Abstract
The distribution of the gene cluster encoding type 3 fimbriae among various isolates of the family Enterobacteriaceae was investigated by using 112 clinical and nonclinical isolates. Closely related DNA sequences were detected in all Klebsiella strains, in most Enterobacter isolates, in a smaller number of Escherichia coli and Salmonella spp., and in a single isolate each of Yersinia enterocolitica and Serratia liquefaciens but not in isolates of Morganella or Providencia species or Serratia marcescens. Except for E. coli and Salmonella strains, the presence of gene sequences was correlated with the phenotypic expression of either the MR/K hemagglutinin or fimbriae that reacted with specific antibodies. In one isolate of Y. enterocolitica the expression of type 3 fimbriae was plasmid determined. The polyamine spermidine was identified as an inhibitor of MR/K hemagglutinating activity, exhibiting an MIC of 1.2 mM. Spermidine inhibited the hemagglutination of 37 MR/K-positive clinical isolates from various genera. However, one clinical isolate of Enterobacter cloacae and most (four of five) nonclinical Klebsiella isolates were not completely inhibited.
Full text
PDF


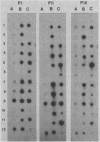
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksić S., Aleksić V. Reindarstellung und physikalisch-chemische Analyse des Fimbrien-Antigens bei zwei verschiedenen Enterobacteriaceae: Salmonella enteritidis und Yersinia enterocolitica. Zentralbl Bakteriol Orig A. 1979 Apr;243(2-3):177–196. [PubMed] [Google Scholar]
- Chatel M., Darcel F., Quemener V., Hercouet H., Moulinoux J. P. Red blood cell polyamines as biochemical markers of supratentorial malignant gliomas. Anticancer Res. 1987 Jan-Feb;7(1):33–38. [PubMed] [Google Scholar]
- Clegg S. Cloning of genes determining the production of mannose-resistant fimbriae in a uropathogenic strain of Escherichia coli belonging to serogroup O6. Infect Immun. 1982 Nov;38(2):739–744. doi: 10.1128/iai.38.2.739-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S., Hull S., Hull R., Pruckler J. Construction and comparison of recombinant plasmids encoding type 1 fimbriae of members of the family Enterobacteriaceae. Infect Immun. 1985 May;48(2):275–279. doi: 10.1128/iai.48.2.275-279.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P. Fimbriae and adhesive properties in Klebsiella strains. J Gen Microbiol. 1959 Aug;21:271–286. doi: 10.1099/00221287-21-1-271. [DOI] [PubMed] [Google Scholar]
- Elsayed N. M. Influence of vitamin E on polyamine metabolism in ozone-exposed rat lungs. Arch Biochem Biophys. 1987 Jun;255(2):392–399. doi: 10.1016/0003-9861(87)90407-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr Res. 1983 Aug 16;120:235–249. doi: 10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- Gerlach G. F., Allen B. L., Clegg S. Molecular characterization of the type 3 (MR/K) fimbriae of Klebsiella pneumoniae. J Bacteriol. 1988 Aug;170(8):3547–3553. doi: 10.1128/jb.170.8.3547-3553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach G. F., Clegg S. Characterization of two genes encoding antigenically distinct type-1 fimbriae of Klebsiella pneumoniae. Gene. 1988 Apr 29;64(2):231–240. doi: 10.1016/0378-1119(88)90338-1. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. F7 and type 1-like fimbriae from three Escherichia coli strains isolated from urinary tract infections: protein chemical and immunological aspects. Infect Immun. 1982 May;36(2):462–468. doi: 10.1128/iai.36.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Tarkka E., Ranta H., Haahtela K. Type 3 fimbriae of Klebsiella sp.: molecular characterization and role in bacterial adhesion to plant roots. J Bacteriol. 1983 Aug;155(2):860–865. doi: 10.1128/jb.155.2.860-865.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer F. J., Chang A. C., Cohen S. N. Indirect selection of bacterial plasmids lacking identifiable phenotypic properties. J Bacteriol. 1975 Oct;124(1):225–231. doi: 10.1128/jb.124.1.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Tenney J. H., Mayrer A. R., Crisp L. J., Penner J. L., Warren J. W. MR/K hemagglutination of Providencia stuartii correlates with adherence to catheters and with persistence in catheter-associated bacteriuria. J Infect Dis. 1988 Feb;157(2):264–271. doi: 10.1093/infdis/157.2.264. [DOI] [PubMed] [Google Scholar]
- Moulinoux J. P., Quemener V., Chambon Y. Evolution of red blood cell polyamine levels in partially hepatectomized rat. Eur J Cancer Clin Oncol. 1987 Feb;23(2):237–244. doi: 10.1016/0277-5379(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Nesbakken T., Kapperud G., Sørum H., Dommarsnes K. Structural variability of 40-50 Mdal virulence plasmids from Yersinia enterocolitica. Geographical and ecological distribution of plasmid variants. Acta Pathol Microbiol Immunol Scand B. 1987 Jun;95(3):167–173. doi: 10.1111/j.1699-0463.1987.tb03107.x. [DOI] [PubMed] [Google Scholar]
- Nowotarska M., Mulczyk M. Serologic relationship of fimbriae among Enterobacteriaceae. Arch Immunol Ther Exp (Warsz) 1977;25(1):7–16. [PubMed] [Google Scholar]
- Old D. C., Adegbola R. A. Antigenic relationships among type-3 fimbriae of Enterobacteriaceae revealed by immunoelectronmicroscopy. J Med Microbiol. 1985 Aug;20(1):113–121. doi: 10.1099/00222615-20-1-113. [DOI] [PubMed] [Google Scholar]
- Old D. C., Adegbola R. A. Haemagglutinins and fimbriae of Morganella, Proteus and Providencia. J Med Microbiol. 1982 Nov;15(4):551–564. doi: 10.1099/00222615-15-4-551. [DOI] [PubMed] [Google Scholar]
- Old D. C., Adegbola R., Scott S. S. Multiple fimbrial haemagglutinins in Serratia species. Med Microbiol Immunol. 1983;172(2):107–115. doi: 10.1007/BF02124511. [DOI] [PubMed] [Google Scholar]
- Old D. C., Tavendale A., Senior B. W. A comparative study of the type-3 fimbriae of Klebsiella species. J Med Microbiol. 1985 Oct;20(2):203–214. doi: 10.1099/00222615-20-2-203. [DOI] [PubMed] [Google Scholar]
- Pere A., Väisänen-Rhen V., Rhen M., Tenhunen J., Korhonen T. K. Analysis of P fimbriae on Escherichia coli O2, O4, and O6 strains by immunoprecipitation. Infect Immun. 1986 Feb;51(2):618–625. doi: 10.1128/iai.51.2.618-625.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podschun R., Heineken P., Sonntag H. G. Haemagglutinins and adherence properties to HeLa and intestine 407 cells of Klebsiella pneumoniae and Klebsiella oxytoca isolates. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Mar;263(4):585–593. doi: 10.1016/s0176-6724(87)80203-1. [DOI] [PubMed] [Google Scholar]
- Przondo Hessek A., Pulverer G. Hemagglutinins of Klebsiella pneumoniae and Klebsiella oxytoca. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Nov;255(4):472–478. [PubMed] [Google Scholar]
- Rhen M., Väisänen-Rhen V., Pere A., Korhonen T. K. Complementation and regulatory interaction between two cloned fimbrial gene clusters of Escherichia coli strain KS71. Mol Gen Genet. 1985;200(1):60–64. doi: 10.1007/BF00383312. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Clinical relevance of polyamines. Crit Rev Clin Lab Sci. 1983;18(3):261–311. doi: 10.3109/10408368209085073. [DOI] [PubMed] [Google Scholar]
- Stauffer G. V., Plamann M. D., Stauffer L. T. Construction and expression of hybrid plasmids containing the Escherichia coli glyA genes. Gene. 1981 Jun-Jul;14(1-2):63–72. doi: 10.1016/0378-1119(81)90148-7. [DOI] [PubMed] [Google Scholar]