Abstract
The nociceptive flexion reflex (NFR) is a physiological tool to study spinal nociception. However, NFR assessment can take several minutes and expose participants to repeated suprathreshold stimulations. These 4 studies assessed the reliability and validity of a brief method to assess NFR threshold that uses a single ascending series of stimulations (Peak 1 NFR), by comparing it to a well-validated method that uses 3 ascending/descending staircases of stimulations (Staircase NFR). Correlations between the NFR definitions were high, were on par with test-retest correlations of Staircase NFR, and were not affected by participant sex or chronic pain status. Results also indicated the test-retest reliabilities for the two definitions were similar. Using larger stimulus increments (4-mA) to assess Peak 1 NFR tended to result in higher NFR threshold estimates than using the Staircase NFR definition, whereas smaller stimulus increments (2-mA) tended to result in lower NFR threshold estimates than the Staircase NFR definition. Neither NFR definition was correlated with anxiety, pain catastrophizing, or anxiety sensitivity. In sum, a single ascending series of electrical stimulations results in a reliable and valid estimate of NFR threshold. However, caution may be warranted when comparing NFR thresholds across studies that differ in the ascending stimulus increments.
Perspective
This brief method to assess NFR threshold is reliable and valid; therefore, it should be useful to clinical pain researchers interested in quickly assessing inter- and intra-individual differences in spinal nociceptive processes.
Keywords: Nociceptive flexion reflex, RIII reflex, threshold, measurement, test-retest, electrocutaneous
Introduction
The nociceptive flexion reflex (NFR) is a polysynaptic spinal reflex that does not require input from supraspinal centers.20–21 To elicit the NFR experimentally, electrocutaneous stimuli are applied over the sural nerve at the ankle. If a stimulus is strong enough to drive A-delta fibers, the reflex can be quantified using electromyographic (EMG) recordings from the ipsilateral biceps femoris muscle of the hamstring. The NFR has become a popular physiological tool to study spinal nociception and descending modulation in persons with and without chronic pain,20–21 largely because the reflex threshold is highly correlated with subjective pain2,10,26–27 and is modulated by pharmacological and psychological influences.20
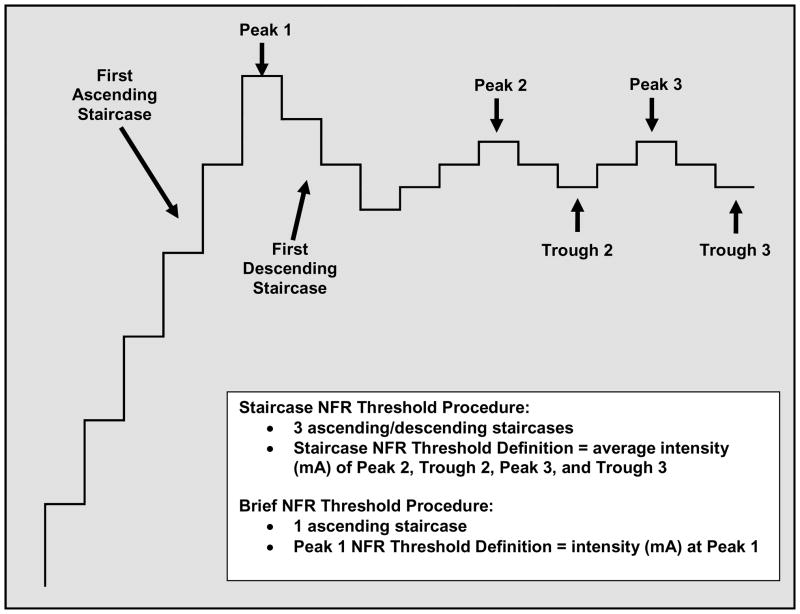
To advance NFR methodology and improve comparisons across studies, attempts have been made to promote a standardized assessment procedure.8,14 One well-validated procedure is based on adaptive testing11 and involves three ascending/descending staircases of electrocutaneous stimuli in which the stimulus intensity is increased (e.g., 4 mA steps) until an NFR is obtained, at which point the stimulus intensity is then decreased in smaller steps (e.g., 2 mA) until the NFR is no longer obtained (Fig. 1). This ascending/descending staircase of stimuli is repeated two more times, usually with smaller stimulus increments (e.g., 1 mA). NFR threshold is defined as the average stimulus intensity of the last two peaks and troughs. Depending on an individual’s NFR threshold, this procedure can take up to 15 minutes and can require participants to tolerate repeated suprathreshold stimulations.
Figure 1.
Illustration of the staircase procedure used to assess the nociceptive flexion reflex (NFR) threshold.
Development of a more streamlined approach to NFR assessment may increase the utility of this paradigm by shortening the testing protocol and reducing the number of electrical stimulations to which participants are exposed, which are changes that could be particularly useful in testing clinical populations. Accordingly, the present study compared the well-validated ascending/descending staircase NFR threshold assessment (Staircase NFR) against a more streamlined definition that identifies NFR threshold from the first reflex exhibited during a single ascending series of stimulations (Peak 1 NFR). The goals were to determine whether the Peak 1 NFR threshold definition: 1) correlates well with Staircase NFR and electrocutaneous pain threshold (external validity), 2) provides similar threshold estimates (no mean differences), 3) shows adequate test-retest reliability, 4) works equally well for men and women (no sex bias), and 5) can be used with chronic pain patients. Moreover, because Staircase NFR is unrelated to anxiety or pain catastrophizing,7,9,15–16 we also verified that the Peak 1 NFR threshold definition was not biased by these variables given that Peak 1 NFR is assessed from the first few noxious stimuli delivered to the participant.
These goals were addressed using existing data from previous studies. In Study 1, three ascending/descending staircases were administered twice on two days 24-hours apart (4 times total). In Study 2, three ascending/descending staircases were administered twice on an initial testing day and then again 1-month later. Finally, Studies 3 and 4 compared the two definitions using a finer resolution of stimulation intensity (i.e., 2 mA versus 4 mA) during the first ascending series, with Study 4 using a sample of individuals with chronic pain. In Studies 2 and 3 electrocutaneous pain threshold was assessed and used as an external correlate to assess validity.
Study 1: Reliability and Validity of a Brief Procedure to Assess NFR Threshold
Materials and Methods
Participants
Participants were 40 healthy young adults (20 men and 20 women) who had their NFR thresholds measured repeatedly as part of an investigation of consistency and individual differences in nociceptive responding.9 All participants were undergraduate students (mean age = 19 years, SD = 1.26) and all received course credit in return for their participation. All participants provided informed consent and all procedures were approved by the Ohio University institutional review board.
Psychological Questionnaires
The 20-item State Anxiety Inventory22 was administered to assess anxiety about NFR testing. Responses were made on a 4-point scale that ranged from 1 (not at all) to 4 (very much so). Items were summed to create a variable ranging from 20 to 80, with higher scores indicating greater state anxiety. Pain catastrophizing was assessed from the Pain Catastrophizing Scale (PCS), a reliable and valid 13-item scale that assesses catastrophic thinking in response to pain.23 Responses were made on a 5-point scale that ranged from 0 (not at all) to 4 (all of the time). For the present study, all items were summed to achieve a PCS total score that ranged from 0 to 52, with higher scores indicating greater pain catastrophizing.
Laboratory testing procedure
The skin at the recording and stimulating electrode sites was cleaned with alcohol and then abraded with Omni Prep electrode paste to achieve an electrode impedance ≤ 10 KΩ. Electromyographic (EMG) activity was recorded using a DelSys, Bagnoli-2 differential EMG amplifier. For the NFR recording, the active electrode was placed over the left biceps femoris muscle 10 cm superior to the popliteal fossa, with a reference electrode attached over the lateral epicondyle of the femur. EMG was recorded and processed using a CED Micro1401 analog-to-digital converter and Spike2 software. To elicit the NFR, a Nicolet bar electrode was attached to the left leg over the retromalleolar pathway of the sural nerve. Electrical stimulation was delivered using a Digitimer DS7A constant-current stimulator.
Participants were asked to refrain from caffeine, nicotine, alcohol, and strenuous exercise for at least 4 h before their arrival at the laboratory, and from analgesic medication for 24 h prior to testing. Throughout all testing sessions, participants were seated in a Hi-Seat rehabilitation chair (model 2000) with a leg rest adjusted to maintain knee flexion at approximately 60 degrees from horizontal. There were two laboratory testing sessions spaced approximately 24 hours apart (±1 hour). At each testing session, baseline state anxiety and pain catastrophizing were measured prior to any electric stimulations, then NFR assessment (3 ascending/descending staircases) was conducted twice, with a 10-minute rest period between each assessment. State anxiety was measured immediately after each NFR assessment (state anxiety A, state anxiety B) on both testing days.
During each NFR assessment, electrical stimulation of the sural nerve was repeated according to a variable interval schedule of 20 s (range 15–25 s) to minimize habituation and predictability. Each stimulation consisted of a train of five 1 ms rectangular pulses with a 3 ms interpulse interval (250 Hz). Using an ascending/descending staircase method (Fig. 1), stimulation intensity was increased in 4 mA increments until an NFR was detected and then was decreased in 2 mA increments until a reflex was no longer detected. NFR occurrence was defined as a mean EMG response in the 90–150 ms post-stimulation interval that exceeded mean EMG activity during the 60 ms pre-stimulation baseline (−65 to −5 ms) interval by at least 1.5 standard deviations (SD). Use of the 90–150 ms interval avoids possible interference from the RII reflex, startle reactions, and voluntary movements.5 Use of the 1.5 SD cut-point leads to 88% specificity and 82% sensitivity to detect an NFR.14 Continuing from this intensity, the procedure was then repeated using 1 mA increments so that the NFR appeared and disappeared two more times.
Data Analysis
Data used for these analyses were collected during four testing occasions: Day 1A, Day 1B, Day 2A, and Day 2B. The dependent variable was the stimulation intensity (mA) that evoked an NFR during the first ascending staircase (Peak 1 NFR), as well as the as the average intensity (mA) of the last two peaks and troughs (Staircase NFR). Thus, there were two ways NFR could be defined: Peak 1 NFR and Staircase NFR. To examine whether there were mean differences in the two NFR definitions, a 4 (Occasion: Day1A, Day1B, Day2A, Day2B) × 2 (NFR definition: Peak 1 NFR, Staircase NFR) repeated measures ANOVA was conducted. Greenhouse-Geisser adjustments were made to correct for violations of sphericity and epsilon corrections (ε) are reported. Bonferroni adjustments were made for multiple mean comparisons (p-values reported have been adjusted). Partial eta-squared (η2) was reported as the effect size.
Pearson’s r (zero-order) correlations were conducted to examine the relationships between Peak 1 NFR and Staircase NFR definitions (external validity), test-retest correlations for the two definitions, and the relationship of psychological variables to each definition. Fisher’s r-to-z analyses were used to compare the magnitude of the external validity coefficients and test-retest correlations across testing occasions and across NFR definitions.
To determine whether the Peak 1 NFR definition worked equally well for men and women, a multiple regression analysis was conducted with the Staircase NFR definition as the criterion and the Peak 1 NFR, participant sex, and a Peak 1 NFR × Sex interaction as predictors. Sex was coded 0 (men) or 1 (women). Peak 1 NFR was centered prior to creating the interaction term to avoid multicollinearity.3 A separate regression analysis was conducted for each testing occasion. For all analyses, significance level was set at p < .05 (2-tailed).
Results
Mean Differences in NFR Definitions
There were no significant effects in the ANOVA model (ps > .43). Thus, the stimulus intensity at Peak 1 NFR (M = 19.75, SD = 8.70) was not significantly different than the NFR threshold defined by Staircase NFR procedure (M = 19.63, SD = 8.94).
Correlations between NFR Definitions (External Validity)
The correlations between the Peak 1 NFR definition and the Staircase NFR definition were high across all testing occasions (Day 1A, r = 0.89; Day 1B, r = 0.86; Day 2A, r = 0.83; Day 2B, r = 0.86; all ps < .01). Results of Fisher’s r-to-z transformations indicated the magnitude of these correlations did not differ between any of the testing occasions (zs < 1.10, ps > .30).
Does the Peak 1 NFR Definition Work Equally Well for Men and Women (No Sex Bias)?
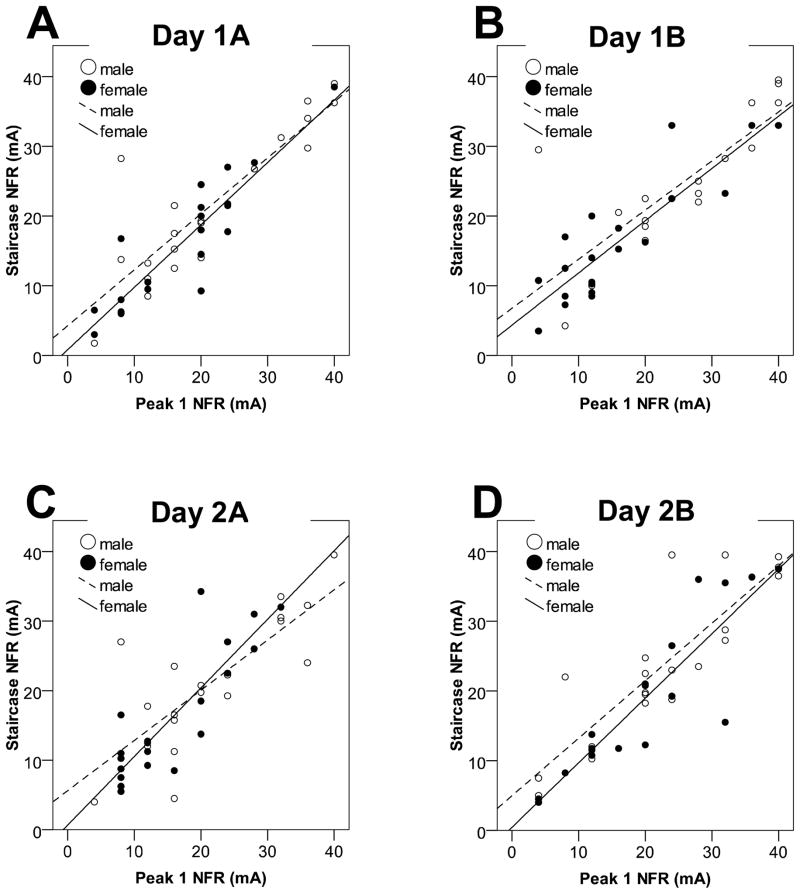
Figure 2 depicts the relationship between the Peak 1 NFR definition and the Staircase NFR definition for men and women across the four testing occasions. The Peak 1 NFR × Sex interaction was non-significant for Day 1A (B = 0.10, p = .51), Day 1B (B = 0.05, p = .76), Day 2A (B = 0.26, p = .18), and Day 2B (B = 0.10, p = .52), indicating that the Peak 1 NFR definition worked equally well for men and women.
Figure 2.
Scatterplots from Study 1 depicting the correlation between the two NFR definitions (Peak 1 NFR vs. Staircase NFR) for men (open circles, dashed line) and women (filled circles, solid line). The top figures depict the correlations for data collected during the Day 1 at Assessment A (Day 1A, Fig 2A) and Assessment B (Day 1B, Fig 2B). The bottom graphs depict the correlation for data collected on Day 2 at Assessment A (Day 2A, Fig 2C) and Assessment B (Day 2B, Fig 2D).
Test-Retest Correlations for NFR Definitions Within and Across Days
The observed test-retest correlations within each day were r = 0.73 (Day 1) and r = 0.51 (Day 2) for Peak 1 NFR, and r = 0.83 (Day 1) and r =0.79 (Day 2) for Staircase NFR. Fisher’s r-to-z analyses indicated that these within-day coefficients did not differ on Day 1 (z = 1.11, p = .26) but were significantly different on Day 2 (z = 2.19, p = .03).
Across-days, the observed correlations were r = .54 (Assessment A) and r = .54 (Assessment B) for Peak 1 NFR, and were r = .56 (Assessment A) and r = .72 (Assessment B) for the Staircase NFR. Fisher’s r-to-z analyses indicated that these across-day coefficients were not significantly different for the two definitions (zs < 1.31, ps < .19).
Correlation with Psychological Variables
Means, standard deviations, and correlations are presented in Table 1. Neither NFR definition was significantly related to state anxiety or pain catastrophizing.
Table 1.
Correlations between psychological variables and NFR definitions (Peak 1 NFR, Staircase NFR) in Study 1.
| Baseline State Anxiety | Post-NFR State Anxiety A | Post-NFR State Anxiety B | Pain Catastrophizing | |
|---|---|---|---|---|
| Day 1 (Mean ± SD) | 32.4 ± 8.3 | 39.0 ± 9.5 | 35.9 ± 9.4 | 16.0 ± 8.0 |
| Peak 1 NFR A | −.01 | .03 | .02 | .01 |
| Staircase NFR A | .03 | .09 | .09 | .02 |
| Peak 1 NFR B | .05 | .02 | .14 | .04 |
| Staircase NFR B | .13 | .06 | .17 | .03 |
| Day 2 (Mean ± SD) | 32.8 ± 8.4 | 36.6 ± 10.3 | 34.8 ± 8.8 | 13.2 ± 8.4 |
| Peak 1 NFR A | −.01 | .03 | .02 | .01 |
| Staircase NFR A | .03 | .09 | .09 | .02 |
| Peak 1 NFR B | .05 | .02 | .14 | .04 |
| Staircase NFR B | .13 | .06 | .17 | .03 |
Note: A and B refer to within-day testing sessions. All correlations are non-significant at p<.05
Study 2: Replication in a Larger Sample with a Longer Test-Retest Delay
Materials and Methods
Participants
Data were obtained from a sample of 122 young adults who completed a larger study examining opiate blockade on nociceptive responding.6 Those who indicated a history of major medical problems or routine use of medication (other than birth control) were excluded. Health status was confirmed by physician examination. Participants were mostly men (n=71, 58%) with a mean age 20 years (SD=1.77). Participants in the larger study were tested on two separate days spaced at least 48 hours apart after consuming either a placebo pill or 50 mg of naltrexone (sessions 1 and 2). Participants were tested again approximately 1 month later without taking placebo or naltrexone (session 3). For the purpose of the present study we only examined data collected on the placebo day (from here on referred to as Day 1) and the 1-month follow-up. Participants received compensation of $20 per hour of testing. All participants provided informed consent and all procedures were approved by the Ohio University and University of Minnesota institutional review boards.
Psychological Questionnaires
No questionnaires were administered in this study.
Laboratory testing procedure
Testing procedures were similar to that used in Study 1, except that on the placebo and naltrexone days, the NFR threshold assessment was repeated three times with a 5 min rest period in between each assessment. During the second NFR assessment, participants played a video game (Nintendo Tetris™) throughout testing; therefore, data from the second assessment were not used for the present study. Immediately following the last NFR threshold assessment, electrocutaneous pain threshold was assessed. To do so, stimulations were applied to the sural nerve using similar parameters as that used during NFR threshold. Immediately following each stimulus, participants made pain ratings on a scale with anchors at 0 (no sensation), 1 (sensory threshold), 25 (uncomfortable), 50 (painful), 75 (very painful), and 100 (maximum tolerable). Stimulations began at 0 mA and increased in 2 mA steps until the participant made a rating of 50 or greater. That stimulus intensity was defined as pain threshold (in mA). One-month later the same procedures were used except that the NFR threshold was assessed only once and electrocutaneous pain threshold was not assessed.
Data Analysis
Analyses were the same as Study 1, except the ANOVA model had to be modified to account for one fewer testing occasion. Thus, a 3 (Occasion: Day1A, Day1B, 1-month) × 2 (NFR definition: Peak 1 NFR, Staircase NFR) repeated measures ANOVA was conducted. Further, zero-order correlations were conducted to examine the relationships between electrocutaneous pain threshold and the two NFR definitions (external validity). Fisher’s r-to-z analyses were used to statistically compare the magnitude of these correlations. Regression analyses were conducted to determine whether the relationship between Peak 1 NFR and pain threshold was similar for men and women (no sex bias).
Results
Mean Differences in NFR Definitions
The two NFR definitions resulted in different stimulus intensities, F(1, 121) = 25.85, p< .001, η2 = .18, with the mean stimulus intensity of Peak 1 NFR (M = 14.42 mA, SD = 6.78) being higher than the Staircase NFR (M = 13.38 mA, SD = 6.80). This may have resulted from the stimulation intensity being increased in 4 mA steps in the first ascending series of stimulation versus 2 mA steps in subsequent ascending and descending series (see Studies 3 and 4 below). All other effects in the model were non-significant (ps >.29).
Correlations between NFR Definitions (External Validity)
The correlations between the stimulus intensity at Peak 1 NFR and the staircase NFR definition were high across all testing occasions (Day 1A, r = 0.94; Day 1B, r = 0.88; 1-month, r = 0.87; all ps < .01). Results of Fisher’s r-to-z transformations indicated the magnitude of these correlations did not differ between Day 1B and 1-month (z = 0.32, p = .74), but the magnitude of the correlation did differ between Day 1A and both Day 1B (z = 2.79, p = .005) and 1-month (z = 3.12, p = .002). Thus, the correlation between the two NFR definitions was strongest on the first testing occasion.
Correlations between Pain Threshold and NFR Definitions (External Validity)
The correlations between electrocutaneous pain threshold and the two NFR definitions were all significant (Table 2). Not surprisingly, the magnitudes of the correlations were stronger when NFR threshold was measured closer in time to pain threshold (i.e., Peak 1 NFR B, Staircase NFR B). Importantly, the magnitude of the correlations did not significantly differ at any time point (Day 1A: z = .43, p = .67; Day 1B: z = .32, p = .74; 1-month: z = .008, p = .99). Therefore, both NFR definitions showed similar external validity with pain threshold.
Table 2.
Correlations between electrocutaneous pain threshold measured on Day 1 and NFR definitions (Peak 1 NFR, Staircase NFR) in Study 2.
| Day 1 Electrocutaneous Pain Threshold (mA) | |
|---|---|
| Day 1 | |
| Peak 1 NFR A | .27 |
| Staircase NFR A | .32 |
| Peak 1 NFR B | .44 |
| Staircase NFR B | .47 |
| 1-month | |
| Peak 1 NFR | .28 |
| Staircase NFR | .28 |
Note: A and B refer to within-day testing sessions. All correlations are significant at p < .05
Does the Peak 1 NFR Definition Work Equally Well for Men and Women (No Sex Bias)?
Figure 3 depicts the relationships between the Peak 1 NFR definition and the staircase NFR definition for men and women across the three testing occasions. The Peak 1 NFR × Sex interaction was non-significant for Day 1A (B =.12, p = .053), Day 1B (B = −.01, p = .91), and 1-month (B = −.11, p = .22), indicating that the Peak 1 NFR definition works equally well for men and women; however, the interaction approached significance on the first testing occasion.
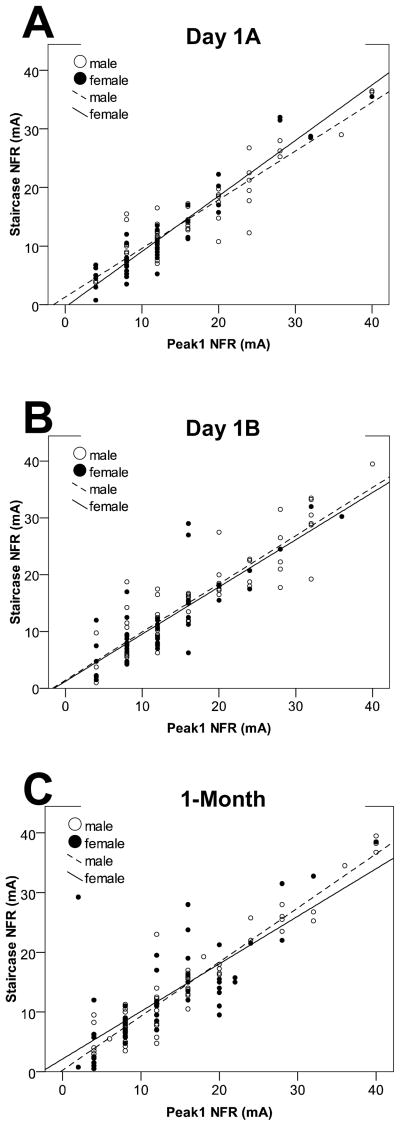
Figure 3.
Scatterplots from Study 2 depicting the correlation between the two NFR definitions (Peak 1 NFR vs. Staircase NFR) for men (open circles, dashed line) and women (filled circles, solid line). Fig 3A depicts the correlation for data collected during assessment A on the placebo day (Day 1A). Fig 3B depicts the correlation for data collected during assessment B on the placebo day (Day 1B). Fig 3C depicts the correlation for data collected at the 1-month follow-up.
Three multiple regression analyses were conducted to determine whether the strength of the relationship between Peak 1 NFR and pain threshold differed between men and women. For these analyses, pain threshold on Day 1 served as the dependent variable. The Peak 1 NFR × Sex interaction was non-significant for Day 1A (B =.14, p = .52), Day 1B (B = .06, p = .77), and 1-month (B = −.07, p = .72), indicating that the strength of the relationship between the Peak 1 NFR definition and pain threshold is similar for men and women.
Test-Retest Correlations for NFR Definitions Within and Across Days
As would be expected, the within-day correlation for Peak 1 NFR was higher (r = 0.72, p < .01) than the correlations across the 1-month interval (Day 1A, r = 0.38; Day 1B, r = 0.40; ps < .01). Similarly, the within-day correlation for Staircase NFR was also higher (r = 0.80, p < .01) than the correlations across the 1-month interval (Day 1A, r = 0.46; Day 1B, r = 0.50; ps < .01). According to Fisher’s r-to-z transformations, the magnitudes of the test-retest correlations for Peak 1 NFR were not different than the test-retest correlations for the Staircase NFR definition (ps > .14).
Study 3: The Influence of Smaller Stimulus Increments during the First Ascending Staircase
Materials and Methods
Participants
Participants were 136 healthy men (n=59, 43%) and women (n=77, 57%), with an average age of 20 years (SD = 2.23) who participated in a study of pain processing in exchange for research credit for their psychology course or a $25 gift card.25 Participants were excluded for the following self-reported conditions: neurological, cardiovascular, or circulatory problems; chronic pain; recent psychological trauma; use of over-the-counter pain medication within 24 hours, or prescription pain medication within 2 weeks of participation; use of antidepressant, anxiolytic, or high blood pressure medications; having a body mass index of 35 or above (due to potential difficulties obtaining an NFR in individuals with high adiposity); and being under the age of 18. All participants provided informed consent and all procedures were approved by the University of Tulsa and Ohio University institutional review boards.
Psychological Variables
The 36-item anxiety sensitivity scale-revised (ASI-R)24 was used to assess fear of anxiety-related symptoms, including somatic sensations like pain. Responses were made on a 5-point scale that ranged from 0 (very little) to 4 (very much). Items were summed to create a variable ranging from 0 to 144, with higher scores indicating greater anxiety sensitivity. The ASI-R was administered with the Pain Catastrophizing Scale23 at the beginning of the testing session.
Laboratory testing procedure
The same procedures were used as in the other studies, except for a few minor differences. For 93 of the 136 participants, EMG activity was recorded using a Grass Technologies amplifier (Model 15LT amplifier with AC Modules 15A54); whereas the equipment for the other 43 were the same as Studies 1 & 2. For all participants, stimulus delivery and physiological data acquisition was controlled by a PC, National Instruments A/D board, and LabVIEW software (National Instruments). Using an ascending/descending staircase method, stimulation intensity was increased in 2 mA increments until an NFR was detected and then was decreased in 1 mA increments until a reflex was no longer detected. NFR occurrence was defined as a mean EMG response in the 90–150 ms post-stimulation interval that exceeded mean EMG activity during the 60 ms pre-stimulation baseline (−60 to 0 ms) interval by at least 1.4 standard deviations (SD). Use of the 1.4 SD cut-point increases sensitivity to detect an NFR to 85%, but slightly lowers specificity to 85% (changing the cut-point should not affect conclusions, however, because both Peak 1 NFR and Staircase NFR definitions will use the same cut-point in this study).14 Continuing from this intensity, the procedure was then repeated using 1 mA increments so that the NFR appeared and disappeared two more times.
Electrocutaneous pain threshold was also measured in this study; however, three ascending/descending staircases were used (8–12 s varying inter-stimulus interval). The first series started at 0 mA and increased in 4 mA steps until pain threshold was reached (rating ≥ 50 on a scale with anchors at 0 [no pain], 50 [painful], and 100 [the most intense pain imaginable]). The current was then decreased in 2 mA steps until the participant rated a stimulus as ≤ 40 on the pain rating scale. The second and third ascending-descending staircases continued with 2 mA steps. Pain threshold was defined as the average intensity (mA) of the four stimuli immediately above and immediately below a rating of 50 on the last two ascending and descending series. The testing order of NFR threshold and pain threshold was counterbalanced across participants. The maximum stimulus intensity was set at 50 mA. Pain threshold was only analyzed for 93 participants, due to differences in the testing procedure used for the other 43.
Data Analysis
A paired samples t-test was used to examine mean differences in NFR Definitions, otherwise analyses were similar to Studies 1 and 2.
Results
The observed Peak 1 NFR threshold (M = 12.38 mA, SD = 8.56) was significantly lower than the Staircase NFR threshold (M = 13.48, SD = 9.22), although the correlation between the two NFR definitions was very high (r = 0.90, p< .001). As can be seen in Figure 4, and confirmed by a non-significant Peak 1 NFR × Sex interaction (B = 0.11, p = .18), the Peak 1 NFR definition works equally well for men and women.
Figure 4.
Scatterplot from Study 3 depicting the correlation between the two NFR definitions (Peak 1 NFR vs. Staircase NFR) for men (open circles, dashed line) and women (filled circles, solid line).
Peak 1 NFR and Staircase NFR were both significantly correlated with electrocutaneous pain threshold (rs = .30 and .30, ps < .01, respectively) and there was no significant difference between the two correlations (z = .05, p = .96). Moreover, the relationship between Peak 1 NFR and electrocutaneous pain threshold was not moderated by sex (Peak 1 NFR × Sex interaction: B = .13, p = .54), suggesting that Peak 1 NFR works equally well for men and women.
The observed mean for anxiety sensitivity was 27.46 (SD =19.56) and for pain catastrophizing was 14.43 (SD = 9.28). Neither NFR definition was significantly correlated with anxiety sensitivity (rs < .07, ps > .52) or pain catastrophizing (rs < .04, ps >.70).
Study 4: Does the Brief Procedure Work Well in a Chronic Pain Sample?
Materials and Methods
Participants
Twenty-five participants (22 females; mean age = 42, SD = 11.26) with chronic pain (fibromyalgia, rheumatoid arthritis) participated in this study.4 Participants were excluded for: <18 years of age, history of cardiac disorders, or recent psychological trauma. Patients were required to have a formal chronic pain diagnosis by a physician to be considered for the study which was verified by medical chart review. Participants were asked to abstain from narcotic analgesics for 2 weeks prior to the experiment and non-narcotic analgesics for 24 hours prior to the experiment. Low dose muscle relaxants and tricyclic antidepressants (15 mg/day) for the treatment of insomnia were permitted. All participants provided informed consent and all procedures were approved by the University of Tulsa institutional review board.
Psychological Questionnaire
The Pain Catastrophizing Scale23 was administered prior to pain testing.
Laboratory testing procedure
The same procedures were used as Study 3, except that NFR occurrence was defined as a 1.0 SD increase in mean EMG response and the last two ascending/descending staircases used 0.5 mA increments. Use of the 1.0 SD cut-point was chosen to reduce the chance that participants with chronic pain would be exposed to unnecessarily high stimulus intensities (given that they are likely to be hyperalgesic). The 1.0 SD cut-point increases sensitivity to detect an NFR to 93%, but decreases specificity to 75%.14 As mentioned earlier, the cut-point change should not affect the comparison of Peak 1 NFR and Staircase NFR definitions because both will be determined using the same 1 SD cut-point in this study.
Data Analysis
Analyses were the same as Study 3, except the multiple regression analysis to examine sex bias was not conducted due to a predominance of female participants.
Results
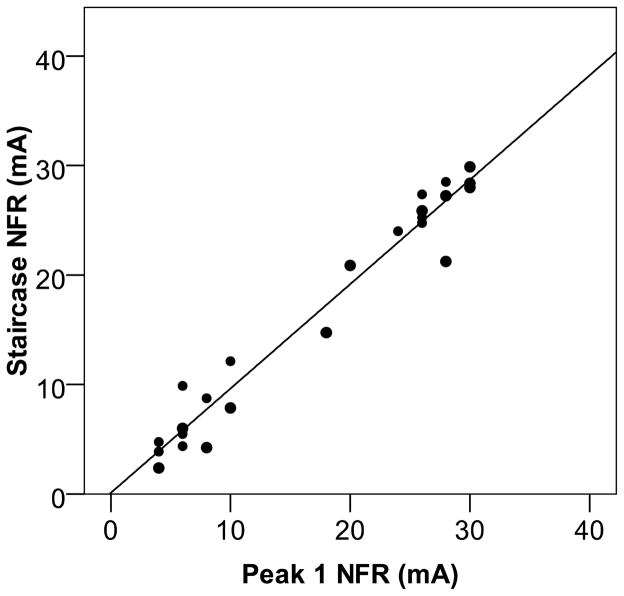
There was no significant difference between mean NFR threshold as defined by the Peak 1 NFR (M = 17.68 mA, SD = 10.53) and the Staircase NFR (M =16.96 mA, SD = 10.24), and as can be seen in Figure 5 the correlation between these NFR thresholds was very high (r = 0.98, p < .001). The observed mean for pain catastrophizing was 15.47 (SD = 10.29) and neither NFR definition was associated with individual differences in pain catastrophizing (rs = −.04 and −.12, ps > .60).
Figure 5.
Scatterplot from Study 4 depicting the correlation between the two NFR definitions (Peak 1 NFR vs. Staircase NFR) in a sample of chronic pain patients.
Discussion
These four studies examined the validity of a brief method to assess the nociceptive flexion reflex (NFR) threshold. Specifically, we examined whether NFR threshold defined as the stimulus intensity that elicits a reflex on a single ascending series of electrocutaneous stimuli (Peak 1 NFR) sufficiently approximated the well-validated method to assess NFR threshold from 3 ascending/descending staircases (Staircase NFR). Results indicated a high correlation between the two definitions (high external validity), with the mean correlation across all studies being r = 0.89 (range = 0.83 to 0.98). Importantly, the correlation between the two definitions is larger in magnitude than the average within-day test-retest correlation for the Staircase NFR definition (mean r = 0.81, range = 0.79 to 0.83). This is likely due to the fact that the Peak 1 NFR and the Staircase NFR definitions were measured closer in time (from the same ascending/descending staircase) than the assessments used to generate the within-day test-retest correlations for Staircase NFR (two different ascending/descending staircases). The ability of the Peak 1 NFR in estimating Staircase NFR was also not affected by participant sex (Studies 1–3) or chronic pain status (Study 4); thus, the brief procedure appears to work well for different populations.
Results from Studies 2 and 3 also suggested that both NFR definitions correlated with electrocutaneous pain threshold and that the magnitude of these correlations did not significantly differ across definitions. Additionally, the associations between Peak 1 NFR and pain threshold were not influenced by participant sex. Thus, when taken together, these external validity data (i.e., correlations with Staircase NFR and pain threshold) support the validity of the Peak 1 NFR definition.
Although the linear relationship between the two NFR assessment approaches was strong across all four studies, comparison of absolute threshold levels (mean differences) indicated that the stimulation intensity increment used during testing may influence the threshold estimate. Of the four studies examined, one found that Peak NFR 1 overestimated NFR threshold relative to Staircase NFR, two found no differences, and one found that Peak NFR 1 underestimated NFR threshold relative to Staircase NFR. From a methodological perspective, it was predictable that the Peak 1 NFR would overestimate the Staircase NFR when 4 mA increments were used in the first ascending series, as the actual threshold could be any intensity that exceeded the preceding stimulation intensity by 4 mA or less.
However, underestimation of Staircase NFR by Peak 1 NFR cannot be easily explained given that the increments used in the first ascending series (2 mA) were not smaller than those used in the subsequent staircase (1 mA). Thus, the observation of underestimation with 2 mA increments in Study 3 may indicate a tendency for the reflex to occur at lower stimulation intensities during the first ascending series. This could pose a problem when comparing Peak 1 NFR threshold values across studies that use different stimulus increments on the first ascending series, but it should not pose a problem when comparing studies using the same stimulus increments due to the high correlation between the Peak 1 NFR and Staircase NFR definitions. Indeed, the correlations between Peak 1 NFR and Staircase NFR were similar, regardless of the increment used. The 4 mA increment produced a mean correlation of r = 0.92 (considering only the first assessment from each study) and the 2 mA increment produced a mean correlation of r = 0.94. When taken together, it appears that each stimulus increment has its pros and cons. Using the 4 mA increment will reach NFR threshold more quickly (fewer steps) and expose participants to fewer stimuli, but the 2 mA increments may be easier for participants to tolerate (steps in intensity are smaller) and should have greater precision for participants with relatively low NFR thresholds (e.g., <8 mA). Given this last reason, we recommend using the 2 mA increment until future studies compare the two increments in the same sample of participants.
Although participants may experience greater anxiety during initial exposure to NFR assessment protocols, it is worth noting that differences in absolute threshold levels are not likely to stem from anxiety, pain catastrophizing, or anxiety sensitivity, because none of these variables were significantly correlated with Peak 1 NFR or Staircase NFR. Moreover, a lack of correlation with these variables suggests that Peak 1 NFR is not biased by psychological factors relative to Staircase NFR.
Finally, both Staircase NFR and Peak 1 NFR definitions appear to demonstrate adequate test-retest reliability. Within-day test-retest coefficients for Staircase NFR averaged r = 0.81 and across-day test-retest coefficients averaged r = 0.56. The absolute magnitude of the test-retest coefficients for Peak 1 NFR tended to be smaller (within = 0.65, across = 0.47); however, the differences were not statistically significant. One exception was the statistically significant difference in the within-day coefficients on Day 2 in Study 1 (rs = 0.51 vs. 0.79). Unfortunately, we did not assess NFR threshold twice on Day 2 (1-month) in Study 2 to determine whether this finding is reliable. Indeed, this may have been a spurious result given the smaller sample size in Study 1. Because NFR threshold may change with repeated testing over multiple days due to habituation/sensitization,1,13 there is a remote possibility that differential test-retest correlations observed for Peak 1 NFR and Staircase NFR definitions may relate to differential susceptibility to habituation. Although future research is needed to address this issue, results from the present study indicate the Peak 1 NFR definition provides adequate test-retest reliability, especially for an outcome that can be modulated up or down over a short period of time (thus having lower stability than trait-like measures).17–19
Limitations
Whereas the relative consistency observed across four different studies is reassuring, it should be noted that the majority of the data was obtained from young, healthy college students; hence there is a need to replicate these findings in larger and more diverse samples. Further, the clinical sample that was used was relatively small; therefore, caution is warranted in generalizing the current findings to chronic pain populations. A final limitation is that we were only able to compare Peak 1 NFR against the standardized Staircase NFR method used in our laboratories, and it is possible that different results would be obtained if the Peak NFR 1 approach was compared to a different definition of NFR threshold. For example, one could compare the single ascending series approach to the average estimate obtained from multiple ascending series to determine whether the latter yielded more reliable NFR threshold estimates over time.
Conclusions
In sum, the present results indicate a single ascending series of electrical stimulations (Peak 1 NFR) can be used to obtain a valid estimate of NFR threshold. Confidence in this conclusion is bolstered by the fact that: 1) results were consistent across studies, including two with relatively large samples (increasing statistical power and generalizability), 2) repeated NFR testing within- and across-days (up to 1-month) was conducted, 3) findings were similar for men, women, and persons with chronic pain, and 4) neither definition was biased by psychological factors (e.g., pain catastrophizing). Thus, we recommend using the Peak 1 NFR method with 2 mA increments when a shorter testing protocol is desired (e.g., assessment time is limited, multiple assessments are needed) or when there is concern that participants may not be able to tolerate repeated exposure to suprathreshold stimuli (e.g., chronic pain patients).
Acknowledgments
This work was partially supported by grants from the National Institutes of Health awarded to Christopher France (NHLBI R01 HL64794) and Jamie Rhudy (NIAMS R03 AR054571).
Footnotes
Disclosures
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jamie L. Rhudy, Department of Psychology, The University of Tulsa, USA
Christopher R. France, Department of Psychology, Ohio University, USA
References
- 1.Bromm B, Scharein E. Response plasticity of pain evoked reactions in man. Physiology & Behavior. 1982;28:109–116. doi: 10.1016/0031-9384(82)90111-1. [DOI] [PubMed] [Google Scholar]
- 2.Chan CW, Dallaire M. Subjective pain sensation is linearly correlated with the flexion reflex in man. Brain Res. 1989;479:145–150. doi: 10.1016/0006-8993(89)91344-9. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3. Mahway, New Jersey: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 4.DelVentura JL, Terry EL, Bartley EJ, Vincent AL, Olech E, Rhudy JL. Emotional modulation of pain and nociception in fibromyalgia and rheumatoid arthritis: Preliminary findings. Paper presented at: Society for Neuroscience; San Diego, CA. 2010. [Google Scholar]
- 5.Dowman R. Possible startle response contamination of the spinal nociceptive withdrawal reflex. Pain. 1992;49:187–197. doi: 10.1016/0304-3959(92)90142-X. [DOI] [PubMed] [Google Scholar]
- 6.France CR, al’Absi M, Ring C, France JL, Brose J, Spaeth D, Harju A, Nordehn G, Wittmers LE. Assessment of opiate modulation of pain and nociceptive responding in young adults with a parental history of hypertension. Biol Psychol. 2005;70:168–174. doi: 10.1016/j.biopsycho.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 7.France CR, France JL, al’Absi M, Ring C, McIntyre D. Catastrophizing is related to pain ratings, but not nociceptive flexion reflex threshold. Pain. 2002;99:459–463. doi: 10.1016/s0304-3959(02)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.France CR, Rhudy JL, McGlone S. Using normalized EMG to define the Nociceptive Flexion Reflex (NFR) threshold: Further evaluation of standardized scoring criteria. Pain. 2009;145:211–218. doi: 10.1016/j.pain.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 9.French DJ, France CR, France JL, Arnott LF. The influence of acute anxiety on assessment of nociceptive flexion reflex thresholds in healthy young adults. Pain. 2005;114:358–363. doi: 10.1016/j.pain.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Guieu R, Blin P, Pouget J, Serratrice G. High level sportsmen and nociceptive flexion reflex of the lower limb. Canadian Journal of Neuroscience. 1992;19:69–71. [Google Scholar]
- 11.Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467–477. [PubMed] [Google Scholar]
- 12.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 13.Rhudy JL, Bartley EJ, Williams AE. Habituation, sensitization, and emotional valence modulation of pain responses. Pain. 2010;148:320–327. doi: 10.1016/j.pain.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: A comparison of different scoring criteria. Pain. 2007;128:244–253. doi: 10.1016/j.pain.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhudy JL, France CR, Bartley EJ, Williams AE, McCabe KM, Russell JL. Does pain catastrophizing moderate the relationship between spinal nociceptive processes and pain sensitivity? The Journal of Pain. 2009;10:860–869. doi: 10.1016/j.jpain.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Rhudy JL, Maynard LJ, Russell JL. Does in-vivo catastrophizing engage descending modulation of spinal nociception? J Pain. 2007;8:325–333. doi: 10.1016/j.jpain.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Rhudy JL, Williams AE, McCabe K, Nguyen MA, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiol. 2005;42:579–587. doi: 10.1111/j.1469-8986.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 18.Rhudy JL, Williams AE, McCabe KM, Russell JL, Maynard LJ. Emotional control of nociceptive reactions (ECON): Do affective valence and arousal play a role? Pain. 2008;136:250–261. doi: 10.1016/j.pain.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Sandrini G, Milanov I, Malaguti S, Nigrelli MP, Moglia A, Nappi G. Effects of hypnosis on diffuse noxious inhibitory controls. Physiol Behav. 2000;69:295–300. doi: 10.1016/s0031-9384(00)00210-9. [DOI] [PubMed] [Google Scholar]
- 20.Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. doi: 10.1016/j.pneurobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans - review article. Pain. 2002;96:3–8. doi: 10.1016/s0304-3959(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD. State-Trait Anxiety Inventory. Palo-Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 23.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 24.Taylor S, Cox BJ. An expanded Anxiety Sensitivity Index: Evidence for a hierarchic structure in a clinical sample. J Anxiety Disord. 1998;12:463–483. doi: 10.1016/s0887-6185(98)00028-0. [DOI] [PubMed] [Google Scholar]
- 25.Terry EL, Bartley EJ, DelVentura JL, Kerr KL, Vincent AL, France CR, Rhudy JL. Assessing hyperexcitability of spinal nociceptive processes in humans: Refining methods for temporal summation of the nociceptive flexion reflex. Society for Neuroscience; San Diego, CA: 2010. [Google Scholar]
- 26.Willer JC. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain. 1977;3:69–80. doi: 10.1016/0304-3959(77)90036-7. [DOI] [PubMed] [Google Scholar]
- 27.Willer JC, Boureau F, Albe-Fessard D. Supraspinal influences on nociceptive flexion reflex and pain sensation in man. Brain Res. 1979;179:61–68. doi: 10.1016/0006-8993(79)90489-x. [DOI] [PubMed] [Google Scholar]