Abstract
Telomeres are DNA-protein complexes that cap linear DNA strands, protecting DNA from damage. When telomeres critically shorten, cells become susceptible to senescence and apoptosis. Telomerase, a cellular ribonucleoprotein enzyme, rebuilds the length of telomeres and promotes cellular viability. Leukocyte telomeres are reportedly shortened in major depression, but telomerase activity in depression has not been previously reported. Further, there are no published reports of the effects of antidepressants on telomerase activity or on the relationship between telomerase activity and antidepressant response. Peripheral blood mononuclear cell (PBMC) telomerase activity was assessed in 20 medication-free depressed individuals and 18 controls. In total, 16 of the depressed individuals were then treated with sertraline in an open-label manner for 8 weeks, and PBMC telomerase activity was reassessed in 15 of these individuals after treatment. Pre- and post-treatment symptom severity was rated with the Hamilton Depression Rating Scale. All analyses were corrected for age and sex. Pretreatment telomerase activity was significantly elevated in the depressed individuals compared with the controls (P = 0.007) and was directly correlated with depression ratings (P< 0.05) across all subjects. In the depressed group, individuals with relatively lower pretreatment telomerase activity and with relatively greater increase in telomerase activity during treatment, showed superior antidepressant responses (P < 0.05 and P < 0.005, respectively). This is the first report characterizing telomerase activity in depressed individuals. PBMC telomerase activity might reflect a novel aspect of depressive pathophysiology and might represent a novel biomarker of antidepressant responsiveness.
Keywords: antidepressant, cell aging, depression, leukocytes, telomeres, telomerase
Introduction
Major depressive disorder (MDD) is among the leading causes of disability worldwide,1 but its underlying pathology is poorly understood. Research is now aimed at characterizing its pathophysiology on a cellular and molecular level.2,3 A novel locus of cellular pathology, leukocyte telomere shortening, has recently been proposed.4–7 Premature telomere shortening could suggest an accelerated rate of cell aging in MDD,4,6–8 which could have important health consequences. Telomeres are DNA-protein complexes that cap the ends of the linear chromosomal DNA, protecting chromosomes from instability. When telomeres shorten to a critical length, cells become susceptible to senescence and apoptosis.9–12 Telomere length is determined by the balance between telomere shortening stimuli (for example, mitotic divisions and exposure to inflammation and oxidation) and telomere lengthening or reparative stimuli.9,10,12–15 A major enzyme responsible for protecting, repairing and lengthening telomeres is telomerase, a ribonucleoprotein enzyme that elongates telomeric DNA, thereby counteracting telomere shortening and maintaining cellular viability. In addition, telomerase has non-telomeric actions, such as regulating the transcription of growth factors and stimulating cell growth in adverse conditions.12,16–22
Recent studies have suggested prematurely shortened leukocyte telomeres in individuals with MDD,4,5,7 chronic stress,8,13,23–26 histories of childhood adversity or maltreatment27,28 and several diseases associated with aging.8,23,29–40 Few studies have characterized telomerase activity in these conditions, and no studies have characterized telomerase activity in MDD. Genetic studies with humans having inherited disease caused by mutated genes encoding telomerase components have shown that low telomerase activity levels can be an important factor limiting leukocyte telomere length.11,41,42 In healthy humans, telomerase activity levels positively correlate with telomere length among different subsets of resting lymphocytes,43 as telomerase promotes longer telomere length. However, it is not known whether this relationship between resting telomerase activity and telomere length observed cross sectionally is modulated by disease states, and/or affects disease processes. Determination of telomerase activity is therefore important to understanding the reported shortening of leukocyte telomeres in MDD to assess whether the reduced telomere length is due to increased shortening or decreased length replenishment by telomerase.
It is possible that either high or low telomerase activity could be associated with shortened telomeres. Low telomerase activity could cause shortened telomeres by limiting the replenishment of shortened telomeres.11,41,42 Conversely, unusually high telomerase activity could represent a compensatory attempt to maintain telomere length in the face of cellular stress.24,44 Further, as telomerase has a number of poorly understood non-telomeric functions,16–21 abnormal telomerase activity in depression could result in unanticipated consequences.
The purpose of this study was to compare peripheral blood mononuclear cell (PBMC) telomerase activity in unmedicated individuals with MDD to matched healthy controls, to determine whether the pre-treatment level of PBMC telomerase activity is altered in depressed individuals, whether it predicts antidepressant response and whether antidepressant-induced changes in PBMC telomerase activity are related to treatment response.
Subjects and methods
Subjects
A total of 20 subjects with MDD, diagnosed using the Structured Clinical Interview for DSM-IV-TR,45 and 18 matched healthy controls were recruited and gave informed consent to participate. The protocol and consent form were approved by the University of California, San Francisco Committee on Human Research. Depressed subjects were all outpatients; they and the controls were recruited by fliers, Craigs-list postings (http://sfbay.craigslist.org), newspaper advertisements and, in the case of depressed subjects, clinical referrals. Subjects were paid for their participation, and depressed subjects received free anti-depressant treatment during the treatment phase of the study (described below). Depressed subjects with psychosis or bipolar histories were excluded, although comorbid anxiety disorders were allowed when the depressive diagnosis was considered to be primary, with the exception of post-traumatic stress disorder, which was excluded as it may have differences in stress hormone regulation.46 Healthy controls were required to have no present or past history of any DSM-IV Axis I or Axis II diagnosis. Potential subjects were also excluded if they met Structured Clinical Interview for DSM-IV-TR criteria for alcohol or substance abuse within 6 months of entering the study. Subjects in both groups were medically healthy and had not had any vaccinations within 6 weeks of entering the study. All subjects (depressed and control) were free of psychotropic medications, including antidepressants, antipsychotics and mood stabilizers, as well as hormone supplements, steroid-containing birth control or other interfering medications (for example, statins) or vitamin supplements above the United States recommended daily allowances, for a minimum of 6 weeks before entry into the study (with the exception of short-acting sedative-hypnotics, as needed, up to a maximum of 3 times per week, but none within 1 week of testing).
Procedure
Subjects were admitted as outpatients to the University of California, San Francisco Clinical and Translational Science Institute at 0800 hours, having fasted (except water) since 2200 hours the night before. On the morning of testing, all subjects were required to test negative on a urine toxicology screen (measuring the presence of drugs of abuse) and in women of childbearing capacity, a urine pregnancy test. After the subjects had sat quietly for 45 min to ensure an unstimulated resting baseline, blood samples were obtained for PBMC telomerase activity. Depressed subjects were then rated with the 17-item version of the observer-rated Hamilton Depression Rating Scale (HDRS),47 and the depressed subjects and controls completed the self-rated Inventory of Depressive Symptomatology48 and Perceived Stress Scale.49
Following this baseline day, 16 depressed subjects completed 8 weeks of open-label outpatient treatment with sertraline. Of the original group of 20 depressed subjects, one withdrew consent before treatment, two were dropped by the investigators because of the development of exclusion criteria (acute medical illnesses), and one dropped out by mutual agreement of the subject and the investigators because of depressive worsening during treatment, leaving 16 subjects completing 8 weeks of treatment. Sertraline dosing began with 50mg per day, increasing to a maximum of 200mg per day, as tolerated and as warranted by clinical response. In two cases, the beginning dose was initially lowered to 25mg per day because of transient side effects. Medication compliance was monitored by pill counts and by plasma antidepressant levels (described below) at week 4 and week 8 of treatment. Following 8 weeks of sertraline treatment, depressed subjects were readmitted as outpatients to the Clinical and Translational Science Institute at 0800 hours and followed a procedure for assessing depression and telomerase activity identical to that used on the baseline day. One subject failed to have telomerase activity assayed at week 8, leaving 15 depressed subjects with week 8 telomerase data.
Assays
PBMC collection and extraction
We performed several pilot studies assessing collection and freezing protocols that yielded consistent telomerase activities. The most reliable methods for obtaining PBMC’s and assessing telomerase activities use Cell Preparation Tubes (Becton-Dickinson, Franklin Lakes, NJ, USA, Vacutainer CPT), which contain a Ficoll separation gradient. Cells are counted using a hemocytometer and are resuspended and lysed with 1× CHAPS buffer (10mM Tris-HCl, pH 7.5, 1mM MgCl2, 1mM EGTA, 0.1mM Benzamidine, 5mM β-mercaptoethanol, 0.5% CHAPS and 10% glycerol). An extract corresponding to 10 000 cells ml−1 was made and was stored frozen at −80 °C until all samples were ready for the telomerase activity assay (to minimize inter-assay variability). Storing lysed cell extracts in CHAPS buffer, rather than storing whole cells live, eliminates the problem of the potentially variable reductions in cell viability that can result from different durations of storage time, and thus results in more accurate telomerase measures.
Telomerase activity assay (telomere repeat amplification protocol)
We optimized the telomerase activity assay on the basis of the commercially available kit TRAPeze (Chemicon, Temecula, CA, USA). Two concentrations (5000 and 10 000 cells) were used for Telomeric Repeat Amplification Protocol (TRAP) reactions to ensure that the assay for each sample is in the linear range. The reaction follows the manufacturer’s protocol. Products are separated on 10% polyacrylamide 8M urea gels, exposed to phosphorimager screen overnight and scanned using a phosphorimager, and quantified using ImageQuant software (GE Healthcare, Piscataway, NJ, USA). In all, 293T cells are used as positive controls. Telomerase activity is defined as 1 unit = the amount of product from one 293T cell/10 000 PBMC’s. Details of the telomerase activity assay method can be found in Lin et al.43 Quality control: In addition to the 293T cancer cell extract as the standard for telomerase activity quantification, we also include two control extracts from resting PBMC’s in each run. Measurement of 24 resting PBMC samples on different days produced an inter-assay CV of 6.8%.
Statistics
We first assessed the impact of age, sex, body mass index, lifetime and current tobacco use and alcohol use as potential confounds. In the controls, but not in the depressed subjects, a negative relationship existed between age and baseline telomerase activity (r =−0.54 and P < 0.05). This relationship showed a non-significant trend across the overall sample (r =−0.27 and P = 0.096). In the control group, but not in the overall sample or in the depressed subjects, there was a non-significant trend for sex to be related to baseline telomerase activity (men greater than women) (r = 0.40 and P = 0.098). To be conservative, all analyses reported here were controlled for both age and sex. Before analyzing the data, distributions were examined for normality, and non-normal distributions were natural log (Ln) transformed.
Between-group comparison of the demographic variables was by independent sample t-tests, Chi square and independent sample Kruskal–Wallis tests. Other between-group data were analyzed by analysis of covariance when the covariates of age and sex were applied, and paired t-tests were used for within-group comparisons. Correlations between variables were assessed by hierarchical linear regression, with age and sex entered as the first independent variables. Within-group correlations were by Pearson or Spearman’s correlation coefficients. All tests were two-tailed with an α = 0.05.
For purposes of characterizing response to anti-depressant treatment, ‘Responders’ were defined as subjects whose week 8 HDRS ratings improved by ≥50% relative to baseline, and ‘Non-responders’ as those with lesser degrees of improvement.
Results
Demographics
The mean lifetime duration of depression in the depressed subjects was 170.6±143.0 months (median: 112.1 months; range: 9–426 months). The depressed and control subjects did not significantly differ in age, sex, body mass index, lifetime and current tobacco use and alcohol use, although average annual household income was higher in the controls than in the depressed subjects (P < 0.02), and average activity and exercise levels (assessed with the Yale Physical Activity Survey50) were lower in the depressed sample than in the controls (P < 0.01). Across groups, annual household income was inversely correlated with baseline telomerase activity (r =−0.50 and P < 0.005) due to the strong relationship between MDD status and annual household income. Within the control and depressed groups separately, annual household income was not significantly correlated with telomerase activity (P > 0.20). Activity and exercise levels were not significantly correlated with baseline telomerase activity or with changes in telomerase activity with antidepressant treatment (P > 0.20). Demographic characteristics of the subjects are provided in Table 1.
Table 1.
Demographic characteristics of depressed and control subjects
| Controls (n = 18) | Depressed (n = 20) | P | |
|---|---|---|---|
| Age (years)±s.d. | 34.8±9.6 | 37.0±10.8 | ns |
| Sex (female, N (%)) | 12 (67) | 13 (65) | ns |
| Ethnicity (N (%)) | ns | ||
| Caucasian | 12 (67%) | 14 (70%) | |
| African-American | 3 (17%) | 2 (10%) | |
| Hispanic | 1 (6%) | 2 (10%) | |
| Asian | 2 (10%) | 2 (10%) | |
| Body mass index (kg m−2) | 24.8±3.9 | 26.2±5.7 | ns |
| Used tobacco ever (N (%)) | 10 (56) | 7 (35) | ns |
| Use tobacco currently (N (%)) | 4 (22) | 6 (30) | ns |
| Alcohol occasions per month | 3.7±4.5 | 4.9±8.1 | ns |
| Alcohol drinks per occasion | 1.9±1.1 | 1.5±1.2 | ns |
| Subjective socioeconomic statusa | 6.50±1.18 | 5.75±1.60 | ns |
| Years of education | 14.8±2.3 | 14.6±2.01 | ns |
| Annual household income ($) | $59 775±32 550 | $29 225±26 005 | 0.02 |
| Physical activity levelb | 3.11±0.90 | 2.10±1.26 | < 0.01 |
Subjective socioeconomic status was measured using a 10-rung ladder version of the MacArthur scale of subjective social status.75
Physical activity level was measured with the Yale Physical Activity Survey (YPAS).50 On this scale, 1 = not very active; 2 = weekend/vacations only; 3 = more than 1–2 times per week; 4 = more than 3 times per week. Other measures on the YPAS, such as ‘Vigorous Activity’ and ‘Duration of Vigorous Activity’ yielded similar differences between groups.
Baseline telomerase activity
Baseline PBMC telomerase activity was significantly elevated in the depressed sample (10.78±5.73 units/10 000 cells) compared with the controls (7.19±5.01 units/10 000 cells) (F = 8.35 and P = 0.007) (Figure 1). With depression and stress severity considered as continuous variables across all subjects, baseline PBMC telomerase activity was positively correlated with Inventory of Depressive Symptomatology (r = 0.36 and P < 0.05) and Perceived Stress Scale ratings (r = 0.36 and P < 0.05). Specifically, individuals with higher depression and stress ratings had higher baseline PBMC telomerase activity.
Figure 1.
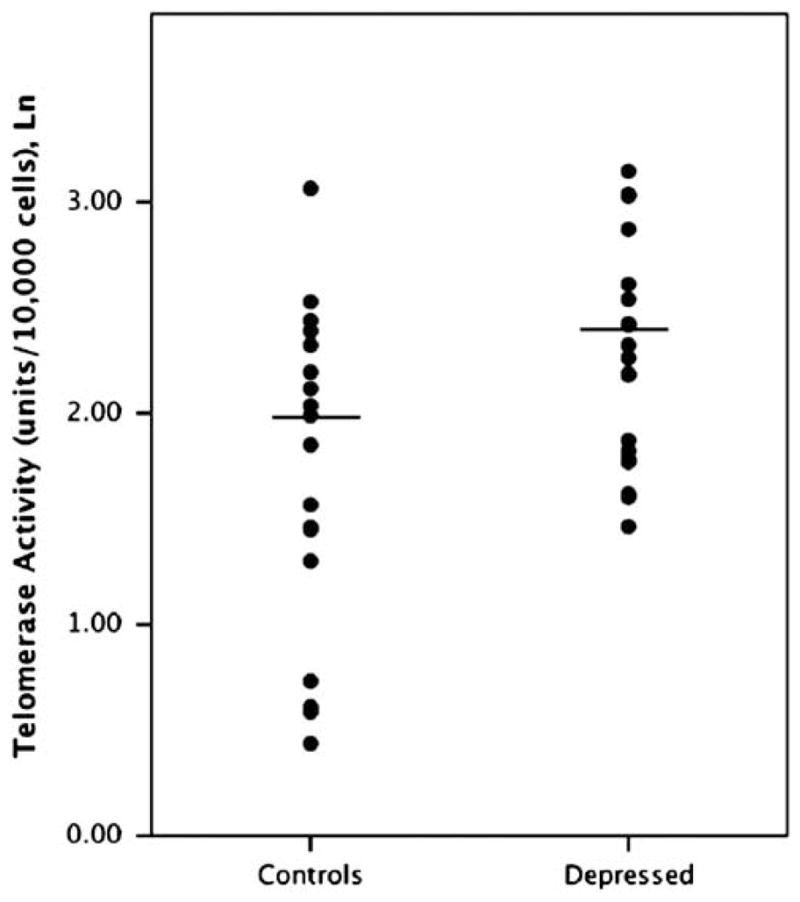
Peripheral blood mononuclear cell (PBMC) telomerase activity (Ln) in unmedicated individuals with major depression vs matched healthy controls (P = 0.007). Horizontal lines indicate the means.
As expected, HDRS ratings significantly declined (improved) over the 8-week course of sertraline treatment (mean±s.d. baseline: 18.50±3.65; week 8: 10.19±4.62; paired t = 5.6 and, P = 0.000), representing changes ranging from 27% worsening to 82% improvement (mean improvement = 42.3±31.0%). As a medication compliance check, plasma antidepressant levels (and their metabolites) were ascertained in the depressed subjects who were treated with sertraline. The mean plasma concentration of (sertraline+N-desmethylsertraline) at week 4 was 46±23 ng ml−1; range: 10–97 ng ml−1, and at week 8 was 67±37 ng ml−1; range: 10–146 ng ml−1. All individuals had plasma concentrations within the range of published steady state concentrations for sertraline at therapeutic doses,51 indicating good compliance with medication treatment. There were no significant correlations between concentrations of sertraline, N-desmethylsertraline, or the combination of (sertraline+N-desmethylsertraline) with changes in HDRS ratings or with changes in PBMC telomerase levels (P>0.30 in all cases).
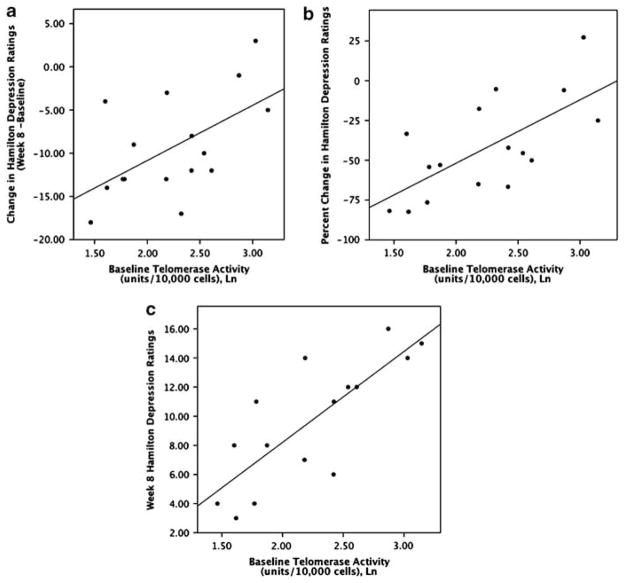
Baseline telomerase activity prediction of antidepressant response
Within the depressed sample, lower baseline (pretreatment) PBMC telomerase activity was directly correlated with decreases (that is, improvements) in HDRS ratings over the course of treatment (week 8 ratings minus baseline ratings), whether considered as the absolute change in HDRS ratings (r = 0.57 and P < 0.03) (Figure 2a) or as the percentage change in HDRS ratings (r =−0.74 and P = 0.002) (Figure 2b). Similarly, lower baseline PBMC telomerase activity was directly correlated with lower absolute HDRS ratings (indicating less depression) at the end of treatment (week 8) (r = 0.80 and P = 0.001) (Figure 2c). When ‘Responders’ and ‘Non-responders’ were compared, the Responders had significantly lower baseline telomerase activity than Non-responders (Responders: 7.67±3.27 units/10 000 cells; Non-responders: 13.69±6.25 units/10 000 cells; F = 8.51; P < 0.02) (Figure 3). These results cumulatively indicate superior antidepressant responses in individuals with lower baseline PBMC telomerase activity. The one depressed subject who prematurely dropped out of the study due to clinical worsening while on sertraline had the second highest pre-treatment PBMC telomerase activity of the depressed group (20.8 units/10 000 cells).
Figure 2.
Correlation between pre-treatment PBMC telomerase activity (Ln) and sertraline treatment-associated: (a) absolute changes in Hamilton Depression Rating Scale (HDRS) ratings (P < 0.03), (b) percent changes in HDRS ratings (P < 0.002) and (c) end-point HDRS ratings after 8 weeks of treatment (P < 0.001). Lower numbers on the Y-axis indicate superior antidepressant responses.
Figure 3.
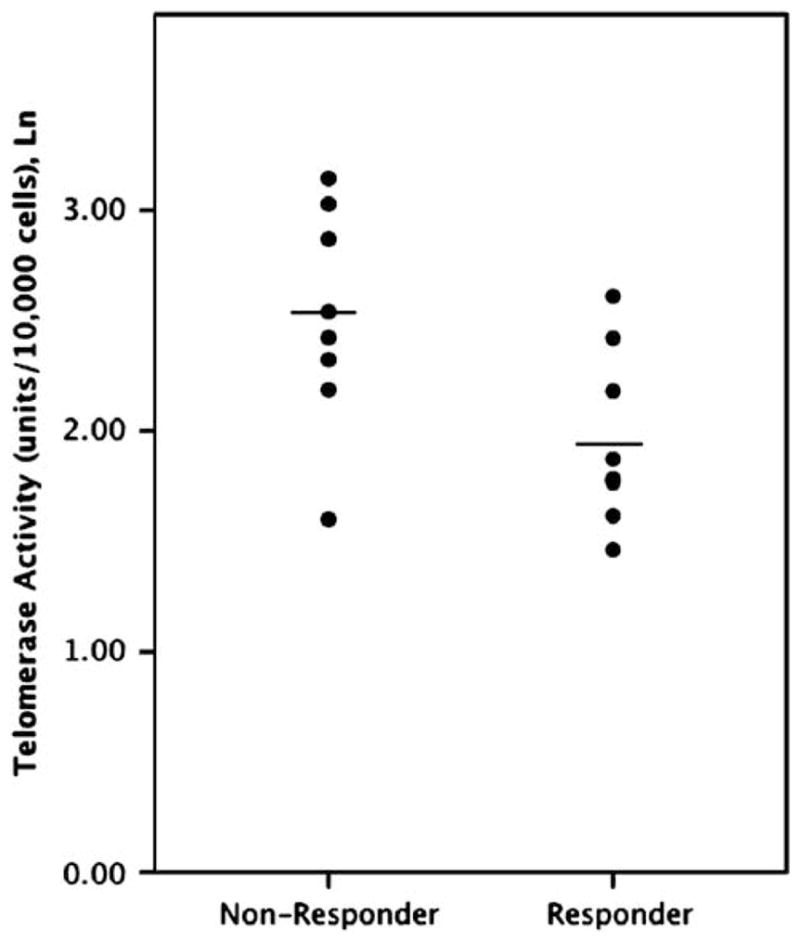
Pre-treatment (baseline) PBMC telomerase activity (Ln) in depressed individuals who were responders vs non-responders after 8 weeks of sertraline treatment. ‘Responders’ are defined as subjects whose week 8 HDRS ratings improved by ≥50% relative to baseline, and ‘Non-responders’ as those with lesser degrees of improvement. Horizontal lines indicate the means.
Antidepressant-associated changes in telomerase activity
Average PBMC telomerase activity did not significantly change with antidepressant treatment (baseline: 10.51±6.10 units/10 000 cells; end of week 8: 9.14±6.97 units/10 000 cells, paired t = 0.75, ns). The range of change in telomerase activity was from a decrease of 13.19 units/10 000 cells to an increase of 13.55 units/10 000 cells. Those who started off with lower telomerase activity tended to show an increase with treatment (r =−0.41 and P = 0.12), but baseline activity levels only accounted for ~17% of the variance in changes in telomerase activity. Treatment-associated changes in PBMC telomerase activity (week 8 activity minus baseline activity) were inversely correlated with changes in HDRS ratings (rs =−0.68 and P = 0.004) (Figure 4). Specifically, greater increases in telomerase activity occurring during the treatment period were associated with larger decreases (improvements) in HDRS ratings.
Figure 4.
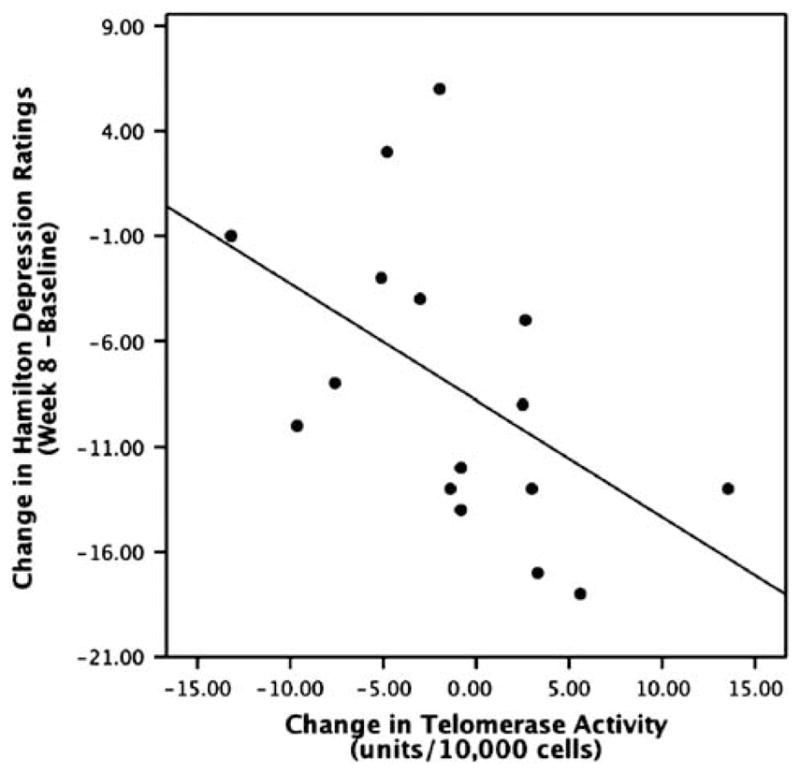
Correlation between treatment-associated changes in PBMC telomerase activity (week 8 minus baseline) and treatment-associated changes in Hamilton Depression Rating Scale (HDRS) ratings (P < 0.004). Treatment consisted of 8 weeks of open-label sertraline treatment. Higher numbers on the X-axis indicate greater increases in telomerase activity. Lower numbers on the Y-axis indicate superior antidepressant responses.
We then assessed the combined effect of having both low baseline telomerase activity and greater treatment-associated increases in telomerase activity. Therefore, we created four groups using median splits on baseline and change in telomerase activity. In addition to lower pre-treatment telomerase activity and greater increases in telomerase activity after 8 weeks of treatment individually predicting superior drug response, individuals with both of these characteristics showed better average antidepressant responses than did individuals with only one or neither characteristic (F = 8.64 and P < 0.02).
Exploratory analyses
To explore possible mechanisms contributing to increased telomerase activity in MDD, we examined correlations between PBMC telomerase activity and peripheral markers of oxidative stress and inflammation, both of which may contribute to changes in telomerase activity.52–57 In the combined groups, as well as in the separate control and depressed groups, there were no significant correlations between PBMC telomerase activity and plasma F2-isoprostanes (an oxidized lipid marker), 8-hydroxydeoxyguanosine (an oxidized DNA marker), ascorbic acid (an anti-oxidant), interleukin-6 or C-reactive protein concentrations (P > 0.20 in all cases).
In another set of exploratory analyses, we assessed the relationship between PBMC telomerase activity and telomere length (the telomere length determination methods and data are reported separately7). We did not have an a priori prediction regarding the relationship between telomerase activity and telomere length, as different results have been reported in the literature,13,24 and telomerase activity and telomere length show markedly different rates of change. (Telomerase activity changes much more rapidly than telomere length40,58). In the combined groups, as well as in the separate control and depressed groups, there was no significant relationship between telomerase activity and telomere length (r = 0.02, 0.05 and 0.01, respectively). As these measures were not significantly correlated in our sample, discussion of differences in telomere length is beyond the scope of this report and are reported separately.7
Discussion
We found that resting (unstimulated) PBMC telomerase activity was significantly higher in unmedicated depressed individuals compared with matched healthy controls. The elevated telomerase activity was more apparent in those depressed individuals who responded more poorly to 8 weeks of antidepressant treatment. We also found that depressed individuals with greater increases in PBMC telomerase activity during the treatment period had better responses to the antidepressant. Specifically, depressed individuals with relatively lower baseline (pre-treatment) telomerase activity, and those whose telomerase activity increased the most with antidepressant treatment, showed the greatest benefit from antidepressant treatment. Given the relatively small sample size, it is not possible to estimate the independence of the effects of baseline telomerase activity and changes in telomerase activity in predicting treatment response. However, individuals with relatively low baseline telomerase activity and relatively greater increases in telomerase activity with treatment showed especially good antidepressant responses. These findings are novel and need to be replicated with a larger sample, but they do raise the possibility of the involvement of telomerase, a cell viability promoting enzyme, in depression and in antidepressant responses.
Our finding of higher PBMC telomerase activity in depressed individuals is consistent with related findings from some studies, but not others, although, to our knowledge, ours is the first study to assess telomerase activity in MDD. In a study of chronically stressed (but generally not depressed) maternal caregivers, decreased PBMC telomerase activity was observed along with shortened leukocyte telomeres.13 However, in another study, stressed caregivers had shortened PBMC telomeres along with elevated telomerase activity.24 The caregivers in that study had higher depression ratings than controls, but it is not known how many, if any, had MDD. The authors of that study interpreted the elevated telomerase activity as ‘an unsuccessful attempt to compensate for the excessive loss of telomeres’.24 The possibility that increased telomerase activity reflects a compensatory response to cellular damage is consistent with preclinical and clinical data suggesting that telomerase preferentially elongates shorter telomeres,59–61 and that telomerase reverse transcriptase (the catalytic subunit of telomerase) is induced in response to certain types of cell injury, such as ischemic injury in brain cells.22,62
The prediction of antidepressant response by PBMC telomerase activity, both before treatment and during treatment, has not previously been reported. We found that relatively low telomerase activity before treatment, and relatively greater increases in telomerase activity with treatment, predicted better response to antidepressants. It is possible that telomerase activation is beneficial in the treatment of depression, as suggested by the observed correlation between telomerase activity increases and degree of antidepressant response. Especially high baseline telomerase activity, however, could indicate more underlying depression-related pathology, which could have induced greater telomerase activation. 22,44,63 Individuals with the highest pre-treatment telomerase activity may have already sustained as much benefit as is possible from endogenous telomerase activation, whereas those individuals with normal or only mildly elevated telomerase activity at baseline may be more likely to benefit from exogenously induced telomerase activation (that is, from medication). Consistent with the possibility that telomerase activation is beneficial in depression, several recent studies have suggested that healthful lifestyle changes,64 moderate physical exercise65,66 and extended periods of mindfulness meditation67 increase PBMC telomerase activity. In the former study, increases in telomerase activity were significantly correlated with decreases in psychological distress.64 For now, explanations of our findings remain speculative, although they lay the foundation for replication studies and for future prospective mechanism-oriented studies.
In our exploratory analysis, we did not find significant relationships between telomerase activity and telomere length. The reasons for this are unknown, but telomerase activity and telomere length change along very different time lines and under different circumstances, and thus may be unrelated. 40,58 Other reasons for the lack of significant correlations between telomere length and telomerase activity have been proposed,68 including the shuttling of telomerase from the nucleus to the mitochondria under conditions of oxidative stress,69,70 and the possibility that telomerase may decrease the number of PBMC’s with ‘short’ telomeres without significantly altering average PBMC telomere length.61 It is possible that depression-related inflammation and/or oxidative stress contributed to our findings, as these may be associated both with shortened telomeres7,15,71 and with stimulatory effects on telomerase activity52–56 (although see ref. 57). Our other exploratory analysis, however, did not support this interpretation. In sum, it is not known whether the increase in telomerase activity seen in depression reflects a salutary process (attempted protection of telomeres and recovery of telomere length), a deleterious one (direct stimulation by cytotoxic processes) or both.
The major strengths of the present study are the use of physically healthy, well characterized subjects, the exclusion of medications that might have influenced our results, the inclusion of a plasma concentration-verified antidepressant treatment component and the use of a sensitive and quantitative telomerase assay that measures activity in unstimulated PBMC’s. The major limitations are the small sample size and the open-label nature of the antidepressant treatment. Another limitation is the lack of determination of telomerase activity in specific PBMC cell types,43 as differences in PBMC telomerase activity could result either from telomerase activity changes on a per-cell basis or from different proportions of circulating PBMC types, with certain cell types having different degrees of telomerase activity than others. This possibility remains to be tested in future studies.
If the present and other emerging data are replicated, the telomere/telomerase system may emerge as a novel site of pathophysiology in MDD. Future studies would then be needed to ascertain the clinical significance of these changes and whether direct manipulation of this system might hold promise for novel therapeutics, as has been proposed for other illnesses involving telomere/telomerase dysregulation.6,42,61,72–74
Acknowledgments
The authors gratefully acknowledge the assistance of Steven Hamilton, MD, PhD (UCSF), who assisted in evaluating the depressed subjects, of Jean Tillie (Stanford), who performed cytokine assays, of Yali Su, PhD (Kronos) and the lab of Jason Morrow, MD (Vanderbilt), who performed oxidation assays, of Alanie Lazaro and Genevieve Manalo (both UCSF), who assisted in study procedures, laboratory collections and assays, and the nursing and other staff of the UCSF CTSI’s Clinical Research Center. This study was funded by an NIMH R01 grant (R01 MH083784), a grant from the O’Shaughnessy Foundation and grants from the UCSF Academic Senate, the UCSF Research Evaluation and Allocation Committee (REAC) and the Bernard and Barbro Osher Foundation. This project was also supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. None of the granting or funding agencies had a role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, or approval of the manuscript. The Principal Investigator, Owen Wolkowitz, MD, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Financial Disclosures
1. Drs Elizabeth Blackburn, Elissa Epel and Jue Lin are co-founders of Telome Health Inc., a diagnostics company related to telomere biology, and they own stock in the company.
2. Drs Elizabeth Blackburn, Elissa Epel, Jue Lin, Owen Wolkowitz and Synthia Mellon, on behalf of the Regents of the University of California (who will be assignees of the patent), have applied for a patent covering the use of cell aging markers (including telomerase activity) as a biomarker of depression.
Clinical Trials Registration: Registry name: Clinical-Trials.gov. URL: http://ClinicalTrials.gov Clinical-Trials.gov Identifier: NCT00285935.
References
- 1.Murray CJ, ADL Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 2.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch gen psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 3.Manji HK, Gottesman II, Gould TD. Signal transduction and genes-to-behaviors pathways in psychiatric diseases. Sci STKE. 2003;2003:pe49. doi: 10.1126/stke.2003.207.pe49. [DOI] [PubMed] [Google Scholar]
- 4.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Lung FW, Chen NC, Shu BC. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet. 2007;17:195–199. doi: 10.1097/YPG.0b013e32808374f6. [DOI] [PubMed] [Google Scholar]
- 6.Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27:327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- 7.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress. 2010. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 10.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 11.Effros RB. Kleemeier Award Lecture 2008–the canary in the coal mine: telomeres and human healthspan. jgerontol. 2009;64:511–515. doi: 10.1093/gerona/glp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr mol med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 15.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 16.Mattson MP, Fu W, Zhang P. Emerging roles for telomerase in regulating cell differentiation and survival: a neuroscientist’s perspective. Mech ageing dev. 2001;122:659–671. doi: 10.1016/s0047-6374(01)00221-4. [DOI] [PubMed] [Google Scholar]
- 17.Gorbunova V, Seluanov A. Telomerase as a growth-promoting factor. Cell cycle (Georgetown, TX) 2003;2:534–537. doi: 10.4161/cc.2.6.515. [DOI] [PubMed] [Google Scholar]
- 18.Zhu H, Fu W, Mattson MP. The catalytic subunit of telomerase protects neurons against amyloid beta-peptide-induced apoptosis. J Neurochem. 2000;75:117–124. doi: 10.1046/j.1471-4159.2000.0750117.x. [DOI] [PubMed] [Google Scholar]
- 19.Calado RT, Chen J. Telomerase: not just for the elongation of telomeres. Bioessays. 2006;28:109–112. doi: 10.1002/bies.20365. [DOI] [PubMed] [Google Scholar]
- 20.Geserick C, Blasco MA. Novel roles for telomerase in aging. Mech ageing dev. 2006;127:579–583. doi: 10.1016/j.mad.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Sung YH, Choi YS, Cheong C, Lee HW. The pleiotropy of telomerase against cell death. Mol Cells. 2005;19:303–309. [PubMed] [Google Scholar]
- 22.Kang HJ, Choi YS, Hong SB, Kim KW, Woo RS, Won SJ, et al. Ectopic expression of the catalytic subunit of telomerase protects against brain injury resulting from ischemia and NMDA-induced neurotoxicity. J Neurosci. 2004;24:1280–1287. doi: 10.1523/JNEUROSCI.4082-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotrschal A, Ilmonen P, Penn DJ. Stress impacts telomere dynamics. Biol Lett. 2007;3:128–130. doi: 10.1098/rsbl.2006.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood Maltreatment and Telomere Shortening: Preliminary Support for an Effect of Early Stress on Cellular Aging. Biol psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PloS one. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serrano AL, Andres V. Telomeres and cardiovascular disease: does size matter? Circ res. 2004;94:575–584. doi: 10.1161/01.RES.0000122141.18795.9C. [DOI] [PubMed] [Google Scholar]
- 30.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 31.Huzen J, de Boer RA, van Veldhuisen DJ, van Gilst WH, van der Harst P. The emerging role of telomere biology in cardiovascular disease. Front Biosci. 2010;15:35–45. doi: 10.2741/3604. [DOI] [PubMed] [Google Scholar]
- 32.Grodstein F, van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH, et al. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses’ health study. PloS one. 2008;3:e1590. doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circ res. 2006;99:1167–1180. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- 34.Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler thromb vasc biol. 2008;28:1379–1384. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 36.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 37.Aviv A. Telomeres and human somatic fitness. j gerontol. 2006;61:871–873. doi: 10.1093/gerona/61.8.871. [DOI] [PubMed] [Google Scholar]
- 38.Adaikalakoteswari A, Balasubramanyam M, Mohan V. Telomere shortening occurs in Asian Indian type 2 diabetic patients. Diabet Med. 2005;22:1151–1156. doi: 10.1111/j.1464-5491.2005.01574.x. [DOI] [PubMed] [Google Scholar]
- 39.Valdes AM, Richards JB, Gardner JP, Swaminathan R, Kimura M, Xiaobin L, et al. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int. 2007;18:1203–1210. doi: 10.1007/s00198-007-0357-5. [DOI] [PubMed] [Google Scholar]
- 40.Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging. 2009:81–88. doi: 10.18632/aging.100007. published on line: 12/19/08 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 42.Effros RB. Telomerase induction in T cells: A cure for aging and disease? Exp gerontol. 2007;42:416–420. doi: 10.1016/j.exger.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. J immunol methods. 2009;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Kong Q, Zhang Z, Ge P, Ba D, He W. Telomere dysfunction of lymphocytes in patients with Alzheimer disease. Cogn Behav Neurol. 2003;16:170–176. doi: 10.1097/00146965-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 45.First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Reserach Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute Biometrics Research; New York: 2002. [Google Scholar]
- 46.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann N Y Acad Sci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 48.Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The inventory for depressive symptomatology (IDS): preliminary findings. Psychiatry res. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 49.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 50.Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med sci sports exerc. 1993;25:628–642. [PubMed] [Google Scholar]
- 51.Mauri MC, Laini V, Cerveri G, Scalvini ME, Volonteri LS, Regispani F, et al. Clinical outcome and tolerability of sertraline in major depression: a study with plasma levels. Prog neuropsychopharmacol biol psychiatry. 2002;26:597–601. doi: 10.1016/s0278-5846(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 52.Akiyama M, Hideshima T, Hayashi T, Tai YT, Mitsiades CS, Mitsiades N, et al. Cytokines modulate telomerase activity in a human multiple myeloma cell line. Cancer Res. 2002;62:3876–3882. [PubMed] [Google Scholar]
- 53.Kawauchi K, Ihjima K, Yamada O. IL-2 increases human telomerase reverse transcriptase activity transcriptionally and posttranslationally through phosphatidylinositol 3′-kinase/Akt, heat shock protein 90, and mammalian target of rapamycin in transformed NK cells. J Immunol. 2005;174:5261–5269. doi: 10.4049/jimmunol.174.9.5261. [DOI] [PubMed] [Google Scholar]
- 54.Bettendorf O, Schmidt H, Eltze E, Rody A, Herchenroder F, Jackisch C, et al. Quantitative measurement of telomerase activity and localization of its catalytic subunit (hTERT) in chronic inflammation of capsule formation around various model implants and in sarcomas in a rat model. J biomed mater res. 2008;85:646–650. doi: 10.1002/jbm.a.31613. [DOI] [PubMed] [Google Scholar]
- 55.Nishikawa T, Nakajima T, Katagishi T, Okada Y, Jo M, Kagawa K, et al. Oxidative stress may enhance the malignant potential of human hepatocellular carcinoma by telomerase activation. Liver Int. 2009;29:846–856. doi: 10.1111/j.1478-3231.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 56.Liu DY, Peng ZH, Qiu GQ, Zhou CZ. Expression of telomerase activity and oxidative stress in human hepatocellular carcinoma with cirrhosis. World J Gastroenterol. 2003;9:1859–1862. doi: 10.3748/wjg.v9.i8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsirpanlis G, Chatzipanagiotou S, Boufidou F, Kordinas V, Zoga M, Alevyzaki F, et al. Serum oxidized low-density lipoprotein is inversely correlated to telomerase activity in peripheral blood mononuclear cells of haemodialysis patients. Nephrology (Carlton) 2006;11:506–509. doi: 10.1111/j.1440-1797.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- 58.Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, et al. Dynamics of telomerase activity in response to acute psychological stress. Brain behav immun. 2010;24:531–539. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hug N, Lingner J. Telomere length homeostasis. Chromosoma. 2006;115:413–425. doi: 10.1007/s00412-006-0067-3. [DOI] [PubMed] [Google Scholar]
- 60.Britt-Compton B, Capper R, Rowson J, Baird DM. Short telomeres are preferentially elongated by telomerase in human cells. FEBS letters. 2009;583:3076–3080. doi: 10.1016/j.febslet.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 61.Harley CB. Telomerase therapeutics for degenerative diseases. Curr mol med. 2005;5:205–211. doi: 10.2174/1566524053586671. [DOI] [PubMed] [Google Scholar]
- 62.Baek S, Bu Y, Kim H, Kim H. Telomerase induction in astrocytes of Sprague-Dawley rat after ischemic brain injury. Neurosci lett. 2004;363:94–96. doi: 10.1016/j.neulet.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 63.Zhang P, Dilley C, Mattson MP. DNA damage responses in neural cells: focus on the telomere. Neuroscience. 2007;145:1439–1448. doi: 10.1016/j.neuroscience.2006.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 65.Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120:2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- 66.Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med sci sports exerc. 2008;40:1764–1771. doi: 10.1249/MSS.0b013e31817c92aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.09.010. (in press) [DOI] [PubMed] [Google Scholar]
- 68.Calcagnile O, Gisselsson D. Telomere dysfunction and telomerase activation in cancer–a pathological paradox? Cytogenet genome res. 2007;118:270–276. doi: 10.1159/000108310. [DOI] [PubMed] [Google Scholar]
- 69.Saretzki G. Telomerase, mitochondria and oxidative stress. Exp gerontol. 2009;44:485–492. doi: 10.1016/j.exger.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, et al. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J cell sci. 2008;121(Part 7):1046–1053. doi: 10.1242/jcs.019372. [DOI] [PubMed] [Google Scholar]
- 71.Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, et al. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 72.Tarkanyi I, Aradi J. Pharmacological intervention strategies for affecting telomerase activity: future prospects to treat cancer and degenerative disease. Biochimie. 2008;90:156–172. doi: 10.1016/j.biochi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Samper E, Flores JM, Blasco MA. Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc−/− mice with short telomeres. EMBO reports. 2001;2:800–807. doi: 10.1093/embo-reports/kve174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harley CB, Liu W, Blasco M, Vera E, Andrews WH, Briggs LA, et al. A Natural Product Telomerase Activator As Part of a Health Maintenance Program. Rejuvenation research. 2010 doi: 10.1089/rej.2010.1085. (e-pub Ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adler N, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]