Abstract
The Pedersen hypothesis was formulated over 50 years ago. Jorgen Pedersen primarily cared for women with type-1 diabetes. He suggested that fetal overgrowth was related to increased transplacental transfer of glucose stimulating the release of insulin by the fetal beta cell and subsequent macrosomia. Optimal maternal glucose control decreased perinatal mortality and morbidity. However, over the ensuing decades, there have been increases in maternal obesity and subsequently gestational (GDM) and type-2 diabetes. The underlying pathophysiology of type- 1 and GDM/type-2 diabetes are fundamentally different: type-1 diabetes being primarily a disorder of beta cell failure and type-2 diabetes/GDM including both insulin resistance and beta cell dysfunction. As such the metabolic milieu in which the developing fetus is exposed may be quite different in type-1 diabetes and obesity. In this review we examine the metabolic environment of obese diabetic women and lipid metabolism affecting fetal adiposity. The importance of understanding these issues relates to the increasing trends of obesity worldwide with perinatal programming of metabolic dysfunction in the offspring.
Keywords: fetal adiposity, lipid metabolism, obesity, pregnancy
Introduction
Over the last two decades there has been a significant increase in obesity in the general population of industrialized countries and a growing concern in the developing world as well (1). In the United States in 2009 approximately sixty-eight percent of the population is overweight (body mass index kg/m2, BMI) defined as a BMI of >25. Thirty-five percent of the adult female population is obese with a BMI >30, and fully 7.2 % have significant or class III obesity, i.e. a BMI >40 (2), (3). The increase in obesity is not limited to adults, but affects 15–25% of children as young as 2 years of age. The increase in obesity has disproportionately affected minority populations (4). How do the changes in the demographics of the population affect the manner in which we care for pregnant women?
We clearly recognize that the maternal metabolic environment of women with poorly controlled diabetes at conception increases the risk of congenital anomalies, i.e. fuel mediated teratogenesis (5). However, we often pay little heed to the periconceptual environment in the woman who is overweight or obese. Obese women are at risk for early pregnancy loss (6) and congenital anomalies, such as neural tube defects (7). However, there is also increasing evidence that the maternal metabolic environment may have long term effects on the developing fetus, i.e. perinatal metabolic programming. The effects of this obesogenic environment on long term childhood development may be more subtle than phenotypic congenital anomalies. For example epigenetic changes in the feto-placental unit affecting the risk of chronic disease such as hypertension, diabetes and cardiovascular dysfunction, may become clinically manifest only decades later. Hence, this review will focus on how the obesity epidemic has altered our understanding of the maternal metabolic environment, causing us to rethink and critically examine our beliefs and practices relating to fetal growth.
The Pedersen Hypothesis
Although first formulated in the 1920's, Jorgen Pedersen is generally given credit for the hyperglycemia-hyperinsulinemia hypothesis or as it is more commonly referred to today; the Pedersen hypothesis (8). The hypothesis as stated by Pedersen is as follows: “maternal hyperglycaemia results in foetal hyperglycaemia and, hence, in hypertrophy of foetal islet tissue with insulin-hypersecretion. This again means a greater foetal utilization of glucose. This phenomenon will explain several abnormal structure and changes found in the newborn” (9) Today the Pedersen hypothesis is most commonly associated with the concept of fetal overgrowth or macrosomia.
There are abundant data to support the Pedersen's hypothesis. For example umbilical cord insulin concentrations are strongly correlated with fetal growth in both human and animal studies. Schwartz et al reported that fetal size was significantly correlated with umbilical total insulin, free insulin and C-peptide (10). The recently completed hyperglycemia and adverse pregnancy outcome study (HAPO) showed a linear relationship between increasing maternal glucose and cord C-peptide with birth weight (11).
Several in vivo animal models also support the Pedersen's hypothesis. Twelve hours after injection of insulin into rat fetuses, total fetal weight, both wet and dry mass, significantly increased as compared with saline injected controls (12). In an elegant experiment, using in utero insulin infusion via an osmotic pump implanted into a fetal rhesus monkey for 21 days, Susa et al reported a 34% increase in body weight particularly in the liver, heart and spleen in the experimental model as compared with controls despite maternal euglycemia. Interestingly, there was no significant increase in the lipid, protein, DNA and RNA concentrations. The authors concluded that increased fetal insulin, even in the presence of normal maternal substrate concentrations, was growth promoting in the non-human primate (13). Last, the corollary experiment with injecting streptozocin to the fetal sheep resulted in beta cell destruction with subsequent hypoinsulinemia (14). Fetal body weight was decreased by 21%, particularly protein content in carcass, liver and kidney. There were no significant changes in fetal lipid accretion (14). Hence based on the available data, it has become widely accepted that fetal insulin is a primary in utero growth factor. However, is the concept developed by Pedersen reflecting the entire story?
The metabolic environment of pregnant women is evolving
In the 1950's when Pedersen cared for pregnant women with preexisting diabetes, the overwhelming majority of these women had type-1 diabetes. One of the primary goals of management was to maintain optimal glucose control and decrease the risk of ketoacidosis. Hence these women were treated using insulin along with a diet of between 1800 to 2000 calories (90 g of protein, 80 g of fat and 180–200 g of carbohydrate distributed over 6 meals (15). Many of these women were quite thin, as this was the common phenotype of women with type 1 diabetes at that time.
Concurrent with the current epidemic of obesity, there is also increasing incidence a of type 2 diabetes. The Center for Disease Control and Prevention (CDC) estimates that in 2007, 23.6 million people or 7.8% of the population had diabetes (3). The majority of women of reproductive age with pre existing diabetes now consist of women with type-2 rather than type-1 diabetes, Figure 1. Along with the increase of type-2 diabetes in the population, Reaven coined the term “metabolic syndrome” to describe the constellation of symptoms associated with this increase in obesity and diabetes in the non-pregnant population (16). The metabolic syndrome encompasses a myriad of seemingly unrelated disorders such as hypertension, hyperlipidemia, atherosclerosis, and inflammation. During pregnancy these disorders may be referred to using different names, such as gestational diabetes (GDM), pregnancy associated hypertension, and preeclampsia, but most likely represent a similar pathophysiology. The common metabolic thread being increased insulin resistance and hyperinsulinemia. As this concept has developed, research has shown that adipose tissue, originally thought to be simply a storage depot for triglycerides, actually serves a wide array of metabolic functions. The current understanding is that the adipocyte and adjacent stroma are metabolically active tissues; having metabolic, endocrine and immune functions (reviewed in 17). In short, with increasing obesity there evolves an inflammatory milieu, where cytokine production by macrophages in the adipose tissue affects post receptor insulin signaling. This disturbance of insulin signaling results in increased insulin resistance (18). Pregnancy is also in and of itself an inflammatory condition, very possibly initiated to allow immuno-tolerance of the fetus by the mother (19). Inflammation, therefore has become an important factor for our understanding of the mechanism for the increased insulin resistance of pregnancy.
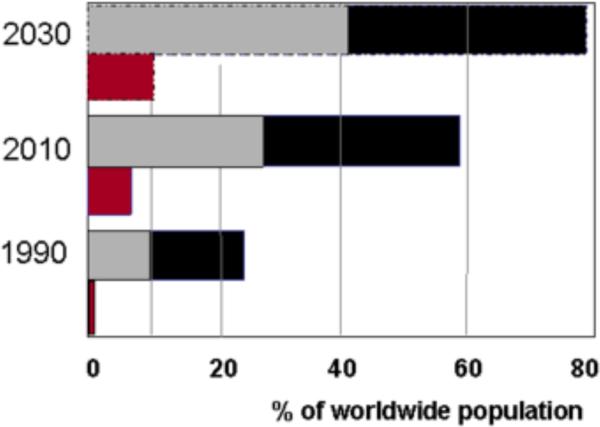
Figure 1.
Longitudinal trends of the diabetes and obesity epidemics, encompassing the 20th and 21st centuries.
Trends in obesity and diabetes from 1990 through 2030: the projected numbers showing a parallel increase from the last century through 2030. Black bars: overweight individuals (BMI>25) including obese (BMI>30). Grey bars: obese individuals, red bars: type-2 and type-1 individuals with diabetes; with type-2 representing >90 % of all the population with diabetes. Adapted from: Refs 76, 75 and www.iotf.org/database
In summary, the obese woman begins pregnancy with greater insulin resistance as compared with her normal weight counterpart. The 50 to 60% increase in insulin resistance of pregnancy further increases this metabolic stress in these women. These metabolic changes create a metabolic environment of excess nutrients and cytokines in which the developing feto-placental unit develops.
Fetal macrosomia despite excellent glucose control; a role for maternal lipids?
Clinically, despite what is apparently excellent glucose control, some pregnant women with diabetes of any classification GDM, type 1 or type 2 have a large or macrosomic fetus (20). We often attribute this to unrecognized hyperglycemia despite of intermittent home blood glucose monitoring values within the normal range. Continuous glucose monitoring in pregnant women with diabetes has provided data supporting the concept that improved glucose control, as estimated by HbA1c in later pregnancy, can decrease the risk of macrosomia when compared to frequent glucose self monitoring. However, although rates of macrosomia have been decreased with continuous glucose monitoring, they remain 3.5 times higher than in the general population (21). Additionally, Langer and Yariv have shown that the risk of having a macrosomic baby in women with well controlled GDM was a function of their pregravid BMI. Overweight women with well controlled GDM on diet alone had a 50% greater risk of having a macrosomic baby as compared with normal weight woman with GDM. The risk of macrosomia increased 2 fold if the women was obese. In women with poorly controlled GDM, the risk of macrosomia increased 3 fold. Of interest, overweight or obese women with well controlled GDM on insulin had no increased risk of macrosomia in comparison to the reference group (22).This effect of insulin to decrease the risk of macrosomia may be related to the effects of insulin on lipid as well as glucose metabolism. Whether or not oral agents would have a similar effect on fetal growth is not known and remains speculative.
It is critical to remember, that insulin resistance or diabetes per se are disorders affecting more than just glucose metabolism (23). Increased free fatty acids and triglycerides are hallmarks of insulin resistance, particularly in the obese individual. In this context, whether factors other than glucose to modulate fetal overgrowth and adiposity in overweight and obese women is a relevant question.
Is macrosomia or increased birth weight a sufficient measure of fetal overgrowth?
In the past 5 years there has emerged solid clinical data based, on randomized clinical trials, that treatment of women with GDM can improve outcomes, in particular limiting fetal overgrowth. Both the ACHOIS and the Maternal Fetal Medicine Network (MFMU) trials have shown that treatment of GDM with lifestyle measures such as diet, decreases the rate of macrosomia and in the case of the MFMU decrease fetal adiposity (24, 25).
At birth, the human has one of the largest percentage of body fat in comparison with other mammalian species; approximately 12–15 percent depending on the methodology used (26). In contrast, murine models have on the order of 1–3% body fat at birth (27). Even the non-human primate, such as the rhesus monkey, has only 3–5 percent body fat (28). As such, factors which are well known to affect fetal growth may differentially affect the various components of fetal body composition. The in utero metabolic environment is thought to primarily affect fetal fat mass and not lean body mass (29). Previous studies have reported that neonates of women with well controlled GDM have significantly increased fat mass but not lean body mass. This increase in fat mass persists even when birth weights are appropriate for gestational age, race and gender (30). Similarly, it is well recognized that neonates of overweight and obese women are significantly heavier at birth as compared with lean or average weight women (31–32). These neonates are heavier because of an increase in fat and not lean body mass. (33).
In summary, the in utero metabolic environment affects primarily growth of adipose and not lean body mass. The increase in birth weight in overweight/obese women, and even in women with well controlled GDM is because of increased fat and not lean body mass. Since increased adiposity at birth is related to both obesity and metabolic dysfunction even in children (31), it is imperative to understand which factors are related to and amenable to preventive treatment during pregnancy. .
Metabolic adaptations to pregnancy: role of lipids
There is a decreased ability of insulin to suppress lipolysis in late pregnancy (35). In addition to the increase in glucose after an oral glucose challenge or post-meal, there are significant increases in circulating lipids during human pregnancy. In the third trimester obese women have higher triglyceride, VLDL-cholesterol and lower HDL concentrations as compared with lean women (36, 37, 38, 39). Similarly, women with GDM have significantly higher triglyceride concentrations as compared with women with normal glucose tolerance (40). Additional evidence for the insulin resistance relating to lipid metabolism during pregnancy was reported using euglycemic clamp studies. Infused insulin in women with gestational diabetes does not decrease free fatty acids (FFA) to the degree observed in a weight matched normal glucose tolerant group (41). This is a mechanism which may increase the availability of FFA available for placental transport to the fetus.
Several clinical studies have suggested the contribution of maternal lipids to fetal growth in particular adiposity. Knopp et al, in 1985 reported that triglycerides hydrolyzed by placental lipoprotein lipase to free fatty acids were able to cross the placenta. These FFA become incorporated into fetal lipids in normal pregnancy and exaggerated in women with GDM (42). Circulating triglyceride concentrations had a significant positive correlation with birth weight, independent of maternal obesity and glucose concentrations (43). Similarly the serum triglycerides and pre-pregnancy BMI of women with a positive glucose screen but normal glucose tolerance both correlate with birth weight at term (40). Non-fasting maternal triglycerides measured at 9–12 weeks gestation were significantly correlated with neonatal birth weight ratio (44). Last, in a well controlled GDM population, maternal FFA concentrations were correlated with ultrasound estimates of neonatal abdominal circumference and neonatal fat mass at birth (45). Taken together, these data suggest that in women with evidence of decreased insulin sensitivity, increased maternal lipids, in particular triglycerides may account for a significant proportion of fetal adiposity. These data support the original work by Freinkel that while glucose was an important component relating to fetal growth, fetal overgrowth is a function multiple nutritional factors in addition to glucose (46).
Perinatal metabolic programming
Why is it important to investigate issues related to maternal obesity and fetal overgrowth? Certainly from a clinical perspective, fetal macrosomia affects our clinical management of the obese pregnant women. There is the increased risk of, spontaneous abortion, congenital anomalies, stillbirth shoulder dystocia, and cesarean delivery in obese pregnant women (31). Furthermore, because of the increased prevalence of obesity, as many as 15–20% of pregnant women may soon be classified as having GDM. These data are based on the results of the HAPO study and recommendations of the International Association of Diabetes in Pregnancy Study Groups (47). Obese women are also at increased risk to develop the chronic medical diseases associated with the metabolic syndrome, both during pregnancy and later in life.
However, from a public health perspective, an equal and potentially more important issue is the increased risk for the offspring of these women. Offspring of obese women have an increased risk of developing obesity and metabolic dysfunction in childhood, thereby perpetuating a viscous cycle of obesity and diabetes (48). There are now multiple studies reporting that the infants of women diabetes are at increased risk of obesity and glucose intolerance as children and adolescents. Hillier et al, also reported that increased glucose concentrations, less than currently used to define GDM are associated with an increased risk of childhood obesity in a Kaiser population (49). Maternal pre-gravid obesity, even in women with well controlled GDM, is the strongest risk factor for childhood obesity and metabolic dysfunction (50). The next steps in this review are to investigate potential mechanisms relating to fetal obesity, in order to develop prevention and treatment strategies.
Molecular mechanisms accounting for metabolic programming of the offspring
Based on the clinical evidence reviewed above, changes in maternal lipid metabolism in pregnancy may account for an increased availability of lipid substrates for fetal growth and nutrition. The question then is what are the mechanisms which make the fetus fatter when maternal homeostasis becomes unbalanced, as with diabetes and obesity? Can increased fetal fat accretion be solely a result of excess maternal derived energy substrates? Are other factors increased in obesity such as inflammatory mediators facilitating fetal fat accretion?
Impact of maternal metabolic homeostasis on fetal lipid metabolism
The factors regulating lipid accumulation in adipose tissue need to be considered in order to understand the contribution of increased energy substrates to fetal adiposity. Triglycerides represent the primary component of lipid stores in fetal as well as in adult adipose tissue. There are two primary sources for triglyceride synthesis in mature adipocytes: 1- circulating FFA and 2- non-lipid precursors such as carbohydrates. Fatty acids are directly esterified into triglycerides, whereas carbohydrates derived from the diet enter the pathways of de novo lipid synthesis (DNLS).
There is considerable species difference in the ability of adipose tissue to perform DNLS from glucose. In humans and rodents, adipose tissue is a secondary site for DNLS from dietary carbohydrates. These data suggest that adipose tissue triglyceride storage may primarily rely upon the esterification of exogenous FFA (51, 52). There is evidence for DNLS from glucose in adipose tissue of the human fetus however, the relative contribution of DNLS to total triglyceride synthesis is not known (53, 54). During pregnancy the lipogenic substrates delivered to the fetus are by necessity of maternal origin. Glucose and FFA derived from maternal diet and metabolism are transferred to the fetal circulation through the placenta, Figure 2. It is thus conceivable that changes in the maternal metabolism, modifying the availability of nutrients, will impact fetal fat accretion. A diabetogenic or obesogenic environment will alter the balance of glucose vs. lipids in the maternal circulation and subsequently the amount transferred into the fetal circulation. The ratio of glucose vs. lipid in the fetal circulation may then differentially impact the pathways of lipogenesis into the fetal adipocyte.
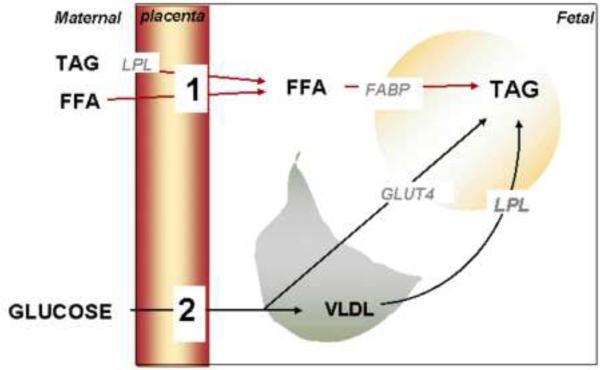
Figure 2.
energy substrates for lipogenesis in fetal adipocytes.
Tri-acylglycerides (TAG) represent the main storage form of lipids in the fetal adipocyte. Triglycerides can be formed from either glucose or free fatty acids (FFA) entering the adipocyte. FFA (1) are derived from the maternal circulation through direct placental transfer or after hydrolysis of TAG. Glucose (2) is directly transferred from the maternal circulation across the placenta for hepatic and adipose de novo lipogenesis. Very low density lipoproteins (VLDL) are also delivered from the fetal liver to adipose tissue to serve as FFA precursors upon hydrolysis by lipoprotein lipase (LPL). GLUT4: glucose transporter. FABP: fatty acid binding protein.
Although there is no direct information on the molecular pathways stimulated by insulin in the fetal adipocyte, multiple lines of evidence suggest that fetal hyperinsulinaemia is facilitating the uptake of glucose and its conversion into FFA. The mechanisms by which high glucose and insulin result in DNLS include increase glucose uptake through GLUT4 translocation, and incorporation into fatty acid through increased expression of the rate limiting enzymes fatty acid synthase and acetylcoA carboxylase (55).
In contrast to glucose, the role of lipids in the regulation of fetal growth has received relatively little attention. The pioneering studies by Szabo et al, proposed that FFA are transferred from mother to fetus and contribute to fetal macrosomia in a diabetic environment (56). However, the role of FFA as an energy fuel for adipose tissue development has received little attention. The potential role of FFA in deposition of fetal adipose stores has been primarily tempered by the long held belief that lipid transport mechanisms in the human placenta are inefficient. As a result of the paucity of studies, the pathways for fatty acid esterification in fetal adipose tissue are still not well described. The characterization of fatty acid binding proteins, lipid transporters, and enzyme for fatty acid esterification in the human placenta has now improved our view of how maternal lipids may contribute to fetal anabolism (57). Our hypothesis that lipids contribute to fetal fat accretion is supported by data showing that placental cells incubated in vitro with oleate as a substrate accumulate lipids (Figure 3). Because placental lipid pools serve as a relay towards transport to the fetus, this is an indirect indication that the placental lipid fluxes may be increased by fatty acids. Further support for this concept relies on an up-regulation of pathways regulating lipid transport and synthesis in the placenta of obese/GDM women (Figure 4 and 5). Furthermore oleate is a better energy substrate than glucose for the lipid storage in placental cells (Figure 4). In the mature adipocyte, the combination of high insulin concentrations with high glucose facilitates triglyceride synthesis whereas the esterification of fatty acids into triglycerides does not require high insulin levels (58).
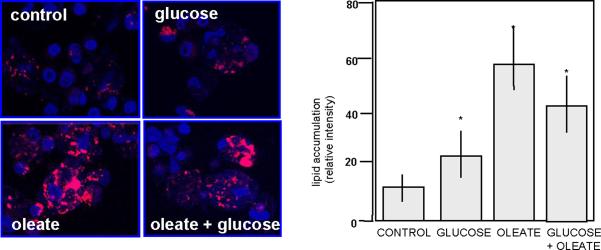
Figure 3.
Nutrient regulation of lipid content in the human placenta.
Quantification of lipid accumulation in human term trophoblast cells incubated for 48 hrs with glucose (10 mMol/L), oleate (400 nMol/L) or the combination of both. Left panel: immunohistochemistry, right panel: scanning densitometry of 4–6 independent (mean +SEM, * p< 0.0001). Adapted from: Ref 63
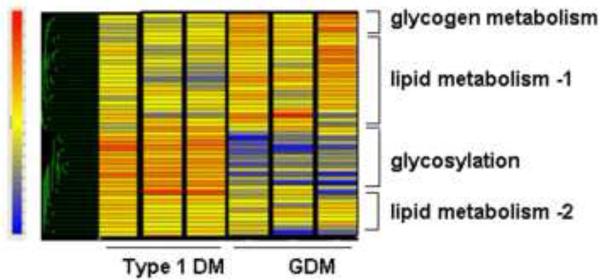
Figure 4.
Placental metabolic genes differentially regulated in diabetic pregnancy.
Expression data for the metabolic genes modified in pregnancy with GDM and type 1diabetes classified by hierarchical clustering. The prominent functional categories clusters shown on the right are based on the putative functional characteristics of the genes. The color tag and intensity represent the expression level from lowest (blue) to highest (red). Type 1 DM: Type 1 diabetes mellitus, GDM: gestational diabetes. Shown are data from 6 representative arrays run in duplicate. Adapted from: Ref 77
Figure 5.
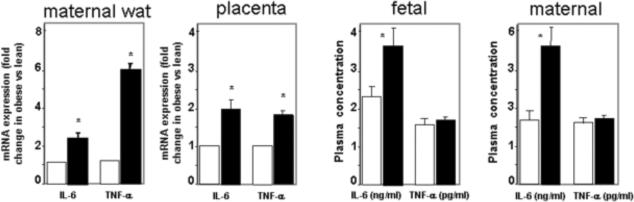
Increased IL-6 at the maternal-fetal interface in pregnancy of obese women relative to lean controls.
The expression of IL-6 is increased in both maternal adipose tissue and the placenta of obese women as well as in the systemic maternal and umbilical circulation. TNF-alpha is increased at the tissue level but not in plasma suggesting that is primary effect may be through paracrine/autocrine mechanisms. Tissue gene expression in stromal vascular fraction of adipose tissue and macrophage enriched fraction of term placenta is quantified by realtime RT-PCR. Results are expressed as mean + SE for 12 obese and 8 lean women. WAT: white adipose tissue. Open bars: lean, dark bars: obese. Adapted from: Refs 63, 64 and 71
Taken together these data suggest that lipogenesis in the fetus will be modulated by the characteristics of metabolic and endocrine environment. However, it is important to recognize that additional evidence-based data are critically needed to bridge the gaps in our understanding of fetal fat accretion in both healthy human pregnancy and those complicated by maternal obesity.
Impact of maternal inflammation on fetal lipid metabolism
The energy-rich condition of obesity is associated with the activation of inflammatory pathways present in adipose tissue. The combined metabolic and immune dysregulation results in an increased in circulating chemokines, cytokines and inflammatory mediators. In pregnancy complicated with obesity the maternal adipose tissue and the placenta both contribute to increase systemic inflammation (60). The chronic inflammatory milieu in pregnancy, associated with obesity and GDM, has provided the basis for the hypothesis: that if the mother is in an inflammatory condition, must not the fetus also be exposed to such an environment?
We propose that the maternal metabolic inflammation of obese women does not directly translate into inflammation in the fetal compartment. Experimental data from our group and others do not support this concept. In pregnancy complicated by obesity, the concentration of inflammatory markers and mediators is typically increased in the maternal but not in the fetal circulation (61). Most maternal cytokines are not readily transferred across the placenta in humans (62). Several cytokines such as Il-6 or leptin, synthesized within the placenta itself, are preferentially released into the maternal rather than into the fetal circulation (63).The environment of elevated IL-6 in systemic maternal and cord blood, as well as in maternal adipose tissue and the placenta, may play a role in metabolic inflammation. The role of hyperinsulinemia as a stimulus of Il-6 production by adipose tissue of obese women further underlies the close interactions of immune and metabolic systems (64). This is in contrast to other cytokines such as TNF-alpha which may primarily act locally (Figure 5).
The metabolic inflammation of pregnancy (sometimes termed as meta-inflammation) is not to the degree associated with severe infection or sepsis. Hence meta-inflammation may play an indirect role at the maternal fetal interface to modify the availability of energy substrates for fetal needs. The pattern of low grade chronic metabolic inflammation, being a common denominator for obesity and other diseases associated with insulin resistance, has emerged from the molecular cross-talks between metabolic and immune systems (65). Adipose tissue is actively involved in innate sensing. For example, adipose tissue is the major source of systemic IL-6 when systemic inflammation is induced by lipopolysaccharides (LPS) injection in mice (66) Toll like receptors, a family of membrane receptors recruited in innate sensing are the first line of pathogen recognition activated by environmental stimuli, such as (LPS) (67). Toll like receptor 4 (TLR4) responds to LPS by activation of intracellular signals which result in the increased synthesis of IL-6 and other pro-inflammatory cytokines (68). The ability of FFA to also activate TLR4 in adipose cells, also underlines the relationship between the immune and metabolic systems in disorders of lipid metabolism (69).
In pregnancies complicated by obesity, there is an increased expression and activation of TLR4 in maternal adipose tissue suggesting a response to immune stimuli (70). The contribution of the placenta to the increased inflammatory environment of obese pregnancy is supported by the observation that placenta perfused with LPS release increased amounts of cytokines (71). How or why circulating LPS is increased in obesity is not yet clear. There are correlations between gut pathogens, obesity and inflammation (72). Changes in diet and associated microbiota have recently been proposed as potential contributors to increased LPS and metabolic inflammation through TLR4 (73). We have reported that obese women have increased circulating levels of LPS and that LPS induces TLR4 receptors in adipose tissue (70). These data are consistent with changes in the microbiota of obese vs. lean pregnant women, which would enrich the environment with LPS (74).
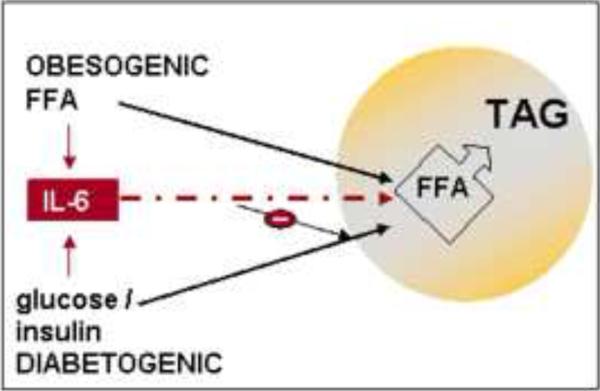
Based on the metabolic and immune interactions between maternal adipose tissue and the placenta, we hypothesize that obesogenic-diabetogenic challenges in the mother result in excess of either glucose or lipid availability for fetal adipose tissue lipogenesis (Figure 6). Depending on whether the maternal environment has evidence of increased glucose plus insulin or elevated lipid concentrations, either source of substrates may contribute to fetal fat accretion. Increased in fetal IL-6 may also contribute to increase insulin resistance before birth. Definite proof of our integrative hypothesis is awaiting experimental support.
Figure 6.
homeostatic regulation of fetal adipose tissue growth
The obesogenic environment with hyperlipidemia and moderate hyperinsulinemia facilitates FFA delivery and entry into the fetal adipocyte. The diabetogenic environment with both hyperglycemia and hyperinsulinemia favors the entry of glucose into the fetal adipocyte and pathways of DNLS into FFA. Either pathway ultimately leads to synthesis and storage of TAG in the lipid droplet. Additionally the increase in fetal systemic IL-6 may contribute to favor FFA over glucose entry into the fetal adipocyte through GLUT4 inhibition. DNLS: De novo lipid synthesis, FFA: free fatty acids, TAG: tri-acylglycerol, GLUT4: Glucose transporter 4.
CONCLUSION
The Pedersen hypothesis has generated an incredible stimulus for investigating and understanding diabetes related pregnancy disorders in the second half of the 20th century. The obesity epidemic in the 21st century is unfortunately opening new opportunities through which to envision in utero perinatal metabolic programming. We are hopeful that the combination of additional knowledge accumulating from ongoing research may result in a better understanding of the mechanisms resulting in fetal adiposity and metabolic dysfunction. For it is only by an understanding of the underlying mechanisms that will we be able to develop rational interventions leading to beneficial preventive strategies.
Condensation.
Because of the increased prevalence of obesity in women of reproductive age, we need to reconsider and address non-glucose related factors relating to fetal overgrowth.
Table 1.
Plasma lipoprotein lipids and apolipoproteins in women during pregnancy, postpartum and post lactation
| 1st Trimester | 2nd Trimester | 3rd Trimester | Postpartum | Post Lactation | |
|---|---|---|---|---|---|
| Total triglyceride | 60 ± 5 | 117 ± 9 | 1.84 ± 14 | 81 ± 9 | 63 ± 7 |
| Total cholesterol | 170 ± 5 | 234 ± 8 | 254 ± 9 | 234 ± 9 | 192 ± 14 |
| VLDL-Tg | 22 ± 4 | 44 ± 6 | 83 ± 11 | 38 ± 8 | 31 ± 6 |
| VLDL – Chol | 4 ± 1 | 8 ± 1 | 18 ± 2 | 7 ± 1 | 6 ± 1 |
| LDL-Tg | 20 ± 2 | 44 ± 3 | 62 ± 5 | 28 ± 2 | 19 ± 2 |
| LDL-Chol | 89 ± 5 | 136 ± 8 | 153 ± 8 | 155 ± 9 | 119 ± 13 |
| HDL-Tg | 12 ± 2 | 26 ± 2 | 29 ± 2 | 8 ± 1 | 6 ± 1 |
| HDL-Chol | 68 ± 3 | 82 ± 3 | 71 ± 3 | 66 ± 3 | 56 ± 4 |
| ApoA-1 | 136 ± 5 | 163 ± 7 | 162 ± 6 | 138 ± 5 | 135 ± 4 |
| ApoA-100 | 83 ± 5 | 126 ± 6 | 158 ± 9 | 125 ± 9 | 94 ± 9 |
Values are + SE of 25 women studied throughout pregnancy and post partum; 11 were also studied post lactation.
Adapted from Alvarez (Ref 37)
Acknowledgments
Supported by NIH-NICHD HD22965 and CTSA UL-1 RR 024989
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization Obesity: Preventing and management of global epidemic. World Health Organization Technical Report Ser. 2000;984:1–4. [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Center for Disease Control and Prevention Department of Health and Human Services. National Diabetes Fact Sheet. 2007 [Google Scholar]
- 4.Flegal KM, Ogden CL, Yanowski JA, et al. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91:1020–6. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freinkel N. Diabetic embriopathy and fuel-mediated organ teratogenesis: Lessons from animal models. Horm Metab Res. 1988;20:463–75. doi: 10.1055/s-2007-1010861. [DOI] [PubMed] [Google Scholar]
- 6.Lashen H, Few K, Sturdee DW. Obesity is assocated with increased first trimester miscarriage: Matched core-control study. Human Reprod. 2004;19:1644–6. doi: 10.1093/humrep/deh277. [DOI] [PubMed] [Google Scholar]
- 7.Strothard KJ, Tennant BWG, Bill R, Ramin J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA. 2009;301:636–50. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen J. Diabetes and pregnancy: Blood sugar of newborn infants (Ph.D. Thesis) Danish Science Press; Copenhagen: 1952. p. 230. [Google Scholar]
- 9.Pedersen J. The pregnant diabetic and her newborn: Problems and management. William & Wilkins; Baltimore, MD: 1967. pp. 128–37. [Google Scholar]
- 10.Schwartz R, Gruppuso PA, Petzold K, Brambilla D, Hiilesmaa V, Teramo KA. Hyperinsulinemia and macrosomia in the fetus of the diabetic mother. Diabetes Care. 1994;17:640–8. doi: 10.2337/diacare.17.7.640. [DOI] [PubMed] [Google Scholar]
- 11.HAPO Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcomes. N Engl J Med. 2008;358:1996–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 12.Ogata ES, Collins JW, Jr., Finley S. Insulin injection in the fetal rat accelerated intrauterine growth and altered fetal and neonatal glucose homeostasis. Metab. 1988;37:649–55. doi: 10.1016/0026-0495(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 13.Susa JB, Neave C, Sehgal P, Singer DB, Zeller WP, Schwartz R. Chronic hyperinsulinemia in the fetal rhesus monkey. Effects of physiologic hyperinsulinemia on fetal growth and composition. Diabetes. 1984;33:656–660. doi: 10.2337/diab.33.7.656. [DOI] [PubMed] [Google Scholar]
- 14.Phillips AF, Rosenkrantz TS, Clark RM, Knox I, Chaffin DG, Raye Effects of fetal insulin deficiency on growth in fetal lambs. Diabetes. 1991;40:20–7. doi: 10.2337/diab.40.1.20. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen J. The pregnant diabetic and her newborn: Problems and management. William & Wilkins; Baltimore, MD: 1967. pp. 138–49. [Google Scholar]
- 16.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1600. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 17.Ahima RS, Flier JS. Adipost tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–32. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 18.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation and insulin resistance. Gastroenterology. 2007;132:2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 19.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 20.Evers IM, de Valk HW, Mol BWJ, ter Braak EWMT, Visser GHA. Macrosomia despite good gylcaemic control in type 1 diabetic pregnancy; results of a nationwide study in the Netherlands. Diabetologia. 2002;45:1484–9. doi: 10.1007/s00125-002-0958-7. [DOI] [PubMed] [Google Scholar]
- 21.Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes in randomized clinical trials. Br Med J. 2008;337:a1680. doi: 10.1136/bmj.a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer O, Yogev Y, Xenakis EMJ, Brustman L. Overweight and obese gestational diabetes: Impact on pregnancy outcome. Am J Obstet Gynecol. 2005;192:1768–76. doi: 10.1016/j.ajog.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 23.McGarry JD. Banting Lecture 2001. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 24.Crowther CA, Hillier JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 25.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment of mild gestational diabetes. N Engl J Med. 2009;361:1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widdowson EM. Chemical composition of newly born mammals. Nature. 1950;66:626–8. doi: 10.1038/166626a0. [DOI] [PubMed] [Google Scholar]
- 27.Russo AR, Ausman LM, Gallina DL, Hegsted DM. Developmental body composition of the squirrel monkey (Saimiri sciureus) Growth. 1980;44:271–86. [PubMed] [Google Scholar]
- 28.Ausman LM, Powell EM, Mercado DL, Samonds KW, Lozy M, Galina DL. Growth and developmental body composition of the cebus monkey (Cebus Albifrons) Am J Perinatol. 1982;3:211–2. doi: 10.1002/ajp.1350030119. [DOI] [PubMed] [Google Scholar]
- 29.Sparks J. Human intrauterine growth and nutrient accretion. Semin Perinatol. 1984;18:74–93. [PubMed] [Google Scholar]
- 30.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: A very sensitive marker of abnormal in-utero development. Am J Obstet & Gynecol. 2003;189:1698–704. doi: 10.1016/s0002-9378(03)00828-7. [DOI] [PubMed] [Google Scholar]
- 31.Catalano PM. Management of obesity in pregnancy. Obstetrics and Gynecology. 2007;109:419–33. doi: 10.1097/01.AOG.0000253311.44696.85. [DOI] [PubMed] [Google Scholar]
- 32.Chu SY, Callaghan WM, Bisch CC, D'Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004–2005: Fueling future obesity. Am J Obstet Gynecol. 2009;200:271–7. doi: 10.1016/j.ajog.2008.09.879. [DOI] [PubMed] [Google Scholar]
- 33.Sewell MF, Huston-Presley L, Super DM, Catalano PM. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–3. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–16. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 35.Sivan E, Homko CJ, Chen X, Reece EA, Boden G. Effect of insulin on fat metabolism during and after normal pregnancy. Diabetes. 1999;48:834–8. doi: 10.2337/diabetes.48.4.834. [DOI] [PubMed] [Google Scholar]
- 36.Fahraeus L, Larsson-Cohn U, Wallentin L. Plasma lipoproteins including high density lipoprotein subfractions during normal pregnancy. Obstet Gynecol. 1985;66:468–72. [PubMed] [Google Scholar]
- 37.Alvarez JJ, Montelongo A, Iglesias A, Lasuncion MA, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res. 1996;37:299–308. [PubMed] [Google Scholar]
- 38.Sattar N, Tan CE, Han TS, et al. Associations of indices of adiposity with atherogenic lipoprotein subfractions. Intern J Obes. 1998;22:432–9. doi: 10.1038/sj.ijo.0800604. [DOI] [PubMed] [Google Scholar]
- 39.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87:4231–7. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 40.Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med. 2005;22:21–5. doi: 10.1111/j.1464-5491.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- 41.Catalano PM, Nizielski SE, Shao J, Presley L, Qiao L, Friedman JE. Downregulated IRS-1 and PPARγ in obese women with gestational diabetes: Relationship to free fatty acids during pregnancy. Am J Physiol Endocrine Metab. 2002;282:E522–33. doi: 10.1152/ajpendo.00124.2001. [DOI] [PubMed] [Google Scholar]
- 42.Knopp RH, Bergelin RO, Wahl PW, Walden CE. Relationships of infant birth size to maternal lipoproteins, apoproteins, fuels, hormones, clinical chemistries, and body weight at 36 weeks gestation. Diabetes. 1985;34:71–7. doi: 10.2337/diab.34.2.s71. [DOI] [PubMed] [Google Scholar]
- 43.Katajima M, Satoshi O, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24-32 weeks' gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol. 2001;97:776–80. doi: 10.1016/s0029-7844(01)01328-x. [DOI] [PubMed] [Google Scholar]
- 44.Nolan CJ, Riley SF, Sheedy MT, Walstab JE, Beischer NA. Maternal serum triglyceride, glucose tolerance, and neonatal birth weight ratio in pregnancy. Diabetes Care. 1995;18:1550–6. doi: 10.2337/diacare.18.12.1550. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008;31:1858–63. doi: 10.2337/dc08-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freinkel N. Banting Lecture of 1980: Of Pregnancy and Progeny. Diabetes. 1980;29:1023–35. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 47.International Association of Diabetes and Pregnancy Study Groups Consensus Panel International Association of Diabetes and Pregnancy Study Groups Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catalano PM. Obesity and pregnancy – The propagation of a viscous cycle? J Clin Endocrinol Metab. 2003;88:3505–6. doi: 10.1210/jc.2003-031046. [DOI] [PubMed] [Google Scholar]
- 49.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles M-A, Petitt DJ. Childhood obesity and metabolic imprinting. Diabetes Care. 2007;30:2287–92. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 50.Catalano PM, Farrell K, Huston-Presley L, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–13. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr. 2005;135:2499–2502. doi: 10.1093/jn/135.11.2499. [DOI] [PubMed] [Google Scholar]
- 52.Patel MS, Owen OE, Goldman LI, Hanson RW. Fatty acid synthesis by human adipose tissue. Metabolism. 1975;24:161–173. doi: 10.1016/0026-0495(75)90017-7. [DOI] [PubMed] [Google Scholar]
- 53.Dunlop M, Court JM. Lipogenesis in developing human adipose tissue. Early Hum Dev. 1978;2:123–130. doi: 10.1016/0378-3782(78)90004-x. [DOI] [PubMed] [Google Scholar]
- 54.Roux JF, Takeda Y, Grigorian A. Lipid concentration and composition in human fetal tissue during development. Pediatrics. 1971;48:540–546. [PubMed] [Google Scholar]
- 55.Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001;2(4):282–6. doi: 10.1093/embo-reports/kve071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szabo AJ, Szabo O. Placental free-fatty-acid transfer and fetal adipose-tissue development: an explanation of fetal adiposity in infants of diabetic mothers. Lancet. 1974;2:498–499. doi: 10.1016/s0140-6736(74)92020-0. [DOI] [PubMed] [Google Scholar]
- 57.Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth--a review. Placenta. 2002 Apr;23(Suppl A):S28–38. doi: 10.1053/plac.2002.0791. [DOI] [PubMed] [Google Scholar]
- 58.Nye C, Kim J, Kalhan SC, Hanson RW. Reassessing triglyceride synthesis in adipose tissue Trends in Endocrinology & Metabolis. 2008;19:356–361. doi: 10.1016/j.tem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Radaelli T, Lepercq J, Varastehpour A, Basu S, Catalano PM. Hauguel-de Mouzon S Differential regulation of genes for feto-placental lipid pathways in pregnancy with gestational and type 1 diabetes. Am J Obstet Gynecol. 2009;201:e201–209. doi: 10.1016/j.ajog.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Challier JC, Bintein T, Basu S, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–81. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes care. 2009;32:1076–80. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol. 2005;106(4):802–7. doi: 10.1097/01.AOG.0000178750.84837.ed. [DOI] [PubMed] [Google Scholar]
- 63.Malek R, Sager, Schneider H. Effect of Hypoxia, Oxidative Stress and Lipopolysaccharides on the Release of Prostaglandins and Cytokines from Human Term Placental Explants Placenta. 2001;22:S45–S50. doi: 10.1053/plac.2001.0635. [DOI] [PubMed] [Google Scholar]
- 64.Siklova-Vitkova M, Polak J, Klimcakova E, et al. Effect of hyperinsulinemia and very-low-calorie diet on interstitial cytokine levels in subcutaneous adipose tissue of obese women. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00086.2009. [DOI] [PubMed] [Google Scholar]
- 65.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;14(444):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 66.Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009;64(7):723–30. doi: 10.1093/gerona/glp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kopp A, Buechler C, Neumeier M, et al. Innate immunity and adipocyte function: ligand-specific activation of multiple toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity. 2009;17:648–656. doi: 10.1038/oby.2008.607. [DOI] [PubMed] [Google Scholar]
- 68.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Basu S, Haghiac M, Surace P, et al. Pre-gravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity. doi: 10.1038/oby.2010.215. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holcberg G, Amash A, Sapir O, Sheiner E, Levy S, Huleihel M. Perfusion with lipopolysaccharide differently affects the secretion of tumor necrosis factor-alpha and interleukin-6 by term and preterm human placenta. J Reprod Immunol. 2007;74:15–23. doi: 10.1016/j.jri.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 72.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 73.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 74.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88(4):894–9. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 75.Haffner SM. Relationship of metabolic risk factors and development of cardiovascular disease and diabetes. Obesity. 2006;(Suppl 3):121S–127S. doi: 10.1038/oby.2006.291. [DOI] [PubMed] [Google Scholar]
- 76.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Radaelli T, Lepercq J, Varastehpour A, Basu S, Catalano PM, Hauguel-de Mouzon S. Differential regulation of genes for feto-placental lipid pathways in pregnancy with gestational and type 1 diabetes. Am J Obstet Gynecol. 2009;201:e201–209. doi: 10.1016/j.ajog.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]