli activators in a manner that can be overcome by Hh, much as Su(fu) affects Ci levels and activity in flies (Wilson and Chuang, 2010). However, in mammalian Hh signaling there is no satisfactory mechanistic model connecting Smo activation and SUFU antagonism. We found that mouse SUFU can substitute for all of the activities of Su(fu) in flies, including a dependence on both Fu and CK1 for Hh to antagonize silencing of Ci-155. These findings, and the observation that Drosophila Su(fu) can partially substitute for murine SUFU in mouse embryo fibroblasts (Chen et al., 2009), suggest that SUFU silencing of Gli proteins in mice is also likely to be sensitive to analogous changes in phosphorylation produced by at least one Hh-stimulated protein kinase. Even though the murine protein kinase most similar in sequence to Drosophila Fu is not required for Hh signaling at least three other protein kinases (MAP3K10, Cdc2l1 and ULK3) have been found to contribute positively to Hh responses in cultured mammalian cells (Evangelista et al., 2008; Maloverjan et al., 2010; Varjosalo et al., 2008; Wilson and Chuang, 2010). It will be of great interest to see if these or other protein kinases are activated by Hedgehog ligands, perhaps promoted by association with Smo-Kif7 complexes in a positive feedback loop, and whether they can antagonize mSUFU to activate Gli proteins, and perhaps even stabilize Gli proteins via Kif7 phosphorylation.
EXPERIMENTAL PROCEDURES
Mutagenesis and Cloning
Gateway Technology (Invitrogen) was used to make Entry clones by TOPO cloning and to transfer coding sequences to destination vectors for P-element germline transformation and tissue culture cell transfection as described previously (Smelkinson et al., 2007). Mutations and deletions were made in Entry clones by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene), followed by sequencing entire coding regions. Coding sequence for GAP-Fu-CFP was made by PCR from a GAP-Fu-CFP plasmid (gift from Dr. Anne Plessis, CNRS Paris, France) and for mouse SUFU from mouse SUFU cDNA plasmid (gift from Dr. Pao-Tien Chuang (UC San Francisco, USA). pUAST destination vectors for Fu constructs for P-element germline transformation added N-terminal triple HA and triple Flag tags plus a C-terminal triple Myc tag. Fu 1–473 variants used only that portion of coding sequence, whereas Fu-EE 1–305 used a stop codon to truncate the protein and hence has no C-terminal Myc tag. GAP-Fu transgenes used no epitope tags, while Su(fu) and mSUFU transgenes used a C-terminal GFP tag. Destination vectors with an actin5C promoter and either triple HA or triple Flag N-terminal tags were additionally used to create transgenes used for Kc cell transfection.
Biochemistry
Kc cells were transfected in six well plates by using Effectine Transfection Reagent (Qiagen). For expression of high levels of Fu together with Cos2 and Su(fu), 100ng Actin-Gal4, 80ng pUAST-Flag-Cos2, 180ng pUAST-Flag or HA-Fu and 35ng pUAST-Su(fu)-GFP (when used) were transfected. Cells were collected three days after transfection to generate extracts for Western blots as described previously (Smelkinson et al., 2007). To express lower amounts of Fu with or without Hh stimulation we used 200ng MK33-Hh plasmid (gift from Dr. Lawrence Lum, University of Texas Southwestern Medical Center, USA), 140ng Actin-Flag-SmoD1–3, 20ng Actin-HA-Cos2, and 40ng Actin-HA-or Flag-Fu. On the next day, 0.5mM Cu2+ was added to the media to induce Hh expression. Two days after Hh stimulation, cells were collected to generate extracts. For wing discs extracts, 40 wing discs were dissected and boiled in 40μl of 2x sample buffer for 10 minutes before Western Blot analysis.
Immunohistochemistry
Wing disc dissection, fixation and staining were performed as previously described (Smelkinson et al., 2007). Primary antibodies used were rabbit anti-β-galactosidase (Promega 1:4000), mouse Cos2 S572P phospho-epitope antibody (1:200, gift from Dr. Pascal Therond, CNRS Nice, France), mouse anti-Ptc monoclonal (1:20, Developmental Studies Hybridoma Bank (DSHB), University of Iowa, USA), mouse anti-En monoclonal (1:5, DSHB), rat 2A1 monoclonal (1:2) for full length Ci, mouse monoclonal clone 12CA5 (1:1000) for the HA epitope, mouse monoclonal clone M1 (1:1000, Sigma) for the Flag epitope, rabbit polyclonal anti-Fu (gift from Dr. David Robbins, University of Miami Medical School, USA) and mouse monoclonal anti-Su(fu) (1:15, DSHB). Secondary antibodies were Alexaflour-488, 594 or 647 (1:1000, Invitrogen).
Fly crosses
yw hs-flp ; smo2 FRT42D P[smo+] ubi-GFP ; C765 ptc-lacZ/TM6B females were crossed to yw; smo2 FRT42D cos22 UAS-GAP-Fu/CyO; males to generate negatively marked smo cos2 double mutant clones in wing discs expressing GAP-Fu throughout.
yw hs-flp ; ubi-GFP FRT40A; C765 ptc-lacZ/TM6B females were crossed to yw; smo2 FRT40A UAS-GAP-Fu/CyO or yw; smo2 pkaB3 FRT40A UAS-GAP-Fu/CyO males to generate negatively marked smo or smo pka mutant clones in wing discs expressing GAP-Fu throughout.
yw hs-flp UAS-GFP ; smo2 FRT42D P[smo+] tub-Gal80; C765 hh-lacZ /TM6B females were crossed to yw; smo2 FRT42D cos22/CyO; UAS-FuEE,UAS-Cos2 (WT or S572A) males to generate positively marked MARCM smo cos2 double mutant clones expressing Fu-EE and Cos2 transgenes only within the clones.
yw hs-flp fumH63; FRT42D P[Fu+] P[y+]/Cyo ; C765 ptc-lacZ/TM6B females were crossed to UAS-Fu males to test the rescue activity of Fu transgenes in male CyO progeny.
yw hs-flp fumH63; FRT42D P[Fu+] P[y+]/Cyo;C765 hh-lacZ/TM6B females were crossed to FRT42D ubi-GFP/CyO ; UAS-Ci males to generate fumH63 clones in wing discs expressing wild type Ci to assay for Ci-75 repressor.
yw hs-flp fumH63; FRT42D P[Fu+] P[y+]/CyO ; C765 ptc-lacZ/TM6B females were crossed to Su(fu)LP males to generate larvae lacking Fu kinase activity in the absence of Su(fu) protein.
yw hs-flp ; Su(fu)LP C765 ptc-lacZ/TM6B females were crossed to yw; Su(fu)LP /TM6B males to generate larvae lacking only Su(fu).
yw hs-flp MS1096; FRT82B Su(fu)LP slimb1 / TM6B females were crossed to UAS-GAP-Fu; FRT82B ubi-GFP males to generate Su(fu)LP slimb1 double mutant clones in wing discs expressing GAP-Fu.
Supplementary Material
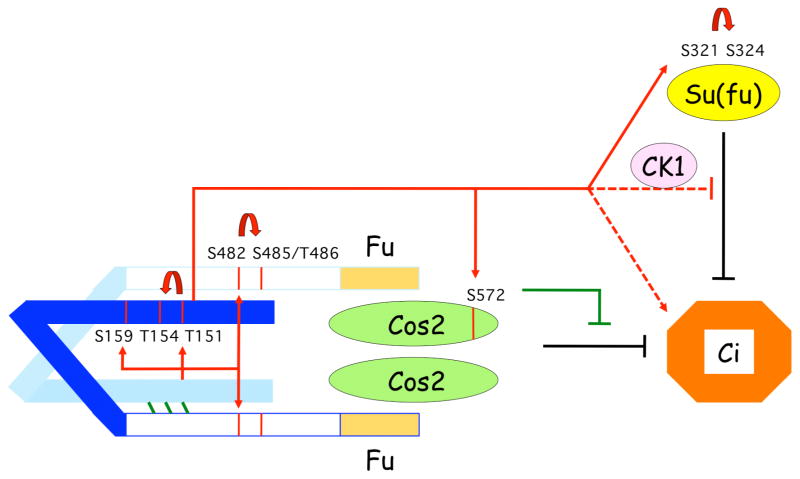
Figure 7. Summary model of Fu activation mechanism and activities.
We postulate that a low affinity interaction between the catalytic (light blue and dark blue for upper and lower Fu molecules) and non-catalytic domains of two Fu molecules (indicated by green diagonal lines) promotes cross-phosphorylation (directly or perhaps via an unknown intermediate kinase) only if association with a Hh-activated Smo-Cos2 complex, or artificially high local Fu concentration, augments this association. Primary, reciprocal cross-phosphorylation in the activation loop and non-catalytic domain (red arrows, illustrated only for activity of the light blue kinase domain) promotes secondary phosphorylation (attributed to CK1; curved arrows) and leads to full Fu activation and phosphorylation of downstream substrates (red arrows, shown only for dark blue kinase domain). Cos2 phosphorylation on S572 inhibits Ci-155 processing, leading to higher levels of Ci-155, while Su(fu) phosphorylation on S321 and S324 is unimportant. Phosphorylation of additional unidentified substrates (indicated by dashed lines) leads to Su(fu)-independent activation of Ci-155 and, in collaboration with CK1, inhibition of antagonism of Ci-155 by Su(fu).
HIGHLIGHTS.
Activation loop phosphorylation controls the Drosophila Fused protein kinase
Hedgehog signaling, CK1, and Fused itself contribute to this phosphorylation
Fused stabilizes Ci-155 by phosphorylating Cos2 S572
Fused activates stabilized Ci-155 by antagonizing Su(fu) and by other mechanisms
Acknowledgments
We thank Anne Plessis (CNRS Paris, France), Pao-Tien Chuang (UC San Francisco, USA), Jianhang Jia (University of Kentucky, USA), Jin Jiang and Lawrence Lum (University of Texas Southwestern Medical Center, USA), Pascal Therond (CNRS Nice, France), David Robbins (University of Miami Medical School, USA), the Developmental Studies Hybridoma Bank (University of Iowa, USA) and the Bloomington Stock Center (Indiana University, USA) for supplying critical reagents, and Pui-Leng Ip for generation of transgenic flies. This work was supported by NIH grant RO1 GM41815 (D.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apionishev S, Katanayeva NM, Marks SA, Kalderon D, Tomlinson A. Drosophila Smoothened phosphorylation sites essential for Hedgehog signal transduction. Nature Cell Biology. 2005;7:86–92. doi: 10.1038/ncb1210. [DOI] [PubMed] [Google Scholar]
- Ascano M, Jr, Nybakken KE, Sosinski J, Stegman MA, Robbins DJ. The carboxyl-terminal domain of the protein kinase fused can function as a dominant inhibitor of hedgehog signaling. Mol Cell Biol. 2002;22:1555–1566. doi: 10.1128/mcb.22.5.1555-1566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Wilson CW, Li YJ, Law KK, Lu CS, Gacayan R, Zhang X, Hui CC, Chuang PT. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 2009;23:1910–1928. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret S, Sanial M, Plessis A. Evidence for a novel feedback loop in the Hedgehog pathway involving Smoothened and Fused. Curr Biol. 2007;17:1326–1333. doi: 10.1016/j.cub.2007.06.059. [DOI] [PubMed] [Google Scholar]
- Evangelista M, Lim TY, Lee J, Parker L, Ashique A, Peterson AS, Ye W, Davis DP, de Sauvage FJ. Kinome siRNA screen identifies regulators of ciliogenesis and hedgehog signal transduction. Sci Signal. 2008;1:ra7. doi: 10.1126/scisignal.1162925. [DOI] [PubMed] [Google Scholar]
- Favelyukis S, Till JH, Hubbard SR, Miller WT. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat Struct Biol. 2001;8:1058–1063. doi: 10.1038/nsb721. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Watanabe-Fukunaga R, Fujisawa K, Nagata S, Fukunaga R. The fused protein kinase regulates Hedgehog-stimulated transcriptional activation in Drosophila Schneider 2 cells. J Biol Chem. 2001;276:38441–38448. doi: 10.1074/jbc.M105871200. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KS, Suyama K, Fish M, Scott MP. Differential regulation of Hedgehog target gene transcription by Costal2 and Suppressor of Fused. Development. 2005;132:1401–1412. doi: 10.1242/dev.01689. [DOI] [PubMed] [Google Scholar]
- Hooper JE, Scott MP. Communicating with Hedgehogs. Nature Reviews Molecular Cell Biology. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jia J, Tong C, Wang B, Luo L, Jiang J. Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature. 2004;432:1045–1050. doi: 10.1038/nature03179. [DOI] [PubMed] [Google Scholar]
- Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D. Hedgehog signaling: a smoothened conformational switch. Curr Biol. 2008;18:R64–66. doi: 10.1016/j.cub.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Kornev AP, Haste NM, Taylor SS, Eyck LF. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc Natl Acad Sci U S A. 2006;103:17783–17788. doi: 10.1073/pnas.0607656103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cao X, Jiang J, Jia J. Fused-Costal2 protein complex regulates Hedgehog-induced Smo phosphorylation and cell-surface accumulation. Genes Dev. 2007;21:1949–1963. doi: 10.1101/gad.1557407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L, Zhang C, Oh S, Mann RK, von Kessler DP, Taipale J, Weis-Garcia F, Gong R, Wang B, Beachy PA. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Molecular Cell. 2003;12:1261–1274. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- Maloverjan A, Piirsoo M, Kasak L, Peil L, Osterlund T, Kogerman P. Dual function of UNC-51-like kinase 3 (Ulk3) in the Sonic hedgehog signaling pathway. J Biol Chem. 2010;285:30079–30090. doi: 10.1074/jbc.M110.133991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpel S, Claret S, Sanial M, Brigui A, Piolot T, Daviet L, Martin-Lanneree S, Plessis A. The last 59 amino acids of Smoothened cytoplasmic tail directly bind the protein kinase Fused and negatively regulate the Hedgehog pathway. Dev Biol. 2007;303:121–133. doi: 10.1016/j.ydbio.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Methot N, Basler K. Suppressor of fused opposes hedgehog signal transduction by impeding nuclear accumulation of the activator form of Cubitus interruptus. Development. 2000;127:4001–4010. doi: 10.1242/dev.127.18.4001. [DOI] [PubMed] [Google Scholar]
- Monnier V, Ho KS, Sanial M, Scott MP, Plessis A. Hedgehog signal transduction proteins: contacts of the Fused kinase and Ci transcription factor with the kinesin-related protein Costal2. BMC Developmental Biology. 2002;2:4. doi: 10.1186/1471-213X-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen B, Taylor S, Ghosh G. Regulation of protein kinases; controlling activity through activation segment conformation. Mol Cell. 2004;15:661–675. doi: 10.1016/j.molcel.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Ohlmeyer JT, Kalderon D. Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature. 1998;396:749–753. doi: 10.1038/25533. [DOI] [PubMed] [Google Scholar]
- Preat T. Characterization of Suppressor of fused, a complete suppressor of the fused segment polarity gene of Drosophila melanogaster. Genetics. 1992;132:725–736. doi: 10.1093/genetics/132.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preat T, Therond P, Limbourg-Bouchon B, Pham A, Tricoire H, Busson D, Lamour-Isnard C. Segmental polarity in Drosophila melanogaster: genetic dissection of fused in a Suppressor of fused background reveals interaction with costal-2. Genetics. 1993;135:1047–1062. doi: 10.1093/genetics/135.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MA, Kalderon D. Proteolysis of cubitus interruptus in Drosophila requires phosphorylation by protein kinase A. Development. 1999;126:4331–4339. doi: 10.1242/dev.126.19.4331. [DOI] [PubMed] [Google Scholar]
- Raisin S, Ruel L, Ranieri N, Staccini-Lavenant L, Therond PP. Dynamic phosphorylation of the kinesin Costal-2 in vivo reveals requirement of fused kinase activity for all levels of hedgehog signalling. Dev Biol. 2010;344:119–128. doi: 10.1016/j.ydbio.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–234. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- Ruel L, Gallet A, Raisin S, Truchi A, Staccini-Lavenant L, Cervantes A, Therond PP. Phosphorylation of the atypical kinesin Costal2 by the kinase Fused induces the partial disassembly of the Smoothened-Fused-Costal2-Cubitus interruptus complex in Hedgehog signalling. Development. 2007;134:3677–3689. doi: 10.1242/dev.011577. [DOI] [PubMed] [Google Scholar]
- Ruel L, Rodriguez R, Gallet A, Lavenant-Staccini L, Therond PP. Stability and association of Smoothened, Costal2 and Fused with Cubitus interruptus are regulated by Hedgehog. Nature Cell Biology. 2003;5:907–913. doi: 10.1038/ncb1052. [DOI] [PubMed] [Google Scholar]
- Smelkinson MG, Zhou Q, Kalderon D. Regulation of Ci-SCFSlimb binding, Ci proteolysis, and hedgehog pathway activity by Ci phosphorylation. Dev Cell. 2007;13:481–495. doi: 10.1016/j.devcel.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therond PP, Knight JD, Kornberg TB, Bishop JM. Phosphorylation of the fused protein kinase in response to signaling from hedgehog. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4224–4228. doi: 10.1073/pnas.93.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjosalo M, Bjorklund M, Cheng F, Syvanen H, Kivioja T, Kilpinen S, Sun Z, Kallioniemi O, Stunnenberg HG, He WW, et al. Application of active and kinase-deficient kinome collection for identification of kinases regulating hedgehog signaling. Cell. 2008;133:537–548. doi: 10.1016/j.cell.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Vervoort M. hedgehog and wing development in Drosophila: a morphogen at work? Bioessays. 2000;22:460–468. doi: 10.1002/(SICI)1521-1878(200005)22:5<460::AID-BIES8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wang G, Wang B, Jiang J. Protein kinase A antagonizes Hedgehog signaling by regulating both the activator and repressor forms of Cubitus interruptus. Genes & Development. 1999;13:2828–2837. doi: 10.1101/gad.13.21.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QT, Holmgren RA. Nuclear import of cubitus interruptus is regulated by hedgehog via a mechanism distinct from Ci stabilization and Ci activation. Development. 2000;127:3131–3139. doi: 10.1242/dev.127.14.3131. [DOI] [PubMed] [Google Scholar]
- Wilson CW, Chuang PT. Mechanism and evolution of cytosolic Hedgehog signal transduction. Development. 2010;137:2079–2094. doi: 10.1242/dev.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Williams EH, Guo Y, Lum L, Beachy PA. Extensive phosphorylation of Smoothened in Hedgehog pathway activation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17900–17907. doi: 10.1073/pnas.0408093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhao Y, Tong C, Wang G, Wang B, Jia J, Jiang J. Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus.[see comment] Developmental Cell. 2005;8:267–278. doi: 10.1016/j.devcel.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tong C, Jiang J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature. 2007;450:252–258. doi: 10.1038/nature06225. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Apionishev S, Kalderon D. The contributions of protein kinase A and smoothened phosphorylation to hedgehog signal transduction in Drosophila melanogaster. Genetics. 2006;173:2049–2062. doi: 10.1534/genetics.106.061036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Kalderon D. Costal 2 interactions with Cubitus interruptus (Ci) underlying Hedgehog-regulated Ci processing. Dev Biol. 2010;348:47–57. doi: 10.1016/j.ydbio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.