Abstract
Central nervous system (CNS) physiology requires special chemical, metabolic and cellular privileges for normal function, and blood brain barrier (BBB) structures are the anatomic and physiologic constructs that arbitrate communication between the brain and body. In the vertebrate BBB two primary cell types create CNS exclusion biology, a polarized vascular endothelium (VE) and a tightly associated single layer of astrocytic glia (AG). Examples of direct action by the BBB in CNS disease are constantly expanding, including key pathophysiologic roles in multiple sclerosis, stroke and cancer. In addition, its role as a pharmacologic treatment obstacle to the brain is long standing, thus molecular model systems that can parse BBB functions and understand the complex integration of sophisticated cellular anatomy and highly polarized chemical protection physiology are desperately needed. Compound barrier structures that use two primary cell types (i.e. functional bicellularity) are common to other humoral/CNS barrier structures. For example, invertebrates use two cell layers of glia, perineurial and subperineurial, to control chemical access to the brain, and analogous glial layers, fenestrated and pseudocartridge, to maintain the blood-eye barrier (BEB). In this article we summarize our current understanding of brain-barrier glial anatomy in Drosophila, demonstrate the power of live imaging as a screening methodology for identifying physiologic characteristics of BBB glia, and compare the physiologies of Drosophila barrier layers to the VE/AG interface of vertebrates. We conclude that the many unique BBB physiologies are conserved across phyla and suggest new methods for modeling CNS physiology and disease.
Keywords: Blood-brain barrier, blood-eye barrier, surface glia, perineurial glia, subperineurial glia, chemical protection, neuroinflammation, transporters
INTRODUCTION
The brain must maintain instantaneous function for perceptual integration and response and long standing cellular interactions to sustain memory for intelligent choice decisions. While neurons are the immediate temporal drivers of CNS outputs, glia provide essential functions that can directly modulate behavior, neurotransmitter metabolism, and CNS immuno-competency (Dong and Benveniste 2001; Halassa and Haydon 2010). These complex interactions are energetically costly, requiring many genetic inputs and precise maintenance of cellular architecture to perform. As a consequence, CNS physiology requires numerous specializations and exaggerations of normal cellular physiology making it exceptionally sensitive to energy balance (e.g. hypoxia and nutrient deprivation), environmental insults (e.g. toxic substances and drugs), and clearance of infectious agents and cellular debris. These physiologies are a direct consequence of the relationship between the CNS and the humoral space; therefore, to guard against and connect the brain to the external milieu, evolution has established an elaborate barrier protection system known as the blood brain barrier (BBB) or blood brain interface (BBI). Indeed, special chemical isolation from the body was one of the first observations in early CNS studies as water soluble dyes would not penetrate viable brain tissue (Ehrlich 1885). Many new functions have been ascribed to the BBB, and it now plays a central role in immune function and a host of CNS diseases including neurodegeneration, stroke, and cancer (Zlokovic 2008). Furthermore, new studies on the BBB focus on its response to pathogenesis and in some cases implicate it as the primary culprit of disease (i.e. MS) (Alvarez et al. 2010). These findings make the BBB of increasing interest as a site of diagnosis and treatment. In this article, we will discuss related properties of vertebrate and invertebrate barrier glia and consider how methods unique to invertebrate systems can elucidate the varied roles of glia in vertebrate CNS barrier structures.
Barrier Structures and Functions
Vertebrate Glia Participate in CNS Barrier Physiology
Maintenance of CNS function requires exceptional humoral connection to the body, as the highest blood flow per mass of any tissue is reserved for the brain (Zlokovic 2008). To utilize this substantial investment in cardiac output, the BBB uses a very large surface area (~20m2) (Begley and Brightman 2003) and close parenchymal approximation to capillaries (<5–10µm). Blood flow itself can be regulated by the barrier, which is functionally integrated with surrounding brain tissue in what is called the neurovascular unit (Anderson and Nedergaard 2003; McCarty 2005; Zlokovic 2008). The complete neurovascular unit, moving from humoral space to CNS interstitial space, is comprised of capillary vascular endothelium (VE), a basement membrane, and astrocytic glia (AG), and also includes CNS specific pericytes and nearby neurons (Fig. 1A) (Ballabh et al. 2004). Many studies suggest that the AG has inductive properties on the VE in development and can regulate VE responses to metabolic need (Abbott et al. 2006). Because of the tremendous importance of the BBB in health and disease, substantial efforts are underway to develop in vitro model systems to study chemoresponsive physiologies and identify regulatory controls.
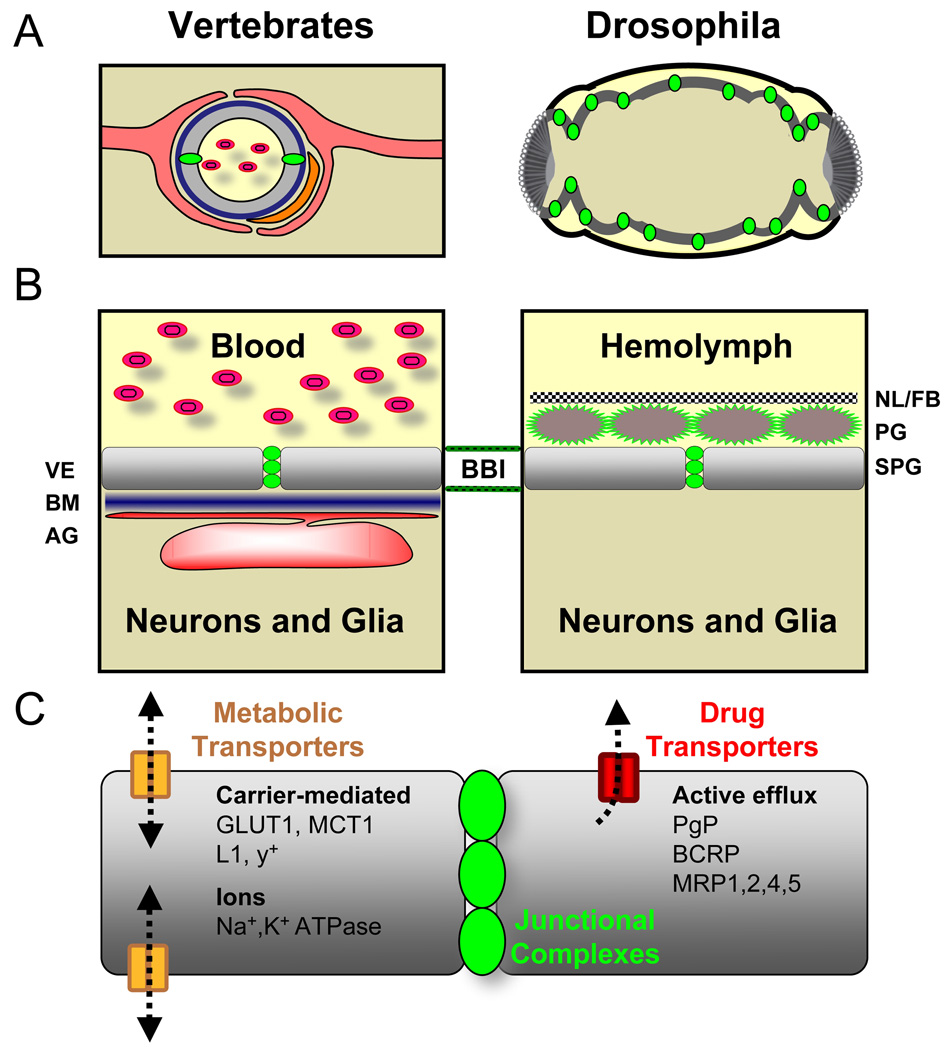
Figure 1. Blood-brain barrier anatomy in vertebrates and flies.
(A) The vertebrate barrier (left) consists of the vascular endothelium (VE, grey), which possess tight junctions (green). The basement membrane (BM, blue) immediately surrounds the endothelium, which itself is surrounded the endfeet processes of astrocytic glia (AG, red). Pericytes (orange) are modulatory cells interspersed between the AG and VE. This compound barrier structure isolates the central nervous system (beige) from the blood (yellow, containing red blood cells). The Drosophila barrier (right) is oriented differently; the brain (beige) is surrounded by hemolymph (yellow), and a single epithelial layer, the subperineurial glia (SPG, grey), forms the passive diffusion barrier by utilizing chemically tight septate junction complexes (green).
(B) On the left is a schematic representation of humoral to CNS (apical to basal) components of the vertebrate BBB. In order to enter the CNS, a substance must pass the VE (colors are the same as in A), the BM, and a closely opposed AG layer to reach neurons. On the right is a proposed barrier layer model for the Drosophila BBB. In this case a substance must pass the neural lamella and fat body layer (NL/FB, checkered line), perineurial glia (PG, maroon), and SPG to reach the CNS.
(C) Numerous essential transport systems are active at the chemical protection interfaces of the VE. Shown here are vertebrate VE transporters, which include metabolic transporters that operate in both directions via carrier-mediated transport, and drug transporters that mainly efflux xenobiotics away from the CNS.
Compound Barrier Structures
The AG form a continuous circumferential cell layer around and in close association with the VE (Fig. 1A). Together these cells isolate the vascular and CNS interstitial spaces so that any molecule moving from one space to another must contact these cell types. The AG’s direct role in chemical exclusion is unclear as it is less than a micron thick and lacks tight junctions at cell-cell contacts to prevent lateral diffusion (Abbott et al. 2006). Furthermore, genomic data shows a much lower level of xenobiotic transporter and junctional protein expression in the AG compared to VE (Cahoy et al. 2008; Daneman et al. 2010a). It is hypothesized that the AG’s role in chemical protection may be to sense chemical, metabolic and inflammatory stresses, which require coordination to maintain proper physiologic balance for neurons (Zlokovic 2008). While this cellular relationship is dominant in vertebrates, it is not universal in chordates. Cartilaginous fish have tight diffusion barriers in the AG layer while their VE is more diffusion permissive (Abbott 2005). Thus, while compound cellular structures are maintained at humoral barriers, physiologic function may be parsed differently between cell types (see below). Furthermore the question remains: how do we discover BBB physiologies, and how are they generated and regulated?
Chemical Isolation Physiology
The primary driver of chemical separation in vertebrates is the highly polarized single-cell layer VE. At this interface, strong selective pressures have produced the integration of two very different cell biologic mechanisms to prevent free movement of small molecules between the humoral and CNS interstitial compartments (Abbott 2005; Daneman and Barres 2005; Neuwelt et al. 2008; Zlokovic 2008). Exceptionally tight lateral border junctions (Reese and Karnovsky 1967) and a diverse array of apically (i.e. vascular) facing efflux drug transporters, including P-gp, Mrp1, and BCRP, work together to maintain chemical protection (Fig. 1C). The functional importance of these transporters to partition drugs in the brain has been studied using gene-specific genetic null mice (“knock-outs”) (Enokizono et al. 2008; Lorico et al. 1996; Schinkel et al. 1994; Vlaming et al. 2009; Wijnholds et al. 1997). Many fold increases in CNS penetration of specific substrates, including chemotherapeutics, are found in mice possessing ABC transporter null alleles (Schinkel 1998). That ABC transporters can so profoundly affect brain drug concentration by their presence or absence in a single endothelial layer suggests a plausible mechanism for increased drug sensitivity of the CNS in transporter-associated pharmacogenomic studies (Park et al. 2007). Furthermore, these studies promote the idea of specifically targeting xenobiotic transporter function to more readily concentrate drugs in the brain for treating CNS illnesses. Indeed, avoiding Mdr1/P-gp transport is the overwhelmingly dominant paradigm in pharmaceutical science for the identification of chemical leads, i.e. new drug candidates for CNS diseases (Neuwelt et al. 2008).
Surface Glia in Adult Drosophila
The Blood-Brain Barrier
Drosophila has emerged as a model system for the study of BBB physiology because the blood barriers of Drosophila share many similarities with the VE/AG interface. In the context of describing the fly BBB, we will highlight these similarities and show that chemical isolation physiology is also present at the CNS/humoral interface of Drosophila. Central to this discussion are the surface glia at the fly BBI, which include two subtypes, the perineurial glia (PG) and the subperineurial glia (SPG) (Fig. 1B). These cell layers form two seemingly complete layers of ensheathing glial cells that surround the CNS in order to shield neurons from the high concentration of potassium ions in the hemolymph, which are high enough to depolarize neurons and disable the nervous system (Hoyle 1952). Just as the VE/AG interface can be considered a compound barrier structure where physiologic functions are parsed differently between cell types, so can the PG/SPG interface of Drosophila.
PG develop from the dorsal neuroectoderm (Schmidt et al. 1997) post-embryonically where they proliferate and enlarge as the brain surface itself grows (Awasaki et al. 2008). The SPG are generated during embryonic development from the ventrolateral neuroectoderm and subsequently migrate to the surface of the developing CNS (Awasaki et al. 2008; Ito et al. 1995; Schmidt et al. 1997). At the nerve cord surface, the SPG spread out until they form a continuous sheet of cells that completely ensheaths the nerve cord (Carlson et al. 2000; Edwards et al. 1993; Schwabe et al. 2005), thus forming a blood-nerve barrier via the formation of septate junctions (Schwabe et al. 2005; Stork et al. 2008). These observations hint at one essential similarity of BBB structures – that tissues derived from different developmental origins must interact to form the bridge between the humoral space and the brain.
The PG form the outermost surface glial layer surrounded only by the neural lamella, a dense network of extracellular matrix. PG display a flattened stellate morphology with protrusions that do not extend past the perineurial layer (Stork et al. 2008). The PG are understudied, and thus there are few PG-specific genetic markers. Awasaki et al. (2008) found the enhancer trap strain NP6293 to be suitable for labeling PG, although a subset of neurons is also labeled. However, the PG can be marked readily and specifically in vivo by hemolymph injected fluor-labeled dextrans. Of note fluorescently marked dextrans are extremely useful size constraint makers that can differentiate cellular barrier properties (see Fig. 6). Even the smallest size dextrans (3kDa) are prevented from penetrating the brain by the SPG cell layer, but collect in the PG (discussed below) (Mayer et al. 2009). We also observe fluorescent-lectin labeling of the PG layer (data not shown), which suggests that the PG express membrane glycoproteins that can be exploited for targeting purposes (Table 1).
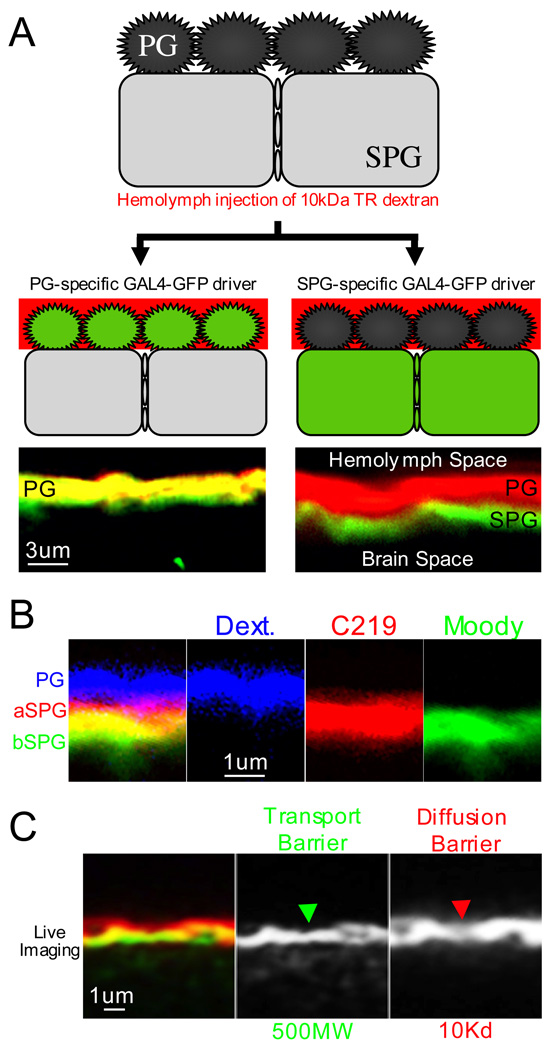
Figure 6. High resolution confocal microscopy of the physiologic barrier.
(A) Following crosses between BBB-specific P-GAL4 lines and UAS-GFP, progeny were injected with 10kDa red dextran. Two main GAL4 expression patterns emerged: 1) on the left, an example of a PG expressing line in which GFP expression overlaps with dextran signal; 2) on the right, an example of an SPG expressing line in which GFP and dextran fluorescence show little signal overlap.
(B) With antibodies to Moody and Mdr65, apical and basal expression of proteins expressed in the SPG can be discerned. In this example, the PG layer is labeled by 3kDa cascade blue dextran, the apical SPG membrane by Mdr65 antibody (C219, red), and the basal SPG membrane by Moody beta antibody (green).
(C) A co-injection of dextran and small molecule fluors and subsequent live confocal microscopy allows simultaneous live visualization of the chemical transport barrier (TB) and the diffusion barrier (DB) in a wild type animal. The TB is demarcated by the accumulation of BODIPY-labeled Prazocin (green), a transportable substrate. The DB is delineated by the accumulation of 10kDa red dextran.
Table 1.
Useful reagents to specifically label the cellular (SPG, PG, fat body) and acellular (neural lamella) layers of Drosophila BBB.
| Physiologic Markers |
Antibody | GAL4 / UAS-GFP | Genetrap | |
|---|---|---|---|---|
| Neural Lamella | Lectin | -- | -- | Trol (G00022) |
| Fat Body | >100kDa dextran (histiocytes) Nile red (triglycerides) | -- | Croquemort-GAL4 (FBst0025041) | -- |
| Perineurial Glia | <100kDa dextran | VMAT B5 | UAS-moesin-GFP1 | -- |
| UAS-tau-GFP2 | ||||
| UAS-actin-GFP1 | ||||
| Subperineurial Glia | -- | C219 (Mdr65)3 | SPG-GAL44 | Nrg (G00305) |
| C494 (unknown)5,6 | Nrx-IV (CA06597) | |||
| Moody7 | Dlg1 (CC01936) |
(Bainton, unpublished),
Abundant homotypic junctional complexes, septate junctions, are the basis of the cell-cell contacts between SPG cells that constitute the insect tight diffusion barrier (Juang and Carlson 1992). This positions the SPG as the principle cellular layer where chemical protection physiology is manifest, and numerous studies show strong conservation in the molecular constituents of tight diffusion barriers (Wu and Beitel 2004). The SPG are basal to the PG and form a thin layer (~1µm) of ensheathing cells 40–80µm in width (see Fig. 5) (Bainton et al. 2005; Schwabe et al. 2005; Stork et al. 2008). For a comprehensive study of molecular markers of surface-associated glia in embryos see (Beckervordersandforth et al. 2008). Numerous SPG-specific molecular markers exist including enhancer trap strains and antibodies for the proteins Moody (Bainton et al. 2005; Schwabe et al. 2005) and Mdr65 (Mayer et al. 2009) (Table 1).
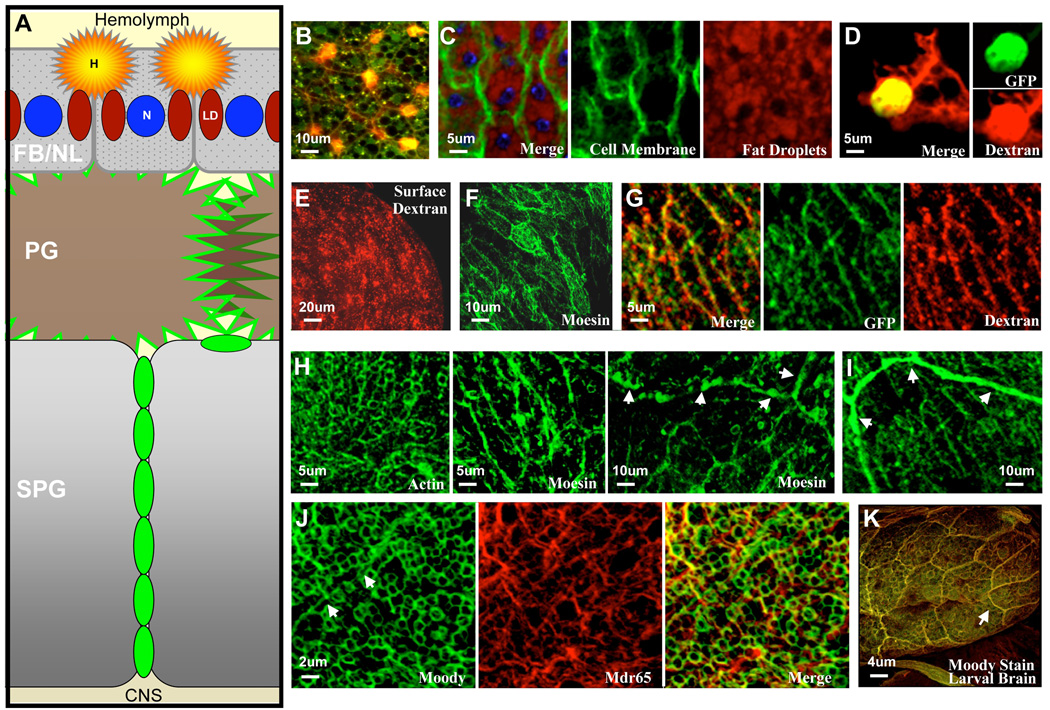
Figure 5. Screen Summaries and Surface Anatomy.
(A) Genes that modify BBB function are found in multiple cellular layers depicted in a cartoon image (not drawn to scale). FB/NL= fat body neural lamella, N=nucleus, LD=lipid droplets, PG=perineurial glia, and SPG=subperineurial glia. Gene expression patterns of the Drosophila humoral interface were assayed in several ways. Brain surface images were obtained by crossing the P-GAL4 to various GFP markers, and adult brains were fixed in situ and then mounted with the posterior medulla shown in the X-Y plane (in our hands the posterior medulla is best preserved for obtaining surface morphology).
(B) In this image the posterior medulla is covered by a fat body and histiocytes best seen by hemolymph co-injection of 3kDa FITC dextran and 2000kDa TMR dextran in a wild type animal. Both dextrans are taken up by histiocytes (yellow) while the 3kDa FITC penetrates the FB to the PG layer.
(C) A fat body specific driver and putative BBB modulator, 11–165 GAL4, is crossed to CD8 GFP (membrane bound GFP). GFP outlines the cell bodies (green), co-stained with Nile Red, a neutral lipid localizing dye (red) and DAPI (blue). Individual green and red channels are shown to the right.
(D) Hemolectin-GAL4 crossed to soluble GFP confirms that dextran-accumulating cells (red) in the fat body are of hemocyte origin (Irving et al. 2005).
(E) Lysine-fixable 10kDa Texas Red dextran accumulating on the surface of PG cells (in the absence of a fat body) marks the brain surface in a uniform grainy pattern.
(F) A PG specific line, 10–72 GAL4, is crossed to Moesin-GFP (a membrane-organizing extension spike protein that is a cross linker between the actin cytoskeleton and the plasma membrane). GFP marks the PG cells in a striated pattern with a uniform periodicity on the brain surface.
(G) A similar striated pattern is shown in Nrg-GFP (green). Interestingly 10kDa dextran accumulates in the same pattern at PG cell borders (green and red merge).
(H) Moody enhancer SPG-GAL4 crossed to different cytoskeletal GFP reporters marks the SPG cells and reveals SPG morphology. These reagents are helpful to recognize the SPG morphology. SPG-GAL4 crossed to actin-GFP (the monomeric subunit of two types of filaments in cells) marks all cells that express Moody in the SPG layer. Note actin-GFP does not mark pleated septate junctions, but maintains a pattern similar to Moody staining (see J). SPG-GAL4 crossed to Moesin-GFP produces two distinct cytoskeletal patterns. Pleated septate junctions are well marked, but appear discontinuous (Moesin, left) and a prominent striated pattern is also seen of similar periodicity to the PG layer (Moesin, right).
(I) Nrg-GFP demonstrates very high contrast signal at the pleated septate junctions in the adult fly brain.
(J) Moody beta antibody marks the basal membrane of the SPG layer (Mayer et al. 2009) demonstrating a prominent circular pattern molding to neuronal cell bodies below. Moody does not distinctly mark homotypic septate junctions in adult animals, but can be found at cell edges upon close inspection (arrows). C219, an Mdr65 transporter antibody, shows the apical membrane cell morphology of the SPG layer. A merged image demonstrates no co-localization of the two antibodies in the SPG layer (also see Fig. 6B).
(K) Co-staining of the larval ventral nerve cord of the two forms of the Moody GPCR with alpha Moody and beta Moody antibody (Green Moody beta, red Moody alpha). The two antibodies show exact co-localization and demonstrate prominent septate junction localization. Similar prominent SJ localization can be found in the adult brain in BBB mutants (data not shown).
The Blood-Eye Barrier
In vertebrates, the blood-retinal barrier restricts transport of molecules between the vitreous/retina and systemic circulation. This is true of the Drosophila BEB; however, the BEB also acts to electrically isolate each cartridge (i.e. the complement of R1–R6 photoreceptors and monopolar L1–L3 neurons associated with the cluster of six adjoining ommatidia that share an identical optical axis) for optimal spatial resolution (Edwards and Meinertzhagen 2010; Shaw 1984). Not surprisingly, the capillary bed of the retinal VE maintains tight junctions and possesses apically facing transporters morphologically similar to the VE of the BBB (Hosoya and Tachikawa 2009). Likewise, in Drosophila, the cell layers at the BEB are morphologically similar to the PG and SPG. Though not explicitly classified as surface glia by Edwards & Meinertzhagen (2010), the fenestrated and pseudocartridge glia in the lamina of the optic lobe share functional commonalities with the PG and SPG. Furthermore, fenestrated and pseudocartridge glia are not in close contact with neuronal cell bodies (sensu cortex glia), nor do they ensheath axonal bundles (sensu neuropile glia). Indeed, the fenestrated and pseudocartridge glia maintain contact with axonal projections, which makes them more similar to the AG of the vertebrate BBB as this is a characteristic lacking in the PG and SPG (Edwards and Meinertzhagen 2010).
Fenestrated glial cells are situated immediately below the basement membrane dividing the retina and lamina. In Musca, they form a monolayer of cells and extend processes that wrap both photoreceptors and tracheae and also penetrate into the retina (Saint Marie and Carlson 1983b). Fenestrated glia can be marked by antibodies and RNA probes to a glial variant of the vesicular monoamine transporter (VMAT-B) (Romero-Calderon et al. 2008). The fenestrated glia are thought to be functionally analogous to the PG given, 1) their location as the externalmost cell layer of the lamina, and 2) that VMAT-B also labels the BBB specifically within the PG layer (Bainton and Krantz, unpublished).
Septate junctions are present in the fenestrated, pseudocartridge, and satellite glial layers of the lamina, and this dense network of cells likely acts as a series of hemolymph barriers (Edwards and Meinertzhagen 2010). However, the pseudocartridge glia (lying basal to the fenestrated glia) form the most septate junctions, which are predominantly homotypic (Saint Marie and Carlson 1983a). Thus, the pseudocartridge glia are likely functionally analogous to the SPG and therefore represent the critical components of the BEB (Edwards and Meinertzhagen 2010). Further evidence of this functional connection is that molecular markers for the SPG-specific protein Moody seem to localize to the pseudocartridge glia (Bainton et al. 2005).
Molecular Components Establish the Physiology of BBB and BEB in Drosophila
Septate Junction Formation
Molecularly and physiologically similar to the tight junctions of the VE, the septate junctions formed between surface glia in Drosophila are central to barrier function (see Edwards & Meinertzhagen (2010) for a thorough review of both septate junction components and the proteins known to regulate septate junction formation). We will focus on those genes that affect septate junction development and lead to decreased barrier function (as measured by uptake of labeled dextrans). NeurexinIV and Coracle bind to each other (Ward et al. 2001) and are believed to construct the electron-dense septae that give septate junctions their name; thus, it is no surprise that neurexinIV and coracle loss of function (LOF) mutants produce a defective BBB in late stage Drosophila embryos (Baumgartner et al. 1996; Schwabe et al. 2005; Stork et al. 2008). In fact, neurexinIV and coracle mutants allow chemical fluor penetration into the CNS of mature embryos on levels comparable to gcm LOF mutants, which lack glial cells altogether (Hosoya et al. 1995; Stork et al. 2008). neurexinIV, coracle, and gcm mutants, together with nervana2 (Stork et al. 2008) represent the strongest BBB defective phenotypes so far reported in the literature. Nervana2, a Na+/K+ ATPase pump, localizes to septate junctions and is required for paracellular barrier formation (Genova and Fehon 2003; Paul et al. 2003; Schwabe et al. 2005).
Numerous other mutants have dextran penetration phenotypes at levels less than the group of mutants mentioned above. These include mutants of neuroglian, contactin, moody, sinuous, and megatrachea (Stork et al. 2008). Neuroglian forms a complex with Nervana2 and Gliotactin, and this complex is essential for septate junction formation (Genova and Fehon 2003). In addition, neuroglian mutants have a defective BBB in mature embryos (Schwabe et al. 2005). Contactin forms a protein complex with Neuroglian and NeurexinIV, which is involved in septate junction organization and paracellular barrier function in late stage embryos (Faivre-Sarrailh et al. 2004). Moody is a GPCR that is required for septate junction and BBB formation via actin cytoskeleton regulation (Bainton et al. 2005; Schwabe et al. 2005). Sinuous (Wu et al. 2004) and Megatrachea (Behr et al. 2003) are claudin-like proteins that localize to septate junctions and are required for septate junction organization and epithelial barrier. More recently, the protein Kune-kune, another claudin-like protein, was shown to localize to septate junctions and be required for septate junction formation and epithelial barrier function (Nelson et al. 2010). The involvement of claudins in septate junction formation points to a shared biochemical and evolutionary basis for tight junctions and septate junctions (Wu and Beitel 2004).
It is interesting that the degree of dextran uptake varies among the numerous mutants discussed above. That neurexinIV and coracle mutants possess the strongest dextran uptake phenotypes is not surprising given that these proteins are thought to form junctional septae. The strong phenotype of nervana2 mutants, however, is intriguing since this protein is not thought to be a septate junction structural component. The functions of Nervana2 and Moody point to SPG cell intrinsic regulation of BBB function. This aspect of fly BBB regulation parallels known processes affecting the vertebrate BBB. For example, the VE use Wnt/β-catenin signaling during development for the formation and maintenance of barrier function (Liebner et al. 2008). Furthermore, Wnt/β-catenin signaling regulates the expression of the glucose transporter GLUT1 (Daneman et al. 2009), which proves that the cells forming the barrier can also regulate chemical isolation physiology.
In contrast to cell intrinsic regulation, cell types other than the VE also regulate vertebrate BBB physiology. For example, the AG induce barrier properties in non-neural endothelial cells (Janzer and Raff 1987). Factors secreted by the AG that induce BBB properties include: apolipoprotein E (Methia et al. 2001) and basic fibroblast growth factor (Sobue et al. 1999). Neural tissue also contains factors that regulate BBB physiology (Stewart and Wiley 1981). Similarly, induction of SPG physiologic properties in Drosophila is likely to be influenced by humoral, PG, and brain-derived factors. For example mutants in the neuronally expressed Rbp9 gene exhibit a leaky BBB, dramatic down-regulation of neurexinIV and gliotactin, and slight down-regulation of neuroglian, coracle, and neurotactin (Kim et al. 2010; Kim and Baker 1993). These results suggest that, consistent with vertebrate systems, regulatory molecules within the Drosophila CNS are involved in the regulation of BBB formation and maintenance.
Chemical Isolation Physiology
In vertebrates, chemical isolation is attained by vascular-facing active efflux transporters, such as the ATP-binding cassette transporter Mdr1/P-gp. In a striking example of evolutionary conservation, the fly homolog of Mdr1/P-gp, Mdr65, is an essential arbiter of chemical isolation in Drosophila. Mdr65 localizes to the apical surface of the SPG near the humoral boundary (Mayer et al. 2009) (see Fig. 6B). Mdr65 transports substrates into the humoral space and thus is involved in neuroprotection since those substrates might otherwise penetrate into the CNS. mdr65 LOF causes increased small, lipophilic molecule penetration into the CNS. This efflux defect, however, does not diminish diffusion barrier physiology (Mayer et al. 2009). Furthermore, mdr65 expression levels at the BBB correlate with quantitative sensitivity to cytotoxic agents proving for the first time that ABC transporters function cell autonomously at chemical protection interfaces in the animal (Mayer et al. 2009).
The discussion above predominantly deals with the physiology of the BBB highlighting the scarcity of information related to the BEB. While the above studies point to functional and structural homology of the BBB and epithelial barriers during development, can the same be said about the BEB? This question has not been completely answered; however, previous observations have shown that measuring the fluorescence within the retina of dextran-injected flies can effectively corroborate known BBB defects (Bainton et al. 2005; Shaw and Varney 1999). Similarly, the septate junction component NeurexinIV is critical to the development of both a functional BBB in mature embryos and BEB in adults (Banerjee et al. 2008; Baumgartner et al. 1996). Thus, while more work needs to be done to fully understand the relationship between BBB and BEB phenotypes, current data suggest a strong concordance between genetic inputs and physiologic functions at both locations.
THE NEED FOR MODELS OF BBB FUNCTION
There is a trade-off between genetic power and biomedical relevance in any experimental system (Dow and Davies 2003). Human BBB physiology is an observational science that uses tools like magnetic resonance imaging to make gross estimates of brain drug pharmacokinetics or limited tissue explants for disease studies. The range of experimental interventions is exceedingly small, and mutational analysis is based on rare spontaneous findings (pharmacogenomics). Historically, BBB function is modeled in vivo in vertebrate animal systems. Rodent BBB models confirm the essential importance of transporters and junctions at the BBB (Nitta et al. 2003; Schinkel et al. 1994), but substantial limitations hinder progress because vertebrates are cumbersome for the assessment of gene function in an organismal context (Garberg et al. 2005). For example, quantitative assays for measuring drug penetration are expensive, difficult, and cannot be done in real time, thus broad based chemical screens are prohibitive. Furthermore, mechanistic studies that utilize reverse-genetic physiologic sensitization to interrogate chemical protection physiology are extremely limited compared to model organisms, and forward genetic studies are not currently possible.
Recently ex vivo systems using co-cultures limited to the VE and AG are attempting to remake the barrier in the test tube; however, these systems are unlikely to possess all of the cues that an intact animal uses to create, maintain and modulate humoral CNS separation physiology (Abbott 2004). Indeed co-culture studies do not fully recapitulate VE properties, and new evidence suggests that pericytes and neurons provide cues for the induction of barrier properties (Daneman et al. 2009; Daneman et al. 2010b). As very little is known about molecular signals and functional compensations that can be induced in vivo, new methods are required to study live physiology at the BBB. To answer this challenge several groups have begun studies in zebrafish (Eliceiri et al. 2010; Zhang et al. 2010). However, while fish have amazing advantages for live visualization of the development of BBB physiologic function, they have less tractable genetics and fewer tools to target BBB properties than simpler systems. Therefore, CNS specific chemical protection is ripe for analysis in more tractable invertebrate model systems like C. elegans and Drosophila.
Drosophila Offers a Way Forward
As noted above, the insect body-plan might seem too far from vertebrates to be useful for the essential questions about glial function in BBB biology. Insects have a hemocoel, rather than a vascular blood system and a markedly different BBB architecture at the CNS interface (Fig. 1). However, we show above and confirm below that these are not necessarily problems as the glia barrier machinery, by which these tasks are accomplished, is easily recognizable in the Drosophila context. Thus, as invertebrate models go, Drosophila seems clearly the most analogous and functionally complete BBB system. Furthermore, despite phylogenetic differences, the advantages conferred by Drosophila genetics are immense. The genome is relatively small containing 13,500 genes and 70% of Drosophila ORFs have human homologs (Chien et al. 2002). Mutants are publicly available for half of all genes, and in addition to classical and P-element insertional mutants, systematic mutagenesis programs have produced RNAi strains for nearly all Drosophila genes (Dietzl et al. 2007). Furthermore, tissue and cell-type specific drivers offer enormous advantages for organotypic study of gene function. These tools, made possible by the binary GAL4/UAS system, offer the ability to drive transgenic constructs (e.g. reporter GFP fusions, over-expressors, dominant negatives, and RNAi) in cell types at times of the experimenter’s choosing to assess anatomy and interrogate physiology. Finally, the speed and cost of culturing Drosophila allows for multi-generational breeding experiments to create uniquely sensitized strains and epistasis panels of informative genetic backgrounds to be maintained for long-term use. For the rest of this review we focus on new methods and primary data that substantiates Drosophila as a model system for BBB function and extols the virtues of live methodologies for assessing chemical protection physiology.
METHODS TO STUDY BBB AND BEB PHYSIOLOGY IN DROSOPHILA
Classic descriptions of glia and cellular junctions in Drosophila focused on their ultrastructure (e.g. Tepass and Hartenstein 1994). Ultrastructural studies address barrier physiology by investigating septate junction structure and identifying the populations of cells responsible for septate junction formation. These studies remain critical for BBB characterization (e.g. Stork et al. 2008), and future studies are necessary to further describe the system of cellular barriers formed by the fenestrated, pseudocartridge, and satellite glia that comprise the BEB.
Other methods by which BBB physiology can be studied utilize the phenotypes that are manifest in genetic mutants that lack a properly functioning BBB. These phenotypes include: paralysis or uncoordinated movement in embryos since a defective barrier allows motor neurons to be exposed to the hemolymph (Auld et al. 1995; Baumgartner et al. 1996; Schwabe et al. 2005; Strigini et al. 2006); behavioral sensitivity to drugs (Bainton et al. 2005); loss of ommatidial integrity and photoreceptor degeneration (Banerjee et al. 2008); and premature loss of climbing ability and increased stress sensitivity (Kim et al. 2010).
The above phenotypes represent broader physiologic properties that can be manifest in BBB mutants. Direct assessment of barrier function via dye tracers is the most direct method to study live barrier physiology. Early studies of barrier function (e.g. Saint Marie and Carlson 1983a) utilized electron-dense tracers such as colloidal lanthanum followed by electron microscopy. More recent studies have utilized chemical fluors to test transepithelial barrier defects (Lamb et al. 1998). The technique involves injection of labeled dextran into the hemocoel of stage 16 embryos followed by measurement of dextran penetration into the salivary glands, epidermis, hindgut, and trachea. Numerous studies identifying septate junction and barrier components have used this technique (Behr et al. 2003; Faivre-Sarrailh et al. 2004; Genova and Fehon 2003; Paul et al. 2003; Schulte et al. 2003; Ward et al. 2001; Wu et al. 2004), and all of these genes (with the exception of gliotactin) have since been shown to have BBB defects (Stork et al. 2008). Furthermore, Schwabe et al. (2005) developed a dye-penetration assay that measures dextran diffusion into the nerve cord of mature embryos. The remaining methods we will discuss also utilize chemical fluors, but are capable of directly assessing BBB and BEB function in live adult Drosophila.
Chemical Fluor Feeding/Injection Followed by Epifluorescence of Retinas and Brain Dissection
Beginning during our investigations of moody (Bainton et al. 2005), we adapted an assay developed by Shaw and Varney (1999) whereby chemical fluors are injected into the abdomen of adult Drosophila (Fig. 2). Hemolymph delivery is rapid with nearly complete distribution in the animal accomplished during the injection (Fig. 2A) (Pinsonneault et al. 2011). Whole animal distribution of different chemical fluors allows overall delivery to the CNS to be assessed live and in real time. Note that different chemical fluors distribute to different chemical spaces in the body as expected of any drug chemical preference for fat or water-soluble spaces (Fig. 2B). Penetration into the CNS is measured coarsely via epifluorescence microscopy (Fig. 3A) and finely via brain dissections. Injection followed by brain dissection is the most quantitative assay for BBB function. However, live imaging is also a powerful tool to evaluate chemo-protective gene function. For example dissected moody brains have quantitatively increased fluorescence uptake relative to controls (data not shown), but also show markedly increased retinal fluorescence in the live animal (Bainton et al. 2005) (Fig. 3A). Other examples of this methodology include a disrupted diffusion barrier in neurexinIV mutants (Banerjee et al. 2008), and a defective transport barrier in mdr65 mutants and flies treated with Mdr1/P-gp transport inhibitors (Mayer et al. 2009).
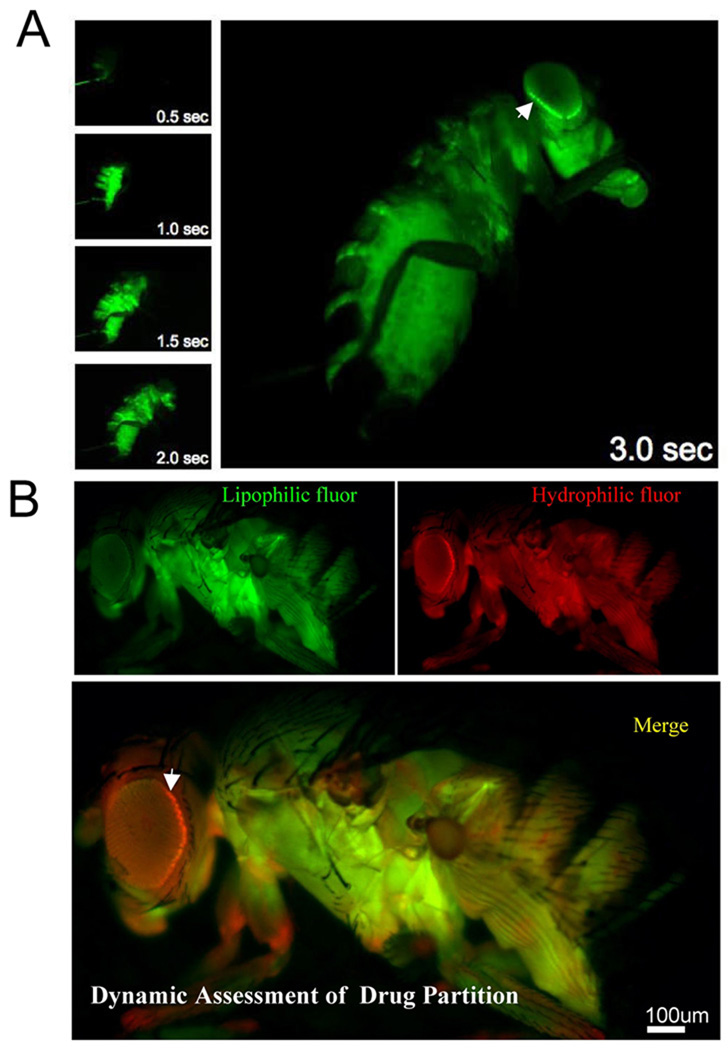
Figure 2. Examples of live whole animal dye injection and chemical partition in the hemolymph compartment.
(A) A series of images taken on a fluorescent dissecting microscope during a live injection of 3kDa FITC dextran. Note the rapid delivery of hemolymph around the entire animal and specific hemolymph partition at the retina/cuticle interface (arrow).
(B) Simultaneous assessment of transport and diffusion barriers by hemolymph co-injection of Rhodamine 123 and 10kDa Texas Red dextran. Images are taken live under CO2 anesthesia 15 min post injection using green and red filter sets, respectively. Hemolymph exclusion from the CNS is seen as a bright line around the retina, referred to as the hemolymph exclusion line (arrow).
Figure 3. Methods for studying barrier physiology in Drosophila.
Imaging through white−/− eyes allows for visualization and quantification of chemical fluor into the fly brain.
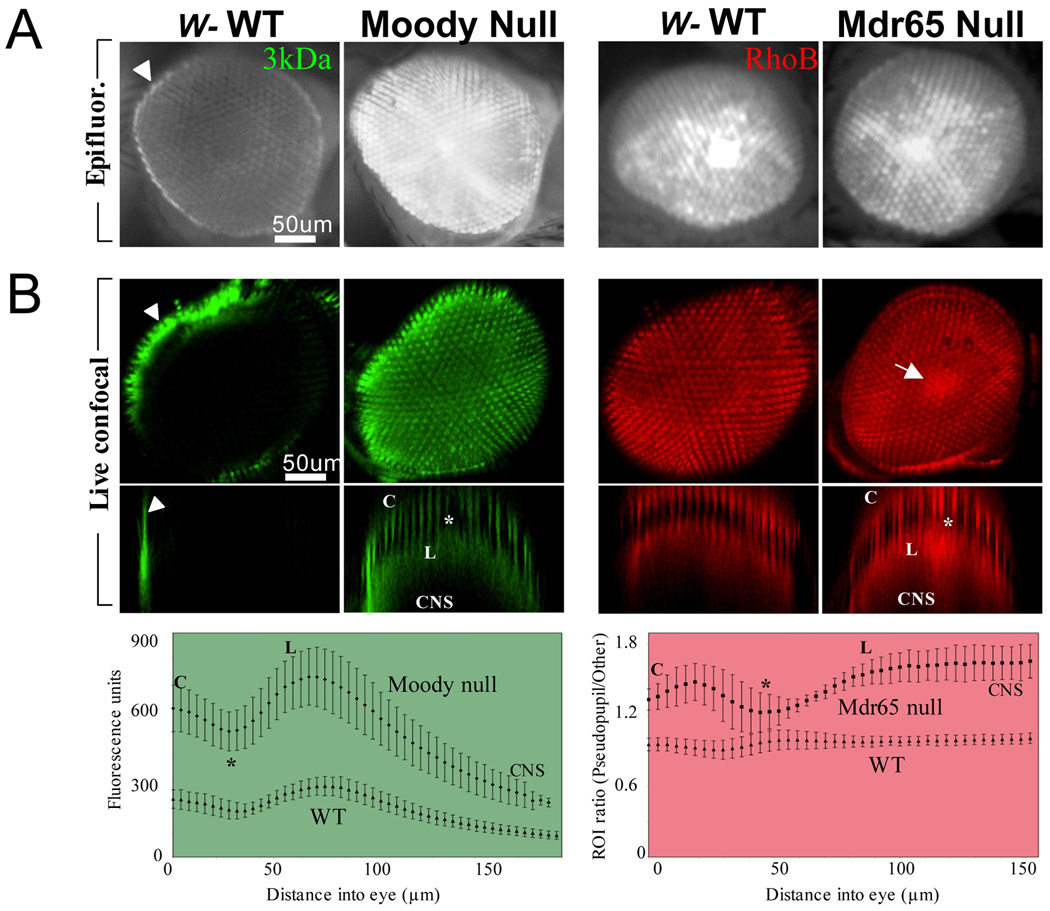
(A) Rapid Retinal Epifluorescence Assay. On the left are retinas of wild type and Moody null (diffusion barrier defective) adult flies injected with 3kDa FITC dextran and imaged 4hr post-injection. In all panels, the arrowhead points to the hemolymph exclusion line (HEL). On the right are retinas of wild type and Mdr65 null (transport barrier defective) adult flies injected with Rhodamine B and imaged 4hr post-injection.
(B) Live Confocal Microscopy Assay. Retinal images are taken in 4µm intervals through the fly head and digital 3D reconstructions are made based on fluorescence signal intensities (Top). The same data is reconstructed in a cross-sectional pixel intensity map from the anatomic center of the eye (Middle). Fluorescence intensities of regions of interest within the retinas (n=5 per genotype) plotted along the Z-axis (Bottom). On the left, confocal imaging recapitulates the diffusion barrier phenotype of Moody null flies seen with epifluorescence. On the right, the transport barrier phenotype of the Mdr65 null is now readily apparent. Note that the quantitation demonstrates better phenotypic differences at deeper depths that correspond to the central brain (see Methods). The arrow points to the pseudopupil, the group of adjacent ommatidia that are in line with the visual axis of the observer. The labels on the 2D cross-sections of mutants correspond with the labels on the bottom panels. C = cornea, * = retina, L = lamina, and CNS = central nervous system.
Differential localization of retinal fluorescence can indicate into which biologic spaces dye has penetrated. For example, a bright, distinct pseudopupil can signify extracellular penetration of dye between microvilli of the cone cells, and diffuse, retina-wide fluorescence can point to further penetration into the cornea (Shaw and Varney 1999). In addition, dye uptake into photoreceptors is possible, which can lead to staining of the lamina cartridge zone (Shaw and Varney 1999). In all cases, dye penetration into the retina mirrors the same process into the CNS. If brain fluorescence occurs due to disrupted septate junctions among the SPG in BBB mutants, then is a similar cellular component responsible for retina fluorescence? Indeed, septate junctions are located among the fenestrated and pseudocartridge glia in the lamina (Banerjee et al. 2008; Edwards and Meinertzhagen 2010), near the cone cell processes and feet at the retinal floor, and between the cone cells themselves and cone cells and pigment cells near the pseudocone cavity (Banerjee et al. 2008). Thus, it appears that mutations affecting septate junction components/regulators that allow dye penetration into the CNS also allow for dye penetration into the retina.
Live Retinal Imaging with Confocal Microscopy
With certain high brain penetrating chemical fluors (Rhodamine B) the retinal epifluorescence assay is not precise enough to corroborate quantitative brain dissection results. For example, mdr65 mutants possess ~2-fold higher brain fluorescence relative to controls 4h post-injection with Rhodamine B; however, a phenotype is not apparent with the retinal epifluorescence assay (Fig. 3A). Epifluorescence images compress all transmitted light into two dimensions thus losing spatial resolution essential for delineating anatomically different brain protection space. To devise a microscopic assay for BBB physiology that is sensitive to these limitations, we began live imaging of retinas with confocal microscopy.
Adult flies are interrogated with large molecular weight dextrans to assess the diffusion barrier, or Rhodamine B to study transport drug phenomena. Confocal microscopy can image multiple chemical fluors as deep as 200µm into the eye and CNS. The results of such an experiment are presented in Fig. 3B. Three-dimensional and cross-sectional reconstructions produce results consistent with previous findings (Bainton et al. 2005; Mayer et al. 2009); namely that the moody null causes loss of junctional integrity, and the mdr65 null causes loss of transport function. These 3D reconstructions now reveal a difference between wild type and the mdr65 null in the pseudopupil that epifluorescence cannot (Fig. 3B, arrow). Cross-sectional reconstructions reveal two distinct layers of fluorescence. The first layer of fluorescence localizes to the top of the ommatidia and thus likely represents corneal fluorescence. The dark layer that is pierced by columns of fluorescence likely represents the retina where photoreceptor neurons have been infiltrated by chemical fluor. The second layer of bright fluorescence corresponds to the depth of the lamina. These developments highlight the opportunity to measure small molecule localization live and in real time in Drosophila, which offers a huge advantage over vertebrate systems when interrogating functional components and regulation of chemical protection.
Anatomic Correlation of BBB Physiology Revealed in Retinal Assays
High resolution confocal microscopic studies on fixed tissue confirm precise anatomic localization and specific physiologic properties of the BBB seen in vivo. In these assays brains are fixed in situ to maintain BBB anatomy, dissected, stained with antibodies, and visualized under multicolor confocal microscopy. By concurrently injecting chemical fluor reporters, highly constrained physiologic properties can be matched with cell biologic components of different cell layers (Mayer et al. 2009). Furthermore, tissue specific transgenic labeling techniques allow targeted gene expression of tagged proteins into the BBB with layer specific GAL4 constructs (see screen below). These tools allow unprecedented precision for probing cell-autonomous physiologic function of specific chemical protection genes and communication between cell layers.
LIVE IMAGING PROVIDES A NOVEL SCREENING TOOL FOR ASSESSING BBB FUNCTION IN DROSOPHILA
Given the correlation between brain and retinal fluorescence in our previous studies (Bainton et al. 2005; Mayer et al. 2009), we realized that a rapid retinal epifluorescence assay might represent a novel screening tool to evaluate the physiology of chemical protection. The added advantages of this methodology include real time responsiveness of chemical barriers, and evaluation of whole animal response to chemical attack (i.e. behavioral effects).
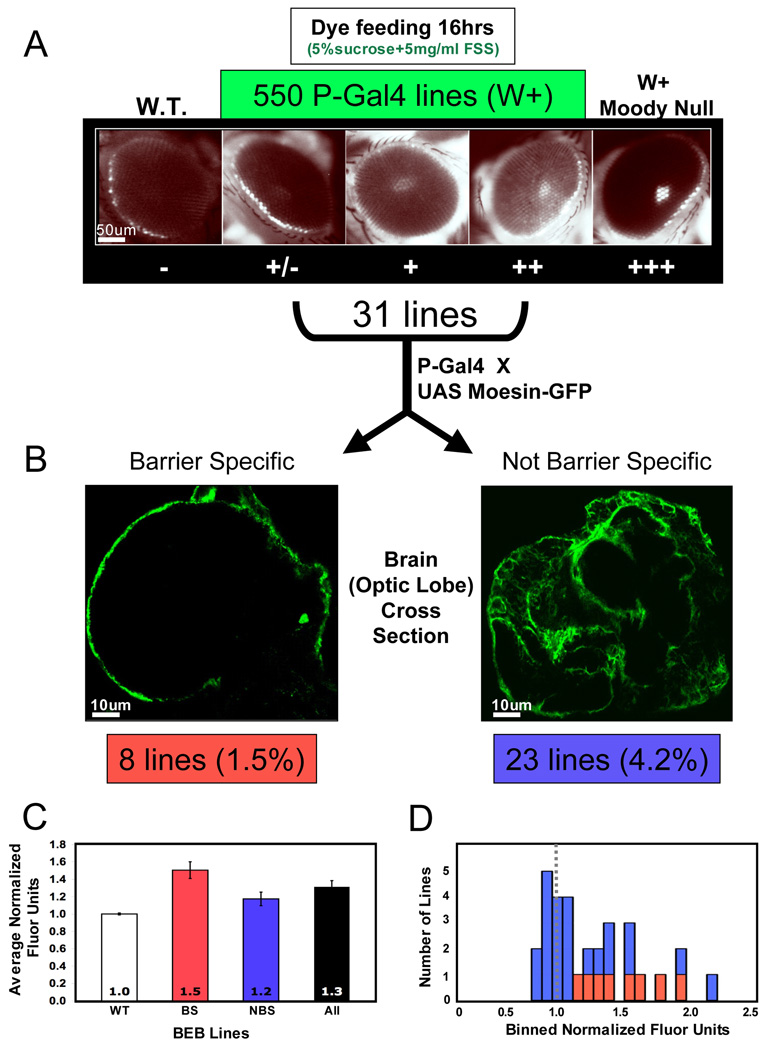
To demonstrate the power of this method, we performed a pilot screen of 550 homozygous viable P-element strains containing GAL4 (Heberlein lab, personal communication) (Brand et al. 1994). P-element screens are powerful because not only do they act as insertional mutagens to assess physiologic function of a gene, but also they contain molecular reporters (e.g. enhancer traps) that allow a rapid interrogation of gene expression and cell biologic localization. In this screen each P-GAL4 strain was fed fluorescein sodium salt (FSS) in a solution of 5% sucrose overnight. The concentration of FSS was non-toxic to animals and sufficient to penetrate their gut and be distributed throughout their body. Whole animal uptake variability was assessed on a dissecting microscope by total body fluorescence (Fig. 2B). At higher magnifications assessment of chemical penetration into the retina was seen as pseudopupil intensity (Fig. 4A). No mutant strains in the screen were as strong as the positive control, the Moody null, but approximately 5% of strains (31) had reproducible pseudopupil enhancements and were thus named BEB-defective strains.
Figure 4. Overview of the screening methodology.
(A) 550 P-GAL4 lines were fed overnight with fluorescein sodium salt (FSS) in 5% sucrose. Flies were viewed on a fluorescent dissecting microscope, and a retinal dye penetration phenotype was scored qualitatively with white+/+ (W+) wild type animals as a negative control (far left) and a W+ version of the Moody null as a positive control (far right). Sample screen lines are shown in between. Thirty-one lines with + or ++ phenotypes were kept for retesting and further analysis.
(B) All putative BBB-defective P-GAL4 strains were crossed to UAS-Moesin-GFP to localize gene expression in larval brains. The eight lines displaying gene expression peripheral to the brain were designated as BBB-specific (left); the remaining 23 lines displayed broad gene expression patterns often including the central nervous system (right).
(C) All 31 lines were also subjected to rigorous injection-dissection assays with 3kDa dextran to confirm diffusion barrier defects. The eight BBB-specific lines (BS) had 1.5-fold greater brain fluorescence relative to controls compared to 1.2-fold for the 23 non-BBB-specific lines (NBS). Averages for the BS and NBS lines were significantly different than the controls (one sample t-tests, BS p < 0.01, NBS p < 0.05) and significantly different from each other (two sample t-test, p < 0.05).
(D) A frequency histogram of normalized brain fluorescence of NBS (blue) and BS (red) lines reveals that the majority of NBS lines had values equal to controls, but two outliers show marked dye penetration. Note that all BS lines were greater than WT controls.
Putative BEB-defective strains were then evaluated by anatomic localization of the enhancer trap via crosses to various GFP reporters. Brains were dissected from third instar larva, and evaluated by confocal microscopy for gene expression on the periphery of optic lobe and ventral nerve cord (Fig. 4B, left). One third of the strains (eight) showed nearly exclusive expression in the CNS periphery, now referred to as BBB specific. This is a ten-fold enrichment over P-GAL4 strains tested at random for gene expression (Heberlein lab, personal communication). Two thirds of strains (23) demonstrated a variety of in vivo expression patterns from no cellular signal to ubiquitous cell expression including glia and neurons (Fig. 4B, right).
As a further secondary screen, in order to validate BEB-defective phenotypes, all BEB-defective strains were subjected to hemolymph injection of 10kDa dextran and average uptake was measured by brain dissection at 16hrs (Bainton et al. 2005; Mayer et al. 2009). The eight BBB specific strains had a higher relative average dextran accumulation (1.5) than the screen as a whole (1.2), and all BBB specific strain diffusion barrier phenotypes were greater than wild type controls (Fig. 4C). This indicates that the visual screen can readily identify putative cell autonomous modulators of BBB physiology as measured by large molecule penetration into the protected BBB chemical space (see below). Non-BBB specific strains had a multi-modal distribution with 15 wild type phenotypes, 6 medium phenotypes and 2 strains with very strong dextran uptake phenotypes (Fig. 4D, right).
As P-elements allow rapid identification of the local insertional loci, all BEB-defective strains were mapped to specific chromosomal locations, and the majority of the P-elements were localized in or near specific genes (Flyblast) (Table 2). The strongest non-BBB specific insertion was in alpha-mannosidase (8–176, alpha-Man-IIb) a Golgi protein (Rabouille et al. 1999) the homolog of which is responsible for a neurodegenerative lysosomal storage disease in vertebrates (Crawley and Walkley 2007). Interestingly, this line had a broad expression pattern, which included the glial barrier, fat body and central brain in adults. Thus, while the screen is designed to identify localized actors in the BBB, it can find more physiologically distributed regulators of barrier function. While still early in analysis, the non-BBB specific strains may provide some of the most salient clues to the systemic molecular cues required by the humoral barrier to modulate homeostasis.
Table 2.
Of the 550 P-GAL4 lines tested in our live retinal imaging screen, the 31 genes listed here showed reproducible retinal phenotypes. The eight barrier-specific genes are grouped by the layers in which they are specifically expressed (as revealed by our follow-up anatomical assays). BLAST homology was determined by inverse PCR, and instances where the P-element inserted in between two loci are noted by a slash. Driver number = Heberlein Lab, personal communication. Fold Δ = fluorescent dextran uptake relative to WT controls.
| Name | Symbol | CG no. | Driver | Fold Δ | Notes | Reference |
|---|---|---|---|---|---|---|
| Fat Body Layer | ||||||
| sarah | sra | CG6072 | 11–165 | 1.4 | Inhibitor of calcineurin | (Chang et al. 2003) |
| Perineurial Glial Layer | ||||||
| I'm not dead yet | Indy | CG3979 | 11–67 | 1.3 | Citrate transmembrane transporter | (Inoue et al. 2002; Knauf et al. 2002) |
| Ranbp 21 / CG14215 | Ranbp 21 / CG14215 | CG12234 / CG14215 | 8–79 | 1.7 | Intracellular protein transport | IPR001494 |
| varicose / CG9328 | vari / CG9328 | CG9326 / CG9328 | 10–12 | 1.8 | Septate junction assembly | (Wu et al. 2007) |
| CG6424 | CG6424 | CG6424 | 10–72 | 1.2 | -- | -- |
| Subperineurial Glial Layer | ||||||
| Gliotactin / CG3793 | Gli / CG3793 | CG3903 / CG3793 | 4–7 | 1.6 | Septate junction assembly | (Auld et al. 1995; Schulte et al. 2003) |
| Neu3 | Neu3 | CG7649 | 2–35 | 1.2 | Metalloendopeptidase activity | IPR001590 |
| snRNA U2 at 38ABb | snRNA:U2:38ABb | CR32878 | 10–37 | 1.9 | Nuclear mRNA splicing | -- |
| Non-Barrier Specific | ||||||
| alpha-Man-IIb | alpha-Man-IIb | CG4606 | 8–176 | 2.2 | Carbohydrate binding, Alpha-mannosidase | IPR011013, UniProt P27046 |
| cactus | cact | CG5848 | 7–67 | 1.0 | I-kappaB homolog | (Geisler et al. 1992) |
| dorsal | dl | CG6667 | 7–11 | 0.9 | NF-kappaB homolog | (Ghosh et al. 1990) |
| Glycerol-3-phosphate dehydrogenase | Gpdh | CG9042 | 9–167 | 1.1 | -- | -- |
| Kruppel homolog 2 | Kr-h2 | CG9159 | 7–61 | 2.0 | Integral to membrane | IPR005344 |
| PNGase-like | Pngl | CG7865 | 2–15 | 1.5 | Carbohydrate binding | (Funakoshi et al. 2010) |
| RNA pol. II 215kD subunit | RpII215 | CG1554 | 6–26 | 1.0 | DNA-directed RNA polymerase | -- |
| CG10778 | CG10778 | CG10778 | 5–55b | 1.1 | Phagocytosis, engulfment | (Stroschein-Stevenson et al. 2006) |
| CG11131 / CG11133 | CG11131 / CG11133 | CG11131 / CG11133 | 4–28 | 1.1 | CG11133 is ATP-dep helicase | IPR011545 |
| CG13624 | CG13624 | CG13624 | 7–63 | 1.6 | Protein homodimerization, Transcription factor | (Fassler et al. 2002), IPR004827 |
| CG1753 | CG1753 | CG1753 | 7–35 | 0.9 | Cysteine biosynthesis | IPR001216 |
| CG32666 | CG32666 | CG32666 | 4–76b | 1.0 | Cell adhesion, Reg’n of cell shape | (Kiger et al. 2003) |
| CG4068 | CG4068 | CG4068 | 4–54a | 1.2 | -- | -- |
| CG5953 | CG5953 | CG5953 | 2–76 | 1.0 | -- | -- |
| CG6785 | CG6785 | CG6785 | 10–69 | 1.0 | -- | -- |
| CG8399 | CG8399 | CG8399 | 5–34a | 0.8 | Dopamine beta-monooxygenase | IPR013050 |
| HMS-Beagle()116 | -- | -- | 5–61 | 1.4 | Transposable element | -- |
| No gene detected | -- | -- | 2–60, 4–10, 5–128, 6–65, 8–194, 9–203 | |||
Analysis of BBB-Specific Strains
We were keen to analyze BBB-specific strains for anatomic detail as cellular contributions to the Drosophila BBB are all currently focused on the SPG. Thus, all BBB-specific strains were crossed to UAS-GFP cytoskeleton reporters and adult animal BBIs analyzed at the brain surface and in cross section by confocal microscopy. We also looked at Moody antibody colocalization (see Fig. 6B) and genetic rescue of the Moody null (data not shown). All BBB-specific strains express in only one cell layer in the adult BBB (see below). Interestingly three separate cellular layers were identified that influence BBB physiology, the SPG, PG, and one strain demarcating a cellularly complex fat body/neural lamella layer. The morphology of these cellular layers is discussed including a summary of useful reagents to differentiate the BBB layers anatomically and physiologically (Table 1).
Humoral Glia Interface
Brain specific fat body (FB) layers have been previously described (Hwangbo et al. 2004), but not much is known about the function of these cells at the brain interface. In our anatomic analysis these cells do not form a complete brain barrier layer, but are directly adherent to the PG layer at multiple different anatomic locations, particularly in the posterior medulla (Fig. 5B). In the adult, the FB layer is best seen in brains fixed in situ and appears in a flagstone pattern as a mass of cells with prominent lipid droplets (Fig. 5B and C). Interspersed among these fat body cells and localized primarily at tricellular contacts are histiocytes with an appetite for the injected dextrans (Fig. 5B). Known hemocyte markers specifically tag histiocytes in the FB layer demonstrating a relationship to other circulating hemocytes (Irving et al. 2005) (Fig. 5D). Little is known about the role of phagocytic cells at the Drosophila humoral barrier and nothing is known about there contact with glia; nevertheless, they show very strong uptake of injected markers from the hemolymph (Fig. 5B and D). Both the fat body and histiocytes trap hemolymph contents adjacent to the PG cells. It is interesting to consider the role of these cells as a chemical sensor and cellular protector in light of neuroinflammatory inputs to many disease models. Our most pertinent example is the fat body expression of BEB-defective strain 11–165. This is a P-insertion in the Drosophila Sra gene, the calcipressin homolog, which has roles in innate neuro-inflammatory processes (Minami et al. 2006) and has been implicated in neurological disorders such as DSCR1-mediated Down’s syndrome (Chang and Min 2009; Chang et al. 2003). The Drosophila fat body has numerous posited roles in metabolism and inflammation, and its proximity to the brain would allow rapid response to changing metabolic conditions or infectious insult. As the surface glia of Drosophila represent the cellular interface with the rest of the body, literally touching immune and metabolic tissues, the fat body may prove to be an interesting locale to investigate modulation of barrier function.
Surface Glia of the BBB
The remainder of the BBB-specific strains were specific to two continuous and tightly adherent cell layers, the previously described PG and SPG (Fig. 5A, E–K). That BEB screens lines point to the SPG layer as an important player in chemical separation of the brain is not surprising, but this is the first direct evidence that PG cells also play a role in regulating barrier physiology. In the next section we detail the anatomic and physiologic signatures of the PG and SPG cell layers that are essential to their identification in situ.
The PG Layer
The PG cells are less than 1µm in thickness and possess many actin-based processes that completely cover the SPG cells. PG cells have intrinsic ability to uptake dextrans from the hemolymph and demonstrate a characteristic uniform surface pattern (Fig. 5E). While no obvious planar polarization is found in the XY dimension, PG cells produce a signature striated pattern on the brain surface with many types of makers (Fig. 5F). Striations are seen with both junctional complexes (Nrg, Nrx, Dlg) and cytoskeletal markers like moesin-GFP (Fig. 5G and F respectively). Striated cytoskeletal contacts are 7–10µm across and 15–20µm long and appear to represent points of contact with the SPG. This is best seen in a Nrg-GFP strain as 10kDa dextran accumulates in the same striated pattern (Fig. 5G) suggesting some trapping of dye along the contact interface between the PG and SPG. Previous EM studies have shown septate junctions between these cells layers, and our results suggest a role for junctional components in tethering the two cell layers together (Carlson et al. 2000; Carlson and Saintmarie 1990; Treherne and Pichon 1972). Indeed, striations of similar localization and periodicity are seen in SPG-specific marked lines (Fig. 5H – SPG-Moesin GFP). Furthermore, one of the PG-specific BBB strains is a putative allele of varicose (10–12), which has effects on septate junction development (Wu et al. 2007). Thus, while this is not definitive evidence of the specific contacts between these cell layers, it is parsimonious with published data and our observations as a whole. Overall, the PG layer has the anatomic localization and uptake properties to be a global hemolymph sensor modulating metabolism or inflammation, and act as buffer for the brain space. Interestingly, one PG-specific strain that hints at these functions is an intragenic insertion in Indy (11–67). Indy is a transporter of citric acid cycle intermediates which has controversial, but long standing roles in modulating metabolism in Drosophila (Knauf et al. 2002). Indy (SLC13A2) is highly expressed in astrocytic glia of vertebrates (Daneman et al. 2010a) suggesting a glia specific role as energy sensor for CNS function, which would be parsimonious with a role in chemical protection phenomena as our studies suggest.
The SPG Layer
The SPG layer is the anatomically and physiologically best understood and most extensively characterized glia barrier layer. The SPG is a diffusion barrier and chemical barrier (Fig. 6) (Mayer et al. 2009). It is a polarized cellular interface at 1µm in thickness with specific functional markers for apical (Mdr65) and basal (Moody) cell membranes (Fig. 6B). The SPG cells are very large in the XY plane (30–50µm on a side) and uniformly form tricellular contacts near the center of the posterior medulla (Fig. 5I). The apical membrane contacts the PG and the basal membrane is thought to contact cortical glia and neurons (Carlson et al. 2000; Carlson and Saintmarie 1990; Treherne and Pichon 1972). Neuronal cell bodies show some influence on basal interface architecture as Moody stains, cytoskeletal markers, and membrane markers suggest that the cell body reaches toward neuronal cell bodies (Fig. 5J and 6B). The most prominent features of the SPG in the XY plane are elaborate homotypic contacts with other SPG cells (Fig. 5I). Many prominent markers for septate junctions demonstrate a high degree of redundancy at the septate junction contact points (Nrg, Nrx, Dlg) and are useful markers for analysis of barrier function mutants (data not shown). Interestingly, one of the SPG-specific BBB lines is Gliotactin (4–7), a gene previously known to function in barrier layers (Auld et al. 1995; Schulte et al. 2003). That this screen identified Gliotactin as a BBB functioning gene, confirms its reach into established pathways of SPG junctional regulation. In addition, the screen can identify putative new modulators of diffusion barrier physiology (i.e. roles for processing of the extracellular matrix by the neuronal specific metalloprotease Neu3, 2–35).
The Barrier in Cross Section
Cross sectional analysis helps interpret surface images particularly in the context of physiologic markers. Similar to histiocytes but at a qualitatively lower extent, PG cells colocalize with fluorescent reporters introduced into the hemolymph layer (Fig. 6). These include dextrans, lectins, and some small molecules. To what extent these tagged molecules are endocytosed or are merely adhering to the elaborate cellular processes of the PG is not known, however the entire cellular space, except the nucleus, is filled with fluorescent agents suggesting that the PG may endocytose and sample the hemolymph compartment continuously. Indeed the source of hemolymph constituents is not known and it is possible that the PG cells contribute to regulating its contents.
The highly polarized nature of the SPG is seen in surface images of junctional molecules that localize to different interfaces (Fig 5J). These separate localizations are best confirmed in cross section as shown in Fig. 6B where the PG (blue dextran), the apical membrane of the SPG (C219 Mdr65) and the basal membrane of the SPG (Moody Ab) can be seen simultaneously and along the extent of the compound cellular interface. Furthermore, physiologic tools can manifest chemical protection function at high resolution – dextrans (PG localized) and transportable small molecule fluors (Prazocin-Bodipy) localize very precisely to the PG/SPG interface (Fig. 6C).
Screen Conclusions
This screen describes the rationale and methodology behind using the BEB as a simpler method to perform forward genetic analysis of BBB physiology. We show its utility in identifying adult barrier modulators and demonstrate that all layers of the barrier can participate in chemical permeation modulation (Fig. 5 and 6). While the screen results only scratch the surface of the types and numbers of genes that control BBB function, they nevertheless confirm that the Drosophila BBB functions as a compound cellular structure that parses physiologies in different cellular layers. In light of these findings, we consider below more expansive use of Drosophila to elucidate novel regulatory mechanisms of BBB function.
NEW DIRECTIONS IN BBB STUDIES
CNS humoral barriers are finely tuned cellular structures that control multiple integrated physiologic concerns simultaneously and must act rapidly to changing conditions of toxic exposure, metabolic energy source, and infectious insult (Zlokovic 2008). Thus, a vast array of cellular and metabolic responses must be balanced efficiently and with great precision. Genetic studies in Drosophila have greatly added to our understanding of the essential components of chemical barriers (Bellen et al. 1998; Schwabe et al. 2005; Stork et al. 2008; Tepass et al. 2001; Wu and Beitel 2004), but thus far most of these studies primarily have identified structurally essential genes for the development of polarized epithelia. Subtle modulators of the chemical barrier were discovered in adult organisms using behavior screens as in the case of the Moody GPCR (Bainton et al. 2005; Mayer et al. 2009). The above physiologic screen suggests that chemical, metabolic, and immune functions of surface glia are woven together in a remarkably diminutive interface and hints at analogous functions to vertebrate BBB cellular layers. Furthermore, the specificity of tissue selective enhancer traps provides the means to test cell autonomous genetic modulators of BBB physiology. This provides a useful framework to examine, in a high throughput manner, hypotheses about gene-specific or layer-specific physiologic perturbations in the homeostatic mechanisms and functional compensations of an intact BBB.
Profiling the BBB: Searching for Mechanistic Overlap
Many recent advances have been made in the description of physiologic function of vertebrate BBB layers. For example, precise genomic profiling of BBB cell layers has lead to the discovery of new regulatory mechanisms in BBB genesis (Daneman et al. 2009; Daneman et al. 2010a). Proteomic analyses of the BBB in health and disease have shown important functions for VE junction components in neuroinflammatory disease (Calabria and Shusta 2006; Cayrol et al. 2008; Terasaki and Ohtsuki 2005). And live imaging methodologies demonstrate real time activation of AG in response to numerous physiologic insults (Neuwelt et al. 2008). Genomic profiling methodologies have yet to be applied broadly to unique cell types in the Drosophila BBB layers, but such experiments are now within reach (Miller et al. 2009). Transphylar genomic biology comparisons will provide a useful mechanistic framework for analysis of the best-conserved functional spaces (e.g. SPG vs. VE) when physiologic constraints must be similarly satisfied. For example, ABC transporters, metabolic transporters, and junctional components are all highly expressed in the VE, but how they are co-regulated is not understood. In Drosophila genetic epistasis studies between ABC transporters and junctional components demonstrate unexpected compensations in the regulation of chemical protection (unpublished observations). Tissue specific drivers are proven tools for specific tissue isolation of RNA, genomic profiling, and gene comparison to other transcriptomes (Miller et al. 2009; Schwabe et al. 2005). With this information we believe that Drosophila will prove to be an intermediate step in physiologic analysis of large vertebrate data sets. Using reverse genetic approaches it will be possible to generate new hypotheses for the regulation of functions and discover novel interactions of cellular layers in compound BBB structures (e.g. PG vs. AG).
Unique Drosophila Tools for Probing BBI Chemical Protection
Despite the methodological advances in studying vertebrate BBB physiology discussed above, a basic working understanding of how the BBB parses control physiologies between the humoral space, AG, VE, neurons, and immune cells remains largely undiscovered. In our view, the large number of putative homeostatic pathways, and the relative paucity of testable hypomorphic genetic conditions and chemical tools with which to target specific functions have hampered these studies. Thus even with the sophistication of current approaches, new models of function and treatment strategies for BBB disease processes remain slow to unfold. Drosophila studies, on the other hand, benefit from a myriad of genetic and chemical tools, such as live brain pharmacokinetics, behavioral assays, and transgenic in vivo markers for physiologic function.
Live Brain Pharmacokinetics (PK)
In order to control the localization of small molecules, cells generate specific chemical space constraints (Hillenmeyer et al. 2008). These constraints are most formidable at the BBB interface where transporters, junctions, and localized metabolic functions create nearly insurmountable chemical barriers (Neuwelt et al. 2008). This is perhaps the biggest problem in all of brain disease treatment biology where numerous groups and companies vie for solutions to brain specific PK (Pardridge 2005). One formidable problem for vertebrate BBB scientists is the quantitative assessment of brain PK since these studies rely on crude bulk evisceration of brain tissue or cumbersome and time-consuming microdialysis experiments (Nag 2011). Thus, one task of invertebrate systems is to establish BBB PK metrics that can be used to quantify and specify chemical protection properties so that meaningful comparisons can be made between evolutionarily different systems. As invertebrates offer the opportunity to measure chemical permeation live and in real time (Fig. 2 and 3), we believe faster methods will lead to broader and better experimental strategies for understanding the BBB’s properties.
Behavioral Assays and BBB Penetration
Behavioral outputs can be a powerful tool to discover BBB control elements, particularly when targets are behind the BBB and can be activated with pharmacologic tools (Guarnieri and Heberlein 2003; Nichols 2006; Rothenfluh and Heberlein 2002; Wolf and Heberlein 2003). Altered behavior due to small molecule localization must be fully queried to understand the CNS interface with the rest of the body. For example, altered pharmacogenomic responses to CNS drugs are attributed to vertebrate BBB PK phenomenon (Park et al. 2007), and some psychiatric disorders are associated with BBB specific genes that alter the chemical barrier (i.e. Claudins and schizophrenia) (Sun et al. 2004). Indeed, the only known signaling system for chemical permeation control was discovered by forward genetic screen for behavioral sensitivity to neuroactive drugs in Drosophila, i.e. cocaine and dopamine reuptake transporters (Bainton et al. 2005). Given that behavioral modification is one of the most sensitive tools to measure chemical insult to the brain, it is likely that novel control mechanisms will be found in glia and the BBI using the relative facility of Drosophila behavioral analysis.
Cell Autonomous Gene Function and Epistasis
Many questions about the integration of the glia’s role in chemical protection systems can only be approached in invertebrates. For example, the minimum complement of xenobiotic transport systems and functional redundancies required to protect the brain require epistasis genetics only available in model organisms. Furthermore, how substrate promiscuous transporters interact with the vast complement of endogenous small molecules (i.e. chemical immunity) (Sarkadi et al. 2006) is of enormous interest to model organism and vertebrate biologists alike. Novel genetic reporters derived from nuclear hormone receptors and endogenous signaling systems can provide insights about targeted localization of in vivo signals (Kozlova and Thummel 2003). Targeted methods to interdict in the communication and coordination of transporters and junctional complexes that function within homeostatic regulatory systems must be found and exploited. Fittingly, tools available for Drosophila allow for the interrogation of endogenous small molecule signaling systems that participate in metabolic and barrier control (Miller 2010). Furthermore synthetic transgenic tools to target the CNS are expanding (e.g. modified targets with novel known ligands – RASSLs), which can be used to interrogate the BBB’s role in drug responses (Conklin et al. 2008).
Chemical Screens
Finally, and perhaps the most exciting utilization of Drosophila as a model system is the opportunity to modify chemical permeation assays into high-throughput screening methodologies for the discovery of novel chemical modulators of BBB function. Chemical modulators offer numerous additional benefits over genetic screening methods including rapid on/off control, easy dose titration, and unmatched portability between systems. Of course, such chemical screens would be useful tools for interrogating BBB function, but could also be used to discover modulators of BBB PK in vivo that would be of enormous clinical value. Furthermore, porting vertebrate genetic targets into the Drosophila CNS for chemical interrogation could prove a useful method for discovery of small molecules that will yield the best in vivo target interactions.
Drosophila and the Study of Other BBB Physiologies
Metabolic Maintenance
If drug-like molecules cannot penetrate the brain, then nutrients and metabolites have similar difficulty in traversing the barrier. Thus, the BBB also functions in metabolic maintenance of the CNS as nutrients must pass through the VE/AG to neurons in order to meet the metabolic demands of the brain. In vertebrates, transporters operating at the VE/AG interface function in the transport of glucose – GLUT1 (Pardridge 1991), monocarboxylates and ketone bodies – MCT1 (Pierre and Pellerin 2005), glutamate – the excitatory amino acid transporters GLT1 and GLAST (Danbolt 2001), and ions – for example the Na+-K+-2Cl− cotransporter (O'Donnell et al. 2006). In Drosophila, the picture is not as clear. While glucose transporters have been identified (e.g. Escher and Rasmuson-Lestander 1999), there is no evidence that they are expressed specifically at the BBB. Also, Drosophila glial cells specifically express excitatory amino acid transporters (Freeman et al. 2003); however, their function in BBB physiology is unknown. In addition, numerous ion transporters are glial-specific (Freeman et al. 2003), and while they probably function in the homeostasis of extracellular ions and small molecules necessary for neuron function, the activity of Nervana2 points to ion transport being necessary for BBB formation (Genova and Fehon 2003; Paul et al. 2003; Schwabe et al. 2005). Further investigation on how the Drosophila BBB is involved in metabolic maintenance of the CNS is necessary in order to make more meaningful comparisons with vertebrates.
Neurodegenerative Diseases
BBB physiology is compromised in many different CNS disease states, and thus glial cells play a major role in new theories about CNS pathogenesis (Zlokovic 2008). For example, BBB transporters are implicated in the accumulation of: 1) beta amyloid during Alzheimer’s disease (LRP1 transporter – Shibata et al. 2000); and 2) toxins contributing to Parkinson’s (MDR1 transporter – Kortekaas et al. 2005). Furthermore, vertebrate immune cells regularly cross the BBB searching for potentially pathogenic entities (Hickey 2001). Since BBB dysfunction likely allows increased penetration of leukocytes into the CNS, the natural process of immunological surveillance provides the foundation for the development of neuroinflammation central to multiple sclerosis and Parkinson’s progression (Zlokovic 2008).
Given that glial cell responses are at the heart of neuroinflammatory processes, the development of Drosophila models of cellular passage and neurodegeneration (coupled with the complement of already existing tools) will facilitate rich discoveries into the molecular and cellular mechanisms of neurodegenerative diseases. To this end, models of tauopathy and Alzheimer’s already exist for Drosophila (Wittmann et al. 2001). In regard to neuroinflammation, very little is known regarding BBB-homing and transiting processes of immune cells in Drosophila. The determinants for T-cell homing to the vertebrate BBB are beginning to be unraveled (Carman and Springer 2004; Cayrol et al. 2008); therefore, functional connections between cellular passage in Drosophila and vertebrates may soon come to fruition. Indeed, several known immunity proteins (i.e. Dorsal and Cactus) were found to affect BBB physiology in the BEB screen. While it is to early to draw strong parallels about neuroinflammatory regulators found in Drosophila, not surprisingly, this hints at connections between chemical isolation physiology and innate immunity.
Final Thoughts
Cellular barrier structures are the sine qua non of metazoan organisms. Cellular specialization favors the development of different cellular milieu and barrier epithelia become the favored method for dividing physiologic space. Unfortunately, mechanistic specialization and homeostatic adaptations that occur across the humoral/CNS space are so complex that progress in understanding the rules that govern physiologic organization of metabolic, chemical and cellular physiologies of the brain have been very difficult to discern. These constraints also make drug discovery to the brain nearly impossible to predict which further complicates the study of CNS metabolic sensitivities and neuroinflammatory remedies of the brain. A model system that allows the investigator to find chemical permeation properties, test hypotheses by reverse genetics, and most importantly, to screen for modifiers with special selectivity for BBB function, provides the essential steps in developing the discovery tools and delivery methods to interrogate the vertebrate CNS. Thus, while not all of vertebrate BBB protection physiologies will be completely recapitulated in insect glial cell barriers, we suggest that in this special issue on invertebrate glia there are already examples of useful comparisons that can inform future studies.
METHODS
Methods relating to fluor injections, brain dissections, retinal epifluorescence microscopy, and immunohistochemistry are described in Bainton et al. (2005) and Mayer et al. (2009). Below are descriptions of novel methods mentioned above and those used in our screen.
Live Retinal Imaging with Confocal Microscopy
Wild type (w- iso), moody null, and mdr65 null adult males were injected with 12.5mg/ml 10kDa FITC dextran or 1.25mg/ml Rhodamine B pH 7.0 according to methods previously described in Bainton et al. (2005). Eyes were imaged 5h post-injection on a Nikon C1si Spectral Confocal microscope with a Plan Fluor 10× water-dipping lens (Nikon Imaging Facility at UCSF). Prior to confocal microscopy, the injected flies were decapitated, their heads mounted in fluorinated grease inside a Petri dish such that one eye was facing up, and the Petri dish filled with water. Using the Nikon EZ-C1 software, 3.7µm stacks were acquired to a final depth of 177.6µm. Three-dimensional reconstructions and two-dimensional slice images were acquired in NIS Elements software.
Quantification of pixel intensities was also performed in NIS Elements. For the green channel (10kDa FITC dextran) a polygon near the center of the retina was defined as the region of interest (ROI) and the average intensity was measured in each stack along the Z-axis. For the red channel (Rhodamine B) a ROI was drawn that outlined the pseudopupil and the average intensity was measured in each Z-stack (Pseudopupil ROI). The same ROI was then moved away from the pseudopupil and the average intensity was measured in each Z-stack (Other ROI). For each Z-stack, we calculated a ratio of the average intensity (Pseudopupil ROI/Other ROI). Hence, in flies where a pseudopupil is observed (e.g. in transport barrier mutants) the ratio is > 1, and in flies lacking a pseudopupil (e.g. wild type) the ratio ~ 1.
Screen Methodology
Fifty flies (males and females) homozygous for each P-GAL4 line were isolated from bottles at 2–4 days post-ecclosion and allowed to recover over night on regular food. Empty plastic fly food vials (10cm height, 2.5cm radius) were covered with 3M filter paper on the sides and bottom. Five ml of 5mM fluorescein sodium salt (Sigma) in 5% sucrose were added to the vials and allowed to completely absorb to the filter paper. Flies were added to the vial at the end of the day by quick transfer (no CO2 anesthesia) and left at RT to feed overnight. The next day the animals were anesthetized with CO2 and fly retinas imaged under an epifluorescence dissecting microscope (Zeiss). Positive and negative controls for dye uptake into the brain were performed in parallel. The screener chose flies that best fed during the night to assess retinal penetration of fluorescein.
ACKNOWLEDGEMENTS
We would like to thank Kurt Thorn and Alice Thwin of the Nikon Imaging Center at UCSF for assistance with confocal microscopy. We would also like to thank Richard Daneman and Fred Wolf for insightful comments on earlier drafts of this work. This work was supported by National Institutes of Health Grant GM081863, the UCSF Anesthesiology and Perioperative Care Department, and the UCSF Sandler Foundation.
REFERENCES
- Abbott NJ. Prediction of blood-brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discovery Today: Technologies. 2004;1(4):407–416. doi: 10.1016/j.ddtec.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25(1):5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26(7):340–344. doi: 10.1016/S0166-2236(03)00141-3. author reply 344-5. [DOI] [PubMed] [Google Scholar]
- Auld VJ, Fetter RD, Broadie K, Goodman CS. Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood-nerve barrier in Drosophila. Cell. 1995;81(5):757–767. doi: 10.1016/0092-8674(95)90537-5. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28(51):13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. 2005;123(1):145–156. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bainton RJ, Mayer N, Beckstead R, Bhat MA. Septate junctions are required for ommatidial integrity and blood-eye barrier function in Drosophila. Dev Biol. 2008;317(2):585–599. doi: 10.1016/j.ydbio.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87(6):1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Beckervordersandforth RM, Rickert C, Altenhein B, Technau GM. Subtypes of glial cells in the Drosophila embryonic ventral nerve cord as related to lineage and gene expression. Mech Dev. 2008;125(5–6):542–557. doi: 10.1016/j.mod.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell. 2003;5(4):611–620. doi: 10.1016/s1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin--novel members of the neurexin family: encounters of axons and glia. Trends Neurosci. 1998;21(10):444–449. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Brand AH, Manoukian AS, Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria AR, Shusta EV. Blood-brain barrier genomics and proteomics: elucidating phenotype, identifying disease targets and enabling brain drug delivery. Drug Discov Today. 2006;11(17–18):792–799. doi: 10.1016/j.drudis.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Carlson SD, Juang JL, Hilgers SL, Garment MB. Blood barriers of the insect. Annual Review of Entomology. 2000;45:151–174. doi: 10.1146/annurev.ento.45.1.151. [DOI] [PubMed] [Google Scholar]
- Carlson SD, Saintmarie RL. Structure and Function of Insect Glia. Annual Review of Entomology. 1990;35:597–621. [Google Scholar]
- Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167(2):377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani AS, Kreymborg K, Krug S, Moumdjian R, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9(2):137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- Chang KT, Min KT. Upregulation of three Drosophila homologs of human chromosome 21 genes alters synaptic function: implications for Down syndrome. Proc Natl Acad Sci U S A. 2009;106(40):17117–17122. doi: 10.1073/pnas.0904397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KT, Shi YJ, Min KT. The Drosophila homolog of Down's syndrome critical region 1 gene regulates learning: implications for mental retardation. Proc Natl Acad Sci U S A. 2003;100(26):15794–15799. doi: 10.1073/pnas.2536696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S, Reiter LT, Bier E, Gribskov M. Homophila: human disease gene cognates in Drosophila. Nucleic Acids Res. 2002;30(1):149–151. doi: 10.1093/nar/30.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin BR, Hsiao EC, Claeysen S, Dumuis A, Srinivasan S, Forsayeth JR, Guettier JM, Chang WC, Pei Y, McCarthy KD, et al. Engineering GPCR signaling pathways with RASSLs. Nat Methods. 2008;5(8):673–678. doi: 10.1038/nmeth.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley AC, Walkley SU. Developmental analysis of CNS pathology in the lysosomal storage disease alpha-mannosidosis. J Neuropathol Exp Neurol. 2007;66(8):687–697. doi: 10.1097/nen.0b013e31812503b6. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106(2):641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Barres BA. The blood-brain barrier--lessons from moody flies. Cell. 2005;123(1):9–12. doi: 10.1016/j.cell.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010a;5(10):e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010b;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36(2):180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Dow JT, Davies SA. Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol Rev. 2003;83(3):687–729. doi: 10.1152/physrev.00035.2002. [DOI] [PubMed] [Google Scholar]
- Edwards JS, Swales LS, Bate M. The Differentiation between Neuroglia and Connective-Tissue Sheath in Insect Ganglia Revisited - the Neural Lamella and Perineurial Sheath-Cells Are Absent in a Mesodermless Mutant of Drosophila. Journal of Comparative Neurology. 1993;333(2):301–308. doi: 10.1002/cne.903330214. [DOI] [PubMed] [Google Scholar]
- Edwards KA, Demsky M, Montague RA, Weymouth N, Kiehart DP. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev Biol. 1997;191(1):103–117. doi: 10.1006/dbio.1997.8707. [DOI] [PubMed] [Google Scholar]
- Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol. 2010;90(4):471–497. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. Eine Farbenanalytishe Studie. Berlin: Herschwarld; 1885. Das Sauerstoff-Bedurfnis des Organismus; pp. 69–72. [Google Scholar]
- Eliceiri BP, Gonzalez AM, Baird A. Zebrafish Model of the Blood-Brain Barrier: Morphological and Permeability Studies. In: Nag S, editor. The Blood-Brain and Other Neural Barriers. Springer; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Ose A, Schinkel AH, Sugiyama Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab Dispos. 2008;36(6):995–1002. doi: 10.1124/dmd.107.019257. [DOI] [PubMed] [Google Scholar]
- Escher SA, Rasmuson-Lestander A. The Drosophila glucose transporter gene: cDNA sequence, phylogenetic comparisons, analysis of functional sites and secondary structures. Hereditas. 1999;130(2):95–103. doi: 10.1111/j.1601-5223.1999.00095.x. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131(20):4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Fassler J, Landsman D, Acharya A, Moll JR, Bonovich M, Vinson C. B-ZIP proteins encoded by the Drosophila genome: evaluation of potential dimerization partners. Genome Res. 2002;12(8):1190–1200. doi: 10.1101/gr.67902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38(4):567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Funakoshi Y, Negishi Y, Gergen JP, Seino J, Ishii K, Lennarz WJ, Matsuo I, Ito Y, Taniguchi N, Suzuki T. Evidence for an essential deglycosylation-independent activity of PNGase in Drosophila melanogaster. PLoS One. 2010;5(5):e10545. doi: 10.1371/journal.pone.0010545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garberg P, Ball M, Borg N, Cecchelli R, Fenart L, Hurst RD, Lindmark T, Mabondzo A, Nilsson JE, Raub TJ, et al. In vitro models for the blood-brain barrier. Toxicol In Vitro. 2005;19(3):299–334. doi: 10.1016/j.tiv.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Geisler R, Bergmann A, Hiromi Y, Nusslein-Volhard C. cactus, a gene involved in dorsoventral pattern formation of Drosophila, is related to the I kappa B gene family of vertebrates. Cell. 1992;71(4):613–621. doi: 10.1016/0092-8674(92)90595-4. [DOI] [PubMed] [Google Scholar]
- Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol. 2003;161(5):979–989. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges E, Bradley G, Gariepy J, Ling V. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci U S A. 1990;87(1):152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Gifford AM, Riviere LR, Tempst P, Nolan GP, Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol. 2003;54:199–228. doi: 10.1016/s0074-7742(03)54006-5. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36(2):118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Hillenmeyer ME, Fung E, Wildenhain J, Pierce SE, Hoon S, Lee W, Proctor M, St Onge RP, Tyers M, Koller D, et al. The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science. 2008;320(5874):362–365. doi: 10.1126/science.1150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya K, Tachikawa M. Inner blood-retinal barrier transporters: role of retinal drug delivery. Pharm Res. 2009;26(9):2055–2065. doi: 10.1007/s11095-009-9930-2. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Takizawa K, Nitta K, Hotta Y. glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell. 1995;82(6):1025–1036. doi: 10.1016/0092-8674(95)90281-3. [DOI] [PubMed] [Google Scholar]
- Hoyle G. High blood potassium in insects in relation to nerve conduction. Nature. 1952;169(4294):281–282. doi: 10.1038/169281a0. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429(6991):562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Inoue K, Fei YJ, Huang W, Zhuang L, Chen Z, Ganapathy V. Functional identity of Drosophila melanogaster Indy as a cation-independent, electroneutral transporter for tricarboxylic acid-cycle intermediates. Biochem J. 2002;367(Pt 2):313–319. doi: 10.1042/BJ20021132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving P, Ubeda JM, Doucet D, Troxler L, Lagueux M, Zachary D, Hoffmann JA, Hetru C, Meister M. New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol. 2005;7(3):335–350. doi: 10.1111/j.1462-5822.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- Ito K, Urban J, Technau GM. Distribution, Classification, and Development of Drosophila Glial-Cells in the Late Embryonic and Early Larval Ventral Nerve Cord. Rouxs Archives of Developmental Biology. 1995;204(5):284–307. doi: 10.1007/BF02179499. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Juang JL, Carlson SD. A Blood-Brain-Barrier without Tight Junctions in the Fly Central-Nervous-System in the Early Postembryonic Stage. Cell and Tissue Research. 1992;270(1):95–103. [Google Scholar]
- Kiger AA, Baum B, Jones S, Jones MR, Coulson A, Echeverri C, Perrimon N. A functional genomic analysis of cell morphology using RNA interference. J Biol. 2003;2(4):27. doi: 10.1186/1475-4924-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim YJ, Kim-Ha J. Blood-brain barrier defects associated with Rbp9 mutation. Mol Cells. 2010;29(1):93–98. doi: 10.1007/s10059-010-0040-0. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Baker BS. The Drosophila gene rbp9 encodes a protein that is a member of a conserved group of putative RNA binding proteins that are nervous system-specific in both flies and humans. J Neurosci. 1993;13(3):1045–1056. doi: 10.1523/JNEUROSCI.13-03-01045.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf F, Rogina B, Jiang Z, Aronson PS, Helfand SL. Functional characterization and immunolocalization of the transporter encoded by the life-extending gene Indy. Proc Natl Acad Sci U S A. 2002;99(22):14315–14319. doi: 10.1073/pnas.222531899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57(2):176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- Kozlova T, Thummel CS. Methods to characterize Drosophila nuclear receptor activation and function in vivo. Methods Enzymol. 2003;364:475–490. doi: 10.1016/s0076-6879(03)64027-9. [DOI] [PubMed] [Google Scholar]
- Lamb RS, Ward RE, Schweizer L, Fehon RG. Drosophila coracle, a member of the protein 4.1 superfamily, has essential structural functions in the septate junctions and developmental functions in embryonic and adult epithelial cells. Molecular Biology of the Cell. 1998;9(12):3505–3519. doi: 10.1091/mbc.9.12.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183(3):409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorico A, Rappa G, Flavell RA, Sartorelli AC. Double knockout of the MRP gene leads to increased drug sensitivity in vitro. Cancer Res. 1996;56(23):5351–5355. [PubMed] [Google Scholar]
- Manning G, Krasnow MA. Development of the Drosophila tracheal system. In: Bate M, Martínez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory Press; 1993. pp. 609–685. [Google Scholar]
- Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J Neurosci. 2009;29(11):3538–3550. doi: 10.1523/JNEUROSCI.5564-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH. Cell biology of the neurovascular unit: implications for drug delivery across the blood-brain barrier. Assay Drug Dev Technol. 2005;3(1):89–95. doi: 10.1089/adt.2005.3.89. [DOI] [PubMed] [Google Scholar]
- Methia N, Andre P, Hafezi-Moghadam A, Economopoulos M, Thomas KL, Wagner DD. ApoE deficiency compromises the blood brain barrier especially after injury. Mol Med. 2001;7(12):810–815. [PMC free article] [PubMed] [Google Scholar]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31(6):246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Robinson KJ, Cleary MD, Doe CQ. TU-tagging: cell type-specific RNA isolation from intact complex tissues. Nat Methods. 2009;6(6):439–441. doi: 10.1038/nmeth.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T, Miura M, Aird WC, Kodama T. Thrombin-induced autoinhibitory factor, Down syndrome critical region-1, attenuates NFAT-dependent vascular cell adhesion molecule-1 expression and inflammation in the endothelium. J Biol Chem. 2006;281(29):20503–20520. doi: 10.1074/jbc.M513112200. [DOI] [PubMed] [Google Scholar]
- Nag S, editor. The Blood-Brain and Other Neural Barriers. 1st ed. New York: Springer; 2011. p. 502. [Google Scholar]
- Nelson KS, Furuse M, Beitel GJ. The Drosophila Claudin Kune-kune is required for septate junction organization and tracheal tube size control. Genetics. 2010;185(3):831–839. doi: 10.1534/genetics.110.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7(1):84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther. 2006;112(3):677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2006;26(10):1234–1249. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Advances in cell biology of blood-brain barrier transport. Semin Cell Biol. 1991;2(6):419–426. [PubMed] [Google Scholar]
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Shinn HK, Ryu SH, Lee HS, Park CS, Kang JH. Genetic polymorphisms in the ABCB1 gene and the effects of fentanyl in Koreans. Clin Pharmacol Ther. 2007;81(4):539–546. doi: 10.1038/sj.clpt.6100046. [DOI] [PubMed] [Google Scholar]
- Paul SM, Ternet M, Salvaterra PM, Beitel GJ. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development. 2003;130(20):4963–4974. doi: 10.1242/dev.00691. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94(1):1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Pinsonneault RL, Mayer N, Mayer F, Tegegn N, Bainton RJ. Novel Models for Studying the Blood-Brain and Blood-Eye Barriers in Drosophila. Methods Mol Biol. 2011;686:357–369. doi: 10.1007/978-1-60761-938-3_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C, Kuntz DA, Lockyer A, Watson R, Signorelli T, Rose DR, van den Heuvel M, Roberts DB. The Drosophila GMII gene encodes a Golgi alpha-mannosidase II. J Cell Sci. 1999;112(Pt 19):3319–3330. doi: 10.1242/jcs.112.19.3319. [DOI] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Calderon R, Uhlenbrock G, Borycz J, Simon AF, Grygoruk A, Yee SK, Shyer A, Ackerson LC, Maidment NT, Meinertzhagen IA, et al. A glial variant of the vesicular monoamine transporter is required to store histamine in the Drosophila visual system. PLoS Genet. 2008;4(11):e1000245. doi: 10.1371/journal.pgen.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfluh A, Heberlein U. Drugs, flies, and videotape: the effects of ethanol and cocaine on Drosophila locomotion. Curr Opin Neurobiol. 2002;12(6):639–645. doi: 10.1016/s0959-4388(02)00380-x. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. Glial Membrane Specializations and the Compartmentalization of the Lamina Ganglionaris of the Housefly Compound Eye. Journal of Neurocytology. 1983a;12(2):243–275. doi: 10.1007/BF01148464. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. The Fine-Structure of Neuroglia in the Lamina Ganglionaris of the Housefly, Musca-Domestica L. Journal of Neurocytology. 1983b;12(2):213–241. doi: 10.1007/BF01148463. [DOI] [PubMed] [Google Scholar]
- Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86(4):1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- Schinkel AH. Pharmacological insights from P-glycoprotein knockout mice. Int J Clin Pharmacol Ther. 1998;36(1):9–13. [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77(4):491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Developmental Biology. 1997;189(2):186–204. doi: 10.1006/dbio.1997.8660. [DOI] [PubMed] [Google Scholar]
- Schulte J, Tepass U, Auld VJ. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J Cell Biol. 2003;161(5):991–1000. doi: 10.1083/jcb.200303192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood-brain barrier formation in drosophila. Cell. 2005;123(1):133–144. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Shaw SR. Early visual processing in insects. J Exp Biol. 1984;112:225–251. doi: 10.1242/jeb.112.1.225. [DOI] [PubMed] [Google Scholar]
- Shaw SR, Varney LP. Primitive, crustacean-like state of blood-brain barrier in the eye of the apterygote insect Petrobius (Archaeognatha) determined from uptake of fluorescent tracers. Journal of Neurobiology. 1999;41(4):452–470. [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, et al. Clearance of Alzheimer's amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106(12):1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, Tsuda T, Katsuya H, Miura Y, Asai K, et al. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res. 1999;35(2):155–164. doi: 10.1016/s0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981;84(1):183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28(3):587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M, Cantera R, Morin X, Bastiani MJ, Bate M, Karagogeos D. The IgLON protein Lachesin is required for the blood-brain barrier in Drosophila. Mol Cell Neurosci. 2006;32(1–2):91–101. doi: 10.1016/j.mcn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Stroschein-Stevenson SL, Foley E, O'Farrell PH, Johnson AD. Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol. 2006;4(1):e4. doi: 10.1371/journal.pbio.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZY, Wei J, Xie L, Shen Y, Liu SZ, Ju GZ, Shi JP, Yu YQ, Zhang X, Xu Q, et al. The CLDN5 locus may be involved in the vulnerability to schizophrenia. Eur Psychiatry. 2004;19(6):354–357. doi: 10.1016/j.eurpsy.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161(2):563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- Terasaki T, Ohtsuki S. Brain-to-blood transporters for endogenous substrates and xenobiotics at the blood-brain barrier: an overview of biology and methodology. NeuroRx. 2005;2(1):63–72. doi: 10.1602/neurorx.2.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treherne JE, Pichon Y. The insect blood-brain barrier. London: Academic; 1972. pp. 257–308. [Google Scholar]
- Vlaming ML, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev. 2009;61(1):14–25. doi: 10.1016/j.addr.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Ward RE, Schweizer L, Lamb RS, Fehon RG. The protein 4.1, ezrin, radixin, moesin (FERM) domain of Drosophila Coracle, a cytoplasmic component of the septate junction, provides functions essential for embryonic development and imaginal cell proliferation. Genetics. 2001;159(1):219–228. doi: 10.1093/genetics/159.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, Beijnen JH, van der Valk M, Krimpenfort P, Borst P. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med. 1997;3(11):1275–1279. doi: 10.1038/nm1197-1275. [DOI] [PubMed] [Google Scholar]
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293(5530):711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Heberlein U. Invertebrate models of drug abuse. J Neurobiol. 2003;54(1):161–178. doi: 10.1002/neu.10166. [DOI] [PubMed] [Google Scholar]
- Wu VM, Beitel GJ. A junctional problem of apical proportions: epithelial tube-size control by septate junctions in the Drosophila tracheal system. Curr Opin Cell Biol. 2004;16(5):493–499. doi: 10.1016/j.ceb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164(2):313–323. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu VM, Yu MH, Paik R, Banerjee S, Liang Z, Paul SM, Bhat MA, Beitel GJ. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development. 2007;134(5):999–1009. doi: 10.1242/dev.02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Piontek J, Wolburg H, Piehl C, Liss M, Otten C, Christ A, Willnow TE, Blasig IE, Abdelilah-Seyfried S. Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion. Proc Natl Acad Sci U S A. 2010 Apr;107:1425–1430. doi: 10.1073/pnas.0911996107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]