Abstract
Background
Allergic contact dermatitis is a typical delayed-type hypersensitivity to sensitizing haptens mediated by T cells. Th1/Tc1 cells are currently considered to be the primary effectors in allergic contact dermatitis. There is little information concerning the role played in allergic contact dermatitis in humans by Th-17/Tc-17 cells, a recently defined subpopulation of effector T cells.
Objectives
In the present report we attempted to characterize Th-17/Tc-17 cells in the infiltrates of the skin in elicitation phase of allergic contact dermatitis.
Patients/Methods
Th-17 as well as Th1/Th2 cytokine gene expression was examined by semi-quantitative real-time polymerase chain reaction in paired samples of positive patch test biopsies and normal skins from 11 patients allergic to 9 different allergens. The in situ characterization of IL-17-producing cells was carried out using anti-RORC and anti-T cell subset antibodies by double immunofluorescence.
Results
Compared to normal paired skins, gene expression of transcription factor for human Th-17 cells, RORC, and Th-17-related cytokines IL-17A, IL-17F and IL-23, was significantly increased in positive patch test biopsies. The mRNA for IFN-γ and IL-4 was also increased. In the dermal infiltrates, about 20 % of the infiltrating cells were IL-17-producing cells as they expressed RORC, and such RORC expressing cells were detected in both CD4+ (~30%) and CD8+ (~20%) subsets.
Conclusions
This is the first demonstration of Th-17/Tc-17 cells in the elicitation phase of human allergic contact dermatitis, showing that they are a regular participant in the immunopathology of this common allergic reaction regardless of the nature of the triggering allergens.
Keywords: Allergic contact dermatitis, IL-17, Th-17 lymphocytes, patch test, RORC
Introduction
Allergic contact dermatitis (ACD) is a cutaneous delayed-type hypersensitivity reaction which is initiated when allergens, usually haptens, penetrate the skin and modify the host's structural proteins. Such altered self proteins are perceived by the host as foreign and are captured and conveyed by antigen-presenting cells (APC) to the draining lymph nodes. There antigen-specific T lymphocytes, recognizing the antigen displayed in the MHC molecules on the surface of the APC and in the presence of co-stimulation provided by APC, undergo activation and clonal expansion.1 The lymphocytes thus activated will then migrate to the peripheral tissue ready for subsequent encounter with the same antigen. In the effector or elicitation phase, the presence of even one antigen-specific T lymphocyte at the site of antigen re-introduction in the skin triggers an antigen-specific immunological response to clear the antigen with inflammation and tissue destruction manifested by ACD symptoms such as erythema, edema, and even blisters.1 However, at the ACD elicitation sites, the frequency of antigen-specific T cells is low despite dense infiltration. Our knowledge concerning the nature of the infiltrating cells in ACD is incomplete, and both the specificity and the mechanisms of action of these cells require further investigation.
Both Th1 and Th2 T cells are involved in ACD,1 but other cells such as NK T cells, NK cells and B1 cells not only contribute to the process but are essential for the efficient execution of the elicitation phase.2, 3 Th-17 cells are a recently defined lineage of effector T cells with the expression of distinctive transcription factor RORγt (RAR-related orphan nuclear receptor γt in mice; RORC, RAR-related orphan nuclear receptor C, in humans), elaboration of a set of signature pro-inflammatory cytokines such as IL-17A, IL-17F, IL-21 and IL-22, and the expression of characteristic chemokine receptors such as CCR6.4 IL-23 is important for the development of the effector functions of Th-17 cells.4 The function of Th-17 cells is the defense against extracellular bacteria and fungal infections, but they are also the main inflammatory cells involved in tissue destruction in autoimmunity.4, 5 Studies have shown that Th-17 cells are involved in contact hypersensitivity (CHS) in murine models. IL-17, the signature cytokine of Th-17 cells, has been detected in human T cell clones specific for nickel,6 and in IL-17 deficient mice immune response to contact allergens was impaired.7 However, there are few studies that directly examine Th-17 cells and their roles in allergic contact dermatitis in humans.5, 8 In the present report we examine Th-17 signature cytokine gene expression and the frequency of IL-17-producing cells in the infiltration of positive patch test reactions to a variety of allergens, and find that IL-17 and some related cytokine gene expression and RORC positive CD4+ and CD8+ T cells are significantly increased in the lesions. These data indicate that Th-17/Tc-17 cells are regular participants in the immunopathology of this allergic reaction regardless of the nature of the triggering allergens.
Materials and Methods
Patients
Patients were recruited from our cutaneous allergy clinic, and were being evaluated for allergic contact dermatitis (see Table 1 for detail). The intensity of reaction to various allergens was determined by the North American Contact Dermatitis Group scoring system.9 Paired biopsy samples of positive patch test sites and normal control skins were collected and snap-frozen in liquid nitrogen before being stored at -80°C for later RNA extraction. Additionally, patch test positive biopsies were obtained from additional patients and frozen in OCT compound for immunocytochemical studies. Two normal adult skins from surgical remnants and one tonsil were used as negative and positive controls for immunocytochemistry. The study was approved by the local institutional review committee and it followed the Declaration of Helsinki Principles for research involving human subjects.
Table 1.
Allergen and Patient Data
| Age/Sex/Race1 | Allergen2 | Scores/Duration | Studies3 |
|---|---|---|---|
| 40/M/W | Balsam | 1+/48h | PCR |
| 69/F/W | MMA | 1+/72h | PCR |
| 49/M/A | Ni | 1+/48h | PCR |
| 63/M/W | Ni | 1+/48h | PCR |
| 57/M/W | Epoxy | 2+/48h | PCR |
| 77/M/W | Bacitracin | 2+/48h | PCR |
| 49/M/A | Ni | 2+/96h | PCR |
| 43/F/AA | Tixocortol | 2+/7d | PCR |
| 53/M/W | Epoxy | 1+/48h | PCR/ICC |
| 49/F/AA | PPD | 1+/48h | PCR/ICC |
| 36/M/W | Gold | 2+/96h | PCR/ICC |
| 71/F/AA | Ni | 2+/48h | ICC |
| 63/M/W | Thimerosal | 2+/96h | ICC |
| 43/F/AA | Tixocortol | 3+/96h | ICC |
Notes:
Race: A: Asians; AA: African-Americans; W, white (Caucasians)
MMA, methylmethacrylate; PPD: para-phenylenediamine
PCR, real-time PCR; ICC, immunocytochemistry
Real-time PCR
Total RNA was isolated from the biopsies using an RNeasy kit (Qiagen, Valencia Ca) after mechanical homogenization. cDNA was synthesized using a reverse transcription kit (Amersham, Piscataway, NJ). Real-time PCR was carried out in LightCycler (Roche Applied Science, Indianapolis, IN) using FastStart DNA Master SYBR Green kit (Roche) according to the manufacturer's instructions and as has been described previously.10 The cytokine mRNA was normalized to the expression of 18S RNA (which showed comparable levels of expression in patch test lesions and normal skins in the present study, data not shown), and the fold-changes were calculated by comparing mRNA levels of positive patch test with those from normal skin of the same patients. The primers for the cytokines and transcription factor are as follows: IL-4 sense: 5’-AGA AGA CTC TGT GCA CCG AGT TGA-3’, anti-sense: 5’-CTC TCA TGA TCG TCT TTA GCC TTT-3’; IFN-γ sense: 5’-GCA TCC AAA GAG TGT GGA G-3’, anti-sense: 5’-GCT CTT CGA CCT CGA AAC-3’; IL17A sense: 5’-ACC AAT CCC AAA AGG TCC TC-3’, anti-sense: 5’-GGG GAC AGA GTT CAT GTG GT-3’; RORC sense: 5’-TTT TCC GAG GAT GAG ATT GC-3’, anti-sense: 5’-CTT TCC ACA TGC TGG CTA CA-3’; IL-17F sense: 5’-TGA AGC TTG ACA TTG GCA TC-3’, anti-sense: 5’-TTC CTT GAG CAT TGA TGC AG-3’; IL-23p19 sense: 5’-CTG ATA GCC CTG TGG GCC AG-3’, anti-sense: 5’-GCT GCC TTT AGG GAC TCA GG -3’; 18S sense: 5’-AAC CCG TTG AAC CCC ATT-3’, anti-sense: 5’-CCA TCC AAT CGG TAG TAG CG-3’. The PCR was run under the following conditions: initial denaturation 10 min at 95°C, and then denaturation at 94°C for 5 sec, annealing/detection at 56°C for 20 sec, and extension at 76°C for 15 sec for 40 cycles. The real-time PCR products were verified for the correct size by agarose gel electrophoresis and melting curve analysis .
Double Immunofluorescence
Skin biopsies in OCT were cut into 5 micron sections and fixed in methanol. The slides were incubated overnight with a rabbit anti-human RORC polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:100 dilutions, followed by goat anti-rabbit IgG FITC conjugate. As negative control, normal rabbit serum was used in place of the first antibody. For double labeling of T cells and T cell subsets, the slides were then incubated with phycoerythrin-labeled monoclonal anti-CD3, CD2, CD4, or CD8 antibodies. The slides were mounted in buffered glycerol containing DAPI. The slides thus stained were examined by a fluorescence microscope and digital images recorded. The cells were counted on the digital pictures after superimposition of the RORC staining (green) and T cell staining (red) images. On average 9 fields from each double-stained section were counted with an average of 130 infiltrating cells per field. RORC+ cells were recognized by the presence of FITC positive stains over the nuclei of the cells that were also positive for a T cell marker.
Data analysis
Mann-Whitney U test was used to analyze cytokine mRNA fold changes. P values less than 0.05 were regarded as statistically significant.
Results
Patient data
Table 1 summarizes the demographic information, the allergens tested, the reaction scores and duration as well as the types of studies performed. Fourteen biopsies from Asians (2), African-Americans (4), and Caucasians (8) were included in the study. These patients were allergic to 9 allergens and the scores ranged from 1 to 3 (1+ in 6 cases; 2+ in 7 cases; and 3+ in 1 case).
Th1, Th2 and Th-17 cytokine gene expression at ACD elicitation sites
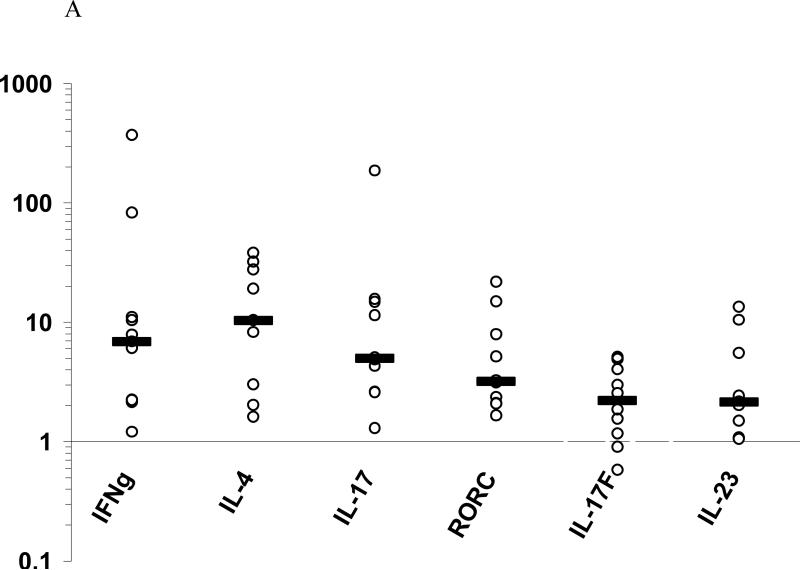
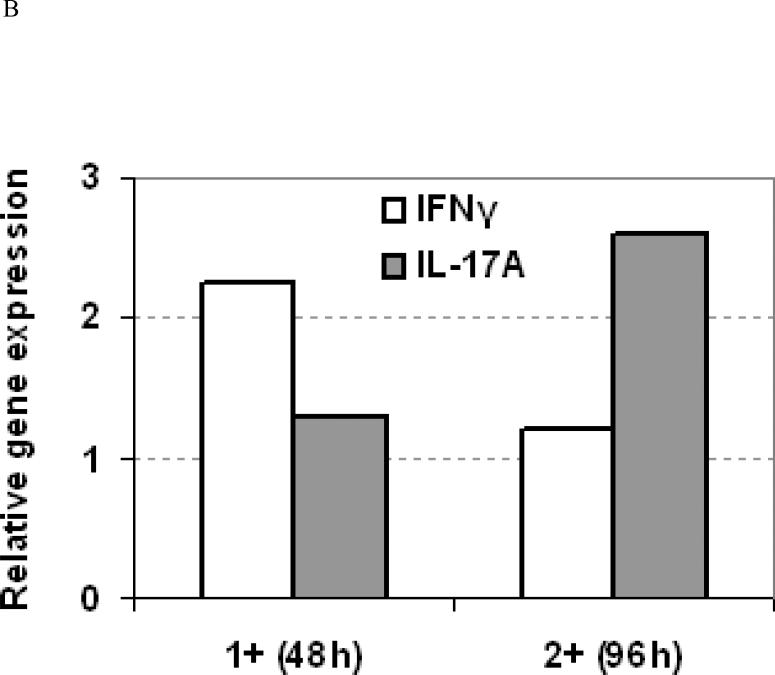
To characterize IL-17-producing Th-17/Tc-17 cells in human ACD, we examined 11 cases of positive patch test biopsies for Th-17 as well as Th1 and Th2 cytokine gene expression. Th1, Th2, and Th-17 cytokine mRNAs were increased in the positive patch test sites compared to the paired normal skins from the same patients. Thus, IFN-γ mRNA was increased 6.9-folds (median, ranged from 1.2 to 370, P < 0.001), IL-4 10.4-folds (median, ranged from 1.6 to 38.4, P < 0.005), IL-17A 5.0-fold (median, ranged from 1.3 to 186.1, P < 0.005), IL-17F 2.2-folds (median, ranged from 0.6 to 5.1, P < 0.05), and IL-23p19 2.1-folds (median, ranged from 1.1 to 13.5, P < 0.005) (Figure 1A). The characteristic Th-17 master transcription factor in humans, RORC, was also increased (median 3.2-fold, P < 0.005) (Figure 1A). No correlation of IL-17 cytokine levels with that of either Th-1 or Th-2 cytokines were detected. However, in one case with nickel allergy, two reaction sites with different scores, 1+ at 48h duration and 2+ at 96h duration, were found to exhibit a decrease in IFN-γ but an increase in IL-17A mRNA expressions as the reaction increased in severity (Figure 1B). A similar correlation pattern between IL-17A and IL-4 gene expression was also observed in this case (data not shown). This seemed to indicate that more IL-17A but less IFN-γ or IL-4 were produced as the reactions aggravated. The above results in this case demonstrate that at the sites of ACD elicitation, there is an increased gene expression of IL-17A and related cytokines apart from increased gene expression of IFN-γ and IL-4, and a probable inverse relationship between IL-17 and Th1/Th2 cytokines exists. Whether this is a generally true for all the cases and allergens remains to be further investigated.
Figure 1. Increased Th1, Th2 and Th-17 cytokine gene expression in ACD positive patch test sites.
A, The relative increase of cytokine mRNAs (medians) is shown in log scales in comparison to their respective normal skins. By Mann-Whitney U test, mRNA increased significantly in the patch test sites over paired controls for IFN-γ (P<0.001), IL-4 (P<0.005), IL-17 (P<0.005), RORC (P<0.005), IL-17F (P < 0.05), and IL-23p19 (P < 0.005). B, Relative fold change in cytokine mRNA expression as the patch test score increases over time in one patient with nickel allergy and a crescendo patch test reaction.
RORC+ IL-17-producing T cells in ACD infiltrates
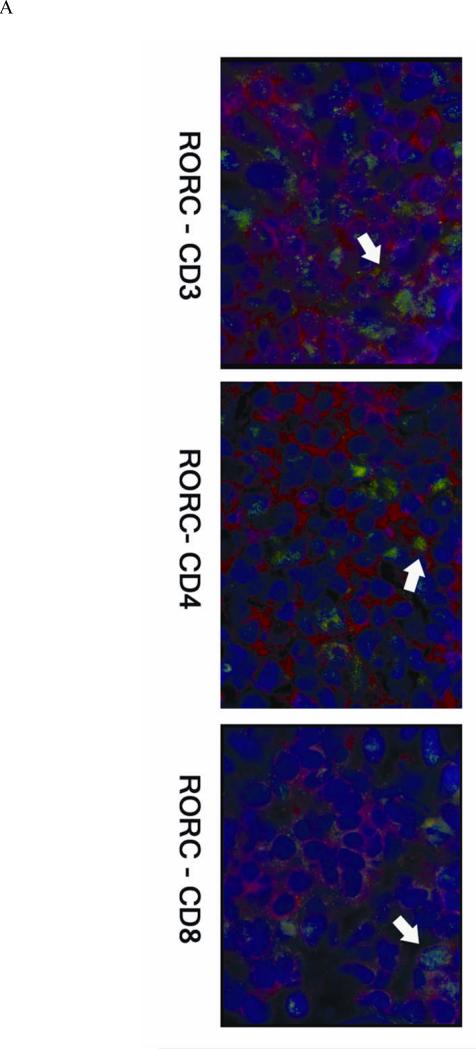
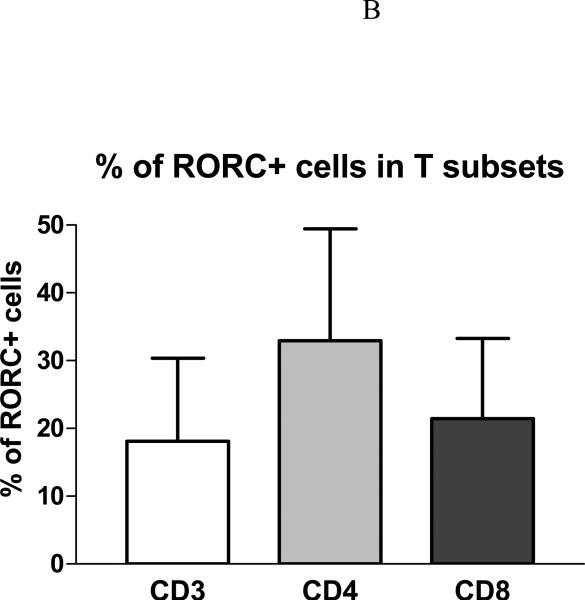
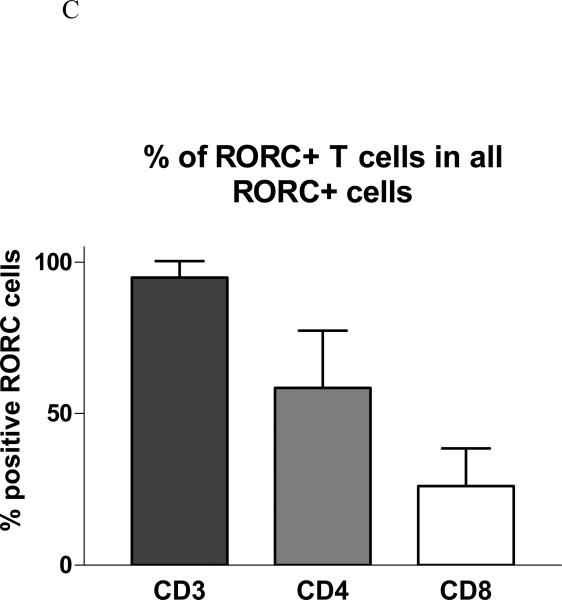
Immunocytochemistry study was also carried out for Th-17 transcription factor RORC and T cell surface markers in positive patch test biopsies from six patients. RORC+ cells were mainly localized in the perivascular infiltrating patches of ACD elicitation sites (Figure 2A). Of the total population of infiltrating CD3+ T cells, 18% were RORC+ (CD3+RORC+ IL-17-producing cells). Of the total population of infiltrating CD4+ T cells, 33% were RORC+ (CD4+RORC+ Th-17 cells). Of the total population of infiltrating CD8+ T cells, 21% were RORC+ (CD8+RORC+ Tc-17 cells) (Figure 2B). Of all the RORC+ cells in the infiltrates, about 95 % were CD3+ T cells, 58 % CD4+ cells, and 26 % CD8+ cells (Figure 2C). There was a small number of CD3-RORC+ cells in the infiltrates. The RORC+ T cells were among the superficial dermal perivascular patches but no RORC positive T cells could be detected in the epidermis. No RORC stained infiltrating cells were detectable in the healthy control skins examined, and there was a high percentage of RORC+ cells (~ 20-30 %) in the tonsil specimen (in the parafollicular areas) we stained (data not shown).
Figure 2. RORC+ cells in ACD.
A, The skin sections are stained with RORC antibody (green) and PE-labeled anti-T cell monoclonals (anti- CD3 or CD4 or CD8) (red). The nuclei are counter-stained by DAPI (blue). B, % RORC+ cells in T subsets. Among the six cases of positive patch test biopsies studied by immunofluorescence, 18.1 ± 12.2 % of RORC+ cells are CD3+ T cells, 32.9 ± 16.5 % RORC+ cells are CD4+, and 21.4 ± 11.9 % are CD8+. C, Of all RORC+ cells, 94.9± 5.4 % are CD3+, 58.5± 18.9 % are CD4+, and 26.2 ± 12.3 % are CD8+.
Discussion
Th-17 cells, a recently defined lineage of T cells distinct from Th1 and Th2 cells, are the main effectors for defense against extracellular bacterial infections and for tissue destruction in inflammation and autoimmunity4. Such Th-17 cell function is achieved through the signature cytokines they produce, such as IL-17A, IL-17F, IL-21 and IL-22.11 Th-17 cells express the characteristic chemokine receptor CCR6 which determines their migration pattern into epithelial sites for defense and inflammation.12,13 In mice Th-17 cells play a pathogenic role in autoimmune disease models such as experimental autoimmune encephalomyelitis and collagen-induced arthritis.4, 14 IL-17-producing cells are now considered to be the main conveyors of autoimmunity previously presumed to be mediated by Th1 cells.4,14 RORγt, RAR-related orphan nuclear receptor γt, or its human equivalent RORC, is the master transcription factor dictating T cell differentiation down the Th-17 rather than the Th1 or Th2 pathways4, 14, 15. TGF-β, together with inflammatory cytokines such as IL-6, IL-1β, or IL-21, has been shown to be critical for the development of Th-17 cells while Th1 and Th2 cytokines, IFN-γ and IL-4, could inhibit Th-17 cell development.4, 11 Such mode of actions between these subsets of T cells satisfies the “self-promoting” yet “mutual regulating” characteristics in defining T lymphocyte lineages, establishing Th-17 cells’ distinct identity. Recent data showed that RORγt could directly bind to the specific ROR responsive element in IL-17 gene promoter region, leading to increased IL-17 expression.16 Indeed the forced expression of RORγt or RORC in naïve T cells could induce Th-17 cell differentiation in mice or in humans.15, 17 These results indicate that RORγt is an appropriate marker for IL-17-producing T cells. RORγ and RORγt differ only at the N terminal AA residues in humans as well as in mice. RORγt does not have the first 24 AA residues of RORγ but, instead, it has an additional 3 AA residues (Met-Arg-Thr) at the very N terminal end. This makes it difficult to raise antibodies with sufficient specificity to distinguish the two RORγ isoforms. The antibody we used in the study recognizes the middle portion of the protein and, thus, both of the isoforms. However, since we only identified the cells positive for both RORγ and a T cell marker, we can be reasonably confident that they were the T cell-specific isoform of the transcription factor.
The current paradigm maintains that Th1 cells are the primary effectors while Th2 cells are minor effectors in ACD/CHS.1 Most chemical haptens, such as DNFB, TNCB, Oxazolone, induce a Th1 response while only some such as FITC and trimellitic anhydride, induce a Th2 dominant CHS response. However, there is also experimental evidence demonstrating that in both IFN-γ- and interferon-γ receptor 1 (IFNGR1)-deficient mice, CHS developed normally to TNCB and DNFB. In contrast, CHS to TNCB and DNFB was reduced in mice deficient in either IL-4 or STAT-6, a Th2 cell-specific transcription factor. These studies indicate that Th2 as well as Th1 cytokines are important in CHS response.8 Characterization of the new Th-17 lineage and the study of their roles in human ACD and murine CHS have helped to re-define the primary effectors in this disease. Thus, nickel-specific CD4+ clones isolated from humans can produce IL-17 which can enhance local inflammation in ACD by recruiting other cells and stimulating keratinocytes to elaborate proinflammatory cytokines.6 In IL-17 deficient mice, experimentally induced CHS response is impaired with reduced ear swelling and cellular infiltration as well as reduced chemokine production and ICAM-1 expression.7 However, T cells from IL-17-deficient mice can still sustain a weakened CHS response7, indicating that either other Th-17 cytokines such as IL-21 and IL-22 are still functional in this situation, or that Th-17 is just one of the participants in CHS. In our study, the presence of significant quantities of RORC+ IL-17-producing T cells (about 20%) of both CD4 and CD8 subsets within the perivascular cellular infiltrates in superficial dermis, the site of ACD onset, indicates that they might be (one of) the effectors in ACD. This increased presence of IL-17-producing cells in the dermis represents a significant enrichment of such cells as IL-17-producing cells consisted of only 1.5% in the regional lymph nodes from allergen-primed mice.18 IL-17 exerts its effects through IL-17 receptors which are widely expressed by both immune and non-immune cells.11 The major functions of IL-17 include the recruitment of inflammatory cells, induction of chemokines (such as CXCL1, CXCL2, CXCL5, CXCL-8) and pro-inflammatory cytokines (such as IL-1, IL-6 and TNF-α) by endothelial and epithelial cells, and the induction of adhesion molecule expression (such as ICAM-1 and VCAM-1).11 The inflammation in ACD is thus likely to be instigated and sustained by the pro-inflammatory effects of IL-17A/IL-17F and other cytokines produced by allergen-specific Th-17/Tc-17 cells in the lesions.
The superficial perivascular dermis is the site of ACD onset during elicitation phase. The lymphocytes as well as other innate and acquired immune cells respond to the challenged antigen in the vicinity of superficial vessels as the immune cells are coming out of the blood into the tissue in an attempt to contain and eliminate the antigen. The epidermal involvement is minimal initially, and severe inflammatory damage in the epidermis only happens as the reaction intensifies. That most RORC+ IL-17-producing cells were found within dermal infiltrates in our cases seems to be concordant with such an immunopathologic scenario. In our cases the prominent infiltration was located in the superficial dermis with less pronounced epidermal changes since the scores of our patch test biopsies were mostly of 1+ to 2+. One of the effects of IL-17 is the recruitment of neutrophils to the inflammatory sites. In contrast to mouse CHS, neutrophil infiltration is not a feature in allergic contact dermatitis of humans due to species differences.19 The effector role of IL-17 in ACD, therefore, must be through its other pro-inflammatory effects. In addition to IL-17A, IL-17F and IL-23p19 were also increased in ACD in our cases. These cytokines could also play a role in ACD, and indeed, IL-23-deficient mice did show impaired hypersensitivity reaction.20 The role played by other Th-17 cytokines in ACD, such as IL-21 and IL-22, remains to be studied.
CD8 T cells are the primary effectors in ACD while CD4 T cells play a regulatory role.18 In mice deficient in CD8 T cells, CHS response was impaired, but in CD4 T cell deficient mice CHS was either detected as normal or enhanced. IL-17 can be produced by both CD4 and CD8 T subsets although disparity exists about the role of these two IL-17-producing subsets in murine CHS. Thus, in IL-17-deficient mice, CD4 but not CD8 cells from the regional lymph nodes had defective proliferation and IFN-γ production,7 yet in another study, in mice depleted of CD4 cells, IL-17 blockade, but not IFN-γ blockage, led to reduced tissue swelling after hapten challenge.18 The situation in the humans is less clear. Our results seem to indicate that both CD4 and CD8 subsets probably contribute to the ACD immune response as they both have the capacity to produce IL-17 and are present in significant numbers in the infiltration. If IL-17 rather than INF-γ is the primary cytokine in driving the inflammatory reactions in ACD in humans as suggested by many recent studies on the function of IL-17-producing cells,4, 5, 8, 11, 14 then both CD4 and CD8 subsets seem to be equally capable of sustaining the response as both can produce IL-17 in view of the master transcription factor they express. Thus, the primary effectors of ACD are probably Th-17/Tc-17 cells rather than Th1/Tc1 cells as stipulated by the current paradigm. ACD is generally construed as being caused by harmless hapten chemicals. However, most of these chemicals have the capacity to modify amino acid residues of cutaneous structural proteins1 which may indeed have acquired altered properties if permitted to persist. Thus, such modification produces in the skin neo-antigens which are recognized by the immune system as foreign. These neo-antigens are, therefore, cleared by IL-17-producing cells in a similar fashion to extracellular bacteria. Previous studies have shown that IL-17 could be produced by nonlymphocytes such as monocytes and macrophages,21 albeit only weakly in a small percentage of them. Our staining of the infiltrates showed some RORC+CD3- cells which, though not further characterized in our study, might represent such cells.
In conclusion, our results indicate that IL-17-producing (Th-17/Tc-17) cells are regular participants in the cellular infiltrates and thus might play an important role in the elicitation phase of ACD in humans as we identified transcripts for Th-17 cytokines and RORC+ lymphocytes in allergic skin reactions to 9 different allergens. The study of these cells and the cytokines they produce may shed light on a new aspect of the immunopathology of the disease, and the present Th1/Th2 paradigm for ACD should thus be broadened to incorporate Th-17/Tc-17 lymphocytes.
Acknowledgments
Sources of funding:
Grant support: this work was funded by NIH Grant R01-AR46108-05 and a VA Merit Award from Department of Veteran Affairs (To AAG)
Abbreviations
- ACD
allergic contact dermatitis
- APC
antigen presenting cells
- CHS
contact hypersensitivity
Footnotes
Confliction of interest: The authors state no conflict of interest.
References
- 1.Gober MD, Gaspari AA. Allergic contact dermatitis. Curr Dir Autoimmun. 2008;10:1–26. doi: 10.1159/000131410. [DOI] [PubMed] [Google Scholar]
- 2.Kerfoot SM, Szczepanik M, Tung JW, et al. Identification of initiator B cells, a novel subset of activation-induced deaminase-dependent B-1-like cells that mediate initiation of contact sensitivity. J Immunol. 2008;181:1717–27. doi: 10.4049/jimmunol.181.3.1717. [DOI] [PubMed] [Google Scholar]
- 3.Gober MD, Fishelevich R, Zhao Y, et al. Human natural killer T cells infiltrate into the skin at elicitation sites of allergic contact dermatitis. J Invest Dermatol. 2008;128:1460–9. doi: 10.1038/sj.jid.5701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettelli E, Korn T, Oukka M, et al. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Beelen AJ, Teunissen MB, Kapsenberg ML, et al. Interleukin-17 in inflammatory skin disorders. Curr Opin Allergy Clin Immunol. 2007;7:374–81. doi: 10.1097/ACI.0b013e3282ef869e. [DOI] [PubMed] [Google Scholar]
- 6.Albanesi C, Scarponi C, Cavani A, et al. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gamma- and interleukin-4-induced activation of human keratinocytes. J Invest Dermatol. 2000;115:81–7. doi: 10.1046/j.1523-1747.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- 7.Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 8.Oboki K, Ohno T, Saito H, et al. Th17 and allergy. Allergol Int. 2008;57:121–34. doi: 10.2332/allergolint.R-07-160. [DOI] [PubMed] [Google Scholar]
- 9.Belsito DV. Patch testing with a standard allergen (“screening”) tray: rewards and risks. Dermatol Ther. 2004;17:231–239. doi: 10.1111/j.1396-0296.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Fishelevich R, Petrali JP, et al. Activation of keratinocyte protein kinase C zeta in psoriasis plaques. J Invest Dermatol. 2008;128:2190–7. doi: 10.1038/jid.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh SP, Zhang HH, Foley JF, et al. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–21. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- 13.Lechner A, Ritter U, Varona R, et al. Protective immunity and delayed type hypersensitivity reaction are uncoupled in experimental Leishmania major infection of CCR6-negative mice. Microbes Infect. 2007;9:291–9. doi: 10.1016/j.micinf.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Ichiyama K, Yoshida H, Wakabayashi Y, et al. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–8. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 17.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–9. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He D, Wu L, Kim HK, et al. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006;177:6852–8. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 20.Ghilardi N, Kljavin N, Chen Q, et al. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J Immunol. 2004;172:2827–33. doi: 10.4049/jimmunol.172.5.2827. [DOI] [PubMed] [Google Scholar]
- 21.Gu Y, Yang J, Ouyang X, et al. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38:1807–13. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]