Abstract
Members of the Krüppel-like family of transcription factors regulate diverse developmental processes in various organs. Previously, we have demonstrated the role of Klf4 in the mouse ocular surface. Herein, we determined the role of the structurally related Klf5, using Klf5-conditional null (Klf5CN) mice derived by mating Klf5-LoxP and Le-Cre mice. Klf5 mRNA was detected as early as embryonic day 12 (E12) in the cornea, conjunctiva and eyelids, wherein its expression increased during development. Though the embryonic eye morphogenesis was unaltered in the Klf5CN mice, postnatal maturation was defective, resulting in smaller eyes with swollen eyelids that failed to separate properly. Klf5CN palpebral epidermis was hyperplastic with 7-9 layers of keratinocytes, compared with 2-3 in the wild type (WT). Klf5CN eyelid hair follicles and sebaceous glands were significantly enlarged, and the meibomian glands malformed. Klf5CN lacrimal glands displayed increased vasculature and large number of infiltrating cells. Klf5CN corneas were translucent, thicker with defective epithelial basement membrane and hypercellular stroma. Klf5CN conjunctiva lacked goblet cells, demonstrating that Klf5 is required for conjunctival goblet cell development. The number of Ki67-positive mitotic cells was more than doubled, consistent with the increased number of Klf5CN ocular surface epithelial cells. Co-ablation of Klf4 and Klf5 resulted in a more severe ocular surface phenotype compared with Klf4CN or Klf5CN, demonstrating that Klf4 and Klf5 share few if any, redundant functions. Thus, Klf5CN mice provide a useful model for investigating ocular surface pathologies involving meibomian gland dysfunction, blepharitis, corneal or conjunctival defects.
Keywords: Klf5, cornea, conjunctiva, meibomian glands, lacrimal glands, eyelids, goblet cells
Introduction
The transparent cornea serves as the chief refractive tissue of the terrestrial vertebrates and a barrier against physical, chemical and biological insults to the eye. Abnormal development and/or defective maintenance of the cornea lead to severe defects in vision (Klintworth, 2003; Vincent et al., 2005). The involvement of various transcription factors in regulating the corneal development has been intensely studied (Adhikary et al., 2005a; Adhikary et al., 2005b; Chiambaretta et al., 2002; Chiambaretta et al., 2006; Davis et al., 2003; Dwivedi et al., 2005; Francesconi et al., 2000; Hough and Piatigorsky, 2004; Lambiase et al., 2005; Nakamura et al., 2004; Nakamura et al., 2005; Sivak et al., 2000; Sivak et al., 2004; Swamynathan et al., 2008; Swamynathan et al., 2007; Ueta et al., 2005). In spite of this progress, knowledge of the genetic network of transcription factors required for maturation and maintenance of the cornea and other components of the ocular surface remains incomplete.
The cornea is protected externally by the eyelids which contain meibomian glands that produce and secrete the lipids to the tear film to reduce evaporative losses from the ocular surface. The meibomian glands consist of a number of lipid producing acini connected to a central lipid conducting duct which releases the secreted meibum to the mucocutaneous junction of the eyelids (Mathers et al., 1996). The mouse meibomian gland development starts around embryonic day 18 (E18), resulting in the formation of mature meibomian glands by postnatal day 15 (PN15) (Nien et al., 2010). Meibomian gland dysfunction (MGD) that perturbs the quantity and/or quality of the secreted lipids is a common cause of evaporative dry eye disorders (Jackson, 2008). In spite of their critical contributions to the ocular surface physiology, little is known regarding the roles of transcription factors regulating the meibomian gland development.
More than 17 members of the Krüppel-like factors (KLF) family have been identified in mammals (Bieker, 2001; Swamynathan, 2010). Several KLFs are expressed in the mammalian ocular surface in varying amounts (Chiambaretta et al., 2004; Nakamura et al., 2004; Norman et al., 2004). Serial analysis of gene expression identified Klf4 and Klf5 as two of the most highly expressed transcription factors in both 9 day and 6 week old mouse cornea (Norman et al., 2004). Conditional deletion of Klf4 in the developing mouse ocular surface resulted in corneal epithelial fragility, stromal edema, altered stromal collagen fibril organization, endothelial vacuolation, loss of conjunctival goblet cells and defective lens (Swamynathan et al., 2008; Swamynathan et al., 2007; Young et al., 2009). Klf4 influenced corneal epithelial barrier function by upregulating the expression of cell junctional proteins and basement membrane components (Swamynathan et al., 2011). Consistent with the increased Klf4CN corneal epithelial cell proliferation and fragility, expression of cell cycle inhibitors and desomosomal components, respectively, was decreased (Swamynathan et al., 2008).
Klf5 and Klf4 are structurally related, but functionally distinct (McConnell et al., 2007). Klf5 is expressed in the proliferating basal epithelial cells of the intestinal crypts, cornea, and epidermis (Chiambaretta et al., 2004; Ohnishi et al., 2000). Klf5, a positive regulator of cell proliferation (Sun et al., 2001), is required for blastocyst development and self renewal of mouse embryonic stem cells (Ema et al., 2008; Parisi et al., 2008), perinatal lung development (Wan et al., 2008), cardiovascular remodeling (Shindo et al., 2002; Suzuki et al., 2009) and adipocyte differentiation (Oishi et al., 2005; Sue et al., 2008). The role of Klf5 in maturation and maintenance of the ocular surface was not studied previously due to embryonic lethality of Klf5 null mice (Shindo et al., 2002). In this report, we have conditionally deleted Klf5 in the ocular surface ectoderm-derived structures of the eye including cornea, conjunctiva, eyelids and lens by mating Klf5-LoxP (Wan et al., 2008) and Le-Cre mice (Ashery-Padan et al., 2000; Dwivedi et al., 2005) to study the function of Klf5 in the ocular surface. The Klf5 conditional null (Klf5CN) mice exhibited multiple anterior ocular defects including abnormal eyelids with malformed meibomian glands and a conjunctiva devoid of mucin producing goblet cells, establishing Klf5 as a critical regulator of anterior eye development.
Materials and Methods
Conditional disruption of Klf5
Derivation and use of Klf5-LoxP (Wan et al 2008) and Le-Cre (Ashery-Padan et al., 2000) mice has been described previously. Klf5loxP/loxP, Le-Cre/- mice were mated with Klf5loxP/loxP mice to obtain equal proportion of Klf5loxP/loxP, Le-Cre/- (Klf5CN) and Klf5loxP/loxP (control) offspring. Genomic DNA isolated from tail clippings of these mice was assayed for the presence of the Klf5-LoxP and Le-Cre transgenes by PCR using specific primers. Klf5loxP/loxP PCR was carried out using primers that can distinguish between the WT allele and the floxed allele (primer 1, CCT GCG TGC AAT CCA TCT TGT TCA ATG GC; primer 2, TCA CCC TCT GCA GAT CTT AGG C; and primer 3, GCT TGG CTC AAA ATT CCG TTC C), as before (Wan et al., 2008). Gestation was determined by identification of a vaginal plug (E0.5). Mice studied here were on a mixed genetic background and maintained in accordance with the guidelines set forth by the Animal Care and Use Committee of the University of Pittsburgh, Pittsburgh and the ARVO statement related to the humane use of animals in experiments.
Histology
Eye tissues from carbon dioxide asphyxiated mice were fixed in freshly prepared 4 % paraformaldehyde (Sigma Chemical Company, St. Louis, MO) in phosphate buffered saline (PBS; pH 7.4) for 24 hours at 4°C and embedded in paraffin. To rule out the inadvertent use of sections from the edges of eyeballs, we started collecting serial sections upon entering the angle tissue on one side, ending while exiting on the other side. We then stained the central sections representing the middle of the eye. 8μm-thick sections were stained with hematoxylin and eosin, or periodic acid-Schiff's (PAS) reagent. For oil red-O staining, 8μm-thick cryosections from OCT embedded adult mouse eyelids were air dried for 60 min, fixed in 4 % paraformaldehyde in PBS for 30 minutes and air dried again. Slides were then placed in absolute propylene glycol for 5 min and incubated in pre-warmed 0.5% Oil-red-O stain for 15 min in 60° C oven, differentiated in 85% propylene glycol, rinsed in two changes of distilled water, counterstained with Meyer's hematoxylin and mounted using aqueous mounting medium. Light microscopy was performed with an Olympus BX60 microscope (Olympus America Inc.) equipped with Spot digital camera (Spot diagnostics instruments Inc., Sterling Heights, CA).
In Situ hybridization
In situ hybridization was performed using 12 μm-thick cryosections from fresh frozen eye tissue in OCT. The sections were fixed in 4% paraformaldehyde, treated with proteinase K (0.2 μg/mL) for 5 minutes, and processed for in situ hybridization as described earlier (Norman et al., 2004). Riboprobes were synthesized using a digoxygenin (DIG) RNA labeling kit (Sp6/T7; Roche Molecular Biochemicals, Indianapolis, IN) with linearized plasmid cDNA templates for Klf4 and Klf5. Color development reaction was allowed to proceed until purple color was visible, (approximately 30 to 60 minutes) and reactions for both the sense and antisense riboprobes were terminated at the same time.
Isolation of total RNA, RT-PCR and real time quantitative RT-PCR
Klf5 mRNA was quantitated in the developing mouse cornea by real time quantitative RT-PCR (Q-RT-PCR) using a standard curve generated with serial dilutions of linearized plasmid pCMVSport6-Klf5. Q-RT-PCR was performed using cDNA synthesized with 100 ng total RNA isolated from dissected corneas. The reagents, equipment and software for TaqMan gene expression assays were obtained from Applied Biosystems, Foster City, CA. Q-RT-PCR assays with pre-standardized gene-specific probes were performed in ABI StepOne Plus thermocycler using 18S rRNA as endogenous control and the results analyzed using the software provided by the manufacturer (Applied Biosystems).
Immunoblots and immunofluorescence
Equal amounts of total protein extracted by homogenizing dissected corneas in 8.0 M urea, 0.08 % Triton X-100, 0.2 % SDS, 3% β-mercapto ethanol and proteinase inhibitors and quantified by bicinchoninic acid method (Pierce, Rockford, IL) were separated by electrophoresis in SDS-PAGE gels, transferred to PVDF membranes and subjected to immunoblot analysis. Rabbit anti-KLF5 antibody (Abcam, Cambridge, MA) and goat anti-actin antibody that recognizes a broad range of actin isoforms (Santa Cruz Biotechnology, Santa Cruz, CA) were used at 1:500 dilution in PBST. Horseradish peroxidase- coupled goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) or donkey anti-goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA) antibody was used at 1:5000 dilution. Immunoreactive bands were identified by chemiluminescence following incubation with Super Signal West Pico solutions (Pierce, Rockford, IL).
For immunofluorescence, 8 μm-thick sections from OCT or paraffin embedded eye tissues were fixed in freshly prepared buffered 4 % paraformaldehyde for 30 minutes, blocked with 10 % goat serum in PBST for 1 h at room temperature in a humidified chamber, washed twice with PBST for 5 minutes each, incubated with 1:150 dilution of rabbit anti-KLF5 antibody, 1:100 dilution of rabbit anti-laminin-332 antibody (Abcam, Cambridge, MA), 1:500 dilution of rabbit anti-αA-crystallin antibody, 1:500 dilution of rabbit anti-αB-crystallin antibody, or 1:25 dilution of rabbit anti-Ki67 antibody (Fisher Scientific, Pittsburgh, PA) for 1 h at room temperature, washed thrice with PBST for 10 minutes each, incubated with second antibody (Alexafluor 555 coupled goat anti-rabbit IgG antibody, Molecular Probes, Carlsbad, CA) at 1:1500 dilution for 1 h at room temperature, washed thrice with PBST for 10 minutes each, mounted with Prolong Gold anti-fade reagent with DAPI (Molecular Probes, Carlsbad, CA) and observed with an Olympus Fluoview 1000 confocal system with an Olympus IX81 microscope.
Results
Expression of Klf5 in the anterior eye during development
Expression of Klf5, detected as early as E13.5, increased with age till 8 weeks of age, the oldest stage tested (Table 1). The number of Klf5 transcripts increased by 7-fold, from 409/ng total RNA at E13.5 to 2841/ng total RNA in 8 week old adult corneas (Table 1). In situ hybridization with Klf5-specific antisense riboprobes confirmed the low expression of Klf5 in the embryonic stages, that increased as the development progressed, reaching the highest expression at PN20, the oldest stage tested (Fig 1A). Klf5 mRNA was largely confined to the epithelial cells, with low levels in stromal cells (Fig 1A). In the conjunctiva, Klf5 mRNA was expressed at low levels in the embryonic stages, gradually increasing in the postnatal stages, with a relatively higher expression in the PN14 and PN20 forniceal epithelium (Fig. 1B). Klf5 mRNA was detected in the early embryonic stages in the palpebral epithelium and postnatally in sebaceous and meibomian glands (Fig. 1C). Klf5 mRNA was more abundant in the external palpebral epidermis compared with inner palpebral conjunctival epithelium (Fig. 1C). Taken together, these results demonstrate that Klf5 is expressed in a developmentally regulated manner throughout the ocular surface (Table 1 and Fig. 1).
Table 1. Developmental changes in corneal expression of Klf5.
Mean number of Klf5 transcripts per ng total RNA ± SEM (n=3, each containing 4 corneas), estimated by real time Q-RT-PCR is shown. One-way Analysis of Variance (ANOVA) was calculated by Tukey's multiple comparison test as a measure of statistical significance.
| Developmental Stage | Number of Klf5 transcripts/ng total RNA (Mean ± SEM) | P Value compared to 8-week levels |
|---|---|---|
| E13.5 | 409.3±31.9 | < 0.001 |
| E16.5 | 542.0±63.8 | < 0.001 |
| PN1 | 685.8±111.9 | < 0.001 |
| PN6 | 896.8±113.5 | < 0.001 |
| PN11 | 1232.1±177.1 | < 0.001 |
| PN21 | 1303.1±159.0 | < 0.01 |
| 8-week | 2841.8±55.6* | Not Applicable |
Figure 1. Developmental expression of Klf5 in the mouse ocular surface.
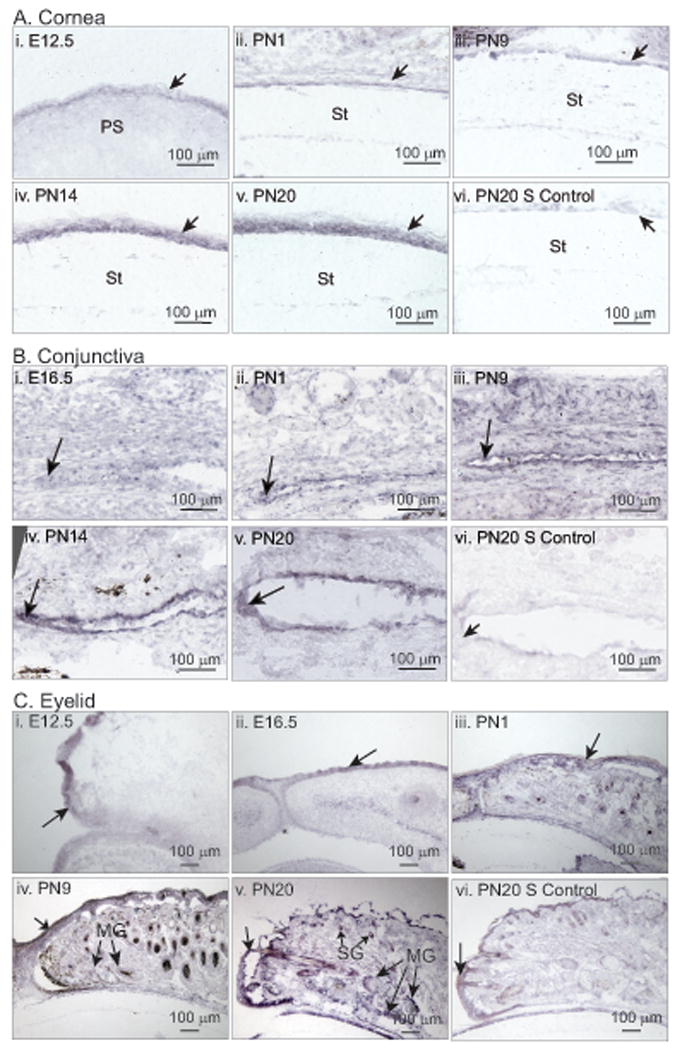
In situ hybridization revealing the expression pattern of Klf5 in the developing mouse cornea (A), conjunctiva (B) and the eyelid (C). For each tissue, PN20 sections processed with Klf5 sense probe served as negative control (PN20 S control) (n=3). Developmental stages tested are listed on top of each panel. St, Stroma; PS, presumptive stroma; SG, sebaceous gland; MG, meibomian gland. Unlabeled arrows point to the epithelium in each tissue.
Conditional disruption of Klf5 in the surface ectoderm derived tissues of the eye
In order to study the function of Klf5 in the ocular surface overcoming the limitation of embryonic lethality of Klf5-null mice (Shindo et al., 2002), we generated the Klf5CN mice that were viable and fertile, by breeding Klf5loxP/loxP, Le-Cre/- mice with Klf5loxP/loxP mice (Ashery-Padan et al., 2000; Dwivedi et al., 2005; Swamynathan et al., 2007; Wan et al., 2008). Real time Q-RT-PCR, immunoblots and immunofluorescence confirmed the loss of Klf5 in the mouse corneas (Fig. 2). Klf5 expression was detected by immunofluorescence in the WT but not the Klf5CN ocular surface epithelia and stroma (Fig. 2D). Together, these results confirm that Klf5 is successfully disrupted in the ocular surface tissues by the Cre-Lox approach using Le-Cre to drive the expression of Cre recombinase.
Figure 2. Klf5 is disrupted in theKlf5 CN ocular surface.
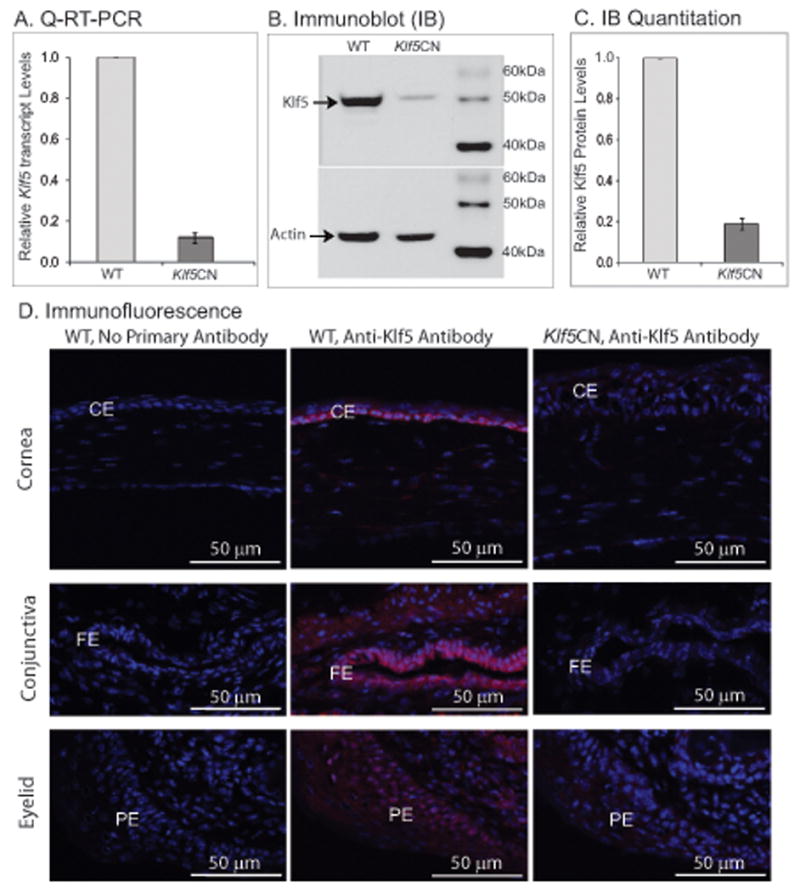
A-D, Confirmation of disruption of Klf5 in the Klf5CN cornea. A, Relative Klf5 transcript levels in 8 week-old WT and Klf5CN corneas measured by real time Q-RT-PCR (n=3). B and C, Relative Klf5 protein levels in 8 week-old mouse corneas measured by immunoblots (B) and their densitometric scan (C), with actin levels as loading controls. D, Confirmation of disruption of Klf5 in the Klf5CN cornea (Top panels), conjunctiva (middle panels) and the eyelid (lower panels) by immunofluorescence with anti-Klf5 antibody (red signal). Left panels, no primary antibody control; middle panels, WT sections probed with anti-Klf5 antibody; right panels, Klf5CN sections probed with anti-Klf5 antibody. Sections have been counterstained with DAPI (blue). CE, Corneal epithelium; FE, forniceal epithelium; PE, palpebral epithelium.
Effect of Klf5 disruption on the ocular surface morphology and histology
Comparison of the PN5, PN8 and PN11 WT and Klf5CN pups by visual examination revealed no major differences in the eyelids (Fig. 3A). While WT eyelids opened at PN12, the Klf5CN eyelids were swollen and remained closed with a small palpebral fissure as late as PN21 (Fig. 3A). The enucleated adult (8 week old) Klf5CN eyeballs were relatively smaller, with a rough and translucent cornea (Fig. 3B). Greater than 80% of the adult Klf5CN eyes displayed a small eye phenotype and contained hypertrophic iris with smaller pupil (n>20).
Figure 3. External appearance of Klf5CN eyes and dissected eyeballs.
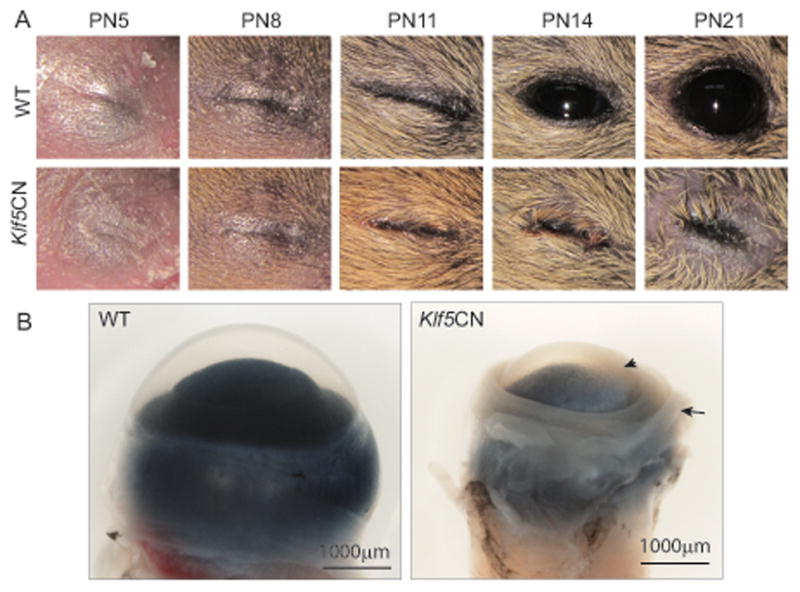
A, WT and Klf5CN eyes imaged at PN5, PN8, PN11, PN14 and PN21. B, Enucleated 8-week old WT and Klf5CN eyeballs imaged sideways to reveal the smaller size, thicker extra-ocular muscle (arrow) and corneal opacity (arrowhead) of the Klf5CN compared with normal WT eyeball.
Histological examination revealed no significant abnormalities in Klf5CN eyes at E13.5, E15.5, E18.5 and PN1, suggesting that Klf5 does not play a major role in early eye development (Fig. 4 A-H). However, PN6, PN11, PN21 and 10 week-old Klf5CN eyes were smaller with swollen eyelids, spongy and deformed lens, and thicker corneas (Fig. 4 I-P). While Klf5CN corneas were unaltered at E13.5, E15.5 and E18.5 (Fig. 5 A-F), postnatal Klf5CN corneal stroma was hypercellular (Fig. 5 G-P). Frequent iridocorneal fusion was observed in Klf5CN eyes (Fig. 5 L and N, asterisks). Morphometric analyses of sagittal sections from 8 week-old WT and Klf5CN eyes confirmed the increase in the central corneal stromal thickness and cell density as well as the decrease in the size of the Klf5CN lens and the eyes (Table 2).
Figure 4. Developmental defects in Klf5CN eyes.
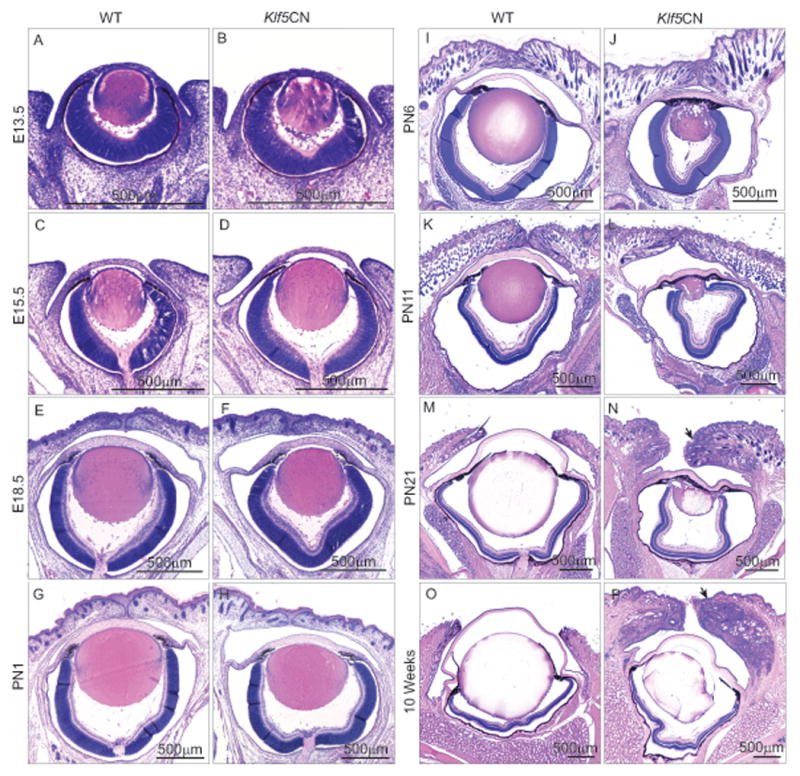
H & E stained sagittal sections through the center of the WT and Klf5CN eyeball in the head tissue from different developmental stages (n=3). Images from different stages have been acquired using different magnifications, to accommodate the whole eye. Arrows (in panels N and P) indicate the swollen Klf5CN eyelids at PN21 and 10 weeks.
Figure 5. Developmental defects in Klf5CN corneas.
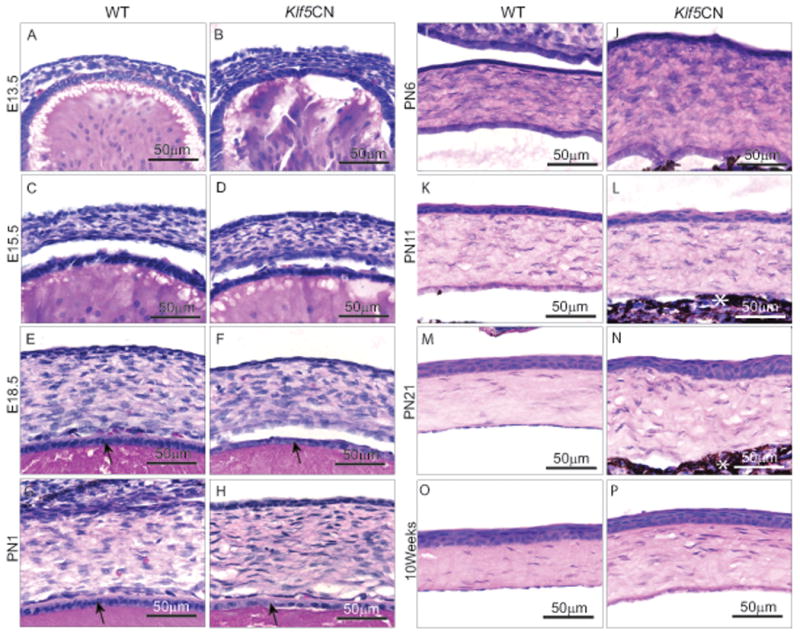
H & E stained sagittal sections through the WT and Klf5CN central corneas from different developmental stages (n=3). White asterisk indicates iridocorneal fusion in Klf5CN eyes (L and N). Arrows indicate lens epithelium (E-H).
Table 2. Morphometric analysis of the sagittal sections of 8 week old WT and Klf5CN eyes.
Mean values ± SEM from 5 independent samples are shown. Student's t-test was used to measure statistical significance.
| Parameters measured | Wild Type | Klf5CN |
|---|---|---|
| Central cornea to optic nerve head (μm) | 2545.4±43.1 | 2236.5±62.3 * |
| Anterior-posterior depth of lens (μm) | 1723.5±22.6 | 1297.2±48.9 ** |
| Equatorial width of lens (μm) | 1769.3±23.6 | 1331.1±51.1 ** |
| Thickness of central corneal stroma (μm) | 47.3±1.4 | 68.7±4.1 ** |
| Corneal stromal cell density (number of cells/mm2) | 3125 ± 137.6 | 7440 ± 365.5 ** |
Significance levels:
P<0.001;
P<0.0001.
Early development of the Klf5CN lens appeared to be relatively normal (Fig. 4, A-D). However, a large fraction (>80%) of the postnatal Klf5CN lenses appeared smaller, with many of them deformed (Fig. 4 I-P). Frequent iridolenticular fusion was observed in the Klf5CN eyes (Fig. 4 J,L and N). The E18.5 and PN1 Klf5CN lenses contained significantly fewer epithelial cells (39±2.1 and 31±1.2 cells per unit area, respectively; n=3) in the central anterior region compared with the WT (72±2 and 64±2 cells per unit area, respectively; n=3) (Fig. 5 E-H, arrows). Late embryonic and neonatal Klf5CN lenses contained fewer, abnormally arranged nuclei compared to WT in the differentiating equatorial region (Supplemental Figure 1). Immunofluorescence detected comparable expression of αA-crystallin and αB-crystallin in the E15.5 and PN1 WT and Klf5CN lenses (Supplemental Figure 2), suggesting that Klf5 does not influence their expression. Abnormal arrangement of nuclei in the E18.5 and PN1 lens equatorial region (Supplemental Figure 1), coupled with postnatal appearance of the Klf5CN lens phenotype suggested that the Klf5CN lens defect is primarily due to malformed secondary fiber cells.
PAS reagent-stained sections revealed that the PN21 and 10 week-old Klf5CN corneal epithelial basement membrane was poorly formed (Fig. 6 A-D, arrows). Immunofluorescence demonstrated decreased expression of laminin-332, confirming that the PN21 and 10-week-old Klf5CN corneal epithelial basement membrane is thin and discontinuous (Fig. 6 E-H). Unlike the Klf4CN corneal stroma that harbored significantly reduced proteoglycans (Swamynathan et al., 2007; Young et al., 2009), Klf5CN stroma was relatively more intensely stained by the PAS reagent, suggesting elevated levels of proteoglycans in the Klf5CN stroma compared with the WT (Fig. 6 A-D). Goblet cells were present in the PN21 and 10 week-old WT (Fig. 7, arrows) but not the Klf5CN conjunctiva (Fig. 7, arrowheads), suggesting that Klf5 is required for conjunctival goblet cell development. Furthermore, the Klf5CN conjunctival epithelium appeared rough and discontinuous, consistent with a disrupted epithelial barrier (Fig. 7, arrowheads).
Figure 6. Defects in the Klf5CN corneal epithelial basement membrane.
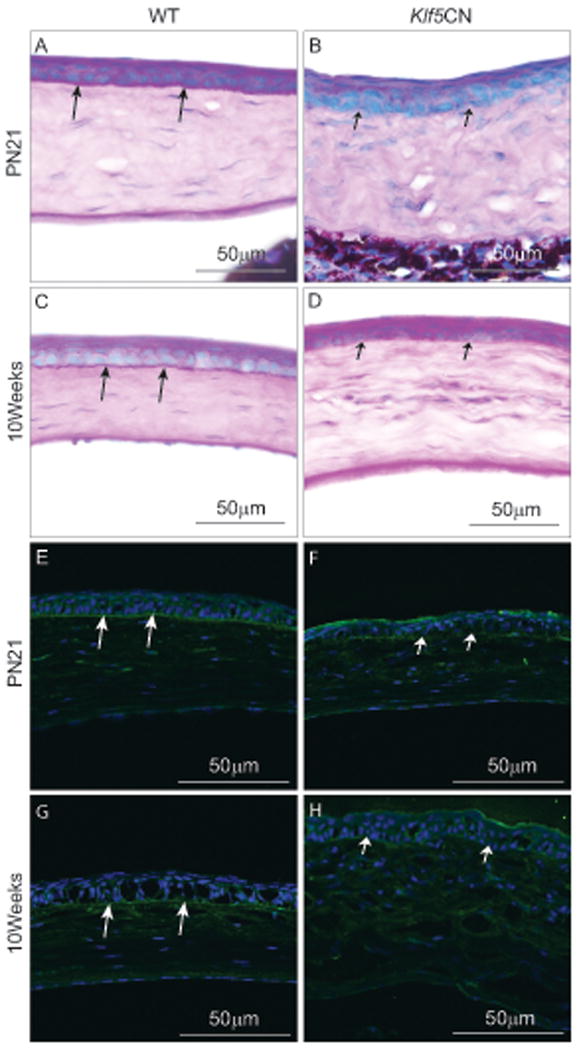
A-D, PAS reagent-stained PN21 and 10 week-old WT and Klf5CN corneal sections indicating well formed basement membrane beneath the WT corneal epithelium (long arrows) but not the Klf5CN corneal epithelium (short arrows) (n=3). E-F, Immunofluorescence of PN21 and 10 week-old WT (E and G) and Klf5CN (F and H) corneal sections with anti-laminin-332 antibody show that the basement membrane is thick and continuous in the WT (long arrows), but malformed and discontinuous in the Klf5CN (short arrows) corneal epithelium.
Figure 7. Effect of disruption of Klf5 on conjunctiva.
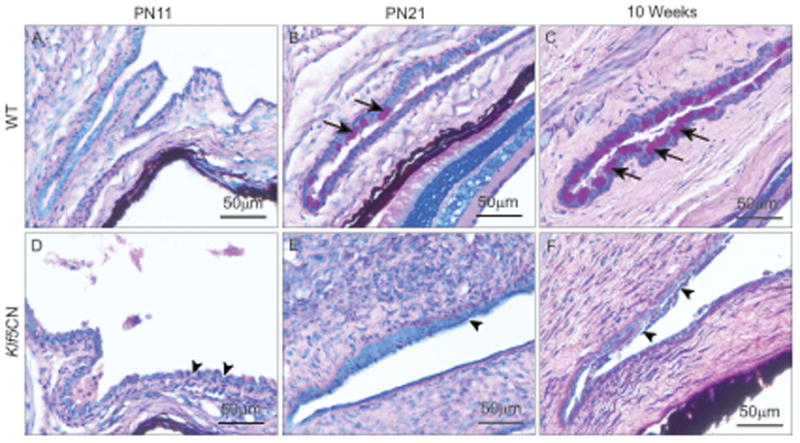
Sagittal sections through the WT (A, B and C) or Klf5CN (D, E and F) PN11 (A and D), PN21 (B and E) and 10 week old (C and F) eyes stained with PAS reagent (n=3). Arrows in B and C point to the conjunctival goblet cells. Arrowheads in D, E and F indicate the rough and discontinuous nature of the Klf5CN conjunctival epithelium devoid of goblet cells.
Effect of Klf5 disruption on eyelids, meibomian and lacrimal glands
Though the embryonic Klf5CN eyelid development and fusion was normal, significant abnormalities were observed postnatally. At PN21, Klf5CN eyelashes were irregularly oriented and the surrounding fur was disorganized, while the palpebral conjunctiva was swollen and inflamed (Fig. 8, A and B, asterisk). The Klf5CN eyelids contained fewer irregularly spaced meibomian gland orifices at the mucocutaneous junction (on average 8/eyelid compared with 12/eyelid in the WT, n= 4 each) (Fig. 8, A and B, white arrows). Even though meibomian gland bud was detected in the PN6 Klf5CN eyelid suggesting timely induction, it was disorganized (Fig. 8 C and D arrows), a feature that became more pronounced at PN11 and PN21 (Fig. 8 E-H arrows). The swollen PN21 Klf5CN eyelids were hypercellular and contained severely malformed meibomian glands with disorganized acini (Fig. 8, G and H, short arrows). Palpebral epidermis was thicker with 7-9 layers of keratinocytes, compared with the normal 2-3 (Fig. 8, G and H). Eyelids contained significantly enlarged hair follicles (Fig. 8 G and H, green arrows) and sebaceous glands (Fig. 8 G-L, arrowheads). Oil red-O stained coronal and sagittal sections through the eyelids confirmed the disorganized nature of the meibomian glands with variably sized acini (Fig. 8, I and J short arrows) and uneven lipid accumulation in the meibomian ducts (Fig. 8, I-L, long arrows). Together, these results show that Klf5 is required for proper maturation and function of the meibomian glands.
Figure 8. Developmental defects in the Klf5CN eyelids and meibomian glands.
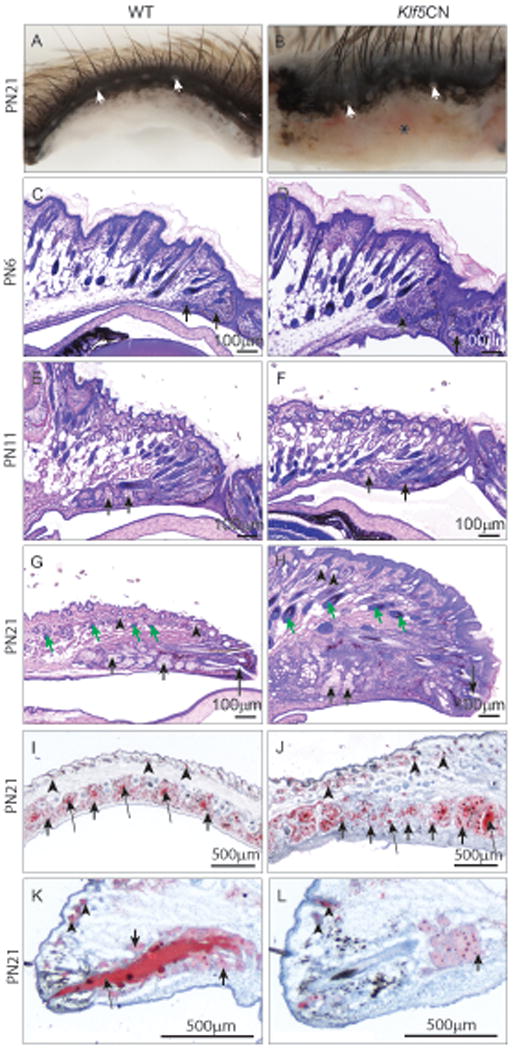
A and B, Dissected PN21 WT (A) or Klf5CN (B) eyelids showing the meibomian gland orifices (white arrows). C-H, H & E stained sagittal sections through PN6, PN11 and PN21 WT (C, E and G) and Klf5CN (D, F and H) eyelids (n=3). I-L, Oil-red-O stained coronal (I-J) or sagittal (K-L) sections through PN21 eyelids showing uniform spacing and lipid accumulation in the WT (I and K) compared with the uneven spacing and variable lipid accumulation in the Klf5CN (J and L) meibomian gland ducts (n=3). Asterisk, swelling and reddishness in Klf5CN palpebral conjunctiva; Long arrows, meibomian gland ducts; Short arrows, meibomian gland acini; Green arrows, hair follicles; Arrowheads, sebaceous glands.
Considering that the lacrimal glands responsible for production of the aqueous component of the tear film originate from the conjunctival forniceal epithelium (Govindarajan et al., 2000; Makarenkova et al., 2000), we compared the 8 week-old WT and Klf5CN mouse lacrimal glands (Fig. 9). Morphological examination revealed interspersed dark spots resembling necrotic spots (Fig. 9 A-B short arrows) and excessive vasculature (Fig. 9 A-B long arrows) in the 8-week old Klf5CN lacrimal glands compared to the normal looking WT. Histological examination confirmed excessive vasculature (Fig. 9 C-D arrowheads) and revealed disrupted acinar organization in the 8-week old Klf5CN lacrimal glands compared to WT. In addition, large numbers of infiltrating cells were observed in the Klf5CN but not WT lacrimal glands (Fig. 9 C-D asterisks). Thus, disruption of Klf5 in the ocular surface results in defective lacrimal glands that display signs of inflammation.
Figure 9. Defects in the Klf5CN lacrimal gland.
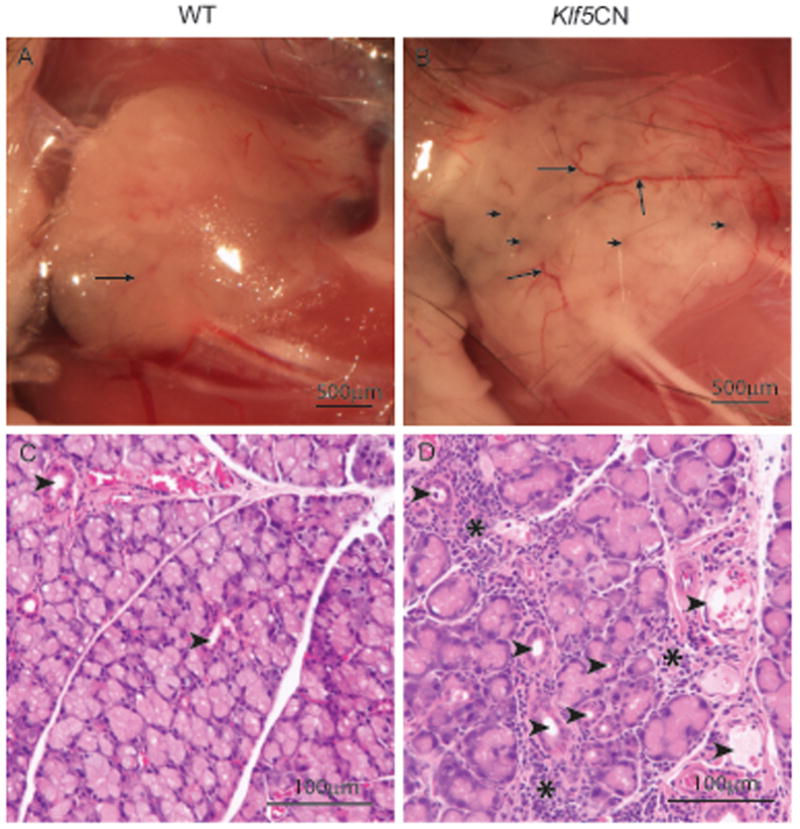
Morphological examination revealed interspersed dark spots (short arrows) and excessive vasculature (long arrows) in the 8-week old Klf5CN lacrimal glands (B), compared to the normal looking WT (A) (n=4). C-D, Histology confirmed excessive vasculature (D; arrowheads) and disrupted acinar organization in the 8-week old Klf5CN lacrimal glands (D) compared to WT (C). Numerous infiltrating cells were observed in the Klf5CN but not the WT lacrimal glands (D; asterisks).
Increased cell proliferation in the postnatal Klf5CN ocular surface
In view of the established ability of Klfs to regulate cell cycle (McConnell et al., 2007; McConnell and Yang, 2010; Swamynathan, 2010), we compared the WT and Klf5CN cell proliferation rates by examining the expression of mitotic cell marker Ki67. While there was no difference in the meibomian gland acinar or ductal epithelial cell proliferation, Ki67-positive cells were significantly increased in palpebral epidermis and eyelid hair follicles (Fig. 10 A and B, arrows). While the Ki67-positive cells were restricted to the basal epithelia in the PN21 WT, they were also present in the spinous cell layers in the PN21 Klf5CN cornea and conjunctiva, suggesting deregulated cell proliferation and differentiation pathways of the ocular surface squamous epithelia (Fig. 10 C-F, arrows). The number of Ki67-positive cells was more than doubled in the PN21 Klf5CN cornea, conjunctiva and the eyelids, compared with the corresponding WT controls (Fig. 10G).
Figure. 10. Effect of conditional disruption of Klf5 on ocular surface cell proliferation.
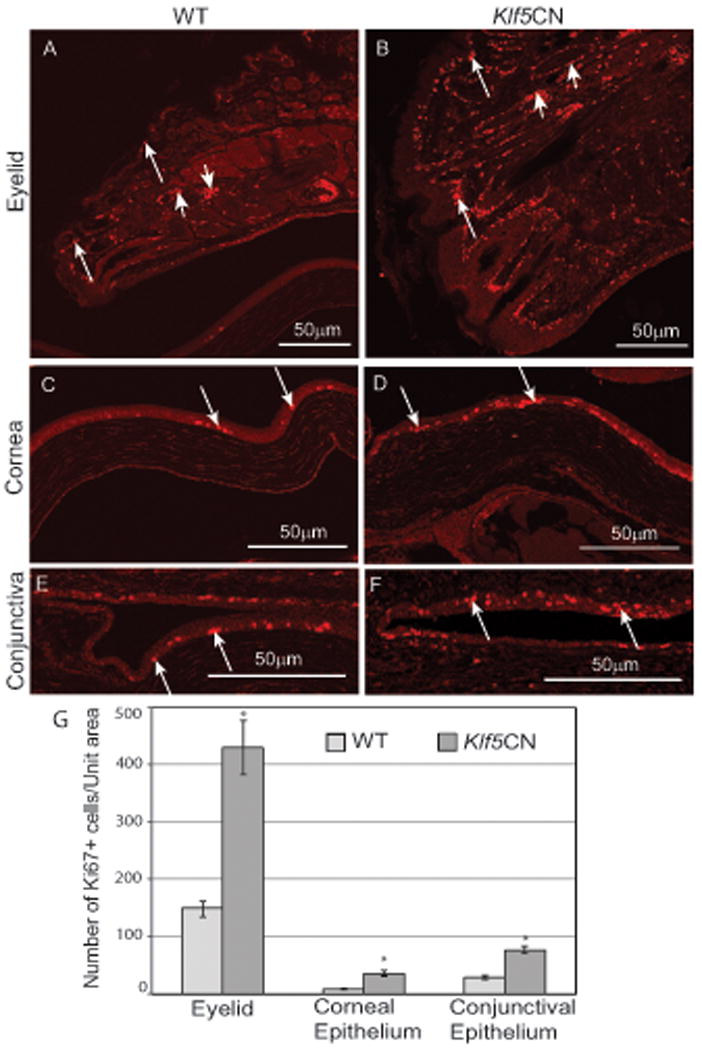
Differences in cell proliferation between PN21 WT and Klf5CN eyelids (A and B), cornea (C and D) and conjunctiva (E and F) examined by immunostaining for Ki67 antigen (n=3). Long arrows, Ki67-positive epithelial cells in the eyelid, cornea, and conjunctiva (A-F); Short arrows, Ki67-positive cells in the hair follicle (A and B). G, Comparison of the number of Ki67-positive cells per unit area in the WT and Klf5CN cornea, conjunctiva and the eyelid. Mean values of Ki67-positive cell counts from three independent experiments (±SEM) are presented.
Co-ablation of Klf4 and Klf5 results in more severe abnormalities
Considering that the structurally related Klf4 and Klf5 are both abundantly expressed in the mouse cornea, we co-ablated them in the ocular surface. Co-ablation of Klf4 and Klf5 resulted in more severe eyelid and corneal abnormalities than those in the Klf4CN (Swamynathan et al., 2007) or Klf5CN eyes (Fig. 11). The Klf4/Klf5dCN eyes failed to open as late as 35 weeks after birth, the oldest stage tested (n=6) (Fig. 11 A-F). The 8 week-old Klf4/Klf5dCN corneal epithelium was thinner than the WT and Klf5CN corneal epithelia, and remained fused to the eyelids. The Klf4/Klf5dCN corneal stroma was thinner with relatively fewer keratocytes compared to WT and Klf5CN corneas (Fig 11 G-I). The Klf4/Klf5dCN conjunctiva lacked goblet cells similar to the Klf4CN and Klf5CN conjunctivae, and possessed thinner epithelium than the WT or Klf5CN (Fig. 11 G-L).
Figure 11. Defects in the Klf4/Klf5 double conditional null (Klf4/Klf5dCN) eyes compared with WT and Klf5CN.
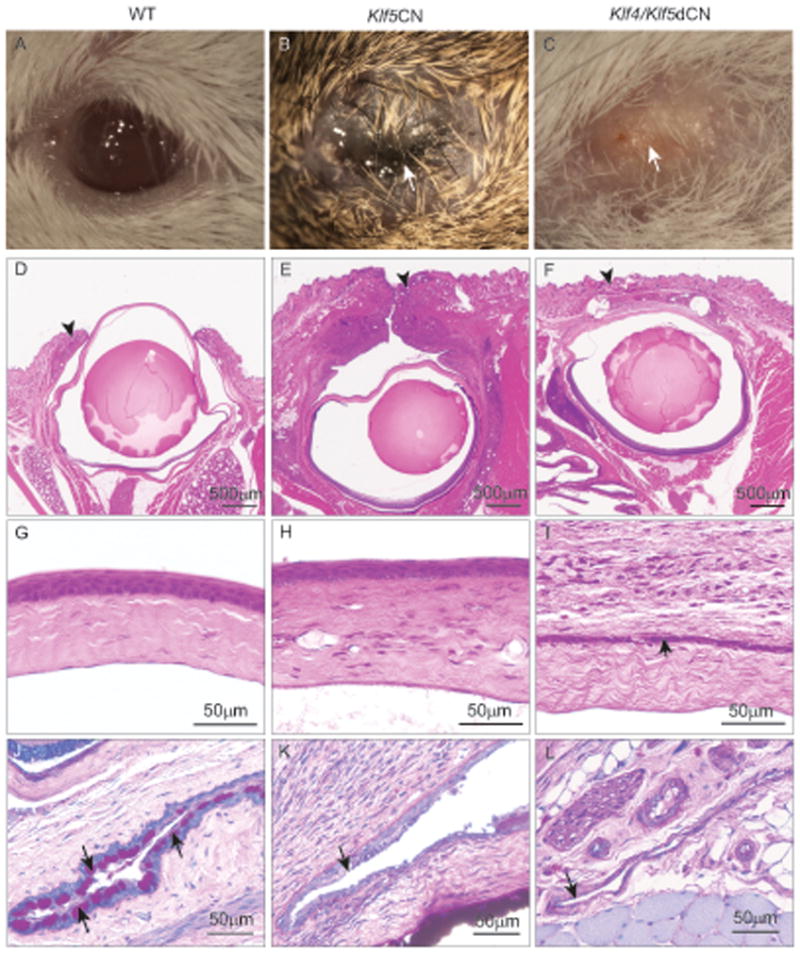
Images of the 35-week old WT (A), Klf5CN (B) and Klf4/Klf5dCN (C) eyes show that the Klf4/Klf5dCN eyes remain closed (n=6). H & E (D-I) or PAS (J-L) stained sagittal sections of 8 week old WT (D, G and J), Klf5CN (E, H and K) and Klf4/Klf5dCN (F, I and L) eye tissue. Arrowheads point to the well formed eyelids in the WT (D), swollen, almost closed eyelids in the Klf5CN (E) and unopened eyelids in the Klf4/Klf5dCN (F) eyes. Short arrow points to the thin layer of corneal epithelial tissue that remains fused to the overlying eyelids in the Klf4/Klf5dCN (I). Long arrows indicate the well formed goblet cells in the WT conjunctiva (J) and the corresponding regions lacking them in the Klf5CN (K) and Klf4/Klf5dCN (L) conjunctivae.
Discussion
We have provided the first detailed description of the developmental expression pattern of Klf5 in the mouse cornea, conjunctiva and the eyelids. By using the Cre-lox approach for selective ablation of Klf5, we identified the critical roles of Klf5, expanding the regulatory network of transcription factors in the ocular surface (Birger et al., 2006; Chen et al., 2009; Collinson et al., 2004; Davis et al., 2003; Dwivedi et al., 2005; Sivak et al., 2000; Sivak et al., 2004; Swamynathan et al., 2007; West-Mays et al., 2003). Even though loss of Klf5 results in multiple ocular surface abnormalities, the human KLF5 locus is not associated with any ocular dystrophies, possibly due to the embryonic lethality of the spontaneous human KLF5 mutants. The Klf5-null mouse embryonic lethality around E8 is consistent with this possibility (Shindo et al., 2002).
The vertebrate eye is a complex organ with multiple tissues and cell types influencing the development and functions of each other. Disruption of Klf4 in the ocular surface resulted in corneal phenotypes overlapping with different dystrophies associated with eye development (Swamynathan et al., 2007). Mutations in FoxC1, PitX2, Pax6 (Hjalt and Semina, 2005), and collagen α1(IV) (Van Agtmael et al., 2005) are associated with Axenfeld-Rieger anomaly, a genetically heterogeneous disease with iridocorneal adhesions and defects in basement membrane. In the experiments reported here, Klf5 was disrupted in the developing lens, conjunctiva and eyelids in addition to the cornea. It is therefore conceivable that while some aspects of the Klf5CN anterior eye phenotype are direct consequences of the absence of Klf5, others may arise as secondary or indirect results of the absence of Klf5 in the neighboring tissues.
Structurally related proteins often compensate for the loss of each other, by virtue of their redundant, overlapping functions. For example, individual knockouts of C/EBPα or C/EBPβ resulted in normal sebaceous and meibomian glands, while their co-ablation severely disrupted sebaceous and meibomian gland development (House et al., 2010). Similarly, individual knockouts of HNF-1α or HNF1β did not have much influence while their simultaneous disruption resulted in a lethal phenotype due to defective intestinal epithelial development (D'Angelo et al., 2010). In contrast, disruption of Klf4 (Swamynathan et al., 2007), or Klf5 (current report) alone resulted in several common changes (e.g., loss of conjunctival goblet cells, disrupted epithelial basement membrane, increased cell proliferation), indicating that these two structurally related factors have essential and non-redundant roles in several aspects of the anterior eye development. Co-ablation of Klf4 and Klf5 resulted in a more severe eyelid and corneal phenotype compared with the phenotype obtained with disruption of Klf4 or Klf5 alone, consistent with the notion that Klf4 and Klf5 share few if any, redundant functions (Fig. 11).
Our observation of increased cell proliferation in the Klf5CN ocular surface differs from the previous reports that found Klf5 to be pro-proliferative (Ghaleb et al., 2005; Sun et al., 2001). However, reduced expression of KLF5 in prostate and breast cancer cells (Chen et al., 2002; Chen et al., 2003), coupled with the ability of KLF5 to work as a tumor suppressor (Bateman et al., 2004; Yang et al., 2005) suggest that Klf5 has an anti-proliferative activity as well, in a context dependent manner. Consistent with this possibility, Klf5 is known to reverse its function, becoming anti-proliferative cofactor for TGFβ (Guo et al., 2009a; Guo et al., 2009b). Mechanistic explanation for the increased cell proliferation in the Klf5CN ocular surface remains to be worked out.
Conclusions
The results presented in this report demonstrate that Klf5 is required for proper postnatal maturation of the ocular surface. Coupled with our previous studies with Klf4 (Swamynathan et al., 2011; Swamynathan et al., 2008; Swamynathan et al., 2007; Young et al., 2009), this study highlights the critical, non-redundant functions of these two structurally related transcription factors in different compartments of the ocular surface. Our results have added Klf5 to the small list of transcription factors known to regulate the relatively understudied meibomian gland (Cascallana et al., 2005; House et al., 2010; Nien et al., 2010) lacrimal gland (Makarenkova et al., 2000; Mattiske et al., 2006) and conjunctival goblet cell (Swamynathan et al., 2007; Ueta et al., 2005; Yoshida et al., 2000) development. Based on these results, we propose that the Klf5CN mouse is a useful model for investigating ocular surface pathologies involving meibomian gland dysfunction, blepharitis, corneal or conjunctival defects.
Supplementary Material
Organization of the nuclei in the E13.5, E15.5, E18.5 and PN1 WT and Klf5CN lens equatorial region was examined in H & E stained sagittal sections (n=3). Klf5CN primary fiber cells appeared normal in E13.5 and E15.5. However, nuclei in the E18.5 and PN1 Klf5CN lens equatorial regions were disorganized, suggesting abnormalities in secondary fiber formation.
Immunofluorescence with anti-αA-crystallin antibody (A-D) or anti-αB-crystallin antibody (E-H) detected comparable expression of αA-crystallin and αB-crystallin in the WT (A, C, E and G) and Klf5CN (B, D, F and H) lenses (n=2).
Highlights.
Klf5-conditional null (Klf5CN) eyes were smaller with swollen inflamed eyelids
Klf5CN eyelid epidermis was hyperplastic, hair follicles & sebaceous glands enlarged
Klf5CN meibomian glands were malformed, and the conjunctiva lacked goblet cells
Klf5CN corneas were translucent with hypercellular stroma & thin basement membrane
Co-ablation of Klf4 and Klf5 resulted in a more severe ocular surface phenotype
Acknowledgments
This work was supported by the NEI K22 Career Development Award EY016875, core grant for vision research (5P30 EY08098-19), Research to Prevent Blindness and the Eye and Ear Foundation, Pittsburgh (SKS), and HL-090156 (JAW). We thank Gloria Limetti and Cindy Stone in Balaban lab, Department of Otolaryngology, University of Pittsburgh for help with histology, Drs Joe Horwitz, University of California, Los Angeles and Eric Wawrousek, NEI, NIH for antibodies, and Kira Lathrop, Imaging Core Module, Department of Ophthalmology, for help with microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikary G, Crish JF, Bone F, Gopalakrishnan R, Lass J, Eckert RL. An involucrin promoter AP1 transcription factor binding site is required for expression of involucrin in the corneal epithelium in vivo. Invest Ophthalmol Vis Sci. 2005a;46:1219–1227. doi: 10.1167/iovs.04-1285. [DOI] [PubMed] [Google Scholar]
- Adhikary G, Crish JF, Gopalakrishnan R, Bone F, Eckert RL. Involucrin expression in the corneal epithelium: an essential role for Sp1 transcription factors. Invest Ophthalmol Vis Sci. 2005b;46:3109–3120. doi: 10.1167/iovs.05-0053. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman NW, Tan D, Pestell RG, Black JD, Black AR. Intestinal tumor progression is associated with altered function of KLF5. J Biol Chem. 2004;279:12093–12101. doi: 10.1074/jbc.M311532200. [DOI] [PubMed] [Google Scholar]
- Bieker JJ. Kruppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- Birger Y, Davis J, Furusawa T, Rand E, Piatigorsky J, Bustin M. A role for chromosomal protein HMGN1 in corneal maturation. Differentiation. 2006;74:19–29. doi: 10.1111/j.1432-0436.2006.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascallana JL, Bravo A, Donet E, Leis H, Lara MF, Paramio JM, Jorcano JL, Perez P. Ectoderm-targeted overexpression of the glucocorticoid receptor induces hypohidrotic ectodermal dysplasia. Endocrinology. 2005;146:2629–2638. doi: 10.1210/en.2004-1246. [DOI] [PubMed] [Google Scholar]
- Chen C, Bhalala HV, Qiao H, Dong JT. A possible tumor suppressor role of the KLF5 transcription factor in human breast cancer. Oncogene. 2002;21:6567–6572. doi: 10.1038/sj.onc.1205817. [DOI] [PubMed] [Google Scholar]
- Chen C, Bhalala HV, Vessella RL, Dong JT. KLF5 is frequently deleted and down-regulated but rarely mutated in prostate cancer. Prostate. 2003;55:81–88. doi: 10.1002/pros.10205. [DOI] [PubMed] [Google Scholar]
- Chen Y, Carlson EC, Chen ZY, Hamik A, Jain MK, Dunwoodie SL, Yang YC. Conditional deletion of Cited2 results in defective corneal epithelial morphogenesis and maintenance. Dev Biol. 2009;334:243–252. doi: 10.1016/j.ydbio.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiambaretta F, Blanchon L, Rabier B, Kao WW, Liu JJ, Dastugue B, Rigal D, Sapin V. Regulation of corneal keratin-12 gene expression by the human Kruppel-like transcription factor 6. Invest Ophthalmol Vis Sci. 2002;43:3422–3429. [PubMed] [Google Scholar]
- Chiambaretta F, De Graeve F, Turet G, Marceau G, Gain P, Dastugue B, Rigal D, Sapin V. Cell and tissue specific expression of human Kruppel-like transcription factors in human ocular surface. Mol Vis. 2004;10:901–909. [PubMed] [Google Scholar]
- Chiambaretta F, Nakamura H, De Graeve F, Sakai H, Marceau G, Maruyama Y, Rigal D, Dastugue B, Sugar J, Yue BY, Sapin V. Kruppel-like factor 6 (KLF6) affects the promoter activity of the alpha1-proteinase inhibitor gene. Invest Ophthalmol Vis Sci. 2006;47:582–590. doi: 10.1167/iovs.05-0551. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Chanas SA, Hill RE, West JD. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6(+/-) mouse. Invest Ophthalmol Vis Sci. 2004;45:1101–1108. doi: 10.1167/iovs.03-1118. [DOI] [PubMed] [Google Scholar]
- D'Angelo A, Bluteau O, Garcia-Gonzalez MA, Gresh L, Doyen A, Garbay S, Robine S, Pontoglio M. Hepatocyte nuclear factor 1alpha and beta control terminal differentiation and cell fate commitment in the gut epithelium. Development. 2010;137:1573–1582. doi: 10.1242/dev.044420. [DOI] [PubMed] [Google Scholar]
- Davis J, Duncan MK, Robison WG, Jr, Piatigorsky J. Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci. 2003;116:2157–2167. doi: 10.1242/jcs.00441. [DOI] [PubMed] [Google Scholar]
- Dwivedi DJ, Pontoriero GF, Ashery-Padan R, Sullivan S, Williams T, West-Mays JA. Targeted deletion of AP-2alpha leads to disruption in corneal epithelial cell integrity and defects in the corneal stroma. Invest Ophthalmol Vis Sci. 2005;46:3623–3630. doi: 10.1167/iovs.05-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Mori D, Niwa H, Hasegawa Y, Yamanaka Y, Hitoshi S, Mimura J, Kawabe Y, Hosoya T, Morita M, Shimosato D, Uchida K, Suzuki N, Yanagisawa J, Sogawa K, Rossant J, Yamamoto M, Takahashi S, Fujii-Kuriyama Y. Kruppel-like factor 5 is essential for blastocyst development and the normal self-renewal of mouse ESCs. Cell Stem Cell. 2008;3:555–567. doi: 10.1016/j.stem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Francesconi CM, Hutcheon AE, Chung EH, Dalbone AC, Joyce NC, Zieske JD. Expression patterns of retinoblastoma and E2F family proteins during corneal development. Invest Ophthalmol Vis Sci. 2000;41:1054–1062. [PubMed] [Google Scholar]
- Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan V, Ito M, Makarenkova HP, Lang RA, Overbeek PA. Endogenous and ectopic gland induction by FGF-10. Dev Biol. 2000;225:188–200. doi: 10.1006/dbio.2000.9812. [DOI] [PubMed] [Google Scholar]
- Guo P, Dong XY, Zhang X, Zhao KW, Sun X, Li Q, Dong JT. Pro-proliferative factor KLF5 becomes anti-proliferative in epithelial homeostasis upon signaling-mediated modification. J Biol Chem. 2009a;284:6071–6078. doi: 10.1074/jbc.M806270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Dong XY, Zhao K, Sun X, Li Q, Dong JT. Opposing effects of KLF5 on the transcription of MYC in epithelial proliferation in the context of transforming growth factor beta. J Biol Chem. 2009b;284:28243–28252. doi: 10.1074/jbc.M109.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalt TA, Semina EV. Current molecular understanding of Axenfeld-Rieger syndrome. Expert Rev Mol Med. 2005;7:1–17. doi: 10.1017/S1462399405010082. [DOI] [PubMed] [Google Scholar]
- Hough RB, Piatigorsky J. Preferential transcription of rabbit Aldh1a1 in the cornea: implication of hypoxia-related pathways. Mol Cell Biol. 2004;24:1324–1340. doi: 10.1128/MCB.24.3.1324-1340.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Zhu S, Ranjan R, Linder K, Smart RC. C/EBPalpha and C/EBPbeta are required for Sebocyte differentiation and stratified squamous differentiation in adult mouse skin. PLoS One. 2010;5:e9837. doi: 10.1371/journal.pone.0009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WB. Blepharitis: current strategies for diagnosis and management. Can J Ophthalmol. 2008;43:170–179. doi: 10.1139/i08-016. [DOI] [PubMed] [Google Scholar]
- Klintworth GK. The molecular genetics of the corneal dystrophies--current status. Front Biosci. 2003;8:d687–713. doi: 10.2741/1018. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Merlo D, Mollinari C, Bonini P, Rinaldi AM, DA M, Micera A, Coassin M, Rama P, Bonini S, Garaci E. Molecular basis for keratoconus: lack of TrkA expression and its transcriptional repression by Sp3. Proc Natl Acad Sci U S A. 2005;102:16795–16800. doi: 10.1073/pnas.0508516102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova HP, Ito M, Govindarajan V, Faber SC, Sun L, McMahon G, Overbeek PA, Lang RA. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development. 2000;127:2563–2572. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- Mathers WD, Lane JA, Sutphin JE, Zimmerman MB. Model for ocular tear film function. Cornea. 1996;15:110–119. doi: 10.1097/00003226-199603000-00002. [DOI] [PubMed] [Google Scholar]
- Mattiske D, Sommer P, Kidson SH, Hogan BL. The role of the forkhead transcription factor, Foxc1, in the development of the mouse lacrimal gland. Dev Dyn. 2006;235:1074–1080. doi: 10.1002/dvdy.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549–557. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Chiambaretta F, Sugar J, Sapin V, Yue BY. Developmentally regulated expression of KLF6 in the mouse cornea and lens. Invest Ophthalmol Vis Sci. 2004;45:4327–4332. doi: 10.1167/iovs.04-0353. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Ueda J, Sugar J, Yue BY. Developmentally regulated expression of Sp1 in the mouse cornea. Invest Ophthalmol Vis Sci. 2005;46:4092–4096. doi: 10.1167/iovs.05-0324. [DOI] [PubMed] [Google Scholar]
- Nien CJ, Massei S, Lin G, Liu H, Paugh JR, Liu CY, Kao WW, Brown DJ, Jester JV. The development of meibomian glands in mice. Mol Vis. 2010;16:1132–1140. [PMC free article] [PubMed] [Google Scholar]
- Norman B, Davis J, Piatigorsky J. Postnatal gene expression in the normal mouse cornea by SAGE. Invest Ophthalmol Vis Sci. 2004;45:429–440. doi: 10.1167/iovs.03-0449. [DOI] [PubMed] [Google Scholar]
- Ohnishi S, Laub F, Matsumoto N, Asaka M, Ramirez F, Yoshida T, Terada M. Developmental expression of the mouse gene coding for the Kruppel-like transcription factor KLF5. Dev Dyn. 2000;217:421–429. doi: 10.1002/(SICI)1097-0177(200004)217:4<421::AID-DVDY9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, Suzuki R, Kitamura T, Akira S, Kadowaki T, Nagai R. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Parisi S, Passaro F, Aloia L, Manabe I, Nagai R, Pastore L, Russo T. Klf5 is involved in self-renewal of mouse embryonic stem cells. J Cell Sci. 2008;121:2629–2634. doi: 10.1242/jcs.027599. [DOI] [PubMed] [Google Scholar]
- Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S, Kawai-Kowase K, Moriyama N, Imai Y, Kawakami H, Nishimatsu H, Ishikawa T, Suzuki T, Morita H, Maemura K, Sata M, Hirata Y, Komukai M, Kagechika H, Kadowaki T, Kurabayashi M, Nagai R. Kruppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med. 2002;8:856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- Sivak JM, Mohan R, Rinehart WB, Xu PX, Maas RL, Fini ME. Pax-6 expression and activity are induced in the reepithelializing cornea and control activity of the transcriptional promoter for matrix metalloproteinase gelatinase B. Dev Biol. 2000;222:41–54. doi: 10.1006/dbio.2000.9694. [DOI] [PubMed] [Google Scholar]
- Sivak JM, West-Mays JA, Yee A, Williams T, Fini ME. Transcription Factors Pax6 and AP-2alpha Interact To Coordinate Corneal Epithelial Repair by Controlling Expression of Matrix Metalloproteinase Gelatinase B. Mol Cell Biol. 2004;24:245–257. doi: 10.1128/MCB.24.1.245-257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sue N, Jack BH, Eaton SA, Pearson RC, Funnell AP, Turner J, Czolij R, Denyer G, Bao S, Molero-Navajas JC, Perkins A, Fujiwara Y, Orkin SH, Bell-Anderson K, Crossley M. Targeted disruption of the basic Kruppel-like factor gene (Klf3) reveals a role in adipogenesis. Mol Cell Biol. 2008;28:3967–3978. doi: 10.1128/MCB.01942-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Chen X, Yang VW. Intestinal-enriched Kruppel-like factor (Kruppel-like factor 5) is a positive regulator of cellular proliferation. J Biol Chem. 2001;276:6897–6900. doi: 10.1074/jbc.C000870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sawaki D, Aizawa K, Munemasa Y, Matsumura T, Ishida J, Nagai R. Kruppel-like factor 5 shows proliferation-specific roles in vascular remodeling, direct stimulation of cell growth, and inhibition of apoptosis. J Biol Chem. 2009;284:9549–9557. doi: 10.1074/jbc.M806230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamynathan S, Kenchegowda D, Piatigorsky J, Swamynathan SK. Regulation of the corneal epithelial barrier function by Kruppel-like transcription factor 4. Invest Ophthalmol Vis Sci. 2011;52:1762–1769. doi: 10.1167/iovs.10-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamynathan SK. Kruppel-like factors: three fingers in control. Hum Genomics. 2010;4:263–270. doi: 10.1186/1479-7364-4-4-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamynathan SK, Davis J, Piatigorsky J. Identification of candidate Klf4 target genes reveals the molecular basis of the diverse regulatory roles of Klf4 in the mouse cornea. Invest Ophthalmol Vis Sci. 2008;49:3360–3370. doi: 10.1167/iovs.08-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27:182–194. doi: 10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta M, Hamuro J, Yamamoto M, Kaseda K, Akira S, Kinoshita S. Spontaneous ocular surface inflammation and goblet cell disappearance in I kappa B zeta gene-disrupted mice. Invest Ophthalmol Vis Sci. 2005;46:579–588. doi: 10.1167/iovs.04-1055. [DOI] [PubMed] [Google Scholar]
- Van Agtmael T, Schlotzer-Schrehardt U, McKie L, Brownstein DG, Lee AW, Cross SH, Sado Y, Mullins JJ, Poschl E, Jackson IJ. Dominant mutations of Col4a1 result in basement membrane defects which lead to anterior segment dysgenesis and glomerulopathy. Hum Mol Genet. 2005;14:3161–3168. doi: 10.1093/hmg/ddi348. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Patel DV, McGhee CN. Inherited corneal disease: the evolving molecular, genetic and imaging revolution. Clin Experiment Ophthalmol. 2005;33:303–316. doi: 10.1111/j.1442-9071.2005.01011.x. [DOI] [PubMed] [Google Scholar]
- Wan H, Luo F, Wert SE, Zhang L, Xu Y, Ikegami M, Maeda Y, Bell SM, Whitsett JA. Kruppel-like factor 5 is required for perinatal lung morphogenesis and function. Development. 2008;135:2563–2572. doi: 10.1242/dev.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Mays JA, Sivak JM, Papagiotas SS, Kim J, Nottoli T, Williams T, Fini ME. Positive influence of AP-2alpha transcription factor on cadherin gene expression and differentiation of the ocular surface. Differentiation. 2003;71:206–216. doi: 10.1046/j.1432-0436.2003.710302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Goldstein BG, Chao HH, Katz JP. KLF4 and KLF5 regulate proliferation, apoptosis and invasion in esophageal cancer cells. Cancer Biol Ther. 2005;4:1216–1221. doi: 10.4161/cbt.4.11.2090. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Hu Y, Karin M. IkappaB kinase alpha is essential for development of the mammalian cornea and conjunctiva. Invest Ophthalmol Vis Sci. 2000;41:3665–3669. [PubMed] [Google Scholar]
- Young RD, Swamynathan SK, Boote C, Mann M, Quantock AJ, Piatigorsky J, Funderburgh JL, Meek KM. Stromal edema in klf4 conditional null mouse cornea is associated with altered collagen fibril organization and reduced proteoglycans. Invest Ophthalmol Vis Sci. 2009;50:4155–4161. doi: 10.1167/iovs.09-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Organization of the nuclei in the E13.5, E15.5, E18.5 and PN1 WT and Klf5CN lens equatorial region was examined in H & E stained sagittal sections (n=3). Klf5CN primary fiber cells appeared normal in E13.5 and E15.5. However, nuclei in the E18.5 and PN1 Klf5CN lens equatorial regions were disorganized, suggesting abnormalities in secondary fiber formation.
Immunofluorescence with anti-αA-crystallin antibody (A-D) or anti-αB-crystallin antibody (E-H) detected comparable expression of αA-crystallin and αB-crystallin in the WT (A, C, E and G) and Klf5CN (B, D, F and H) lenses (n=2).


