Abstract
Stem cells mediate tissue repair throughout the lifespan of an organism. However, the ability of stem cells to mitigate catastrophic damage, such as that sustained after major myocardial infarction is inadequate to rebuild the heart and restore functional capacity. However, capitalizing on the ability of these cells to attenuate damage in the myocardium, various maneuvers that enhance repair mechanisms to improve cardiac structure and function after injury are being investigated. These studies have led to discovery of various factors that mediate cardioprotection and enhance endogenous repair by 1) salvaging surviving myocardium, 2) promoting homing of stem cells and 3) increasing survival and proliferation of stem cell populations at the site of injury. Herein we report upon a downstream target of Akt kinase, named Pim-1, which promotes cardioprotective signaling and enhances cardiac structure and function after pathological injury. The compilation of studies presented here supports use of Pim-1 to enhance long-term myocardial repair after pathological damage.
I. Pim-1 Kinase
The use of cardioprotective molecules to mitigate pathologic injury has been extensively studied in the myocardial context, particularly with respect to the serine/threonine kinase known as Akt [1, 2]. In comparison the cardioprotective actions of Pim-1, a downstream target of Akt activity with substrate specificities similar in many respects to Akt have just begun to be investigated. Pim-1 kinase was initially identified as the Proviral Insertion site for Moloney Murine Leukemia Virus in mouse T cell lymphomas [3]. Pim-1 belongs to a family of constitutively active serine/threonine kinases, including Pim-2 and - 3 [4], which have the ability to auto-phosphorylate at Ser-190, Thr-205 and Ser-4 [5–7]. Pim-1 expression promotes cell cycle progression, proliferation, and survival in the context of hematopoiesis and cancer [8, 9]
Seminal studies describing the function of Pim-1 in vivo were conducted in the hematopoetic system in the context of lymphomagenesis. Initial studies investigating Pim-1 demonstrated that B cell lymphomas [10], erythroleukemias [11], and T cell lymphomas [12, 13] all carry elevated levels of Pim-1. Interestingly, subsequent studies revealed that overexpression of Pim-1 alone is not sufficient to induce transformation [14, 15]. Rather, the cooperation of other oncogenic stimuli must also be present, including BCR/ABL [16], c-Myc, bmi-1 and gfi-1 [17, 18]. High incidence of overexpression in lymphomas as well as some endogenous expression in healthy hematopoetic cells led to an investigation into other cell types that express Pim-1 under normal conditions. In humans, Pim-1 is highly expressed in the liver and spleen during fetal development but down regulated upon maturation [19]. Pim-1 is expressed in the brain and central nervous system during mouse development, thus highlighting the importance of Pim-1 in cellular proliferation and differentiation [14].
In the context of the myocardium, Pim-1 protein and transcript is highly expressed in the neonatal heart and progressively decreases as the heart ages indicative of postnatal developmental regulation [20]. Localization of Pim-1 expression is primarily nuclear in myocytes of young mouse hearts but predominantly cytoplasmic as the mouse ages [20]. Mechanisms governing Pim-1 cellular localization are unknown at present. Some studies suggest that Pim-1 nuclear localization is necessary for its biological function [21], however other studies demonstrate a potent protective effect for Pim-1 by regulating proteins predominantly in the cytoplasm [22]. Furthermore, other studies suggest Pim-1 may promote proliferation in the nuclear compartment while cytoplasmic localization may promote survival [20]. Therefore, it is likely that Pim-1 controls a variety of cellular processes depending on its localization.
Pim-1 protein is ubiquitously expressed throughout the murine myocardium in all cardiac cell types including cardiac progenitor cells (CPC) where it appears to promote proliferation [20, 22–25]. The considerable increase in Pim-1 protein during heart failure led to further investigations of Pim-1 expression following myocardial pathology. Pim-1 levels increase after myocardial infarction (MI) and in hypertensive hearts of both humans and mice [20, 23, 25]. This reactivation is believed to be an attempt by the heart to promote endogenous regeneration or perhaps increase survival and proliferation in remaining cells. Interestingly, Pim-1 accumulates at the peri-nuclear partition in surviving myocytes after MI, which is thought to stimulate the pro-survival effects of Pim-1 and is in contrast to the nuclear localization seen during development [20]. In CPCs Pim-1 is predominantly nuclear after infarction and enhances regeneration after infarction [23]. In the context of pressure overload induced cardiomyopathy produced by trans-aortic constriction (TAC), Pim-1 is reactivated and localized at the peri-nuclear region of surviving myocytes similar to that seen after MI [25]. In contrast to the acute TAC results, Pim-1 is profoundly nuclear in hearts exhibiting chronic dilated cardiomyopathy as seen with transgenic mice overexpressing tropomodulin, suggesting multiple roles of Pim-1 during heart failure [20, 25].
Pim-1 is a pivotal beneficial mediator of cardioprotection activated downstream of Akt. In vitro studies demonstrate stimulation of neonatal rat cardiomyocytes (NRCMs) with the cardioprotective factors LIF, PMA, Dexamethasone or IGF-1 can induce expression of Pim-1 at both the protein and mRNA level. Adenoviral infection of NRCMs with dominant negative Akt attenuated Pim-1 expression after IGF-1 and Dexamethasone treatment, whereas overexpression of nuclear-targeted Akt induced Pim-1 expression. Moreover, hearts of genetically engineered mice that possessed deletion of Pim-1 were more susceptible to infarction damage despite substantial increases in Akt activity. Collectively, these results confirmed that Pim-1 was indeed a downstream target of Akt in the heart [20].
Cardioprotective effects elicited by Pim-1 were demonstrated when overexpression of wild type Pim-1 attenuated doxorubicin as well as deoxyglucose induced cell death. In line with this finding, overexpression of dominant negative Pim-1 induced cell death under basal conditions and exacerbated stress-induced apoptosis in NRCMs [20]. Further analysis of apoptotic signaling pathways in Pim-dominant negative expressing NRCMs revealed increased PARP and caspase-3 cleavage. In contrast, overexpression of Pim-WT in NRCMs upregulated Bcl-XL and Bcl-2 protein levels, and increased BAD phosphorylation on Ser112, whereas the overall abundance of BAD was unchanged [20]. These anti-apoptotic protective effects induced by Pim-1 correlate well with decreased mitochondrial swelling and preserved mitochondrial integrity in NRCM after hydrogen peroxide treatment [22]. Additionally, Pim-1 overexpression has been found to increase proliferation in a variety of cell types. In cardiac progenitor cells (CPCs), Pim-1 overexpression induced proliferation in vitro and in vivo [23, 24]. As well, in vascular smooth muscle cells (VSMC), platelet derived growth factor (PDGF) induced Pim-1 mediated proliferation [26]. In addition to promoting cell survival, Pim-1 also abrogated endothelin-induced hypertrophy in NRCMs in vitro [25].
These in vitro studies delineated a clear role for Pim-1 in the induction of protective signaling by promoting cell survival and proliferation, while inhibiting hypertrophic signaling [20]. To further characterize the potential of Pim-1 as a novel therapeutic to treat cardiovascular disease, transgenic mice were constructed to examine Pim-1 expression and cardioprotective signaling in vivo.
II. Lessons from genetically altered mice
Gain- and loss-in-function studies comprehensively characterized Pim-1 as a kinase responsible for cardioprotection both in vivo and in vitro. Similar to knockout of AKT, deletion of Pim-1 showed no overt cardiac phenotype at baseline and the hemodynamic performances of Pim-1 Knockout (Pim-1 KO) and wild–type (Pim-WT) control mice were comparable at baseline [20]. Mouse lines engineered with deletion of Pim-1 or triple knockouts, deficient for all Pim kinases, are viable without severe phenotypic effects [4]. Inactivation of Pim-1 kinase activity induced apoptosis in vivo in cardiomyocytes, but compensatory upregulation of Pim-2 and Akt appeared to disguise a cardiac phenotype under baseline conditions.
In contrast however, Pim-1 KO mice displayed deficiencies in contractile function and increased apoptotic cell death in response to cardiomyopathic injury such as myocardial infarction (MI) or transaortic constriction (TAC) banding [20, 25]. After TAC banding challenge, Pim-1 KO mice rapidly decompensate and display decreased contractile performance [25]. This could be attributed, at least in part, to increased cardiomyocyte apoptosis, as four weeks after TAC challenge Pim-1 KO mice showed significantly more apoptotic myocytes than their wild-type TAC counterparts. Consistently after infarction, the left ventricular free wall of Pim-1 KO mice exhibited increased infarct size together with deterioration of contractile function compared to control mice.
On the molecular level, under basal conditions deletion of Pim-1 leads to a minor, but significant, increase in TUNEL-positive myocytes in vivo compared to wild-type control mice, an effect that is aggravated after MI [20]. In line with these findings expression and phosphorylation of Bcl-2, Bcl-Xl, Bad and STAT3, key molecules involved in cell survival, were decreased in Pim-1 KO mice compared to wild-type control mice. Increased expression of Pim-2 and Pim-3, may compensate for Pim-1 loss, may explain the lack of a cardiomyopathic phenotype under basal conditions in the Pim-1-KO mice, whereas increased levels of Pim-2 and Pim-3 were insufficient to compensate for Pim-1 deficiency after pathological cardiac injury. In summary, in vivo data suggest that Pim-1 deletion impairs endogenous repair responses to either pressure overload or ischemic injury.
In contrast, cardiac specific overexpression of Pim-1 driven by an α-MHC promoter is cardioprotective after MI or TAC. Mice with cardiac specific overexpression of Pim-1 showed a significant decrease in the left ventricular infarct size after MI compared to non-transgenic hearts [20]. Importantly, contractile performance after MI was maintained in the Pim-1 transgenic mice, highlighting the therapeutic potential for Pim-1 in myocardial repair. Mechanistically, decreased apoptotic cell death after MI, together with enhanced contractile performance on isolated cardiomyocytes due to improved calcium cycling, explains at least in part, the improved cardiac performance in Pim-1 transgenic mice. Consistent with in vitro data, the anti-apoptotic effects of Pim-1 in vivo are linked to phosphorylation of Bad and inhibition of caspase cleavage. Accordingly, cardiomyocytes overexpressing Pim-1 exhibit increased levels of Bcl-2 and Bcl-xl [22].
In addition, mice with cardiac specific overexpression of Pim-1 are protected against pathologic remodeling and displayed preserved contractile function induced by pressure overload in TAC hearts. Pim-1 transgenic hearts after TAC surgery exhibit persistent blunting of cardiac hypertrophy, decreases in apoptosis and hypertrophic signaling markers, and maintain cardiac function for up to 10 weeks.
The protective role of Pim-1 appears to be dependent upon kinase activity, as shown by Pim-1 dominant negative (Pim-DN) transgenic animals [25]. Pim-DN hearts overexpress a kinase dead form of Pim-1 with a mutated ATP binding site [27]; thus only downstream phosphorylation events are inhibited without any compensatory signaling that may occur in Pim-1 null mice. Pim-DN transgenic mice develop a cardiomyopathic phenotype, beginning 3–4 months after birth, characterized by decreased contractility and a two-fold increase in apoptotic cardiomyocytes compared to wild type control mice. Therefore, it is tempting to speculate that the cardiomyopathic phenotype observed in Pim-DN mice at basal levels results from the lack of kinase activity critical to cardiomyoctyte survival, given the lack of a cardiomyopathic phenotype occurring in Pim-1 KO mice, where alternative Pim-2 or Pim-3 genes appear to be up-regulated in compensation for Pim-1 deletion. Alternatively, Pim-1 may serve as a partner in formation of a multimolecular complex, and that overexpression ties up other factors essential for maintenance of myocardial health.
Results from transgenic animal studies clearly demonstrate that Pim-1 is essential for mediating cardioprotection after pathological injury. Overexpression of Pim-1 in the heart results in profound protective effects whereas knock out or inhibition of its kinase activity significantly reduced cardiac functional recovery. Pim-1 overexpression enhances postnatal myocardial hyperplasia with increased cardiomyocyte and cardiac progenitor cell (CPC) cycling [23]. Increased CPC cycling led to a preservation of the cardiac progenitor cell pool, particularly after myocardial infarction [23]. The ability of Pim-1 to increase CPC cycling makes Pim-1 a promising target to engineer CPCs with enhanced capacity to minimize the damage following MI as discussed in the following section.
III. Engineering CPCs with Pim-1
In order to improve cardiac repair after infarction, strategies enlisting the help of stem and/or progenitor cell populations are being investigated. However, improvements appear to be short-lived due to the consistent observation that only a small population of cells remains in the infarcted myocardium. Improving the therapeutic potential of progenitor cells likely involves increasing their ability to survive and proliferate in damaged tissue. Over-expression of Pim-1 kinase has previously been shown to increase cell proliferation and attenuate apoptosis in disease models [20, 24, 25]; therefore making Pim-1 a likely candidate for enhancing stem cell mediated cardiac regeneration.
Viral delivery of Pim-1 kinase to CPCs resulted in increased proliferation as well as enhanced metabolic activity in vitro [24]. After infarction, animals that received Pim-1 modified CPCs had improved cardiac structure and function at 3 and 8 months after pathological challenge compared to animals receiving unmodified cells. Accounting in part for these dramatic benefits was the observation that Pim-1 modified CPCs influenced vascular lineage commitment [24]. These observations were in support of previous studies demonstrating that Pim-1 expression influenced endothelial cell [28] and vascular smooth muscle cell proliferation [26] and lineage commitment.
Interestingly, the number of Pim-1 modified CPCs remaining in the heart was significantly increased, although the number of endogenous CPCs remained unchanged compared to animals receiving unmodified cells [24]. Thus, it appears that the overexpression of Pim-1 in CPCs imparts protective benefits to the myocardium in part from the ability of Pim-1 modified CPCs to proliferate and undergo lineage commitment under the appropriate stimulation [24]. While contributions of endogenous reparative mechanisms are likely to be involved along with paracrine secretion from the adoptively transferred CPC population, the long term presence of Pim-1 engineered cells together with persistent engraftment and differentiation is a feature unique to the Pim-1 engineered stem cells at the present time.
While these key findings support the use of Pim-1 kinase to enhance cardiac regenerative medicine, patient safety regarding the use of a gene with known oncogenic potential is of concern and must be addressed prior to clinical implementation.
IV. Implications and safety of Pim-1 in humans
In humans, Pim-1 is normally expressed in the cytoplasm of cardiomyocytes in adult myocardial tissue and appears to translocate to the nuclear compartment of myocytes stimulated to re-enter the cell cycle in the failing heart [20]. In comparison, cytoplasmic expression of Pim-1 in the normal human myocardium promotes myocyte survival and protection from injury [20]. Previous studies of preferential subcellular localization of Pim-1 correlate with activation of distinctly different signaling pathways in malignant human cells [15, 29]. For example, nuclear expression of Pim-1 leads to the development of Burkitt’s lymphoma through increased mdm2 expression [21], whereas activation of completely different signaling pathways leads to cytoplasmic expression of the kinase and is responsible for the observed tumorigenic properties. Therefore, the understanding and therapeutic manipulation of the signaling pathways and mechanisms regulating the subcellular localization of Pim-1 might open new possibilities to improve cardiac performance under pathological challenge. In addition to the aforementioned therapeutic transfer of Pim-modified CPCs, induction of endogenous Pim-1 in patient's cells might be an additional treatment option (i.e. through application of growth factors). However, to avoid systemic non-cardiac effects of induced Pim-1 in cell types of other organs, delivery systems must be developed to release respective factors at a specific location and duration. Gene therapy implementing viruses delivering Pim-1 under control of a cardiac-specific cardiac specific promoter, which is also active in CPCs [30], represents another therapeutic option. Finally, small molecule activators might be a possibility to activate endogenous Pim-1. However, Pim-1 kinase activity can be regulated other than by expression and localization, i.e. by its phosphorylation status, as described so far only in T cells. Whether this kind of regulation takes place in the cardiac context too, is so far not described yet [31].
In human tissues, Pim-1 has multiple roles in cell proliferation [21], survival, differentiation [32] and apoptosis [33]. Pim-1 minimally induces oncogenic transformation unless aided by participation with proteins such as c-Myc, c-Myb that cooperate to induce cellular proliferation and promote oncogenic potential. Additionally, Pim-1 enhances the activity of cell cycle promoters such as Cdc25A and Cdc25c and inactivates cell cycle inhibitors such as p21 and C-Tak1 [34–36], accelerating the transition of cells between G2 and M phases of the cell cycle. The anti-apoptotic effects of Pim-1 are mediated primarily through bcl-2 and bcl-xl protein and inactivation of Bad by phosphorylation [33]. This constellation of features including proliferative and anti-apoptotic signaling mediated by Pim-1 are an ideal mixture to promote beneficial cellular repair since Pim-1 does not appear to prevent lineage specification and differentiation.
Of course aside from the weak oncogenic potential of Pim-1, the myocardium is notoriously resistant to oncogenic transformation such as rhabdomyosarcoma due to terminal differentiation of myocytes [37]. From a therapeutic perspective, the ability of Pim-1 to promote cell proliferation may have critical impact that enables the myocardium to cope with pathologic damage in the failing or acutely damaged heart. Consistent with the protective role of Pim-1 in the heart, increased endogenous Pim-1 expression has been observed in failing mouse hearts and is likely a consequence of prevalent ischemic conditions promoting activation of the kinase. These observations are consistent with evidence from human studies in the pancreas suggesting hypoxia-mediated upregulation of Pim-1 [38].
Our studies in mouse models suggest that Pim-1 engineered CPCs possess unique properties to mediate beneficial repair in the pathologically damaged heart. One noteworthy aspect of our murine adoptive transfer studies is that the effects of the engineered Pim-1 CPCs reached a plateau and maintained improvement without progressive changes in myocardial structure or performance for several months. Thus, there may be a temporally finite effect of Pim-1 engineering wherein potentiation of aberrant cell growth is self-limiting and oncogenic transformation is not of major concern. These issues are currently under intense investigation by our group to determine how Pim-1 mediates cardioprotection without uncontrolled growth that has been noted with adoptive transfer of primitive cell types such as embryonic or the more recently created inducible pluripotent stem (iPS) cell types. The implications for therapeutic implementation of a molecular solution that promotes cell proliferation and survival without preventing engraftment and commitment to tissue-specific lineages is obvious, not only for myocardial repair but for multiple tissue types that have historically proven to be resistant to repair following damage.
As heart failure is a major cause of morbidity and mortality in the elderly population, it may be that impairment of a stem cell-mediated reparative response is a primary contributory factor to age-related cardiomyopathy. We will need to find strategies for augmenting a reparative and / or regenerative response in elderly patients who have exhausted their endogenous stem cell populations by a combination of increased demand, senescence, and diminished mitotic potential. At the present time the most likely strategy appears to be engineering of the impaired stem cell pool as a means to facilitate cardiac regeneration with minimal risk of host rejection. One could envision a strategy in which the compromised stem cell pool of an elderly or cardiomyopathic patient is harvested, transformed with pro-survival and pro-proliferative molecules such as Pim-1 and re-introduced into the damaged myocardium. As we pursue the goal to enhanced structural and functional cardiac repair, tactics allowing for controlled expression of Pim-1 would allow for increased safety as well as reactivation under appropriate conditions.
As we continue to search for ways to preserve myocardial function and restore hemodynamic capacity to the failing heart, the most desirable outcome would be to find an interventional strategy to “turn back the clock” on cells with diminished regenerative potential. Returning the heart to a more youthful phenotype with concomitant improvement in functional capacity will not be possible with the resident cells alone. An interventional strategy with energized engineered cells such as the CPCs expressing Pim-1 kinase may be just the roadmap we seek to find the long sought after “fountain of youth” to maintain and restore a heart under siege from acute injury or the ravages of time.
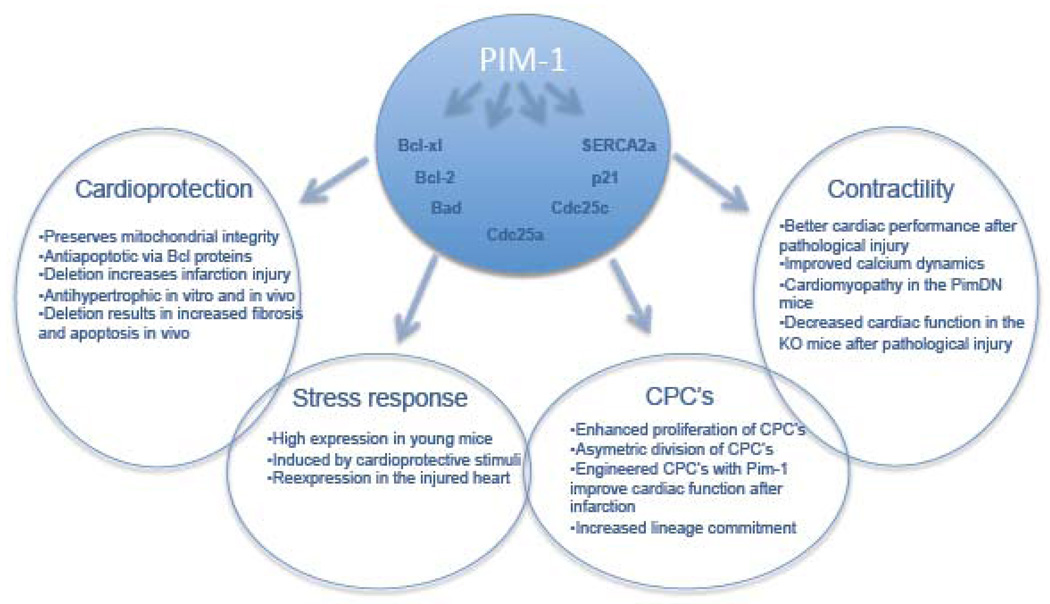
Figure 1.
Summary of the multifaceted effects mediated by Pim-1 in the myocardium as described in the published studies [20, 22–25].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sussman MA. Mitochondrial integrity: preservation through Akt/Pim-1 kinase signaling in the cardiomyocyte. Expert Rev Cardiovasc Ther. 2009 Aug;7(8):929–938. doi: 10.1586/erc.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyamoto S, Rubio M, Sussman MA. Nuclear and mitochondrial signaling Akts in cardiomyocytes. Cardiovasc Res. 2009 May 1;82(2):272–285. doi: 10.1093/cvr/cvp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuypers HT, Selten G, Quint W, Zijlstra M, Maandag ER, Boelens W, et al. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- 4.Mikkers H, Nawijn M, Allen J, Brouwers C, Verhoeven E, Jonkers J, et al. Mice deficient for all PIM kinases display reduced body size and impaired responses to hematopoietic growth factors. Mol Cell Biol. 2004 Jul;24(13):6104–6115. doi: 10.1128/MCB.24.13.6104-6115.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padma R, Nagarajan L. The human PIM-1 gene product is a protein serine kinase. Cancer Res. 1991 May 1;51(9):2486–2489. [PubMed] [Google Scholar]
- 6.Palaty CK, Kalmar G, Tai G, Oh S, Amankawa L, Affolter M, et al. Identification of the autophosphorylation sites of the Xenopus laevis Pim-1 proto-oncogene-encoded protein kinase. J Biol Chem. 1997 Apr 18;272(16):10514–10521. doi: 10.1074/jbc.272.16.10514. [DOI] [PubMed] [Google Scholar]
- 7.Qian KC, Wang L, Hickey ER, Studts J, Barringer K, Peng C, et al. Structural basis of constitutive activity and a unique nucleotide binding mode of human Pim-1 kinase. J Biol Chem. 2005 Feb 18;280(7):6130–6137. doi: 10.1074/jbc.M409123200. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann M, Moroy T. The serine/threonine kinase Pim-1. Int J Biochem Cell Biol. 2005 Apr;37(4):726–730. doi: 10.1016/j.biocel.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005 Oct;115(10):2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martelli M, Ferreri AJ, Johnson P. Primary mediastinal large B-cell lymphoma. Crit Rev Oncol Hematol. 2008 Dec;68(3):256–263. doi: 10.1016/j.critrevonc.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Dreyfus F, Sola B, Fichelson S, Varlet P, Charon M, Tambourin P, et al. Rearrangements of the Pim-1, c-myc, and p53 genes in Friend helper virus-induced mouse erythroleukemias. Leukemia. 1990 Aug;4(8):590–594. [PubMed] [Google Scholar]
- 12.Lin YW, Beharry ZM, Hill EG, Song JH, Wang W, Xia Z, et al. A small molecule inhibitor of Pim protein kinases blocks the growth of precursor T-cell lymphoblastic leukemia/lymphoma. Blood. Jan 28;115(4):824–833. doi: 10.1182/blood-2009-07-233445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong Y, Stewart KD, Thomas S, Przytulinska M, Johnson EF, Klinghofer V, et al. Isoxazolo[3,4-b]quinoline-3,4(1H,9H)-diones as unique, potent and selective inhibitors for Pim-1 and Pim-2 kinases: chemistry, biological activities, and molecular modeling. Bioorg Med Chem Lett. 2008 Oct 1;18(19):5206–5208. doi: 10.1016/j.bmcl.2008.08.079. [DOI] [PubMed] [Google Scholar]
- 14.Eichmann A, Yuan L, Breant C, Alitalo K, Koskinen PJ. Developmental expression of pim kinases suggests functions also outside of the hematopoietic system. Oncogene. 2000 Feb 24;19(9):1215–1224. doi: 10.1038/sj.onc.1203355. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Bhattacharya N, Weaver M, Petersen K, Meyer M, Gapter L, et al. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. J Vet Sci. 2001 Dec;2(3):167–179. [PubMed] [Google Scholar]
- 16.Klejman A, Schreiner SJ, Nieborowska-Skorska M, Slupianek A, Wilson M, Smithgall TE, et al. The Src family kinase Hck couples BCR/ABL to STAT5 activation in myeloid leukemia cells. EMBO J. 2002 Nov 1;21(21):5766–5774. doi: 10.1093/emboj/cdf562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Lohuizen M, Verbeek S, Krimpenfort P, Domen J, Saris C, Radaszkiewicz T, et al. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989 Feb 24;56(4):673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 18.van Lohuizen M, Verbeek S, Scheijen B, Wientjens E, van der Gulden H, Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991 May 31;65(5):737–752. doi: 10.1016/0092-8674(91)90382-9. [DOI] [PubMed] [Google Scholar]
- 19.Amson R, Sigaux F, Przedborski S, Flandrin G, Givol D, Telerman A. The human protooncogene product p33pim is expressed during fetal hematopoiesis and in diverse leukemias. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8857–8861. doi: 10.1073/pnas.86.22.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007 Dec;13(12):1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 21.Ionov Y, Le X, Tunquist BJ, Sweetenham J, Sachs T, Ryder J, et al. Pim-1 protein kinase is nuclear in Burkitt's lymphoma: nuclear localization is necessary for its biologic effects. Anticancer Res. 2003 Jan–Feb;23(1A):167–178. [PubMed] [Google Scholar]
- 22.Borillo GA, Mason M, Quijada P, Volkers M, Cottage C, McGregor M, et al. Pim-1 kinase protects mitochondrial integrity in cardiomyocytes. Circ Res. Apr 16;106(7):1265–1274. doi: 10.1161/CIRCRESAHA.109.212035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cottage CT, Bailey B, Fischer KM, Avitable D, Collins B, Tuck S, et al. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. Mar 19;106(5):891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, et al. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009 Nov 24;120(21):2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muraski JA, Fischer KM, Wu W, Cottage CT, Quijada P, Mason M, et al. Pim-1 kinase antagonizes aspects of myocardial hypertrophy and compensation to pathological pressure overload. Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):13889–13894. doi: 10.1073/pnas.0709135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willert M, Augstein A, Poitz DM, Schmeisser A, Strasser RH, Braun-Dullaeus RC. Transcriptional regulation of Pim-1 kinase in vascular smooth muscle cells and its role for proliferation. Basic Res Cardiol. Mar;105(2):267–277. doi: 10.1007/s00395-009-0055-x. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharya N, Wang Z, Davitt C, McKenzie IF, Xing PX, Magnuson NS. Pim-1 associates with protein complexes necessary for mitosis. Chromosoma. 2002 Jul;111(2):80–95. doi: 10.1007/s00412-002-0192-6. [DOI] [PubMed] [Google Scholar]
- 28.Zippo A, De Robertis A, Bardelli M, Galvagni F, Oliviero S. Identification of Flk-1 target genes in vasculogenesis: Pim-1 is required for endothelial and mural cell differentiation in vitro. Blood. 2004 Jun 15;103(12):4536–4544. doi: 10.1182/blood-2003-11-3827. [DOI] [PubMed] [Google Scholar]
- 29.Xie Y, Xu K, Dai B, Guo Z, Jiang T, Chen H, et al. The 44 kDa Pim-1 kinase directly interacts with tyrosine kinase Etk/BMX and protects human prostate cancer cells from apoptosis induced by chemotherapeutic drugs. Oncogene. 2006 Jan 5;25(1):70–78. doi: 10.1038/sj.onc.1209058. [DOI] [PubMed] [Google Scholar]
- 30.Bailey B, Izarra A, Alvarez R, Fischer KM, Cottage CT, Quijada P, et al. Cardiac stem cell genetic engineering using the alphaMHC promoter. Regen Med. 2009 Nov;4(6):823–833. doi: 10.2217/rme.09.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, Arnold HK, Lilly MB, Sears RC, Kraft AS. Negative regulation of Pim-1 protein kinase levels by the B56beta subunit of PP2A. Oncogene. 2007 Aug 2;26(35):5145–5153. doi: 10.1038/sj.onc.1210323. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt T, Karsunky H, Rodel B, Zevnik B, Elsasser HP, Moroy T. Evidence implicating Gfi-1 and Pim-1 in pre-T-cell differentiation steps associated with beta-selection. EMBO J. 1998 Sep 15;17(18):5349–5359. doi: 10.1093/emboj/17.18.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lilly M, Sandholm J, Cooper JJ, Koskinen PJ, Kraft A. The PIM-1 serine kinase prolongs survival and inhibits apoptosis-related mitochondrial dysfunction in part through a bcl-2-dependent pathway. Oncogene. 1999 Jul 8;18(27):4022–4031. doi: 10.1038/sj.onc.1202741. [DOI] [PubMed] [Google Scholar]
- 34.Bachmann M, Kosan C, Xing PX, Montenarh M, Hoffmann I, Moroy T. The oncogenic serine/threonine kinase Pim-1 directly phosphorylates and activates the G2/M specific phosphatase Cdc25C. Int J Biochem Cell Biol. 2006 Mar;38(3):430–443. doi: 10.1016/j.biocel.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y. Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1-mediated activation of the c-Myc signaling pathway. J Biol Chem. 1999 Jun 25;274(26):18659–18666. doi: 10.1074/jbc.274.26.18659. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Bhattacharya N, Mixter PF, Wei W, Sedivy J, Magnuson NS. Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim-1 kinase. Biochim Biophys Acta. 2002 Dec 16;1593(1):45–55. doi: 10.1016/s0167-4889(02)00347-6. [DOI] [PubMed] [Google Scholar]
- 37.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001 Jun 7;344(23):1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Kobayashi M, Darmanin S, Qiao Y, Gully C, Zhao R, et al. Hypoxia-mediated up-regulation of Pim-1 contributes to solid tumor formation. Am J Pathol. 2009 Jul;175(1):400–411. doi: 10.2353/ajpath.2009.080972. [DOI] [PMC free article] [PubMed] [Google Scholar]