Abstract
Cocaine use during pregnancy induces profound neural and behavioral deficits in both mother and offspring. The present study was designed to compare the effects of cocaine exposure on spine density of postpartum and virgin female rat brains. Timed, pregnant, primiparous rats were injected with either cocaine (30 mg/kg) or saline, once daily, from gestational day 8–20. Twenty four hours after giving birth, dam brains were processed for Golgi-impregnation. Since cocaine effects in female rats have not been determined, virgin females were also injected with the same dose of cocaine or saline for 12 days and sacrificed 24h after the last injection for comparison. Pregnant rats had significantly greater spine density in the medial amygdala (MeA) and medial preoptic area (MPOA) and lower spine density in CA1 than virgin females independent of cocaine treatment. Cocaine significantly increased dendritic spine density on the apical branch of pyramidal cells in the prefrontal cortex (PFC, 15%), both apical (13%) and basal (14.8%) branches of CA1 and cells in the MeA (28%) of pregnant rats. In the MPOA, cocaine administration resulted in a decrease in dendritic spine density (14%) in pregnant rats. In virgin females, cocaine had fewer effects but did increase dendritic spine density on both branches of CA1 neurons and in the MeA. The present study is the first to demonstrate that spine density differs between pregnant and virgin females and that pregnancy makes the brain more vulnerable to cocaine, which has important clinical implications.
Keywords: cocaine, pregnancy, spine, preoptic, hippocampus
Introduction
The use of cocaine during pregnancy is an important health concern that impacts both the mother and the fetus (Thompson et al., 2009). Different regimens of cocaine administration during gestation cause disruption of many aspects of maternal behavior (reviewed Strathearn and Mayes, 2010), including the onset of maternal behavior (Kinsley et al., 1994; Johns et al., 2005) and increased maternal aggression in the post partum rat (McMurray et al., 2008b). Administration of cocaine to virgin rats prior to mating has also been shown to alter maternal behavior (Nephew and Febo, 2010). Little is known about changes in the brain when rat dams are exposed to cocaine, but alterations in oxytocin levels have been reported (Elliot et al., 2001; Johns et al., 2010). Oxytocin is one of the key factors in mediating maternal behavior (Neumann, 2008; Strathearn and Mayes, 2010). In addition, oxytocin receptors and oxytocin mRNA are altered by cocaine in brain regions that are implicated in maternal behavior such as the amygdala and the medial preoptic area (MPOA) (Johns et al., 2004; McMurray et al., 2008a; Jarrett et al., 2006). The behavioral alterations seen following gestational cocaine exposure also occur in subsequent generations, suggesting enduring morphological and neurochemical changes in the brain (McMurray et al., 2008a).
In adult male rats, cocaine increases dendritic spine density in the nucleus accumbens (NAC) and the prefrontal cortex (PFC) (Robinson et al., 2001; Robinson and Kolb, 2004), but its effects in female rats are unknown. When cocaine is administered prenatally to rats, there is increased dendritic spine density in the NAC, the PFC and the CA1 region of the hippocampus (CA1) at postnatal day 21 in both sexes (Frankfurt et al., 2009). Spine density remained elevated in the NAC and the PFC and there was a sex difference in CA1 when rats that received prenatal cocaine were examined in adulthood (Salas-Ramirez, et al., 2010).
Given that pregnancy alone is known to cause profound changes in many aspects of brain function (Kinsley, 2008; Macbeth and Luine, 2010), we wanted to assess possible interactions between pregnancy and cocaine exposure. Therefore, in the present study, we assessed the effects of cocaine on the brains of both dams and virgin female rats. Spine density in the PFC and CA1, as well as in brain regions known to be involved in maternal behavior, the MPOA, the ventromedial hypothalamic nucleus (VMN) and medial amygdala (MeA) were examined.
Materials and Methods
Timed-pregnant Sprague-Dawley rats (colony 208A, Harlan Inc, Frederick, MD) arrived on gestational day (GD) two. Animals were housed singly in a temperature controlled colony room (±21–22°C), under a 12:12 light dark cycle (lights on at 8:00am) with food and water (Harlan Teklab Rodent Chow) available ad libitum. From GD8 to GD20, five of the pregnant dams were treated daily (at approximately 10:00am) with an intraperitoneal (ip) injection of 30 mg/kg of cocaine HCl (Sigma-Aldrich, St. Louis, MO), and five dams received daily saline injections. The cocaine was made fresh every three days. The volume of the injections ranged from 0.24 to 0.39 mls. Within 24 hrs of parturition the dams were sacrificed and the pups born to these mothers were cross-fostered to saline treated dams for other experiments (Salas-Ramirez et al., 2010). Another group of adult female virgins was injected with 30mg/kg cocaine (n =5) or saline (n =5) daily for twelve days and sacrificed 24h after the last injection.
Brains were removed from subjects and cut into an anterior block (anterior to the optic chiasm) and a posterior block (between the optic chiasm and the brainstem) and placed in solutions provided in the Rapid GolgiStain Kit (FD NeuroTechnologies, Ellicott City, MD). Golgi impregnation was performed as previously described (Frankfurt et al 2009).
Secondary basal dendrites and tertiary apical dendrites were analyzed from pyramidal cells from the CA1 region of the dorsal hippocampus and layer II/III of the PFC. Primary dendrites from the VMN, the MPOA and the MeA were also analyzed. Regions were defined according to The Rat Brain Atlas in Stereotaxic Coordinates (Paxinos and Watson, 2007). Six cells per region/brain were quantified for each subject. Cells chosen for analyses had to be well impregnated, clearly distinguishable from adjacent cells and have continuous, unbroken dendrites. The dendrite chosen was the most lateral dendrite for a given dendritic tree. Spines were counted under oil (100x), using a Leitz Diaplan microscope and the entire dendritic length visible measured using Image Pro Plus software (Media Cybernetics) with a Nikon DXM 1200F camera. Spine density was calculated by dividing the number of spines by the length of the dendrite and data expressed as number of spines/10 μm dendrite. Statistical analysis was done for each area examined with a 2-way ANOVA: status (pregnant vs. virgin females) × treatment (control vs. cocaine), followed by post hoc tests where appropriate (Fisher’s LSD tests).
Results
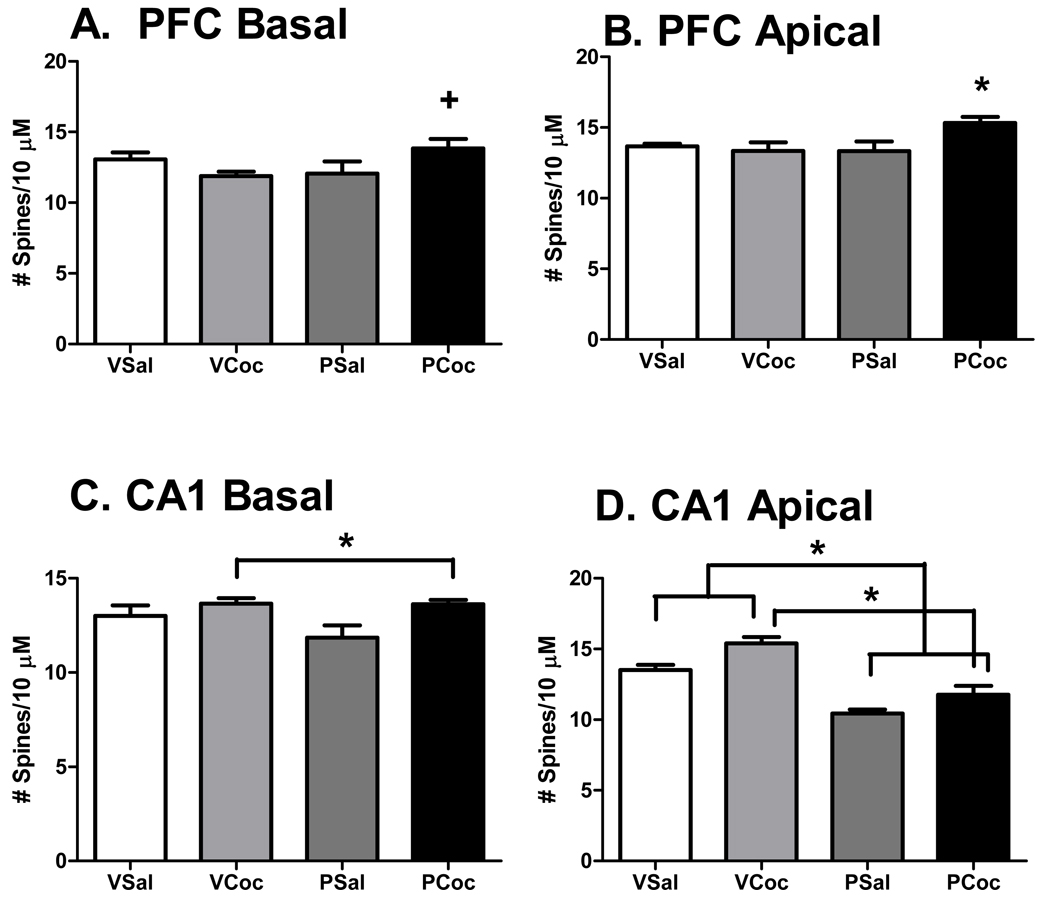
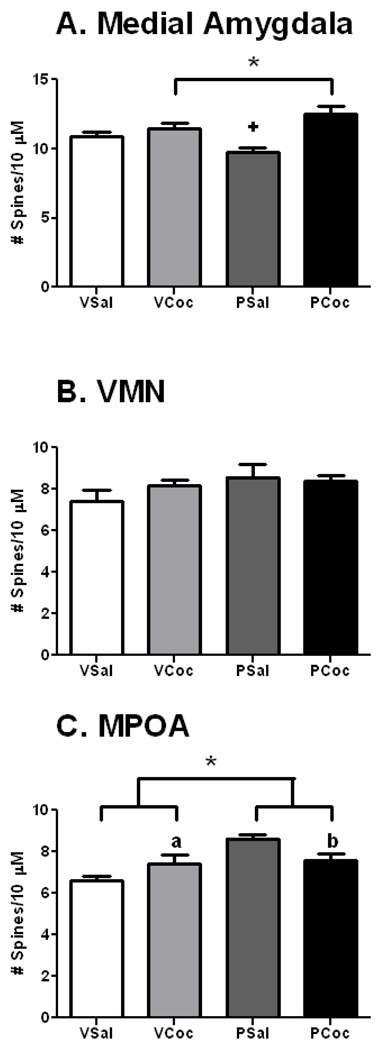
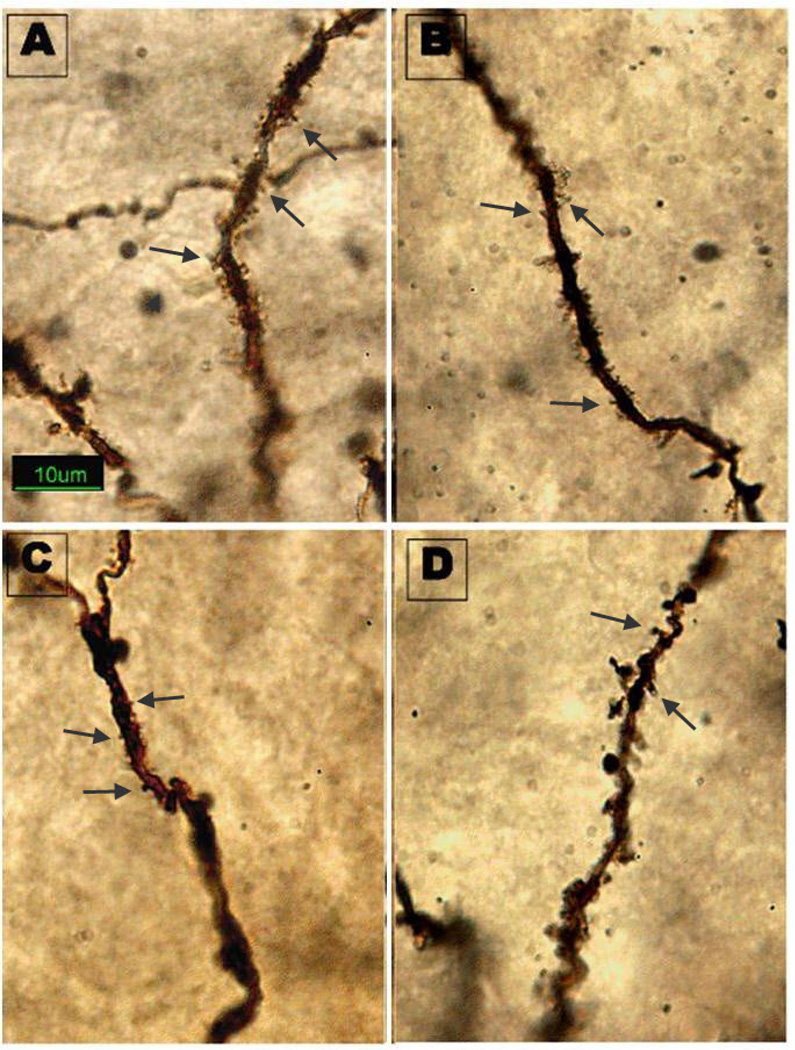
Cocaine treatment did not affect the weight of the dams (mean ± sem: cocaine: 330 ± 8.84; saline: 345 ± 9.87, p = 0.279), virgin females or pups or alter litter sizes (Salas-Ramirez et al., 2010). In general, both status (pregnant or virgin) and treatment (saline or cocaine) significantly altered spine density in brain areas and, in some there were significant interactions between status and treatment. Data for the PFC and CA1 are shown in Figure 1. Data for the MeA, MPOA and VMN are shown in Figure 2. An example of Golgi impregnated dendrites in the MPOA is shown in Figure 3.
Figure 1.
Figure 1: Effects of cocaine on spine density in the PFC and CA1. Mean ± SEM. Data analyzed by two-way ANOVA, status (virgin, pregnant) × treatment (saline, cocaine) and post hoc testing by LSD. Please see results for ANOVA outcomes.
A. PFC Basal, Significant interaction of status and treatment; +, PCoc > VCoc, p < 0.05.
B. PFC Apical, Significant interaction of status and treatment; *, PCoc > than all other groups, p < 0.05.
C. CA1 Basal, Significant effect of treatment; Coc groups > Sal groups
D. CA1 Apical, Significant effect of treatment and status; Coc groups > Sal groups, Virgin groups > pregnant groups.
Figure 2.
Effects of cocaine on spine density in subcortical regions. Mean + SEM. Data analyzed by two-way ANOVA, status (virgin, pregnant) × treatment (saline, Cocaine) and post hoc testing by LSD. Please see results for ANOVA outcomes.
A. MeA, Significant effect of treatment; Coc groups > Sal groups; Significant interaction of status and treatment; +, vir-sal >preg-sal, p < 0.05.
B. VMN, No significant differences.
C. MPOA, Significant effect of status; significant interaction between status and treatment; a, VCoc > Vsal, p < 0.05; b, PSal > PCoc, p < 0.05.
Figure 3.
Golgi impregnated dendrites in the MPOA. Photomicrograph illustrates the difference in dendritic spine density between treatments. Groups: A = Virgin Cocaine B= Pregnant Cocaine, C = Virgin Saline, D = Pregnant Saline. Arrows denote spines
On the apical and basal branches of dendrites on PFC neurons, there was a significant interaction between status and treatment (basal: F (1,16) = 5.794, p = 0.0285; apical: F (1,16) = 4.99, p =0.04). On the apical branch of PFC neurons, cocaine treatment increased spine density by 15 % (MD= 2.6; P = 0.04) in pregnant rats, but no significant differences were observed following cocaine treatment in virgin rats. In contrast to the effects on apical dendrites, cocaine treatment did not increase spine density in virgin (MD = 2.0, p =.08) or pregnant rats on basal dendrites (MD=1.7, p =1.4), but the pregnant cocaine group had a greater spine density than the virgin cocaine group (MD=2.7, P = 0.03). On the apical branch of CA1 neurons, there was a main effect of treatment (F (1,16) = 12.6, p = .0026). Cocaine increased spine density on the apical dendrites in virgin rats by 14% and in pregnant rats by 13 %. In addition, there was a significant status effect (F (1,16) = 54.3, p = 0.0001), where overall spine density of apical CA1 neurons was 32% higher in virgin than pregnant rats. On the basal branch of CA1 neurons, there was a main effect of cocaine (F (1,16) =,6.766, p =0.0193) to increase spine density by 14.8 % in the pregnant rats and by 5% in the virgin animals as compared to saline.
Interestingly, in the MPOA, there was a main effect of status such that pregnancy increased dendritic spine density by 16% as compared to virgins (F(1,16) = 13.5, p = 0.0021) and a significant interaction between treatment and status (F(1,16) = 9.5, p = 0.0072). Cocaine decreased dendritic spine density in the MPOA of pregnant rats by 13% (MD=1.06; p =0.02) but did not alter spine density in virgins (MD= 1.6, p =0.19). In the MeA, there was a main effect of treatment (F(1,16) = 21.56, p= 0003) and an interaction between status and treatment (F(1,16) = 9.4, p = 0075). In the pregnant rats, cocaine increased dendritic spine density by 28% as compared to saline whereas increases in virgins were smaller (13%). Lastly, cocaine did not alter the spine density of VMN neurons in either pregnant or virgin rats.
Discussion
The present study demonstrates novel findings on the effects of cocaine in female rats, both virgin and pregnant. We have found that cocaine treatment and reproductive status (virgin vs. pregnant) influence spine density in several regions of the female rat brain. Moreover, there was an interaction between drug treatment and reproductive status in some brain areas. For example, both pregnant and virgin rats showed increased spine density in CA1and the MeA following cocaine treatment but only pregnant rats had increased spine density in the PFC. In addition, only cocaine-treated, pregnant rats showed a decrease in MPOA spine density. Furthermore, pregnancy alone also affected spine density. Decreased spine density in apical dendrites of CA1 and increased spine density in the MPOA neurons were observed in pregnant rats when compared to virgin females. Thus, overall, spine density was affected by pregnancy, and pregnant rats appeared to have an enhanced morphological response to cocaine treatment in some brain areas, a finding which has important clinical ramifications.
Previous studies have demonstrated that cocaine increases dendritic spine density in the PFC and NAC of adult male rats (Robinson et al., 2001, Robinson and Kolb, 2004). Alterations in spine density are thought to underlie the mechanism(s) of addiction. The effects of cocaine on spine density in adult female rats have not been described in the literature. In the present study we report that cocaine administration generally has the same effect in female rats as has been reported for male rats, which is to increase spine density, with the notable exception of the MPOA of postpartum rats, where we observed a decrease in spine density. However, in our study there are significant differences between pregnant and virgin females. Although we did observe a cocaine induced increase in dendritic spine density in the PFC of pregnant rats, cocaine did not alter spine density in virgin female rats, suggesting that there are sex differences in response to cocaine in some brain regions. It must be noted, however, that because we have previously observed cocaine induced changes in layer II/III and not in layer V (Frankfurt et al 2009), we examined layer II/III in the present study while Robinson et al. (2001) examined layer V.
Sex differences in response to cocaine have been reported in both humans and rodents (Becker and Hu, 2008; Dow-Edwards, 2010; Lukas et al, 1996, Quinones-Jenab, and Jenab 2010). The observation that females are more vulnerable to the addictive effects of cocaine and more prone to relapse has generally been attributed to estrogen (Becker and Hu, 2008, Hu and Becker, 2003, Larson et al. 2005, Watson et al., 2010). Studies manipulating gonadal hormones have reinforced the idea that high estrogen levels make the brain more sensitive to cocaine (Russo et al., 2003; Zhen et al., 2007). In contrast to the paucity of cocaine studies in adult females, there is some data on female rats following prenatal administration of cocaine. When cocaine is given prenatally and spine density is assessed at 21 days postnatal there is an increase in dendritic spine density in CA1, but there is no sex difference (Frankfurt et al., 2009). However, when prenatal cocaine treated rats are examined at 72 days of age, there is a sex difference in dendritic spine density in CA1 (Salas-Ramirez et al., 2010). Female rats that received cocaine in utero had significantly greater spine density on CA1 dendrites than any other group. Morrow et al. (2007) found that rats treated prenatally with cocaine had similar increases in the number of spine synapses in the prelimbic cortex of both male and female rats at an intermediate time (postnatal day 44). Taken together, these studies suggest that cocaine induced morphological changes, which may underlie the known sex differences in cocaine addition, are influenced differently by gonadal hormones during development. Moreover, the transition from adolescence to adulthood is critical.
The maternal brain is capable of remarkable plasticity in order to prepare for lactation and motherhood (Kinsley et al., 2006, Kinsley 2008). In the MPOA, perikarya increase in size with pregnancy (Keyser-Marcus et al., 2001), and the number of astrocytes increases when dams are exposed to their pups (Featherstone et al. 2000). Exposure of lactating pups to their mothers induces alterations in C-fos in the amygdala, MPOA and PFC (Fleming et al. 1996). A recent study in humans, demonstrated that there was an increase in grey matter in mothers who had a positive perception of their offspring (Kim et al. 2010). Given that dendritic spines exhibit plasticity in response to increased estrogen levels in CA1 (Gould et al., 1990; Woolley et al., 1990), the PFC (Chen et al., 2009) and the VMN (Frankfurt et al., 1990), we expected that the high levels of estrogen during pregnancy would result in higher dendritic spine density than virgin females. Indeed, in a recent study, Leuner and Gould (2010) demonstrated that pregnant rats had increased dendritic spine density in both apical and basal branches of neurons in CA1 and the medial PFC 20 days after birth compared to virgin females. In the present study, we saw an increase in spine density in the MPOA when pregnant rats were compared to virgin rats, independent of cocaine treatment. However, we did not see any effect of pregnancy on either branch of the PFC neurons and on the apical branch of CA1 neurons we found that dendritic spine density was decreased with pregnancy when compared to the virgin females. The effects of pregnancy on spine density in CA1 have been determined in several studies with variable results. Kinsley et al. (2006) demonstrated that dendritic spine density on the apical branch of CA1 neurons was greatest in late pregnancy and during lactation (day five) when compared to virgin rats in different stages of estrous. Brusco et al. (2008) demonstrated that, starting at day four postpartum, there were no differences in either spine density or spine type in CA1 between postpartum and virgin Wistar rats. The differences between these studies and our results may be attributed to the fact that we examined the postpartum brains 24h after birth when the effects of estrogen may still be present.
Since gestational cocaine has been shown to cause a disruption of maternal behavior (Johns et al., 2005) and the amygdala and the MPOA (Neumann, 2008; Pereira and Morrell, 2010) are known to be critical for both the onset and maintenance of maternal behavior, we examined the effects of gestational cocaine on these regions as well. In the MeA, cocaine induced an increase in dendritic spine density in the rat dams. Gestational cocaine has previously been shown to increase dopamine and DOPAC levels (Lubin et al., 2003), decrease oxytocin levels (Johns et al., 2010) and result in an up-regulation of oxytocin receptors in the amygdala of rats six days after birth (Johns et al., 2004). In contrast, in the present study cocaine exposure resulted in a decrease in dendritic spine density in the MPOA of dams. Gestational cocaine has been shown to result in a decrease in DOPAC levels in the MPOA (Lubin et al., 2003) and a down-regulation in oxytocin receptors 6 days after birth (Johns et al., 2004). The primary mechanism of action of cocaine seems to occur via the dopaminergic system (Levitt et al., 1997; Ritz et al., 1990) and dopamine is implicated in directing the growth of neurons (Levitt et al., 1997; Van Kampen et al., 2005). Moreover, in a recent study, Martin et al. (2010) showed that cocaine induced increases in dendritic spine density were dependent on the dopamine transporter. Despite this data, it is not possible to determine whether the alterations in spine density are the result of direct effects on a given dendrite, the result of cocaine induced changes in another cell that project to that dendrite or both. However the special hormonal environment of pregnancy appears to alter the brain’s susceptibility to the effects of cocaine.
In conclusion, this study demonstrates that cocaine alters dendritic spine density in the brains of adult female rats, both virgin and postpartum. Notably, the magnitude of change and the number of brain areas involved were greater in the pregnant subjects. In addition, we report that the MPOA, a region critical for maternal behavior, of postpartum rats is the only area examined where cocaine administration results in decreased dendritic spine density. This change may therefore underlie previously reported adverse effects of cocaine on maternal behavior. Taken together, these results further demonstrate that the brain’s response to cocaine varies depending on the endocrine status of female. Lastly, they show that the use of psychoactive drugs during pregnancy results in morphological repercussions for the mother as well as the offspring.
Acknowledgments
We would like thank Juan Gomez and Rita Mogilanski for technical support. Supported by DA 018055 to E.F. and RR 03037 to V.N.L
References
- Becker J, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco J, Wittmann R, de Azevedo MS, Lucion AB, Franci CR, Giovenardi M, Rasia-Filho AA. Plasma hormonal profiles and dendritic spine density and morphology in the hippocampal CA1 stratum radiatum, evidenced by light microscopy, of virgin and postpartum female rats. Neurosci Lett. 2008;438(3):346–350. doi: 10.1016/j.neulet.2008.04.063. [DOI] [PubMed] [Google Scholar]
- Chen JR, Yan YT, Wang TJ, Chen LJ, Wang YJ, Tseng GF. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cereb Cortex. 2009;11:2719–2727. doi: 10.1093/cercor/bhp048. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D. Translational issues for prenatal cocaine studies and the role of environment. Neurotoxicol Teratol. 2010 Jun 30; doi: 10.1016/j.ntt.2010.06.007. 2010, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Elliott JC, Lubin DA, Walker CH, Johns JM. Acute cocaine alters oxytocin levels in the medial preoptic area and amygdala in lactating rat dams: implications for cocaine-induced changes in maternal behavior and maternal aggression. Neuropeptides. 2001;35(2):127–134. doi: 10.1054/npep.2001.0854. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Fleming AS, Ivy GO. Plasticity in the maternal circuit: Effects of experience and partum condition on brain astrocyte number in female rats. Behavioral Neuroscience. 2000;114:158–172. doi: 10.1037//0735-7044.114.1.158. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M. Plasticity in the maternal circuit: Effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behavioral Neuroscience. 1996;110:567–582. doi: 10.1037//0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51(5):530–535. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Wang HY, Marmolejo N, Bakshi K, Friedman E. Prenatal cocaine increases dendritic spine density in cortical and subcortical brain regions of the rat. Dev Neurosci. 2009;31:71–75. doi: 10.1159/000207495. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10(4):1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23(2):693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett TM, McMurray MS, Walker CH, Johns JM. Cocaine treatment alters oxytocin receptor binding but not mRNA production in postpartum rat dams. Neuropeptides. 2006;40:161–167. doi: 10.1016/j.npep.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Elliott DL, Hofler VE, Joyner PW, McMurray MS, Jarrett TM, Haslup AM, Middleton CL, Elliott JC, Walker CH. Cocaine treatment and prenatal environment interact to disrupt intergenerational maternal behavior in rats. Behav Neurosci. 2005;119:1605–1618. doi: 10.1037/0735-7044.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Walker CH, Joyner P, Middleton C, Hofler V, McMurray M. Gestational treatment with cocaine and fluoxetine alters oxytocin receptor number and binding affinity in lactating rat dams. Int J Dev Neurosci. 2004;22:321–328. doi: 10.1016/j.ijdevneu.2004.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, McMurray MS, Joyner PW, Jarrett TM, Williams SK, Cox ET, Black MA, Middleton CL, Walker CH. Effects of chronic and intermittent cocaine treatment on dominance, aggression, and oxytocin levels in post-lactational rats. Psychopharmacology (Berl) 2010;211(2):175–185. doi: 10.1007/s00213-010-1877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser-Marcus L, Stafisso-Sandoz G, Gerecke K, Jasnow A, Nightingale L, Lambert KG, Gatewood J, Kinsley CH. Alterations of medial preoptic area neurons following pregnancy and pregnancy-like steroidal treatment in the rat. Brain Research Bulletin. 2001;55:737–745. doi: 10.1016/s0361-9230(01)00554-8. [DOI] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci. 2010;124(5):695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH. The neuroplastic maternal brain. Horm Behav. 2008;54(1):1–4. doi: 10.1016/j.yhbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49(2):131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Turco D, Bauer A, Beverly M, Wellman J, Graham AL. Cocaine alters the onset and maintenance of maternal behavior in lactating rats. Pharmacol Biochem Behav. 1994;47:857–864. doi: 10.1016/0091-3057(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;82(1):98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. J Neurosci. 2010;30(40):13499–13503. doi: 10.1523/JNEUROSCI.3388-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Cannon JB, Black MC, Brown LE, Johns JM. Effects of chronic cocaine on monoamine levels in discrete brain structures of lactating rat dams. Pharmacol Biochem Behav. 2003;74(2):449–454. doi: 10.1016/s0091-3057(02)01027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharm. 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- MacBeth A, Luine V. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2010;34(3):452–467. doi: 10.1016/j.neubiorev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Martin BJ, Naughton BJ, Thirtamara-Rajamani K, Yoon DJ, Han DD, Devries AC, Gu HH. Dopamine transporter inhibition is necessary for cocaine-induced increases in dendritic spine density in the nucleus accumbens. Synapse. 2010 Oct 8; doi: 10.1002/syn.20865. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MS, Cox ET, Jarrett TM, Williams SK, Walker CH, Johns JM. Impact of gestational cocaine treatment or prenatal cocaine exposure on early postpartum oxytocin mRNA levels and receptor binding in the rat. Neuropeptide. 2008a;42(5–6):641–652. doi: 10.1016/j.npep.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MS, Joyner PW, Middleton CW, Jarrett TM, Elliott DL, Black MA, Hofler VE, Walker CH, Johns JM. Intergenerational effects of cocaine on maternal aggressive behavior and brain oxytocin in rat dams. Stress. 2008b;11(5):398–410. doi: 10.1080/10253890701850239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Hajszan T, Leranth C, Elsworth JD, Roth RH. Prenatal exposure to cocaine is associated with increased number of spine synapses in rat prelimbic cortex. Synapse. 2007;61:862–865. doi: 10.1002/syn.20430. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Febo M. Effect of cocaine sensitization prior to pregnancy on maternal care and aggression in the rat. Psychopharmacology. 2010;209:127–135. doi: 10.1007/s00213-010-1777-z. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin: A key regulator of emotional and social behaviors in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain Atlas in Stereotaxic Coordinates. ed 4. Academic Press; 1998. [Google Scholar]
- Pereira M, Morrell JI. The medial preoptic area is necessary for motivated choice of pup- over cocaine-associated environments by early postpartum rats. Neuroscience. 2010;167:216–231. doi: 10.1016/j.neuroscience.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Hormones and Behavior. 2010;58:22–32. doi: 10.1016/j.yhbeh.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Robinson TG, Gorny G, Mitton E, Kolb B. Cocaine self administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 Suppl 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. 2004. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quiñones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Salas-Ramirez K, Frankfurt M, Luine V, Friedman E. Prenatal cocaine exposure increases anxiety, impairs cognitive function and increases dendritic spine density in adult rats: influence of sex. Neuroscience. 2010;169(3):1287–1295. doi: 10.1016/j.neuroscience.2010.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Mayes LC. Cocaine addiction in mothers: potential effects on maternal care and infant development. Ann N Y Acad Sci. 2010;1187:172–183. doi: 10.1111/j.1749-6632.2009.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10(4):303–312. doi: 10.1038/nrn2598. Epub 2009 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kampen JM, Robertson HA. A possible role for dopamine D 3 receptor stimulation in the induction of neurogenesis in the adult rat substantia nigra. Neuroscience. 2005;136:381–386. doi: 10.1016/j.neuroscience.2005.07.054. [DOI] [PubMed] [Google Scholar]
- Watson CS, Alyea RA, Cunningham KA, Jeng YJ. Estrogens of multiple classes and their role in mental health disease mechanisms. Int J Womens Health. 2010;9(2):153–166. doi: 10.2147/ijwh.s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;12:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Goswami S, Abdali SA, Frankfurt M, Friedman E. Estrogen-modulated frontal cortical CaMKII activity and behavioral supersensitization induced by prolonged cocaine treatment in female rats. Psychopharmacology (Berl) 2007;191:323–331. doi: 10.1007/s00213-006-0648-0. [DOI] [PubMed] [Google Scholar]