Abstract
Sweet taste is a powerful factor influencing food acceptance. There is considerable variation in sweet taste perception and preferences within and among species. Although learning and homeostatic mechanisms contribute to this variation in sweet taste, much of it is genetically determined. Recent studies have shown that variation in the T1R genes contributes to within- and between-species differences in sweet taste. In addition, our ongoing studies using the mouse model demonstrate that a significant portion of variation in sweetener preferences depends on genes that are not involved in peripheral taste processing. These genes are likely involved in central mechanisms of sweet taste processing, reward and/or motivation. Genetic variation in sweet taste not only influences food choice and intake, but is also associated with proclivity to drink alcohol. Both peripheral and central mechanisms of sweet taste underlie correlation between sweet-liking and alcohol consumption in animal models and humans. All these data illustrate complex genetics of sweet taste preferences and its impact on human nutrition and health. Identification of genes responsible for within- and between-species variation in sweet taste can provide tools to better control food acceptance in humans and other animals.
Keywords: sweet, taste, behaviour, genetics, receptor
Introduction
Sweet Taste
The sense of taste has probably evolved to allow animals to choose and consume appropriate food. The most common natural taste stimuli that humans describe as sweet are sugars. Sugars are important nutrients for animals from many different species ranging from insects to mammals. In animals from many species, sugars are recognized by the taste system and evoke appetitive consummatory responses.[1] In addition to sugars, a wide range of other chemicals (referred to here as sweeteners), also evoke the sensation of sweetness in humans and are palatable to many other animals. Numerous studies have shown that the mechanisms of taste perception of sweeteners are similar in humans and non-human mammals. This justifies using laboratory animals, such as mice and rats, as model organisms to study mechanisms of sweet (sucrose-like) taste relevant to humans.
In addition to evoking behavioural responses, sweet taste stimuli can elicit preabsorptive cephalic phase responses, such as insulin release,[2-4] activate endogenous opioidergic, dopaminergic and serotonergic systems[5-12] and produce analgesic effects in children and young animals.[13-17] Taste responses to sweeteners are modulated by post-ingestive feedback and hormones.[18-24] Because ingested sugars evoke sweet taste sensation and also produce rewarding post-ingestive feedback,[25-27] sweet taste preferences can be modified by the experience of consuming sugar. These effects of experience are strong enough to alter initial genetic differences in sweet taste responsiveness.[28-30] Although appetitive responses to sweet taste stimuli are inborn in many animals,[31,32] they are also often modulated by environment and depend on genetic factors.[33,34] The interactive mechanisms of sweet taste suggest that it is a part of a complex ingestive behaviour and is likely to be determined by multiple genes.
Sweet Taste Receptors
In mammals, sweetness perception is initiated when sweeteners interact with taste receptor proteins from the T1R family expressed in taste receptor cells in taste buds of the oral cavity. Thus, sweeteners function as ligands of the G protein coupled T1R receptors. The mammalian T1R gene family consists of three genes named ‘taste receptor, type 1, member 1, 2 or 3’. Corresponding gene symbols abbreviate these names to TAS1R1, TAS1R2 or TAS1R3 (in humans) or Tas1r1, Tas1r2 or Tas1r3 (in rodents and other non-human animals). Corresponding protein symbols are T1R1, T1R2 and T1R3. Species origin of a protein can be indicated as hT1R1 (human T1R1) or mT1R1 (mouse T1R1). For brevity, when we refer to both human (TAS1R) and non-human (Tas1r) genes, we describe them as T1R genes. The three mouse Tas1r genes are located in the distal chromosome 4 in the order: Tas1r2 – Tas1r1 – Tas1r3. Their human orthologues reside in a region of conserved synteny in the short arm of human chromosome 1 (1p36) in the same order: TAS1R2 – TAS1R1 – TAS1R3. The mouse Tas1r genes contain six coding exons that are translated into 842–858 amino acid proteins. The T1R proteins have a predicted secondary structure that includes seven transmembrane helices forming a heptahelical domain, and a large extracellular N-terminus composed of a venus flytrap (VFT) domain and a cysteine-rich domain connected to the heptahelical domain.
There is strong evidence that T1R2 and T1R3 proteins function as sweet taste receptors. Although T1R genes are expressed in several different internal organs (reviewed by Bachmanov and Beauchamp[35]), their main sites of expression are taste receptor cells of the taste buds. In mice and rats, T1R2 and T1R3 are co-expressed in the same taste cells, but some taste cells express only T1R3.[36-38] Co-expression of T1R2 and T1R3 in the same taste cells suggested that they may function as heterodimers, which is believed to commonly occur with GPCRs.[39] Consistent with this, cells heterologously expressing both T1R2 and T1R3 respond to sweeteners,[38,40,41] but T1R3 may also function as a low-affinity sugar receptor alone, probably as a homodimer.[42] Finally, genetically engineered mice with targeted mutations of the Tas1r2 or Tas1r3 genes have diminished taste responses to sweeteners.[42,43]
There is also evidence that sweet taste reception may be not limited only to the T1R-mediated mechanisms. Glucose transporter 4 (GLUT4), sodium-glucose co-transporter (SGLT1), and ATP-gated K+ (KATP) metabolic sensors are present in T1R3-expressing taste cells and may serve as mediators of the T1R-independent sweet taste of sugars in mice.[44] In addition, some sweet-tasting compounds can penetrate the TRC membrane and act on intracellular targets,[45] which in this case could function as intracellular receptors of such compounds.
Behavioural Genetics of Taste
In genetic terms, measurements of the sweet taste preference are considered phenotypes, or traits. Phenotype is defined as the observable characteristics of an organism determined by both genetic make-up and environmental influences. The goal of the genetic analysis is to separate the genetic and environmental effects on phenotype.
In humans, taste phenotypes are usually rating sensation intensity on a scale with verbal descriptors or by reporting a perceptual difference between samples. These techniques allow one to evaluate sensitivity, intensity, quality and hedonic value of the taste sensation. Human sweet taste preference and liking can also be evaluated by measuring consumption or cravings of sweet foods using questionnaires or records of intake.
Studies of model organisms help to understand the genetic mechanisms of variation in sweet taste preferences. Laboratory animals offer an important advantage in these studies because inbred strains are available for several species. Animals within an inbred strain are genetically homogeneous. Therefore, the within-strain variation is due to non-genetic (environmental) factors, but differences between strains represent genetic variation.
Assessment of taste perception in non-human animals relies on a number of different techniques to record behaviour elicited by taste stimuli.[46] These techniques include two-bottle preference tests, brief-access lick recording tests, and approaches that require animal conditioning to examine generalization and discrimination between taste stimuli, and to measure taste thresholds.
Many taste phenotypes are measured using a continuous quantitative scale (e.g. volume of solution consumed, preference score or lick rate) and thus are considered quantitative traits. Genes with allelic variants that underlie variation of quantitative traits reside in chromosomal regions named quantitative trait loci (QTL). Defining these chromosomal regions through genetic linkage analysis is called QTL mapping. QTL mapping helps to identify DNA sequences of genes in the QTL regions and to find genes that are responsible for phenotypical variation. Because this approach to identify genes is based on a chromosomal position of a phenotypical locus, it is called positional cloning. Quantitative traits that depend on multiple genetic and environmental factors are considered complex traits. There is strong evidence that sweet taste preference is a complex trait.
Species Differences in Sweet Taste Preferences
Although many vertebrate and invertebrate animals detect taste of sugars and avidly consume them, receptors for sugars evolved independently in these two lineages. The vertebrate T1R receptors are not found in invertebrates[47] and are not related to a Drosophila taste receptor for a sugar trehalose encoded by the Gr5a gene.[48-53] Numbers of the T1R genes in different vertebrate species range from complete absence in the frog to five in some fishes.[40,47,54-63] Ligands for the T1R receptors have been experimentally confirmed only for a few species (mostly humans and rodents), but it is likely that their orthologues in other species have similar ligand specificities. Therefore, species differences in sweet taste preferences could be due to variation in the T1R genes.
There are several examples of differences in sweet taste preferences among species of vertebrate animals. Despite nearly universal preference for sugars, the chicken and Felidae species (domestic cat, tiger, lion and cheetah) are not attracted to sugars and other sweeteners.[64-69] Mammals also differ in preferences for artificial sweeteners, for example aspartame.[70,71] Species variation in the T1R receptors plays prominent role in these differences in sweet taste preferences.
Sweet Taste Blindness in Cats and Other Species
Domestic cats (Felis silvestris catus) and their wild relatives from the family Felidae are obligate carnivores. They do not show preferences for sweeteners, but otherwise have the normal sense of taste.[64,65,69] We have identified the cat Tas1r2 and Tas1r3 genes.[56] The cat Tas1r3 gene shows high sequence similarity with functional Tas1r3 genes of other species and is expressed in taste buds. However, the cat Tas1r2 is a pseudogene (Figure 1) with no evidence of its expression. The Tas1r2 genes of three other Felidae species, the tiger (Panthera tigris), cheetah (Acinonyx jubatus) and Asiatic lion (Panthera leo persica), are also pseudogenes.[56,69] Because cat Tas1r2 is an unexpressed pseudogene, a functional sweet taste receptor heteromer T1R2+3 cannot form, which explains the molecular origins of sweet taste blindness in cats.
Figure 1.
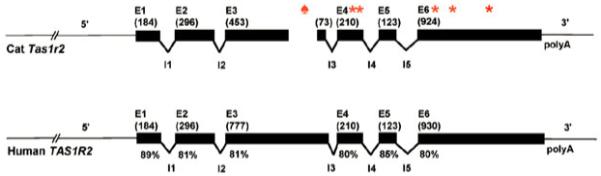
Structures of the cat Tas1r2 and human TAS1R2 genes. The cat Tas1r2 gene has a 247-base pair micro-deletion (♠) in exon 3 and stop codons (*) in exons 4 and 6. The exons are shown as black bars; exon numbers and size (bp; shown in parentheses) are indicated above the bars. The % similarity between corresponding human and cat exons at the nucleotide level are indicated under the human exons. Introns are not scaled proportionally because of their large size. Reproduced from Li et al.[56] with open-access licence from the Public Library of Science (PLoS)
Tas1r2 pseudogenization and lack of sweet taste responsiveness in cats are probably results of these animals being obligate carnivores that do not seek sugars in their food, and thus do not have a selective advantage of having a functional sweet taste receptor that recognizes sugars. Other species of the order of Carnivora (dogs, Canis lupus familiaris, Canidae family; lesser panda, Ailurus fulgens, Ailurus; domestic ferret, Mustela putorious furo, Mustelidae; Haussa genet, Genetta thierry, Viverridae; meerkat, Suricata suricatta, Herpestidae; and yellow mongoose, Cynictis penicillata, Herpestidae) have a functional Tas1r2 structure[56,57,69] and are attracted to sugars.[69,72,73] Thus, Tas1r2 pseudogenization was an important event in the evolution of the cat’s carnivorous behaviour.
Similarly to cats, chickens also lack functional Tas1r2 gene.[57,61] Interestingly, some other birds recognize sugar taste,[74,75] suggesting that they may have a functional T1R2. Thus, pseudogenization of Tas1r2 occurred multiple times in evolution. Loss of Tas1r2 in cats and chickens may be a consequence of their feeding behaviour that does not require a sweet taste receptor for proper food choice. However, a reverse causative relationship is also possible, when a loss-of-function mutation in the Tas1r2 gene resulted in loss of sweet taste sensation, which in turn altered feeding behaviour of these animals.
The role of pseudogenization of T1R genes in evolution of feeding behaviour is also illustrated, by a recent finding that Tas1r1 is a pseudogene in the giant panda (Ailuropoda melanoleuca),[76,77] which deems its umami/amino acid taste receptor dimer T1R1+3 non-functional. Most species of the order Carnivora have an intact Tas1r1. The giant panda also belongs to the order Carnivora, but it eats almost exclusively bamboo, and estimated time of its dietary switch to bamboo coincides with time of its Tas1r1 pseudogenization.[76]
Perception of Aspartame Sweetness in Primates and Other Species
Aspartame is a commercially available low-calorie sweetener widely used in foods and beverages. Aspartame preference and the ability to taste aspartame sweetness vary among mammalian species. Humans, apes and Old World monkeys perceive aspartame as sweet, but other primate species, and most of non-primate species, do not. We analysed the association of aspartame taster/non-taster status and sequence variants of the sweet taste receptor proteins, T1R2 and T1R3, in several species. Nine variant sites in T1R2 and 32 variant sites in T1R3 distinguished aspartame tasters and non-tasters. We next examined whether any of these variant sites disrupt interaction between aspartame and the T1R2+3 receptor. Molecular docking of aspartame to computer-generated models of the T1R2 + T1R3 receptor dimer identified primary active binding sites in the VFT domain of the T1R2 and T1R3 proteins. In addition, previously unknown allosteric sites were identified. Sequence variants at the T1R2 allosteric binding site (Figure 2) likely influence the interaction of aspartame with the primary binding site and ability of aspartame to activate the receptor, and therefore an animals’ ability to taste sweetness of aspartame.[78]
Figure 2.
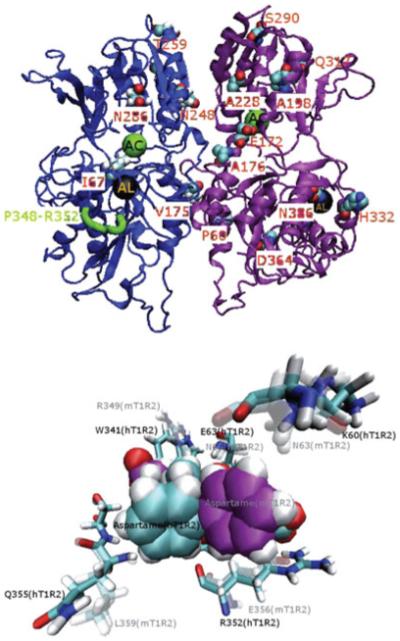
Sequence variants predicted to influence interaction of the T1R2+3 receptor with aspartame. Top panel: VFT domain of the hT1R2 (active–close)-hT1R3 (active–open) heterodimer. The C-alpha trace for hT1R2 is shown as blue ribbon; hT1R3 is shown in purple. Centres of binding regions are shown as green or black spheres. The green spheres (labelled AC) indicate binding regions at the centres of the VFT domains referred to as active sites. Black spheres (labelled AL) indicate binding regions referred to as allosteric sites. Taster/non-taster variant sites are shown as space-filled representation. The hT1R2 segment P348–R352 (PPLSR; shown in green ribbon) is a part of the allosteric site. It is deleted in most aspartame non-tasters and is replaced with PMPNE in the mouse. This segment is important for the spatial arrangement of the putative allosteric site (see bottom panel). Bottom panel: Aspartame (carbon atoms are cyan) bound to the allosteric site of hT1R2 is superposed to aspartame (carbon atoms are purple) bound to the allosteric site of mT1R2. Amino acids within 4.5 Å of bound aspartame in hT1R2 are shown in stick representation (the equivalent amino acids in mT1R2 are shown as shadows). R352 (a part of a polymorphic segment P348–R352) in hT1R2 is predicted to be directly involved in binding of aspartame to the putative allosteric site. Substitution of R352 in hT1R2 with a corresponding residue, E356, in mT1R2 changes orientation of aspartame within the allosteric site and leads to stronger binding of aspartame to the mouse site compared with the human site. This likely interferes with aspartame binding to the active site of mT1R2+3 and prevents receptor activation. Reproduced from Li et al.[78] by permission of the Oxford University Press
Within-species Variation in Sweet Taste Preferences
Humans
Humans differ in their perception of sweet taste.[79-86] One of the best known examples of this variation is a sweet liking phenotype (Figure 3): in ‘sweet-likers’, hedonic ratings of sucrose solutions monotonously increase with increasing concentrations, while in ‘sweet-dislikers’ at higher sucrose concentrations the ratings decrease.[81,86] Mechanisms underlying human variation in sweet taste, including ‘sweet-liker’ and ‘sweet-disliker’ phenotype could be complex: they may involve peripheral or central taste processing and can be genetically determined, acquired or depend on interaction between genetic and environmental factors. Nevertheless, genetic factors explain at least part of variation in sweet taste preferences in humans.[33,34,87-94]
Figure 3.
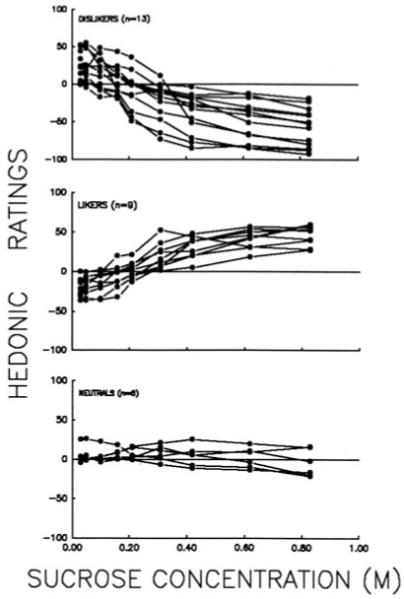
The variation in sweet-liking in humans: individual hedonic ratings of sucrose. ‘Dislikers’ report a general decrease in pleasantness as concentration increases, ‘likers’ report an increase in pleasantness with increasing concentration, and ‘neutrals’ have a minimal affective response to all concentrations of sucrose. Reproduced from Looy and Weingarten[86] by permission of the Oxford University Press
T1R-dependent variation
In humans of African, Asian, European and Native American origin, all three TAS1R genes have multiple polymorphisms, which include those resulting in amino acid changes of the T1R proteins. The majority of amino acid sequence variation occurs in the N-terminus extracellular domain, where taste ligands are likely to bind to the taste receptors. TAS1R2 is particularly diverse compared with other human genes: its rate of polymorphisms is in the top 5–10% of all human genes surveyed. The high rate of TAS1R2 variation was predicted to result in variation in sweet taste perception.[95] This prediction was confirmed in a recent study, which demonstrated association of Ile191Val variant in TAS1R2 with habitual consumption of sugars in overweight and obese individuals.[96]
Another recent study has shown that non-amino acid coding single nucleotide polymorphisms (SNPs) in the TAS1R3 promoter are associated with taste sensitivity to sucrose in humans. These polymorphisms influenced promoter activity in an in vitro luciferase reporter assay, which indicates that they affect TAS1R3 gene transcription.[97]
T1R-independent variation
Genetic factors responsible for variation in human sweet taste preferences are not limited to polymorphisms of the sweet taste T1R2+3 receptor. While human TAS1R2 or TAS1R3 genes reside in chromosome 1, a genome-wide linkage study has detected a QTL for use frequency of sweet foods on chromosome 16.[94] Candidate gene association studies indicate that T1R-independent genetic variation in sweet taste preferences involves both peripheral and central mechanisms. The GNAT3 gene encodes the taste-specific Gα protein subunit gustducin expressed in taste bud cells in the tongue. Several SNPs in the non-coding regions of GNAT3 (upstream of the ATG translation start site and within introns) are associated with human sucrose perception.[98] The dopamine D2 receptor (DRD2) is involved in the rewarding effects of sugar. Glucose transporter type 2 (GLUT2) was suggested to function as a glucose sensor in the brain and to be involved in regulation of food intake. Amino acid coding variants in both of these brain-expressed genes, DRD2 and GLUT2, are associated with habitual consumption of sugars by humans.[99,100]
Mice
Prominent genetic differences in taste responses to sweeteners among inbred strains of mice were shown using different experimental techniques and a variety of sweeteners (e.g. sucrose, glucose, dulcin, saccharin, acesulfame, glycine, d-phenylalanine and l-glutamine). Mice from different strains differ in taste responses to sweeteners assessed using long-term preference tests,[101-113] single-bottle tests,[114] brief-access tests based on lick recording,[115] taste detection thresholds,[116] conditioned taste aversion generalization,[117] and responses of gustatory nerves.[118-120] These studies have shown that responses to many of these sweeteners (e.g. sucrose, glucose, dulcin, saccharin and acesulfame) closely correlate among mouse strains, suggesting a common genetic basis for sweet taste. However, responses to some sweet-tasting amino acids display somewhat different patterns of strain differences.[121]
Some genetic analyses of sweetener consumption by mice yielded evidence that it is influenced by a single locus, named Sac (saccharin preference),[102,106,110,122] whereas other experiments indicated that more than one gene is involved.[107,110,111,123,124] The apparent discrepancy in whether the single-gene or the multi-gene model better describes genetic variation in sweetener preferences is likely due to use of different progenitor strains and types of mapping panels, different sweetener solutions tested, and different quantitative analyses used in these studies.
T1R-dependent variation
Genetic analyses and positional cloning of the Sac locus were instrumental in discovery of the T1R sweet taste receptor genes. Studies using crosses between different inbred mouse strains have mapped the Sac locus to the subtelomeric region of mouse chromosome 4[110,123,125,126] and have shown that it affects sweetener preferences[102,106,110,122,123,125,126] and the afferent responses of gustatory nerves to sweeteners.[126,127] Our positional cloning study has shown that the Sac locus corresponds to the Tas1r3 gene[60,113,128] and that Tas1r3 sequence variants are associated with sweetener preference phenotypes in genealogically diverse mouse strains.[60,113] Additional evidence for identity of Sac and Tas1r3 was obtained in studies using congenic,[60] transgenic[38] and knockout mice.[42,43] Furthermore, allelic variants that confer different sweet taste responsiveness in mice also influenced receptor properties in in vitro assays.[41,129] We analysed association of the Tas1r3 sequence variants with saccharin preferences in a large panel of genealogically diverse inbred mouse strains.[113] This analysis has identified an amino acid substitution of isoleucine to threonine at position 60 (I60T) as a candidate causative variant for phenotypical variation in sweet taste preferences. Because this sequence variant is in the extracellular N-terminus of the predicted T1R3 protein, we proposed that it affects ligand binding.[113] This prediction was subsequently confirmed in an in vitro study showing that a corresponding site-directed mutation changes binding affinity of the T1R3 protein to several sweeteners.[129]
Allelic variation of the Tas1r3 gene influenced taste responsiveness to non-nutritive sweeteners (saccharin, acesulfame-K, sucralose, SC-45647), sugars (sucrose, maltose, glucose, fructose), sugar alcohols (erythritol, sorbitol), and some amino acids (d-tryptophan, d-phenylalanine, l-proline). Tas1r3 genotype did not affect taste responses to several sweet-tasting amino acids (l-glutamine, l-threonine, l-alanine, glycine), glucose polymers (Polycose, maltooligosaccharide), and non-sweet NaCl, KCl, citric acid, HCl, quinine, monosodium glutamate, ammonium glutamate, and inosine 5′-monophosphate. Thus, Tas1r3 polymorphisms affect taste responses to many nutritive and non-nutritive sweeteners (all of which must interact with a taste receptor involving T1R3), but not to all carbohydrates and amino acids.[130,131]
T1R-independent variation
Several studies have shown multigenic inheritance of sweetener preferences.[107,110,111,123,124] Consistent with these findings, several lines of evidence indicate that allelic variation of the mouse Tas1r3 locus does not account for all the genetically determined differences in sweetener preferences. Analysis of multiple inbred mouse strains has shown that the Tas1r3 genotype explains only 78% of genetic variation in saccharin preference.[113] In the B6 × 129 F2 cross, the Tas1r3 genotype explained 64–96% of genetic variation in preference scores for different sweeteners, but only 10–35% of genetic variation in sweetener intakes.[126,130] Responses to sweeteners in brief-access tests differ among mouse strains but do not seem to be associated with Tas1r3 alleles.[115] Thus, a substantial part of the genetic variation in taste responses to sweeteners among mouse strains is attributed to loci other than Tas1r3. Taste responses to glycine provide a remarkable example: although mouse strains differ in responses to glycine,[110,132] this variation is not attributed to the Tas1r3 genotypes.[116,130]
One of the genetic loci affecting sweet taste responses is dpa (d-phenylalanine aversion), which affects ability of mice to generalize conditioned taste aversion between d-phenylalanine and sucrose, inferring that dpa affects ability to detect the sweetness of d-phenylalanine. The dpa locus also affects responses of sucrose-sensitive fibres of the chorda tympani nerve to d-phenylalanine. The dpa locus was mapped to proximal chromosome 4, a region distinct from the subtelomeric chromosome 4 harboring the Tas1r genes.[133-136] It was suggested that the dpa locus can also affect responses to sweeteners in two-bottle tests.[111] Consistent with this, a locus on proximal chromosome 4, in the dpa region, was found to be suggestively linked to consumption of, and chorda tympani responses to, sucrose.[126] An epistatic interaction between effects on sucrose intake of this locus and the Tas1r3 locus suggests that these two loci may encode interacting components of sweet taste transduction.[126]
To study the non-Tas1r genes involved in sweet taste, we began selective breeding of mouse lines divergent in sweetener consumption. To eliminate the Tas1r3 effects, we crossed B6 inbred mice with 129.B6–Tas1r3 congenic mice. As a result, all mice in this cross had only the B6 Tas1r3 allele. Despite genetic identity at the Tas1r3 locus, mice from the F2 generation varied widely in consumption of 20 mm saccharin and 30 mm glycine, but there was no correlation between these two traits. We therefore began selective breeding of mouse lines with high and low saccharin intakes, and with high and low glycine preferences.[55,137] The large divergence between the selected strains demonstrates that much of genetic variation in mouse sweet taste responses depends on genes other than Tas1r3. To map these genes, we genotyped mice from the selected strains and found linkages on four chromosomes in mice selected for glycine preference, and linkages on five chromosomes for mice selected for saccharin intake. We have found the complex genetic architecture of sweetener preferences, with each progenitor strain contributing loci increasing or decreasing a trait value, and with two distinct sets of genes for glycine and saccharin consumption.[138-140] Consistent with our genetic mapping studies, mice with mutations in genes other than taste receptors also have altered behavioural responses to sweeteners.[141-145] Furthermore, taste responses to complex carbohydrates (malto oligosaccharide and Polycose) are not affected by allelic variants of the Tas1r3 gene.[131,146,147]
Other Species
Although most research on genetics of sweet taste was conducted in mice, strain differences in sweetener preferences have been reported for rats[148-152] and hamsters.[153] Rat T1R3 is a part of the taste receptor responding to saccharin.[38,40,154] However, rat strains with different saccharin preferences do not differ in protein sequence of T1R3.[155] Consistent with this, QTLs for saccharin preference in the rat were mapped to chromosomes 3, 16 and 18, but not to chromosome 5 where rat Tas1r3 resides.[156] Therefore, rat strain differences in saccharin preferences depend on genes other than Tas1r3.
Sweet Taste Preferences and Alcohol Intake
Humans perceive certain concentrations of alcohol (ethanol) as sweet.[157] In rodents, perception of the sweet taste component of ethanol was shown in behavioural and neurophysiological experiments (reviewed by Bachmanov et al.[158]). In behavioural studies, conditioned taste aversion generalized between ethanol and sucrose.[159-162] Electrophysiological recordings indicate that lingual application of ethanol activates sweetener-responsive neural fibres in the gustatory nerves[163,164] and sweetener-responsive units in the nucleus of the tractus solitarius;[165,166] this activity is blocked by application of gurmarin, a peripheral antagonist of sweet taste.[166]
In addition to activation of peripheral mechanisms of sweet taste by ethanol, central mechanisms that determine hedonic responses to ethanol and sweeteners also overlap and involve opioidergic, serotonergic and dopaminergic brain neurotransmitter systems.[167-172] These neural pathways are also implicated in drug addiction, and there is evidence that in humans and rodents sweetener preference correlates with administration of drugs, such as cocaine and heroin.[173,174]
Several studies have shown that in humans sweet liking is associated with proclivity to drink more alcohol,[175-179] but genes responsible for this association are still unknown. Sweet taste phenotypes have a potential to be used as biological markers for diagnosing predisposition to alcoholism.[150,176,177,180]
Studies with rodents elucidated some genetic factors for the association between sweet taste and alcohol. In mice and rats, positive correlations between preferences for ethanol and sweeteners were found among various strains and in segregating crosses.[105,112,123,124,149,150,181-189] Genetic analysis of a cross between mice from a high ethanol- and sweetener-preferring C57BL/6ByJ strain and a low ethanol- and sweetener-preferring 129P3/J strain suggested that the strain differences in sweetener and ethanol consumption depend on relatively small and partially overlapping sets of genes.[124]
T1R-dependent Mechanisms
One of the genetic loci influencing alcohol preference in mice, Ap3q (alcohol preference 3 QTL), maps to a region of chromosome 4 overlapping with the Tas1r3 gene.[190] This suggests that the Tas1r3 gene is identical to the Ap3q locus. Consistent with this, allelic variation of the Tas1r3 gene in congenic and knockout mice has pleiotropic effects on ingestive responses to sweeteners and ethanol in the long-term and brief-access tests, and influences taste quality perception of ethanol.[191-193] These data suggest that Tas1r3 alleles influence perception of the sweet taste component of ethanol flavour. As a result, mice with a more sensitive variant of the sweet taste receptor perceive stronger sweetness from ethanol, which makes it more hedonically attractive and promotes ethanol intake.
T1R-independent Mechanisms
In addition to the Tas1r3 gene, rodents have other genetic loci with pleiotropic effects on ethanol and sweetener intake.[194,195] To study T1R-independent mechanisms of association between ethanol and sweetener preferences, we used mice selectively bred for high and low saccharin intake, which have identical allele of the Tas1r3 gene.[55,137] Mice selected for high saccharin intake had higher ethanol intakes and preferences than mice selected for low saccharin intake. Because these mice do not differ in peripheral taste input, we hypothesized that effects on sweetener and ethanol preference are mediated by the central mechanisms. Consistent with this, mice from the two strains differed in number of urocortin 1-containing cells in the brain Edinger–Westphal nucleus, which is involved in the regulation of ethanol consumption.[196] These differences are consistent with the involvement of central mechanisms in selection for sweetener intake and in correlated divergence in ethanol consumption. Therefore, this study has shown that genetic association between consumption of ethanol and sweeteners depends not only on the Tas1r3 locus, but also on at least one other locus, which is involved in central mechanisms regulating ethanol and sweetener intake.
Concluding Remarks
The data presented in this review demonstrate that sweet taste has a complex genetic architecture. Variation of the sweet taste receptor genes contributes to differences in sweet taste perception within and between species. In addition to the sweet taste receptors, a number of other genes influence sweet taste responses. These genes are involved in different stages of sweet taste processing pathway, including peripheral and central mechanisms. There is evidence that responses to different sweeteners are affected by different sets of genes. Individual differences in sweet taste preferences are associated with predisposition to alcoholism.
In recent years, genetics has experienced dramatic progress, with genome sequencing completed for several species, including the mouse and the human. These advances in genomic resources tremendously facilitate genetic studies and make them an even more powerful approach for understanding mechanisms of sweet taste.
Acknowledgements
This study was supported by grants from NIH R01DC00882 (A.A.B. and G.K.B.), R01AA11028 (A.A.B.), R03TW007429 (A.A.B. and V.A.Z.) and Ajinomoto Amino Acid Research Program (A.A.B.).
Footnotes
This article is part of the Special Issue of Flavour and Fragrance Journal entitled “Proceedings of the Procida Workshop on Taste (April 14–17 2010)” edited by Pierandrea Temussi and Gabriella Morini.
References
- 1.McCaughey SA. Neurosci. Biobehav. Rev. 2008;32:1024. doi: 10.1016/j.neubiorev.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teff KL, Devine J, Engelman K. Physiol. Behav. 1995;57:1089. doi: 10.1016/0031-9384(94)00373-d. [DOI] [PubMed] [Google Scholar]
- 3.Grill HJ, Berridge KC, Ganster DJ. Am. J. Physiol. 1984;246:R88. doi: 10.1152/ajpregu.1984.246.1.R88. [DOI] [PubMed] [Google Scholar]
- 4.Malaisse WJ, Vanonderbergen A, Louchami K, Jijakli H, Malaisse-Lagae F. Cell. Signal. 1998;10:727. doi: 10.1016/s0898-6568(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto T, Sako N, Maeda S. Physiol. Behav. 2000;69:345. doi: 10.1016/s0031-9384(99)00252-8. [DOI] [PubMed] [Google Scholar]
- 6.Marks-Kaufman R, Hamm MW, Barbato GF. J. Am. Coll. Nutr. 1989;8:9. doi: 10.1080/07315724.1989.10720272. [DOI] [PubMed] [Google Scholar]
- 7.Melchior JC, Rigaud D, Colas-Linhart N, Petiet A, Girard A, Apfelbaum M. Physiol. Behav. 1991;50:941. doi: 10.1016/0031-9384(91)90418-n. [DOI] [PubMed] [Google Scholar]
- 8.O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neuron. 2002;33:815. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 9.Cooper SJ, Barber DJ. Pharmacol. Biochem. Behav. 1994;47:541. doi: 10.1016/0091-3057(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 10.Muscat R, Kyprianou T, Osman M, Phillips G, Willner P. Pharmacol. Biochem. Behav. 1991;40:209. doi: 10.1016/0091-3057(91)90541-9. [DOI] [PubMed] [Google Scholar]
- 11.Schneider LH. Ann. NY Acad. Sci. 1989;575:307. doi: 10.1111/j.1749-6632.1989.tb53252.x. [DOI] [PubMed] [Google Scholar]
- 12.Yirmiya R, Lieblich I, Liebeskind JC. Brain Res. 1988;438:339. doi: 10.1016/0006-8993(88)91360-1. [DOI] [PubMed] [Google Scholar]
- 13.Blass EM, Watt LB. Pain. 1999;83:611. doi: 10.1016/S0304-3959(99)00166-9. [DOI] [PubMed] [Google Scholar]
- 14.Blass EM, Shah A. Chem. Senses. 1995;20:29. doi: 10.1093/chemse/20.1.29. [DOI] [PubMed] [Google Scholar]
- 15.Calcagnetti DJ, Holtzman SG. Brain Res. Bull. 1992;29:859. doi: 10.1016/0361-9230(92)90156-r. [DOI] [PubMed] [Google Scholar]
- 16.Carbajal R, Chauvet X, Couderc S, Olivier-Martin M. BMJ. 1999;319:1393. doi: 10.1136/bmj.319.7222.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ors R, Ozek E, Baysoy G, Cebeci D, Bilgen H, Turkuner M, Basaran M. Eur. J. Pediatr. 1999;158:63. doi: 10.1007/s004310051011. [DOI] [PubMed] [Google Scholar]
- 18.Laeng B, Berridge KC, Butter CM. Appetite. 1993;21:247. doi: 10.1006/appe.1993.1043. [DOI] [PubMed] [Google Scholar]
- 19.Hajnal A, Takenouchi K, Norgren R. J. Neurosci. 1999;19:7182. doi: 10.1523/JNEUROSCI.19-16-07182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atchley DP, Weaver KL, Eckel LA. Physiol. Behav. 2005;86:265. doi: 10.1016/j.physbeh.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Curtis KS, Stratford JM, Contreras RJ. Physiol. Behav. 2005;86:281. doi: 10.1016/j.physbeh.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Simon SA, Liu L, Erickson RP. Am. J. Physiol. 2003;284:R1494. doi: 10.1152/ajpregu.00544.2002. [DOI] [PubMed] [Google Scholar]
- 23.Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. Endocrinology. 2004;145:839. doi: 10.1210/en.2003-0602. [DOI] [PubMed] [Google Scholar]
- 24.Mook DG. J. Comp. Physiol. Psychol. 1963;56:645. [Google Scholar]
- 25.Sclafani A. In: Neural and Metabolic Control of Macronutrient Intake. Berthoud HR, Seeley RJ, editors. CRC Press; Boca Raton, FL: 1999. p. 93. [Google Scholar]
- 26.Ramirez I. Am. J. Physiol. 1994;266:R682. doi: 10.1152/ajpregu.1994.266.3.R682. [DOI] [PubMed] [Google Scholar]
- 27.Tordoff MG. In: Chemical Senses: Appetite and Nutrition. Friedman MI, Kare MR, Tordoff MG, editors. Vol. 4. Marcel Dekker; New York: 1991. p. 239. [Google Scholar]
- 28.Sclafani A. Physiol. Behav. 2006;87:745. doi: 10.1016/j.physbeh.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Sclafani A. Physiol. Behav. 2007;90:602. doi: 10.1016/j.physbeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Sclafani A. Physiol. Behav. 2006;89:525. doi: 10.1016/j.physbeh.2006.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Neurosci. Biobehav. Rev. 2001;25:53. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 32.Berridge KC. Neurosci. Biobehav. Rev. 2000;24:173. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 33.Reed DR, McDaniel AH. BMC Oral Health. 2006;6(Suppl 1):S17. doi: 10.1186/1472-6831-6-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed DR, Tanaka T, McDaniel AH. Physiol. Behav. 2006;88:215. doi: 10.1016/j.physbeh.2006.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachmanov AA, Beauchamp GK. Annu. Rev. Nutr. 2007;27:389. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Nat. Genet. 2001;28:58. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 37.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. Nat. Neurosci. 2001;4:492. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- 38.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Cell. 2001;106:381. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 39.Pin JP, Galvez T, Prezeau L. Pharmacol. Ther. 2003;98:325. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Proc. Natl Acad. Sci. U.S.A. 2002;99:4692. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. Nature. 2002;416:199. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 42.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. Cell. 2003;115:255. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 43.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Science. 2003;301:850. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 44.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Proc. Natl Acad. Sci. U.S.A. 2011;108:5431. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naim M, Nir S, Spielman AI, Noble AC, Peri I, Rodin S, Samuelov-Zubare M. In: Chemistry of Taste: Mechanisms, Behaviors, and Mimics, ACS symposium series 825. Given P, Parades D, editors. American Chemical Society; Washington, DC: 2002. [Google Scholar]
- 46.Spector AC. In: Handbook of Olfaction and Gustation. 2nd edition Doty RL, editor. Marcel Dekker; New York: 2003. p. 861. [Google Scholar]
- 47.Bjarnadottir TK, Fredriksson R, Schioth HB. Gene. 2005;362:70. doi: 10.1016/j.gene.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 48.Chyb S, Dahanukar A, Wickens A, Carlson JR. Proc. Natl Acad. Sci. U.S.A. 2003;100(Suppl 2):14526. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. Nat. Neurosci. 2001;4:1182. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 50.Hallem EA, Dahanukar A, Carlson JR. Annu. Rev. Entomol. 2006;51:113. doi: 10.1146/annurev.ento.51.051705.113646. [DOI] [PubMed] [Google Scholar]
- 51.Isono K, Morita H, Kohatsu S, Ueno K, Matsubayashi H, Yamamoto MT. Chem. Senses. 2005;30(Suppl 1):i275. doi: 10.1093/chemse/bjh221. [DOI] [PubMed] [Google Scholar]
- 52.Inomata N, Goto H, Itoh M, Isono K. Genetics. 2004;167:1749. doi: 10.1534/genetics.104.027045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueno K, Ohta M, Morita H, Mikuni Y, Nakajima S, Yamamoto K, Isono K. Curr. Biol. 2001;11:1451. doi: 10.1016/s0960-9822(01)00450-x. [DOI] [PubMed] [Google Scholar]
- 54.Boughter JD, Bachmanov AA. In: Olfaction and Taste. Firestein S, Beauchamp GK, editors. Vol. 4. Elsevier/Academic Press; San Diego: 2008. p. 371. [Google Scholar]
- 55.Bachmanov AA. In: Sweetness and Sweeteners: Biology, Chemistry and Psychophysics. Weerasinghe DK, DuBois GE, editors. American Chemical Society; Washington, D.C.: 2008. p. 18. [Google Scholar]
- 56.Li X, Li W, Wang H, Cao J, Maehashi K, Huang L, Bachmanov AA, Reed DR, Legrand-Defretin V, Beauchamp GK, Brand JG. PLoS Genet. 2005;1:27. doi: 10.1371/journal.pgen.0010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi P, Zhang J. Mol. Biol. Evol. 2006;23:292. doi: 10.1093/molbev/msj028. [DOI] [PubMed] [Google Scholar]
- 58.Liao J, Schultz PG. Mamm. Genome. 2003;14:291. doi: 10.1007/s00335-002-2233-0. [DOI] [PubMed] [Google Scholar]
- 59.Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Cell. 1999;96:541. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- 60.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Chem. Senses. 2001;26:925. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lagerstrom MC, Hellstrom AR, Gloriam DE, Larsson TP, Schioth HB, Fredriksson R. PLoS Comput. Biol. 2006;2:e54. doi: 10.1371/journal.pcbi.0020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishimaru Y, Okada S, Naito H, Nagai T, Yasuoka A, Matsumoto I, Abe K. Mech. Dev. 2005;122:1310. doi: 10.1016/j.mod.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schioth HB. Genomics. 2006;88:263. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Beauchamp GK, Maller O, Rogers JG. J. Comp. Physiol. Psychol. 1977;91:1118. [Google Scholar]
- 65.Bartoshuk LM, Jacobs HL, Nichols TL, Hoff LA, Ryckman JJ. J. Comp. Physiol. Psychol. 1975;89:971. doi: 10.1037/h0077172. [DOI] [PubMed] [Google Scholar]
- 66.Halpern BP. Am. J. Physiol. 1962;203:541. doi: 10.1152/ajplegacy.1962.203.3.541. [DOI] [PubMed] [Google Scholar]
- 67.Ganchrow JR, Steiner JE, Bartana A. Dev. Psychobiol. 1990;23:103. doi: 10.1002/dev.420230202. [DOI] [PubMed] [Google Scholar]
- 68.Kare MR. In: Physiological and Behavioral Aspects of Taste. Kare MR, Halpern BP, editors. The University of Chicago Press; Chicago: 1961. p. 6. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Glaser D, Li W, Johnson WE, O’Brien SJ, Beauchamp GK, Brand JG. J. Hered. 2009;100(Suppl 1):S90. doi: 10.1093/jhered/esp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glaser D, Tinti JM, Nofre C. Chem. Senses. 1995;20:573. doi: 10.1093/chemse/20.5.573. [DOI] [PubMed] [Google Scholar]
- 71.Bachmanov AA, Tordoff MG, Beauchamp GK. Chem. Senses. 2001;26:905. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grace J, Russek M. Physiol. Behav. 1969;4:553. [Google Scholar]
- 73.Ferrell F. Neurosci. Biobehav. Rev. 1984;8:199. doi: 10.1016/0149-7634(84)90041-1. [DOI] [PubMed] [Google Scholar]
- 74.Matson KD, Millam JR, Klasing KC. Zoo Biol. 2001;20:1. doi: 10.1002/zoo.1001. [DOI] [PubMed] [Google Scholar]
- 75.Matson KD, Millam JR, Klasing KC. Appl. Anim. Behav. Sci. 2000;69:313. doi: 10.1016/s0168-1591(00)00130-1. [DOI] [PubMed] [Google Scholar]
- 76.Zhao H, Yang JR, Xu H, Zhang J. Mol. Biol. Evol. 2010;27:2669. doi: 10.1093/molbev/msq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li R, Fan W, Tian G, Zhu H, He L, Cai J, et al. Nature. 2010;463:311. doi: 10.1038/nature08696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X, Bachmanov AA, Maehashi K, Li W, Lim R, Brand JG, Beauchamp GK, Reed DR, Thai C, Floriano WB. Chem. Senses. Vol. 36. 2011. p. 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gent JF, Bartoshuk LM. Chem. Senses. 1983;6:265. [Google Scholar]
- 80.Looy H, Weingarten HP. Physiol. Behav. 1992;52:75. doi: 10.1016/0031-9384(92)90435-5. [DOI] [PubMed] [Google Scholar]
- 81.Looy H, Callaghan S, Weingarten HP. Physiol. Behav. 1992;52:219. doi: 10.1016/0031-9384(92)90261-y. [DOI] [PubMed] [Google Scholar]
- 82.Beauchamp GK, Moran M. Appetite. 1982;3:139. doi: 10.1016/s0195-6663(82)80007-x. [DOI] [PubMed] [Google Scholar]
- 83.Desor JA, Greene LS, Maller O. Science. 1975;190:686. doi: 10.1126/science.1188365. [DOI] [PubMed] [Google Scholar]
- 84.Greene LS, Desor JA, Maller O. J. Comp. Physiol. Psychol. 1975;89:279. doi: 10.1037/h0076802. [DOI] [PubMed] [Google Scholar]
- 85.Falciglia GA, Norton PA. J. Am. Diet. Assoc. 1994;94:154. doi: 10.1016/0002-8223(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 86.Looy H, Weingarten HP. Chem. Senses. 1991;16:123. [Google Scholar]
- 87.McDaniel AH, Reed DR. In: Genomics and Proteomics in Nutrition. Berdanier CD, Moustaid-Moussa N, editors. Marcel Dekker; New York: 2003. p. 51. [Google Scholar]
- 88.Reed DR, Li X, Bachmanov AA, Mascioli K, Beauchamp GK. In: Progress in Obesity Research. Medeiros-Neto G, Halpern A, Bouchard C, editors. Vol. 9. John Libbey Eurotext Ltd; London: 2003. p. 304. [Google Scholar]
- 89.Reed DR, Bachmanov AA, Beauchamp GK, Tordoff MG, Price RA. Behav. Genet. 1997;27:373. doi: 10.1023/a:1025692031673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drayna D. Annu. Rev. Genomics Hum. Genet. 2005;6:217. doi: 10.1146/annurev.genom.6.080604.162340. [DOI] [PubMed] [Google Scholar]
- 91.Kim UK, Breslin PA, Reed D, Drayna D. J. Dent. Res. 2004;83:448. doi: 10.1177/154405910408300603. [DOI] [PubMed] [Google Scholar]
- 92.Keskitalo K, Tuorila H, Spector TD, Cherkas LF, Knaapila A, Silventoinen K, Perola M. Am. J. Clin. Nutr. 2007;86:1663. doi: 10.1093/ajcn/86.5.1663. [DOI] [PubMed] [Google Scholar]
- 93.Keskitalo K, Tuorila H, Spector TD, Cherkas LF, Knaapila A, Kaprio J, Silventoinen K, Perola M. Am. J. Clin. Nutr. 2008;88:263. doi: 10.1093/ajcn/88.2.263. [DOI] [PubMed] [Google Scholar]
- 94.Keskitalo K, Knaapila A, Kallela M, Palotie A, Wessman M, Sammalisto S, Peltonen L, Tuorila H, Perola M. Am. J. Clin. Nutr. 2007;86:55. doi: 10.1093/ajcn/86.1.55. [DOI] [PubMed] [Google Scholar]
- 95.Kim UK, Wooding S, Riaz N, Jorde LB, Drayna D. Chem. Senses. 2006;31:599. doi: 10.1093/chemse/bjj065. [DOI] [PubMed] [Google Scholar]
- 96.Eny KM, Wolever TM, Corey PN, El-Sohemy A. Am. J. Clin. Nutr. 2010;92:1501. doi: 10.3945/ajcn.2010.29836. [DOI] [PubMed] [Google Scholar]
- 97.Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D. Curr. Biol. 2009;19:1288. doi: 10.1016/j.cub.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fushan AA, Simons CT, Slack JP, Drayna D. Chem. Senses. 2010;35:579. doi: 10.1093/chemse/bjq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eny KM, Corey PN, El-Sohemy A. J Nutrigenet Nutrigenomics. 2009;2:235. doi: 10.1159/000276991. [DOI] [PubMed] [Google Scholar]
- 100.Eny KM, Wolever TM, Fontaine-Bisson B, El-Sohemy A. Physiol. Genomics. 2008;33:355. doi: 10.1152/physiolgenomics.00148.2007. [DOI] [PubMed] [Google Scholar]
- 101.Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Physiol. Behav. 2005;85:546. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Fuller JL. J. Hered. 1974;65:33. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- 103.Nachman M. J. Comp. Physiol. Psychol. 1959;52:451. doi: 10.1037/h0048853. [DOI] [PubMed] [Google Scholar]
- 104.Hoshishima K, Yokoyama S, Seto K. Am. J. Physiol. 1962;202:1200. doi: 10.1152/ajplegacy.1962.202.6.1200. [DOI] [PubMed] [Google Scholar]
- 105.Rodgers DA, McClearn GE. Q. J. Stud. Alcohol. 1964;25:26. [PubMed] [Google Scholar]
- 106.Lush IE. Genet. Res. 1989;53:95. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- 107.Ramirez I, Fuller JL. Physiol. Behav. 1976;16:163. doi: 10.1016/0031-9384(76)90300-0. [DOI] [PubMed] [Google Scholar]
- 108.Pelz WE, Whitney G, Smith JC. Physiol. Behav. 1973;10:263. doi: 10.1016/0031-9384(73)90308-9. [DOI] [PubMed] [Google Scholar]
- 109.Stockton MD, Whitney G. J. Comp. Physiol. Psychol. 1974;86:62. doi: 10.1037/h0035929. [DOI] [PubMed] [Google Scholar]
- 110.Lush IE, Hornigold N, King P, Stoye JP. Genet. Res. 1995;66:167. doi: 10.1017/s0016672300034510. [DOI] [PubMed] [Google Scholar]
- 111.Capeless CG, Whitney G. Chem. Senses. 1995;20:291. doi: 10.1093/chemse/20.3.291. [DOI] [PubMed] [Google Scholar]
- 112.Belknap JK, Crabbe JC, Young ER. Psychopharmacology. 1993;112:503. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 113.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. J. Neurosci. 2004;24:938. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kotlus BS, Blizard DA. Physiol. Behav. 1998;64:37. doi: 10.1016/s0031-9384(98)00016-x. [DOI] [PubMed] [Google Scholar]
- 115.Dotson CD, Spector AC. Chem. Senses. 2004;29:489. doi: 10.1093/chemse/bjh051. [DOI] [PubMed] [Google Scholar]
- 116.Eylam S, Spector AC. Chem. Senses. 2004;29:639. doi: 10.1093/chemse/bjh068. [DOI] [PubMed] [Google Scholar]
- 117.Ninomiya Y, Higashi T, Katsukawa H, Mizukoshi T, Funakoshi M. Brain Res. 1984;322:83. doi: 10.1016/0006-8993(84)91183-1. [DOI] [PubMed] [Google Scholar]
- 118.Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Chem. Senses. 2001;26:915. doi: 10.1093/chemse/26.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frank ME, Blizard DA. Physiol. Behav. 1999;67:287. doi: 10.1016/s0031-9384(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 120.Ninomiya Y, Mizukoshi T, Higashi T, Katsukawa H, Funakoshi M. Brain Res. 1984;302:305. doi: 10.1016/0006-8993(84)90244-0. [DOI] [PubMed] [Google Scholar]
- 121.Bachmanov AA, Li S, Li X, Lu K, Tordoff MG, West DB, Ohmen JD, Reed DR, Beauchamp GK. Chem. Senses. 2002;27:A95. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Belknap JK, Crabbe JC, Plomin R, McClearn GE, Sampson KE, O’Toole LA, Gora-Maslak G. Behav. Genet. 1992;22:81. doi: 10.1007/BF01066794. [DOI] [PubMed] [Google Scholar]
- 123.Phillips TJ, Crabbe JC, Metten P, Belknap JK. Alcohol. Clin. Exp. Res. 1994;18:931. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 124.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Behav. Genet. 1996;26:563. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blizard DA, Kotlus B, Frank ME. Chem. Senses. 1999;24:373. doi: 10.1093/chemse/24.4.373. [DOI] [PubMed] [Google Scholar]
- 126.Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, Price RA, Beauchamp GK. Mammal. Genome. 1997;8:545. doi: 10.1007/s003359900500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li X, Inoue M, Reed DR, Huque T, Puchalski RB, Tordoff MG, Ninomiya Y, Beauchamp GK, Bachmanov AA. Mammal. Genome. 2001;12:13. doi: 10.1007/s003350010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li X, Bachmanov AA, Li S, Chen Z, Tordoff MG, Beauchamp GK, de Jong PJ, Wu C, Chen L, West DB, Ross DA, Ohmen JD, Reed DR. Mammal. Genome. 2002;13:5. doi: 10.1007/s0033501-2109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Curr. Biol. 2005;15:1948. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 130.Inoue M, Reed DR, Li X, Tordoff MG, Beauchamp GK, Bachmanov AA. J. Neurosci. 2004;24:2296. doi: 10.1523/JNEUROSCI.4439-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Physiol. Genomics. 2007;32:82. doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bachmanov AA, Tordoff MG, Beauchamp GK. Chem. Senses. 2001;26:905. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ninomiya Y, Sako N, Katsukawa H, Funakoshi M. In: Genetics of Perception and Communication. Wysocki CJ, Kare MR, editors. Vol. 3. Marcel Dekker; New York: 1991. p. 267. [Google Scholar]
- 134.Ninomiya Y, Higashi T, Mizukoshi T, Funakoshi M. Ann. NY Acad. Sci. 1987;510:527. [Google Scholar]
- 135.Ninomiya Y, Nomura T, Katsukawa H. Brain Res. 1992;596:349. doi: 10.1016/0006-8993(92)91571-u. [DOI] [PubMed] [Google Scholar]
- 136.Shigemura N, Yasumatsu K, Yoshida R, Sako N, Katsukawa H, Nakashima K, Imoto T, Ninomiya Y. Chem. Senses. 2005;30(Suppl 1):i84. doi: 10.1093/chemse/bjh125. [DOI] [PubMed] [Google Scholar]
- 137.Bachmanov AA, Bosak NP, Beauchamp GK. Chem. Senses. 2005;30:A171. [Google Scholar]
- 138.Bosak NP, Lin C, Li X, Theodorides ML, Smith Z, Reed DR, Beauchamp GK, Bachmanov AA. Chem. Senses. 2007;32:A26. [Google Scholar]
- 139.Bosak NP, Theodorides MI, Lin C, Smith Z, Beauchamp GK, Bachmanov AA. Chem. Senses. 2008;33:S39. [Google Scholar]
- 140.Bosak NP, Theodorides ML, Lin C, Smith Z, Beauchamp GK, Bachmanov AA. Chem. Senses. 2009;34:A43. [Google Scholar]
- 141.Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Am. J. Physiol. 2005;289:R1798. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- 142.Sclafani A, Rinaman L, Vollmer RR, Amico JA. Am. J. Physiol. 2007;292:R1828. doi: 10.1152/ajpregu.00826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. J. Neurosci. 2003;23:9395. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sakic B, Denburg JA, Denburg SD, Szechtman H. Brain Res. Bull. 1996;41:305. doi: 10.1016/s0361-9230(96)00190-6. [DOI] [PubMed] [Google Scholar]
- 145.Sakic B, Szechtman H, Braciak T, Richards C, Gauldie J, Denburg JA. Brain Res. Bull. 1997;44:155. doi: 10.1016/s0361-9230(97)00107-x. [DOI] [PubMed] [Google Scholar]
- 146.Treesukosol Y, Blonde GD, Spector AC. Am. J. Physiol. 2009;296:R855. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. Am. J. Physiol. 2009;296:R866. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dess NK, Minor TR. Anim. Learn. Behav. 1996;24:105. [Google Scholar]
- 149.Overstreet DH, Rezvani AH, Parsian A. Alcohol Alcohol. 1999;34:378. doi: 10.1093/alcalc/34.3.378. [DOI] [PubMed] [Google Scholar]
- 150.Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Alcohol Alcohol. 1999;34:386. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- 151.Tordoff MG, Alarcon LK, Lawler MP. Physiol. Behav. 2008;95:308. doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tordoff MG. Chem. Senses. 2010;35:473. doi: 10.1093/chemse/bjq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frank ME, Wada Y, Makino J, Mizutani M, Umezawa H, Katsuie Y, Hettinger TP, Blizard DA. Behav. Genet. 2004;34:465. doi: 10.1023/B:BEGE.0000023651.99481.d5. [DOI] [PubMed] [Google Scholar]
- 154.Winnig M, Bufe B, Meyerhof W. BMC Neurosci. 2005;6:22. doi: 10.1186/1471-2202-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lu K, McDaniel AH, Tordoff MG, Li X, Beauchamp GK, Bachmanov AA, VanderWeele DA, Chapman CD, Dess NK, Huang L, Wang H, Reed DR. Chem. Senses. 2005;30:231. doi: 10.1093/chemse/bji019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Foroud T, Bice P, Castelluccio P, Bo R, Ritchotte A, Stewart R, Lumeng L, Li TK, Carr L. Behav. Genet. 2002;32:57. doi: 10.1023/a:1014459912935. [DOI] [PubMed] [Google Scholar]
- 157.Scinska A, Koros E, Habrat B, Kukwa A, Kostowski W, Bienkowski P. Drug Alcohol Depend. 2000;60:199. doi: 10.1016/s0376-8716(99)00149-0. [DOI] [PubMed] [Google Scholar]
- 158.Bachmanov AA, Kiefer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk LM, Mennella JA. Alcohol. Clin. Exp. Res. 2003;27:220. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kiefer SW, Lawrence GJ. Chem. Senses. 1988;13:633. [Google Scholar]
- 160.Kiefer SW, Mahadevan RS. Chem. Senses. 1993;18:509. [Google Scholar]
- 161.Lawrence GJ, Kiefer SW. Chem. Senses. 1987;12:591. [Google Scholar]
- 162.Blizard DA, McClearn GE. Alcohol. Clin. Exp. Res. 2000;24:253. [PubMed] [Google Scholar]
- 163.Hellekant G, Danilova V, Roberts T, Ninomiya Y. Alcohol. 1997;14:473. doi: 10.1016/s0741-8329(96)00215-7. [DOI] [PubMed] [Google Scholar]
- 164.Sako N, Yamamoto T. Am. J. Physiol. 1999;276:R388. doi: 10.1152/ajpregu.1999.276.2.R388. [DOI] [PubMed] [Google Scholar]
- 165.Di Lorenzo PM, Kiefer SW, Rice AG, Garcia J. Alcohol. 1986;3:55. doi: 10.1016/0741-8329(86)90071-6. [DOI] [PubMed] [Google Scholar]
- 166.Lemon CH, Brasser SM, Smith DV. J. Neurophysiol. 2004;92:536. doi: 10.1152/jn.00097.2004. [DOI] [PubMed] [Google Scholar]
- 167.Levine AS, Kotz CM, Gosnell BA. Am. J. Clin. Nutr. 2003;68:834S. doi: 10.1093/ajcn/78.4.834S. [DOI] [PubMed] [Google Scholar]
- 168.Gosnell BA, Majchrzak MJ. Pharmacol. Biochem. Behav. 1989;33:805. doi: 10.1016/0091-3057(89)90474-7. [DOI] [PubMed] [Google Scholar]
- 169.George SR, Roldan L, Lui A, Naranjo CA. Alcohol. Clin. Exp. Res. 1991;15:668. doi: 10.1111/j.1530-0277.1991.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 170.Hubell CL, Marglin SH, Spitalnic SJ, Abelson ML, Wild KD, Reid LD. Alcohol. 1991;8:355. doi: 10.1016/0741-8329(91)90573-f. [DOI] [PubMed] [Google Scholar]
- 171.Pucilowski O, Rezvani AH, Janowsky DS. Psychopharmacology. 1992;107:447. doi: 10.1007/BF02245174. [DOI] [PubMed] [Google Scholar]
- 172.Fortuna JL. J. Psychoactive Drugs. 2010;42:147. doi: 10.1080/02791072.2010.10400687. [DOI] [PubMed] [Google Scholar]
- 173.Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Behav. Pharmacol. 2008;19:435. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- 174.Janowsky DS, Pucilowski O, Buyinza M. J. Psychiatr. Res. 2003;37:35. doi: 10.1016/s0022-3956(02)00063-8. [DOI] [PubMed] [Google Scholar]
- 175.Yamamoto ME, Block GD, Ishii E. Alcohol. Clin. Exp. Res. 1991;15:359. [Google Scholar]
- 176.Kampov-Polevoy AB, Garbutt JC, Khalitov E. Alcohol. Clin. Exp. Res. 2003;27:1743. doi: 10.1097/01.ALC.0000093739.05809.DD. [DOI] [PubMed] [Google Scholar]
- 177.Kampov-Polevoy AB, Eick C, Boland G, Khalitov E, Crews FT. Alcohol. Clin. Exp. Res. 2004;28:1291. doi: 10.1097/01.alc.0000137808.69482.75. [DOI] [PubMed] [Google Scholar]
- 178.Kampov-Polevoy AB, Garbutt JC, Davis CE, Janowsky DS. Alcohol. Clin. Exp. Res. 1998;22:610. doi: 10.1111/j.1530-0277.1998.tb04300.x. [DOI] [PubMed] [Google Scholar]
- 179.Kampov-Polevoy AB, Garbutt JC, Janowsky D. Am. J. Psychiatr. 1997;154:269. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- 180.Mennella JA, Pepino MY, Lehmann-Castor SM, Yourshaw LM. Addiction. 2010;105:666. doi: 10.1111/j.1360-0443.2009.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bachmanov AA, Tordoff MG, Beauchamp GK. Alcohol. Clin. Exp. Res. 1996;20:201. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Murelle L, Halikas JA, Janowsky DS. Alcohol. Clin. Exp. Res. 1993;17:366. doi: 10.1111/j.1530-0277.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 183.Stewart RB, Russell RN, Lumeng L, Li T-K, Murphy JM. Alcohol. Clin. Exp. Res. 1994;18:375. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 184.McClearn GE, Rodgers DA, Stud QJ. Alcohol. 1959;20:691. [Google Scholar]
- 185.Rodgers DA, McClearn GE. Q. J. Stud. Alcohol. 1962;23:26. [PubMed] [Google Scholar]
- 186.Belknap JK, Crabbe JC, Young ER. Psychopharmacology (Berlin) 1993;112:503. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 187.Stewart RB, Russell RN, Lumeng L, Li TK, Murphy JM. Alcohol. Clin. Exp. Res. 1994;18:375. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 188.Stewart RB, Bice P, Foroud T, Lumeng L, Li TK, Carr LG. Alcohol. Clin. Exp. Res. 2003;27:49A. [PubMed] [Google Scholar]
- 189.Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Alcohol. 1998;16:275. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- 190.Bachmanov AA, Reed DR, Li X, Li S, Beauchamp GK, Tordoff MG. Genome Res. 2002;12:1257. doi: 10.1101/gr.129702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Murovets VO, Zolotarev VA, Margolskee RF, Bachmanov AA. Chem. Senses. 2009;34:A41. [Google Scholar]
- 192.Bachmanov AA, Li X, Reed DR, Murovets VO, Lin C, Bosak NP, Margolskee RF, Beauchamp GK, Zolotarev VA, Tordoff MG. Chem. Senses. 2008;33:S147. [Google Scholar]
- 193.Murovets VO, Zolotarev VA, Bachmanov AA. Dokl. Biol. Sci. 2010;432:181. doi: 10.1134/S001249661003004X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Terenina-Rigaldie E, Moisan MP, Colas A, Beauge F, Shah KV, Jones BC, Mormede P. Pharmacogenetics. 2003;13:543. doi: 10.1097/01.fpc.0000054120.14659.8c. [DOI] [PubMed] [Google Scholar]
- 195.Terenina-Rigaldie E, Jones BC, Mormede P. Genes Brain Behav. 2003;2:125. doi: 10.1034/j.1601-183x.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 196.Bachmanov AA, Avigdor M, Datta R, Smith Z, Theodorides ML, Ryabinin AE. Alcohol. Clin. Exp. Res. 2006;30:123A. [Google Scholar]


