Abstract
The expression of expert motor skills typically involves learning to perform a precisely timed sequence of movements (e.g., language production, music performance, athletic skills). Research examining incidental sequence learning has previously relied on a perceptually-cued task that gives participants exposure to repeating motor sequences but does not require timing of responses for accuracy. Using a novel perceptual-motor sequence learning task, learning a precisely timed cued sequence of motor actions is shown to occur without explicit instruction. Participants learned a repeating sequence through practice and showed sequence-specific knowledge via a performance decrement when switched to an unfamiliar sequence. In a second experiment, the integration of representation of action order and timing sequence knowledge was examined. When either action order or timing sequence information was selectively disrupted, performance was reduced to levels similar to completely novel sequences. Unlike prior sequence-learning research that has found timing information to be secondary to learning action sequences, when the task demands require accurate action and timing information, an integrated representation of these types of information is acquired. These results provide the first evidence for incidental learning of fully integrated action and timing sequence information in the absence of an independent representation of action order, and suggest that this integrative mechanism may play a material role in the acquisition of complex motor skills.
Keywords: sequence learning, implicit memory, timing, integration, motor control
Every year, millions of fans watch professional baseball players swinging a narrow wooden stick at a small, rapidly moving ball. Successfully making contact requires executing a complex and precisely timed sequence of motor actions. Once a pitch is initiated, the batter must estimate the speed and trajectory of the ball in order to properly time the swinging of the bat such that it will intercept the ball as it passes over the plate. Mistiming the swing by just a few milliseconds can be the difference between a towering homerun and a weak foul ball. In order to properly time the swing to the pitched ball, the timing between every component movement of the swing must be executed precisely and consistently. Much of the job of the pitcher is to unexpectedly vary the delivery speed of the pitch to attempt to disrupt this precisely timed sequence of motor actions (e.g., a devastatingly slow change-up following a scorching fastball). How such a precisely timed sequence of motor actions is learned and represented in the human brain is not well-understood. In fact, despite having extensive expertise, professional baseball players are not necessarily effective at explaining and improving their swing, as evidenced by the need for hitting coaches and immense amounts of regular practice. The relative unavailability of explicit knowledge of the swing suggests that implicit learning mechanisms are likely to be critical for acquisition of these precisely timed motor sequences.
Prior studies of implicit learning of perceptual-motor sequences have relied heavily on the serial reaction time (SRT) task paradigm (e.g., Nissen & Bullemer, 1987; Robertson, 2007). In this paradigm, participants respond to a perceptual cue appearing in one of four locations by pressing the corresponding key as quickly as possible. Unbeknownst to the participants, the order of cue locations follows a repeating sequence, evoking a repeating sequence of motor responses. With practice, reaction times decrease. Sequence-specific learning for the repeating sequence is shown by an increase in reaction time when the order of cue locations is changed to no longer follow the repeating sequence.
The SRT task is based on making responses as quickly as possible. Recently, we reported a novel Serial Interception Sequence Learning (SISL) task (Sanchez, Gobel, & Reber, in press) that extends the SRT task to require precisely timed motor responses to intercept moving cues, which move vertically upward from the bottom of the screen towards a marked target zone (Figure 1). The corresponding motor response must be timed to coincide with the cue intercepting its target, making precise timing essential to successful task performance. Rather than reaction time or error magnitude, the key dependent variable describing performance is the binary accuracy of the response (correct/incorrect). A correct response is defined as pressing the single key that corresponds to the target being crossed by the cue. To enable participants to make responses separated by short intervals (e.g., as little as 350ms, less than typical in the SRT), multiple cues are always moving concurrently on the screen, allowing for future responses to be anticipated and planned. These task demands better capture real-world skills in which responses are not made as soon as possible, but are timed to relevant cues in the environment. During practice with the SISL task, participants are cued to make responses following a repeating sequence without being informed about the existence of the repeating sequence, just as in the SRT. Timing information can be embedded into the sequences, and learning in this task appears to frequently occur without awareness in healthy participants (Sanchez et al., in press).
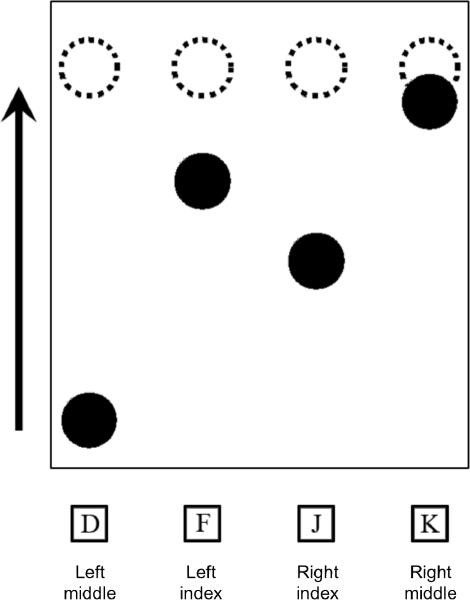
Figure 1.
The SISL paradigm. Four dashed rings, or targets, are assigned to the four motor responses (keys D, F, J, and K pressed with the left middle, left index, right index, and right middle fingers, respectively) and remain stationary on the screen. Filled circles, or cues, scroll upwards on the screen at a constant speed. Participants press the corresponding key when a cue is centered within a target.
The SISL task combines the SRT with elements of tracking tasks that have also been used to examine learning of spatiotemporal motor sequences (Shea, Wulf, Whitacre, & Park, 2001; Wulf & Schmidt, 1997; but see Perruchet, Chambaron, & Ferrel-Chapus, 2003). By embedding a repeating sequence of target locations to be tracked, implicit learning is observed via faster execution (Shea, Park, & Braden, 2006) and decreased error (Boyd & Winstein, 2004) during repeated than during random movement sequences. Separate scoring of spatial and temporal accuracy can attempt to separately assess learning and performance of these two aspects in tracking tasks (e.g., Boyd & Winstein, 2004). However, since the learned order (spatial locations) and timing sequences cannot be manipulated independently, due to biomechanical and task-related constraints, the contribution of timing to sequence learning is difficult to assess in these tracking tasks.
To test how well-integrated the learning of action and timing sequences are, the repeating sequence of actions to be learned and timing intervals between them can be separately manipulated in the SISL task. Prior research with the SRT task (O'Reilly, McCarthy, Capizzi, & Nobre, 2008; Shin & Ivry, 2002) has found some evidence for partial integration. In O'Reilly et al. (2008), changing the inter-trial timing led to only some loss of performance, indicating partial transfer of ordinal sequence knowledge. Shin and Ivry (2002) also found evidence for significant but incomplete disruption of performance when the timing pattern correlated with the learned action sequence was changed or shifted. Both studies provide evidence for integration of order and timing, since the best performance occurred with the correlated practiced sequence. However, partial transfer of ordinal sequence knowledge indicates that there was an independent representation of cue order information, so this integration was not complete. Those studies did not find evidence for independent learning of the timing sequence, i.e., learning of a response rhythm, independent of any ordinal sequence of response locations. Salidis (2001) found reliable implicit learning of a timing sequence using a single-button reaction time task, but since there was only one response, it is unclear if rhythm was represented independently of response locations. However, since the SRT task requires immediate reaction to a cue rather than precise timing of the motor response, the above studies may have been unable to fully estimate the degree to which timing information is learned and integrated into perceptual-motor action sequences when they require precisely timed responses.
In Experiment 1, sequence-specific learning is shown to occur with the SISL task for a repeating sequence of cues without an embedded timing pattern. Sanchez et al. (in press) demonstrated sequence learning in the SISL task with such a pattern. This experiment will show that sequence learning is seen in this task with constant inter-trial timing. In Experiment 2, after training, the order and timing dimensions were separately manipulated to determine if knowledge of order and correlated timing are represented separately from each other or integrated into a common representation. Sequence-specific improved performance in the SISL task may be supported by separate representations supporting selection of the next action and planning a precisely timed motor response. These representations may be fully independent or show partial integration along with an independent ordinal representation (e.g., O'Reilly et al., 2008; Shin & Ivry, 2002). Alternately, because the SISL task incorporates the learning of timing together with action order, the representation of sequence knowledge may depend on fully integrated action and timing information.
Experiment 1
Method
Participants
Participants were randomly selected from the introductory psychology participant pool at Northwestern University. All 63 participants (38 female, 25 male) gave informed consent and received course credit for their participation in the study. Nine (seven female, two male) were excluded from analysis due to inability to perform the task (overall correct trials less than 51 percent), and one female participant's data was lost due to computer failure, leaving 53 participants (30 female, 23 male).
Procedure
The display, as shown in Figure 1, consisted of four unfilled, dashed gold rings (“targets”) of 1 cm diameter, centered on a horizontal 20 cm from the bottom of the screen on a 17-inch (43.18 cm) monitor. At the bottom of the screen were filled blue circles (“cues”) of the same size as the targets, lined up vertically beneath the targets. From left to right, the targets were assigned to the D, F, J, and K keys, corresponding to the left middle, left index, right index, and right middle fingers, respectively. Participants were seated at an approximate viewing distance of 50–60 cm.
SISL task
Participants were instructed to press the corresponding key when a cue was centered in its target. When the task began, one cue initially started scrolling straight up toward one of the four targets near the top of the screen. Additional cues soon began to scroll concurrently so that there were always three other on-screen cues moving toward the targets.
Cues scrolled at a constant velocity of 10 cm/s, from the bottom to top of the screen (a distance of 25 cm) in 2.5 s, reaching their targets in 2.0 s. Each trial, defined as the passage of a cue through its target, was separated by an inter-trial interval (ITI) of 500ms. When any keypress response (correct or incorrect) was made, the corresponding dashed gold target briefly flashed bright green as visual feedback. To avoid ambiguity in assigning responses to trials, a response was scored as correct if the appropriate key was pressed for the cue closest to the target zone at that time. Incorrect keys, multiple responses, and non-responses were counted as errors.
Participants completed a total of 1440 trials with self-terminated breaks after every 480 trials (4 min, 15 s). Performance was scored as percent correct in blocks of 60 trials (24 blocks). For 21 of these blocks, the cues followed a 12-item second-order conditional (SOC) repeating sequence. A SOC sequence contains balanced item and first-order conditional frequency, assuring that each possible transition occurs exactly once, as recommended in Reed and Johnson (1994) for the SRT task. Half the participants trained with the sequence F-J-F-K-D-J-D-K-J-KF-D and the other half K-J-F-K-F-J-D-F-D-K-D-J, where the letters refer to the appropriate keypress responses. On blocks 7, 15, and 23, cues followed an unfamiliar pseudorandom order constructed by randomly ordering a set of 15 novel 12-item SOC sequences for each participant.
Explicit recognition test
Following training, participants performed a recognition test for explicit knowledge of the repeating sequence. For this test, participants were shown five 12-item SOC sequences (performing the SISL task during display): the practiced sequence and 4 novel foils. After each sequence, participants were asked to rate their confidence, on a scale of 1-100, that they had practiced that sequence during the task (1 = sequence was novel, 50 = unsure, 100 = sequence was encountered during training). The same five sequences were presented in a random order for each subject. An explicit recognition score was calculated for each participant as the rating given to the practiced sequence minus the average of the four foils (positive scores reflect recognition).
Free recall test
After the recognition test, participants were informed that there had been a repeating sequence and were asked to reproduce the sequence. The screen was shown with the four targets but no cues, and participants were asked to press the keys in the order of the repeating sequence. The targets flashed green as the corresponding keys were pressed. Participants were encouraged (but not required) to continue until they had pressed 12 keys. Their responses were scored by identifying the longest matching subsequence to the target sequence. To control for baseline recall performance, the recalled sequence was also scored for the longest matching subsequence to the four foils (explicit sequence knowledge is reflected as a longer matching subsequence to the target than the foils).
Results
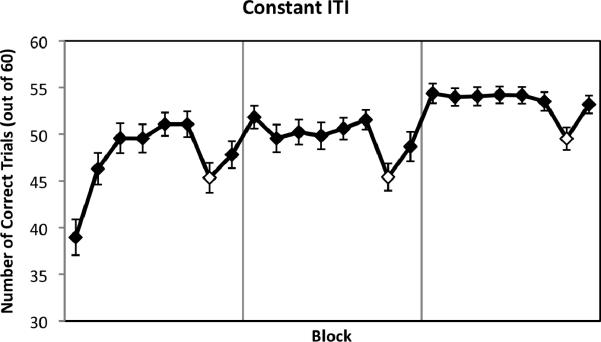
Learning during SISL practice is shown by significant drops in performance observed in all three periods where the cues no longer followed the repeating sequence (Figure 2). Participants made significantly fewer correct responses during these pseudorandom blocks than during the repeating sequence blocks immediately before and after (averaged together), reflecting sequence-specific learning, M = 4.21 additional errors (SD = 4.41), averaged across all assessments, t(52) = 6.95, p < .001 (for each assessment, in order, t(52) = 3.93; t(52) = 4.99; t(52) = 4.80; all ps < .001). There was no significant difference in these learning scores across the assessments, F(2,104) = 0.27, p = .77, suggesting that most of the sequence-specific learning took place during the first 8 blocks (4 min, 15 s).
Figure 2.
Performance and implicit learning with a constant 500 ms ITI. Performance was assessed by the number of correct trials out of 60 for each block, with repeating sequence blocks shown as filled diamonds and pseudorandom order blocks as open diamonds. The number of correct trials for each pseudorandom block was compared to the mean of its flanking blocks to find the decrease in performance when switching from the repeating sequence to a pseudorandom cue order, an index of implicit learning of the sequence. Participants performed significantly worse during the pseudorandom blocks, both overall and for each run. Error bars represent standard errors.
The average recognition score, M = 12.9 (SD = 26.4), was reliably greater than zero, t(52) = 3.56, p < .001, d = .49, indicating that participants had some explicit recognition knowledge of the repeating sequence. Likewise, the average recall score for the repeating sequence was M = 4.62 items (SD = 1.82), slightly but reliably higher than the match to the foils, M = 3.70 items (SD = 0.59), t(52) = 3.54, p < .001, d = .68. By both measures, participants exhibited some explicit sequence knowledge. However, regression analyses found that the training sequence used (of 2) and recognition score did not reliably predict the amount of implicit learning (whole-model r2 = .011, ts < 1.00 for both predictors), nor did training sequence and recall score (whole-model r2 = .013, ts < 1.00). Furthermore, participants scoring below the mean on either recognition (n = 21) or recall (n = 21) exhibited reliable implicit learning, t(20) = 3.49 and t(20) = 5.26, respectively, both ps < .002, suggesting that SISL task performance was not dependent on explicit sequence knowledge.
Discussion
In Experiment 1, significant learning of a repeating sequence was exhibited during SISL task performance with cues spaced by a constant ITI (no sequential inter-trial timing information). Sequence-specific learning was reflected in better performance (fewer errors) during the trained sequence than during a pseudorandom order of cues. While some participants developed some explicit sequence knowledge (as in the SRT, e.g., Reber & Squire, 1994; Willingham, Greely, & Bardone, 1993), explicit scores did not predict implicit learning and participants with minimal explicit knowledge exhibited reliable learning (as in Sanchez et al., in press).
A second experiment examined the effect of manipulating the embedded timing and/or order information within the sequence. Since successful SISL task performance requires precise timing of motor responses, we predict that knowledge of the pattern of timing intervals between optimal responses should improve task performance. Participants learned an order of responses along with a correlated pattern of inter-trial intervals, and transfer to conditions that selectively modified the order or timing information was assessed.
Experiment 2
Method
Participants
Participants were randomly selected from the introductory psychology participant pool at Northwestern University. All 20 participants (8 female, 12 male) gave informed consent and received course credit for their participation in the study.
Procedure
The SISL task was similar to Experiment 1, with participants instructed to respond to vertically moving cues as they crossed the target zone at the top of the screen. The cues followed a repeating 12-item SOC sequence for the majority of training, using the same repeating orders as in Experiment 1. However, half of the ITIs were 350 ms and half were 700 ms in a fixed pattern, e.g. F350J700F700K350D350J700D350K700J700K350F350D700 (where the subscripted numbers indicate the time in ms between trials). The timing sequence was constrained such that the same interval could not occur more than twice in a row. The velocity of cue movement was constant and identical to Experiment 1 (10 cm/s). Differences in timing were visible on the screen as differing distances between the cues as they moved vertically up the screen, similar to a music roll on a player piano. Participants performed 1440 total trials during training, scored as 24 blocks of 60 trials. During blocks 7, 15, and 23, cues followed a pseudorandom order (novel SOC sequences as in Experiment 1, but with embedded timing intervals opposite to the training pattern, i.e., long and short intervals were swapped, which also changed the vertical spacing between given stimuli). Participants had a self-terminated break after every 480 trials.
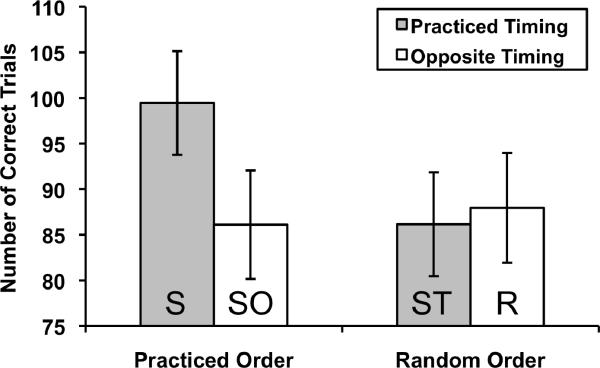
Immediately following training, a transfer test was administered that contained four conditions manipulating the order and timing information of the learned sequence. The cues followed either the same order as the training pattern or a pseudorandom order (using a set of 20 novel 12-item SOC sequences, randomly ordered for each participant). Timing was either identical to or opposite to the training pattern. Therefore, the four conditions were Sequenced (S; practiced order and practiced timing), Same Order (SO; practiced order and opposite timing), Same Timing (ST; pseudorandom order and practiced timing), and pseudoRandom (R; pseudorandom order and opposite timing). Each participant completed all four conditions in a random order twice, giving two blocks (120 trials) of each condition.
Following the transfer test, all participants performed both the recognition and recall tests of explicit sequence knowledge as in Experiment 1. Timing information was removed from the recognition test (targets and foils were presented with a constant 500 ms ITI). During the recall test, participants were required to continue until 12 keys were pressed.
Results
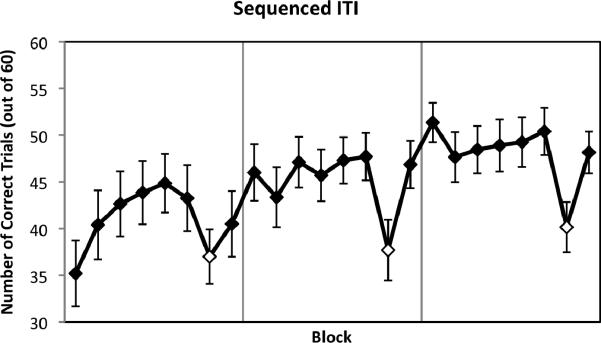
During the training runs of the SISL task, participants learned the repeating sequence (Figure 3). Sequence-specific learning was exhibited by reliably fewer correct trials in each pseudorandom block than the mean of its flanking repeating sequence blocks, M = 7.86, SD = 5.81, t(19) = 6.05, p < .001 (in order of assessment, t(19) = 2.65, p = .016; t(19) = 5.04, p < .001; t(19) = 5.56, p < .001). There was a marginal trend for the amount of learning expressed to be higher for the second assessment than the first, t(19) = 2.01, p = .059, suggesting much but not all of the sequence-specific learning occurred prior to the first assessment.
Figure 3.
Performance and implicit learning during training with a correlated pattern of ITIs. Performance was assessed by the number of correct trials out of 60 for each block, with blocks containing the repeating sequence shown as filled diamonds and pseudorandom blocks (pseudorandom order with opposite timing) as open diamonds. Participants showed significant implicit learning, both overall and in all three runs, as shown by the performance reduction during the pseudorandom blocks compared with the flanking repeating sequence blocks. Error bars represent standard errors.
The effect of changing the timing and order of the repeating cues during the transfer test was assessed using a 2×2 within-subjects ANOVA on the number of correct trials. There were significant main effects of order, F(1,19) = 10.03, p = .005, ηp2 = .35, and timing, F(1,19) = 9.60, p = .006, ηp2 = .34, and a significant order × timing interaction, F(1,19) = 15.87, p = .001, ηp2 = .46, reflecting the fact that performance was best when neither the order nor the timing was changed (Figure 4). All three effects are driven by performance during the repeating sequence condition being superior to the three transfer conditions, ts(19) > 4.05, ps ≤ .001. No significant differences were found between any of the transfer conditions, F(2,38) = 0.34, p = .71.
Figure 4.
Performance during the transfer test. After implicitly learning the practiced sequence, participants executed the task in four conditions: Sequenced (S; practiced order and practiced timing), Same Order (SO; practiced order and opposite timing), Same Timing (ST; pseudorandom order and practiced timing), and pseudoRandom (R; pseudorandom order and opposite timing). Performance (number of correct trials out of 120) during the S condition was significantly better than for the other three conditions (SO, ST, and R), none of which significantly differed from each other. Error bars represent standard errors.
The average recognition score, M = 6.70 (SD = 22.3), was not significantly greater than zero, t(19) = 1.34, p = .20, suggesting poor recognition memory for the practiced sequence (but this may have been influenced by the removal of timing information). The average recall score for the repeating sequence, M = 4.85 items (SD = 1.79), was slightly but reliably higher than the match to the foils (M = 3.86, SD = 0.59), t(19) = 2.13, p = .047, d = .74. However, regression analyses found that the training sequence used (of 2) and recognition score did not reliably predict the amount of implicit learning (whole-model r2 = .092, ts < 1.00 for both predictors), nor did training sequence and recall score (whole-model r2 = .075, t(17) = 1.16 for sequence and t(17) = −0.305 for recall). Furthermore, the subset of participants who scored fewer or the same number of consecutive matches to the practiced sequence than to the foils (n = 7) showed reliable implicit learning, t(6) = 3.06, p = .022, suggesting that explicit sequence knowledge did not entirely support performance.
Discussion
After learning a repeating sequence with a timing pattern embedded within the order of motor actions, participants did not show any transfer of sequence knowledge when the order was selectively disrupted (with timing maintained), suggesting that participants did not form an independent representation of the timing pattern. More notably, simply changing the inter-trial timing also led to performance levels similar to SISL task performance with a completely novel cue order and timing sequence, indicating that an independent representation of the ordinal sequence also was not formed. This failure to transfer sequence knowledge to either of the altered conditions suggests that the sequence knowledge representation is based on fully integrated timing and order information. The inability to transfer the integrated representation to conditions where it could be partially useful indicates that the sequence knowledge obtained was specific and inflexible.
General Discussion
In both experiments, participants exhibited robust learning of perceptual-motor sequences within the SISL task, whether the cues were spaced with a constant ITI (Experiment 1) or with an embedded timing pattern (Experiment 2). A significant performance improvement was found that was specific for the practiced repeating sequence. As in many previous reports of perceptual-motor sequence learning tasks with healthy participants (e.g., Willingham et al., 1993), some explicit sequence knowledge was obtained during training. However, explicit scores did not predict implicit learning and participants who exhibited very poor explicit sequence knowledge also showed reliable improvement in performance, suggesting that the learning was implicit, as in Sanchez et al. (in press).
In Experiment 2, when the repeating sequence of actions was embedded with a specific pattern of timing intervals, participants did not exhibit any evidence of transfer to sequences of the same order with different timing or sequences with the same timing pattern and a random order of responses. The lack of transfer in the SO condition – suggesting the absence of an independent ordinal representation – contrasts with previous research using the SRT task (O'Reilly et al., 2008; Shin & Ivry, 2002), which found partial transfer when timing information was changed but cue order was maintained. Our results indicate that when precise timing is necessary for task performance, sequence learning depends on a fully integrated representation of sequential action and inter-action timing information. Shin and Ivry (2002) also found that phase-shifting the temporal sequence impaired performance to some degree, demonstrating relational learning of the two sequences in their research as well. While we did not examine the effect of phase-shifting the two sequences with the SISL task, this technique could provide another test for the existence of separate time and order representations, e.g., through savings in relearning, although the current results argue against this.
The difference between the previous results and our current finding likely emerges from differing task demands. In the SRT task, the motor response is initiated as quickly as possible in response to cue appearance. To make an interception response in the SISL task, it is necessary to identify the velocity of the moving cue and its distance from the target zone, and then plan the motor response with precise timing to complete the action as the cue crosses the zone. By making response timing integral to successful task performance, it appears that timing information becomes fully integrated with the representation of the repeating action sequence. A possible mechanism of temporal integration with order selection would be if short sequences of responses, perhaps 2–3 responses separated by the shorter intervals, were learned as “chunks” for motor planning purposes (e.g., “performance units” in Graybiel, 1998). Therefore, changing the inter-item timing might make the same response order unrecognizable as known chunks to the motor system, resulting in baseline level performance.
It should also be noted that because multiple scrolling cues are visible simultaneously during the SISL task, the embedded timing information is reflected in the visual pattern of the cues as different inter-cue vertical spacing. The change in visuospatial information during the transfer conditions allows for the possibility that the failure to transfer sequence knowledge to the SO condition indicates some dependency on the visuospatial cuing details. The current results do not rule out this alternate hypothesis, but studies with visual and response mapping manipulations in the SRT task suggest that response-based learning within the motor system plays a major role in perceptual-motor sequence learning (Willingham, 1999; Willingham, Wells, Farrell, & Stemwedel, 2000). Observing the same pattern of results with a slightly modified SISL paradigm, such that only one cue is visible at any given time (thus participants are only able to plan a response to a single cue at any time) would indicate that integration is not due to the visuospatial pattern of stimulus presentation. However, many complex motor skills are guided by visual information and feedback, and future research may indicate that visual information is also integrated into the representation of sequential motor action planning.
The integration of information among representations of action order and timing here poses some questions about the neural basis of perceptual-motor sequence learning. Prior studies with the SRT task have suggested that the medial temporal lobe memory system (supporting explicit memory) is not necessary for sequence learning (Nissen & Bullemer, 1987; Reber & Squire, 1994; Reber & Squire, 1998). Rather, this learning may depend on cortiostriatal connections between the basal ganglia and motor cortical regions (e.g., Doyon, Penhune, & Ungerleider, 2003). Patients with Parkinson's disease demonstrate impaired implicit motor sequence learning (Jackson, Harrison, Henderson, & Kennard, 1995), likely due to dysfunction in the basal ganglia, although this deficit might only occur when stimuli are spatially compatible with responses (Werheid, Ziessler, Nattkemper, & von Cramon, 2003). Neuroimaging studies of the SRT task have reported learning-related activity increases in the basal ganglia (especially the putamen), the supplementary motor area (SMA), and other motor areas of the frontal cortex (Bischoff-Grethe, Goedert, Willingham, & Grafton, 2004; Grafton, Hazeltine, & Ivry, 1995; Poldrack et al., 2005; Rauch et al., 1997; Willingham, Salidis, & Gabrieli, 2002). These corticostriatal circuits have been more directly linked with action selection and initiation than timing (Grahn, Parkinson, & Owen, 2009). The current results suggest either that timing information is integrated into the representation of action sequences in these circuits (e.g., via chunking) or that timing information from another brain region (e.g., the cerebellum) is integrated in a convergence zone, such as a cortical area involved in motor control with connections to both the basal ganglia and cerebellum (e.g., the SMA).
Familiar sequences of motor actions that are used in everyday life frequently depend on accurate timing between movements. This is clear in expertly trained sequential behaviors such as sports and music performance, which are explicitly guided at first but gradually become automatic through practice, and also in more basic processes such as walking and speaking. The results reported here show that when timing is intrinsic to the task – as is the case with most real-life motor skills – timing is tightly integrated with order in the learned sequence representation. Thus, examining sequential motor learning with the SISL task, which makes timing intrinsic to performance, may provide better insight into the learning mechanisms and neural systems supporting skilled motor performance than simpler cue-response reaction time tasks. While our finding – that disrupting timing leaves participants unable to apply their sequence knowledge of the repeating sequence – may seem surprising based on prior SRT work, it would probably not be surprising to a baseball player such as Hall of Fame pitcher Warren Spahn, who said, “Hitting is timing. Pitching is upsetting timing.”
Acknowledgments
This research was supported by the National Institutes of Health training grant T32 NS047987 awarded to Eric W. Gobel from the National Institute of Neurological Disease and Stroke and was partially supported by a Research Grant from the University Research Grants Committee at Northwestern University. The authors would like to acknowledge Dr. Michael Ziessler for insightful comments during the review of the manuscript and the game designers at RedOctane/Harmonix (Guitar Hero, Rock Band) and Konami (Dance Dance Revolution) for inspiration in design of the interface to the SISL task.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/xlm
References
- Bischoff-Grethe A, Goedert KM, Willingham DT, Grafton ST. Neural substrates of response-based sequence learning using fMRI. Journal of Cognitive Neuroscience. 2004;16:127–138. doi: 10.1162/089892904322755610. doi:10.1162/089892904322755610. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ. Cerebellar stroke impairs temporal but not spatial accuracy during implicit motor learning. Neurorehabilitation and Neural Repair. 2004;18:134–143. doi: 10.1177/0888439004269072. doi: 10.1177/0888439004269072. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. doi:10.1016/S0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. Journal of Cognitive Neuroscience. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. doi:10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The role of the basal ganglia in learning and memory: neuropsychological studies. Behavioral Brain Research. 2009;199:53–60. doi: 10.1016/j.bbr.2008.11.020. doi:10.1016/j.bbr.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Basal ganglia and chunking of action repertoires. Neurobiology of Learning and Memory. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. doi:10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Jackson GM, Jackson SR, Harrison J, Henderson L, Kennard C. Serial reaction time learning and Parkinson's disease: evidence for a procedural learning deficit. Neuropsychologia. 1995;33:577–93. doi: 10.1016/0028-3932(95)00010-z. doi:10.1016/0028-3932(95)00010-Z. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cognitive Psychology. 1987;19:1–32. doi:10.1016/0010-0285(87)90002-8. [Google Scholar]
- O'Reilly JX, McCarthy KJ, Capizzi M, Nobre AC. Acquisition of the temporal and ordinal structure of movement sequences in incidental learning. Journal of Neurophysiology. 2008;99:2731–2735. doi: 10.1152/jn.01141.2007. doi:10.1152/jn.01141.2007. [DOI] [PubMed] [Google Scholar]
- Perruchet P, Chambaron S, Ferrel-Chapus C. Learning from implicit learning literature: Comment of Shea, Wulf, Whitacre, and Park, (2001) Quarterly Journal of Experimental Psychology A. 2003;56:769–778. doi: 10.1080/02724980244000657. doi:10.1080/02724980244000657. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. The neural correlates of motor skill automaticity. Journal of Neuroscience. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. doi:10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Savage CR, Curran T, Kendrick A, Brown HD, Rosen BR. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Human Brain Mapping. 1997;5:124–132. doi:10.1002/(SICI)1097-0193(1997)5:2<124∷AID-HBM6>3.0.CO;2–5. [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Parallel brain systems for learning with and without awareness. Learning & Memory. 1994;1:217–229. doi:10.1101/lm.1.4.217. [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Encapsulation of implicit and explicit memory in sequence learning. Journal of Cognitive Neuroscience. 1998;10:248–263. doi: 10.1162/089892998562681. doi:10.1162/089892998562681. [DOI] [PubMed] [Google Scholar]
- Reed J, ohnson P. Assessing implicit learning with indirect tests: determining what is learned about sequence structure. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:585–594. doi:10.1037/0278-7393.20.3.585. [Google Scholar]
- Robertson EM. The serial reaction time task: implicit motor skill learning? Journal of Neuroscience. 2007;27:10073–10075. doi: 10.1523/JNEUROSCI.2747-07.2007. doi:10.1523/JNEUROSCI.2747-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salidis J. Nonconscious temporal cognition: learning rhythms implicitly. Memory & Cognition. 2001;29:1111–1119. doi: 10.3758/bf03206380. [DOI] [PubMed] [Google Scholar]
- Sanchez DJ, Gobel EW, Reber PJ. Performing the unexplainable: Implicit task performance reveals individually reliable sequence learning without explicit knowledge. Psychonomic Bulletin & Review. 17(6) doi: 10.3758/PBR.17.6.790. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea CH, Park J, Braden HW. Age-related effects in sequential motor learning. Physical Therapy. 2006;86:478–488. [PubMed] [Google Scholar]
- Shea CH, Wulf G, Whitacre CA, Park J. Surfing the implicit wave. Quarterly Journal of Experimental Psychology A. 2001;54:841–862. doi: 10.1080/713755993. doi:10.1080/713755993. [DOI] [PubMed] [Google Scholar]
- Shin JC, Ivry RB. Concurrent learning of temporal and spatial sequences. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:445–457. doi: 10.1037//0278-7393.28.3.445. doi:10.1037/0278-7393.28.3.445. [DOI] [PubMed] [Google Scholar]
- Werheid K, Ziessler M, Nattkemper D, von Cramon DY. Sequence learning in Parkinson's disease: The effect of spatial stimulus-response compatibility. Brain and Cognition. 2003;52:239–249. doi: 10.1016/s0278-2626(03)00076-9. doi:10.1016/S0278-2626(03)00076-9. [DOI] [PubMed] [Google Scholar]
- Willingham DB. Implicit motor sequence learning is not purely perceptual. Memory and Cognition. 1999;27:561–572. doi: 10.3758/bf03211549. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Greeley T, Bardone AM. Dissociation in a serial response time task using a recognition measure: comment on Perruchet and Amorim, (1992) Journal of Experimental Psychology: Learning, Memory and Cognition. 1993;19:1424–1430. doi:10.1037/0278-7393.19.6.1424. [Google Scholar]
- Willingham DB, Salidis J, Gabrieli JDE. Direct comparison of neural systems mediating conscious and unconscious skill learning. Journal of Neurophysiology. 2002;88:1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Wells LA, Farrell JM, Stemwedel ME. Implicit motor sequence learning is represented in response locations. Memory and Cognition. 2000;28:366–375. doi: 10.3758/bf03198552. [DOI] [PubMed] [Google Scholar]
- Wulf G, Schmidt RA. Variability of practice and implicit motor learning. Jounral of Experimental Psychology: Learning, Memory, & Cognition. 1997;23:987–1006. doi:10.1037/0278-7393.23.4.987. [Google Scholar]