Abstract
Background
Physiologic processes during aging leading to multi-morbidity and diseases that increase risk of premature death may be influenced by aging-associated changes in endogenous hormone production.
Objective
To evaluate the decline in sex steroid hormone levels across age and estimate the number of US men 40+ years old who may have low hormone levels.
Design
We measured serum testosterone, estradiol, and sex hormone binding globulin by immunoassay in 1,351 men 20+ years old in NHANES III. We estimated free hormones by mass action.
Results
Free testosterone declined most rapidly with age (a 2% decline in geometric mean concentration occurred after aging 1.3 years), followed by total testosterone (2.4 years), free estradiol (4.1 years), and total estradiol (8.1 years). These hormone changes with age translated into 25.0% and 30.2% of men 70+ years old having low total (which we defined as <10.4 nmol/L) and free (<0.17 nmol/L) testosterone, respectively, and 8.3% and 23.9% having low total (<73.4 pmol/L) and free (<2.2 pmol/L) estradiol. Using population size projections between the 2000 and 2010 Censuses, we estimated that 8.4 (95% CI 4.7-12.2), 6.2 (3.1-9.2), and 6.0 (3.1-9.0) million 40+ year old men may have low total testosterone, free testosterone, and free estradiol, respectively. The prevalences were only modestly lower in men without prevalent chronic diseases.
Conclusion
Although no consensus exists for defining low hormone levels in aging men, a substantial number of US men may have low sex steroid hormone levels, possibly putting them at risk for adverse health consequences and pre-mature death.
Keywords: NHANES III, testosterone, men, aging
Introduction
We previously reported in the Third National Health and Nutritional Examination Survey (NHANES III), a US nationally representative study, that men who do not currently have a diagnosis of cardiovascular disease or cancer, but who have low free testosterone or low free estradiol subsequently have higher all-cause and cardiovascular disease mortality rates than men with higher levels.1 Men with low free testosterone also had a higher risk of cancer death. Our study was consistent with some studies, for example,2, 3 but other studies did not observe this association.4, 5
Physiologic processes during aging that lead to multi-morbidity and diseases that increase risk of premature death, such as cardiovascular disease and cancer, are partly thought to be influenced by aging-associated changes in endogenous hormone production. It is well-known that the blood concentrations of sex steroid hormones in males change over the lifetime. Most prominent is the decrease in circulating testosterone concentration with age, which is partly due to an age-related decline of the Leydig cell mass in the testicles6 and/or a dysfunction in the hypothalamic-pituitary-gonadal homeostatic regulatory mechanism. Aging may reduce the capacity of endogenous luteinizing hormone pulses to stimulate Leydig cell steroidogenesis and, thus, testosterone secretion.7 In addition to the effects of aging itself, it has to be taken into account that body composition changes over men's lifetime,8 which, in turn, affects the production and metabolism of sex steroid hormones.9, 10
Given that populations in the Western World are aging – between 1900 and 2000, the percentage of men who were older than 60 years increased from 6.4% to 14.2% in the US11 – the number of men who may be at higher risk for morbidity and mortality related to aging-related hormone changes is rising. Thus, the goals of this study were to estimate cross-sectionally in a nationally representative study the extent of the declines in total and free testosterone and total and free estradiol, and the extent of the increase in sex hormone binding globulin (SHBG) across age, and to estimate the number of US men 40+ years old who may be at higher risk of morbidities and death because of low hormone levels.
Material and Methods
Study population
NHANES III is a cross-sectional study conducted by the National Center for Health Statistics between 1988 and 1994.12 It was designed as a multistage stratified, clustered probability sample of the US civilian non-institutionalized population at least two months old. Mexican-Americans, non-Hispanic blacks, and the elderly were over-sampled to generate more precise estimates for these subgroups of the US population.12
NHANES III was conducted in two phases (1988-1991 and 1991-1994). Unbiased national estimates of health and nutrition characteristics can be independently produced for each phase. Within each phase, subjects were randomly assigned to participate in either the morning or afternoon/evening examination session. In total, 30,818 people were interviewed in NHANES III and had a physical examination and a blood sample taken. Body height and weight were measured and body fat percentage was estimated from bioelectrical impedance analysis conducted during the medical examination. Cigarette smoking, alcohol consumption, and physical activity were assessed using questionnaires. Of the 14,781 males with an examination, 7,772 were at least 20 years old. Serum surplus specimens were available for 1,470 of the 1,998 men who participated in the morning session of phase I. Assays were only conducted on specimens from those participants in the morning sample to minimize extraneous variation due to the diurnal production of testosterone. We further excluded men without an estimate of body fat percentage, leaving 1,351 men for analysis. After applying sampling weights, key characteristics that we used in our analysis (age, percent body fat, smoking behavior, alcohol consumption, and physical activity per age and race/ethnicity category) were comparable between the subsample with surplus serum and all men 20+ years old in the morning examination session of Phase I.
The protocols for the conduct of NHANES III were approved by the institutional review board of the National Center for Health Statistics, Centers for Disease Control and Prevention. Informed consent was obtained from all participants. The assay of stored serum specimens for the Hormone Demonstration Program was approved by the Institutional Review Boards at the Johns Hopkins Bloomberg School of Public Health and the National Center for Health Statistics, Centers for Disease Control and Prevention.
Sex steroid hormones and SHBG measurement
Blood was drawn after an overnight fast for all participants of the morning sample. All surplus specimens were separated into their components and stored at -70°C. Sex steroid hormones and SHBG have been reported to be stable when exposed to multiple freeze-thaw cycles.13 Concentrations of testosterone, estradiol, androstanediol glucuronide (a metabolite of dihydrotestosterone), and SHBG were measured at the Children's Hospital Boston, MA. Competitive electrochemiluminescence immunoassays on the 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN) were used to quantify serum testosterone, estradiol, and SHBG concentrations. Androstanediol glucuronide was measured by an enzyme immunoassay (Diagnostic Systems Laboratories, Webster, TX). The samples were randomly ordered for testing and the laboratory technicians were blinded to the men's identities and ages. The lowest detection limits of the assays were: testosterone 0.07 nmol/L, estradiol 18.4 pmol/L, androstanediol glucuronide 0.70 nmol/L, and SHBG 3 nmol/L. Quality control specimens with known hormone concentrations spanning higher and lower concentrations were used to determine reliability. The coefficients of variation for these quality control specimens included during the analyses of the NHANES III specimens were as follows: testosterone 5.9% and 5.8% at 8.6 and 19.1 nmol/L, respectively; estradiol 6.5% and 6.7% at 0.38 nmol/L and 1.7 nmol/L, respectively; androstanediol glucuronide 9.5% and 5.0% at 6.2 and 21.6 nmol/L, respectively; and SHBG 5.3% and 5.9% at 5.3 and 16.6 nmol/L, respectively. In a separate run, we tested quality control samples with a mean estradiol concentration of 144.6 pmol/L, which is in the range of typical adult male estradiol concentration; the CV% was 2.5%. Due to limited serum volume, all markers have been measured only once. Free testosterone concentration was estimated from measured testosterone, SHBG, and albumin (already available in the NHANES III public use database).14, 15 free estradiol was calculated from total estradiol, SHBG, and albumin.14, 16 Because of limited volume serum testosterone concentration could not be measured for 7, estradiol for 4, androstanediol glucuronide for 14, and SHBG for 10 men.
Statistical analysis
All statistical analyses were performed with SUDAAN17 as implemented using SAS v.9.1 (Cary, NC). Sampling weights for NHANES III phase 1 were used to account for sampling variability and to adjust for differential probability of selection of persons.12 To examine differences in hormone and SHBG concentrations by age, individuals were divided into six age categories (20-29, 30-39, 40-49, 50-59, 60-69, and 70+ years old).
Because non-endogenous factors that influence hormone levels can vary by age, we used linear regression to estimate geometric mean hormone concentrations across age adjusted for cigarette smoking (never smoker, former smoker, current smoker <35 cigarettes/day, current smoker ≥35 cigarettes/day), alcohol consumption (never drinker, <1 drink/week, ≥1 drink/week to <1 drink/day, ≥1 drink/day), and physical activity (moderate or vigorous physical activity on 0, 1-2, 3-4, 5-6 or ≥7 days/week). Percent body fat was calculated from bioelectrical impedence analysis, height, weight, and age. To take into account body fat mass, we adjusted for percent body fat (continuous) instead of body mass index (BMI) because BMI may also be an indicator of muscle mass, especially in younger men.18 To further examine the association between age and total and free testosterone, total and free estradiol, and SHBG concentrations, we fit restricted cubic spline models for age and adjusted for race/ethnicity and percent body fat (continuous). We specified knot positions according to quintiles of the hormone levels, respectively. To be able to compare the steepness of the slopes for the association between age and each hormone, given that the range of the distribution of concentrations differ among the hormones, we calculated the change in age associated with a 2% change in geometric mean hormone concentrations. These estimates were calculated based on geometric mean hormone levels for white non-smoking men in the second category of drinking and exercise with 24.9% body fat.
We estimated the proportion of men 40 years old and older and 95% confidence intervals after applying the sampling weights by decade of age that had low hormone levels or high SHBG levels; we report unadjusted proportions, which are thus reflective of the total burden in population. We did not include men 20-29 years old in the prevalence estimates because so few had low testosterone and the reasons for their low levels are more likely due to congenital hypogonadism rather than due to aging. We defined low concentrations using as cut points <10.4 nmol/L for total testosterone,19, 20 <0.17 nmol/L for free testosterone,19 <73.4 pmolL for total estradiol,21 and <2.2 pmol/L (10th percentile) for free estradiol. We used ≥66 nmol/L (90th percentile) as the cut point for high SHBG. There is no consensus on the optimal cut point for defining low total and free testosterone20 and no guidelines exist for low free estradiol and high SHBG. Using population size projections between the 2000 and 2010 Censuses and the estimated proportions and their standard errors for low hormones and high SHBG, we estimated the number of men (and 95% confidence interval) in each decade of age and also men 40+ years old who may have low hormone or high SHBG levels.
We repeated these analyses after excluding men with a diagnosis of cardiovascular disease (n=111), cancer (n=37), or diabetes (n=88) (told by a doctor or using medications to treat diabetes). All tests were two-sided; p-values <0.05 were considered to be statistically significant.
Results
Selected baseline characteristics of the men are presented in Table 1. The distribution of racial/ethnic groups differed by age categories with a higher percentage of Mexican-Americans in the younger age groups. Median percentage of body fat increased from 22.6% in men 20-29 years old to 26.8% in men 70+ years old. The prevalence of chronic diseases increased across decades of age.
Table 1. Characteristics (weighted) of 1,351 male participants, NHANES III (Phase 1), 1988-1991.
| Age group (years) | ||||||
|---|---|---|---|---|---|---|
| 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70+ | |
| N (unweighted) | 276 | 258 | 232 | 178 | 181 | 226 |
| Race/ethnicity (%) | ||||||
| Non-Hispanic white | 75.8 | 76 | 78 | 79.5 | 84.2 | 84.4 |
| Non-Hispanic black | 11.5 | 9.9 | 7.3 | 8.2 | 7 | 7.9 |
| Mexican-American | 7.4 | 5 | 4.3 | 3.8 | 2.6 | 2.1 |
| Other | 5.3 | 9 | 10.5 | 8.5 | 6.2 | 5.7 |
| Percent body fat | ||||||
| Median | 22.6 | 24.7 | 25.7 | 26.8 | 26.8 | 26.8 |
| 25th percentile | 19.4 | 21.3 | 22.6 | 22.7 | 23.9 | 22.6 |
| 75th percentile | 25.9 | 28.5 | 29.7 | 29.6 | 29.5 | 30.4 |
| Smoking status (%) | ||||||
| Never | 50.6 | 35.17 | 28.6 | 17.6 | 27.1 | 31 |
| Former | 13.7 | 21.3 | 40.2 | 46.4 | 51.1 | 57.2 |
| Current | 35.7 | 43.5 | 31.2 | 36 | 21.8 | 11.8 |
| Alcohol consumption (%) | ||||||
| Never | 25.7 | 22 | 33.6 | 33.3 | 40.5 | 53.5 |
| > 0 to < 1/week | 18 | 19.8 | 14.6 | 19.7 | 17.3 | 9 |
| ≥ 1/week to < 1/day | 40.1 | 47 | 28.4 | 26.4 | 22.4 | 18.4 |
| ≥ 1/day | 16.3 | 11.2 | 23.4 | 20.7 | 19.7 | 19.1 |
| Moderate or vigorous physical activity (times per week; %)* | ||||||
| 0 | 6.6 | 7 | 7.7 | 14.5 | 7.2 | 11.3 |
| 1 to 2 | 19.3 | 24.7 | 25.8 | 22 | 20.6 | 20.6 |
| 3 to 4 | 17.2 | 9.8 | 18.4 | 18.9 | 14.2 | 12.8 |
| 5 to 6 | 9.9 | 11.5 | 8.3 | 8.6 | 5.7 | 5.8 |
| ≥ 7 | 47 | 47 | 39.9 | 35.9 | 52.3 | 49.6 |
| Prevalence of chronic diseases (%) | ||||||
| Cardiovascular disease | 0 | 0.7 | 2.4 | 10 | 16.2 | 23.2 |
| Cancer | 0.9 | 0 | 1.2 | 0.8 | 3.2 | 13.7 |
| Diabetes | 0.5 | 1.2 | 3.9 | 8.4 | 7.3 | 9.5 |
After taking into account race/ethnicity, percent body fat, smoking, alcohol consumption and physical activity, the highest total testosterone and free testosterone concentrations were seen in the third decade of life (Table 2). Both decreased continuously with age without indication of an inflection point, although the decline was steeper for free testosterone (Figure 1A). Circulating androstanediol glucuronide levels also decreased significantly with age (Table 2). Circulating total and free estradiol concentrations were highest in the youngest age groups and decreased with age (Figure 1B). In contrast to the hormones, circulating SHBG concentrations increased with age (Table 2 and Figure 1C). We estimated by how much men of the same age and characteristics would have to age to result in 2% lower (or higher in the case of SHBG and molar ratio of estradiol to testosterone) geometric mean concentrations assuming that the associations between age and the hormones are causal (Table 2). The decline in total testosterone was more strongly related to age than the decline in total estradiol and the decline in free hormones was more strongly related to age than the decline in total hormone concentrations. For example, a 2% decline in the geometric mean total testosterone would be observed if men aged 2.4 years, whereas the same decline in free testosterone would be observed if the men aged only 1.3 years. In contrast, a 2% decline in the geometric mean total estradiol concentration would be observed if the men aged 8.1 years (4.1 years for free estradiol). For SHBG, a 2% increase in geometric mean concentration would be observed if the men aged 1.6 years. These results did not differ materially after excluding men with prevalent diseases at baseline, with the possible exception of total testosterone for which a man without prevalent chronic disease would have to age 3.1 (instead of 2.6 years) to experience the same decline in total testosterone (Table 2).
Table 2. Serum concentrations (geometric mean and 95% confidence intervals*) of sex steroid hormones and sex hormone binding globulin by age, 1,351 male participants, NHANES III, 1988-1991.
| Age group (years) | Number of years of age corresponding to a 2% change in geometric mean hormone concentration** | |||||||
|---|---|---|---|---|---|---|---|---|
| 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70+ | All men | Excluding men with prevalent disease at recruitment | |
| Total testosterone (nmol/L) | 20.68 (19.54-21.93) |
18.77 (17.97-19.61) |
16.66 (15.20-18.25) |
15.89 (14.85-17.00) |
15.62 (14.71-16.55) |
12.28 (10.38-14.57) |
2.4 | 2.7 |
| Free testosterone (nmol/L) | 0.458 (0.433-0.456) |
0.402 (0.385-0.420) |
0.326 (0.295-0.357) |
0.298 (0.274-0.323) |
0.264 (0.246-0.281) |
0.180 (0.146-0.219) |
1.3 | 1.4 |
| Total estradiol (pmol/L) | 142.54 (138.07-147.17) |
133.29 (127.38-139.46) |
124.56 (117.40-132.16) |
128.19 (120.96-135.90 |
125.11 (116.81-133.99) |
120.89 (111.52-130.98) | 8.1 | 7.6 |
| Free estradiol (pmol/L) | 3.74 (3.60-3.89) |
3.49 (3.30-3.67) |
3.16 (2.97-3.38) |
3.27 (3.08-3.49) |
3.01 (2.83-3.19) |
2.75 (2.50-3.05) |
4.1 | 4.1 |
| Androstanediol glucuronide (nmol/L) | 31.7966 (30.11-33.59) |
25.03182 (23.11-27.12) |
24.541 (22.34-26.95) |
22.64174 (19.42-26.38) |
20.01692 (17.16-23.35) |
18.84322 (16.86-21.06) |
-1.6 | -1.7 |
| SHBG (nmol/L) | 29.01 (26.58-31.66) |
30.87 (29.04-32.83) |
35.68 (33.14-38.42) |
38.31† (35.16-41.74) |
45.21 (41.82-48.87) |
56.21 (52.81-59.83) |
1.6 | 1.6 |
| Molar ratio of estradiol (nmol/L) to testosterone (nmol/L) | 6.88 (6.47-7.31) |
7.1 (6.73-7.48) |
7.47 (6.65-8.39) |
8.07 (7.50-8.68) |
8.01 (7.53-8.52) |
9.82 (8.81-10.96) |
-3.4 | -4.0 |
Adjusted for race/ethnicity, percent body fat, physical activity, smoking, and alcohol consumption
Based on white non-smoking men in the second category of drinking and exercise with 24.9% body fat
Figure 1.
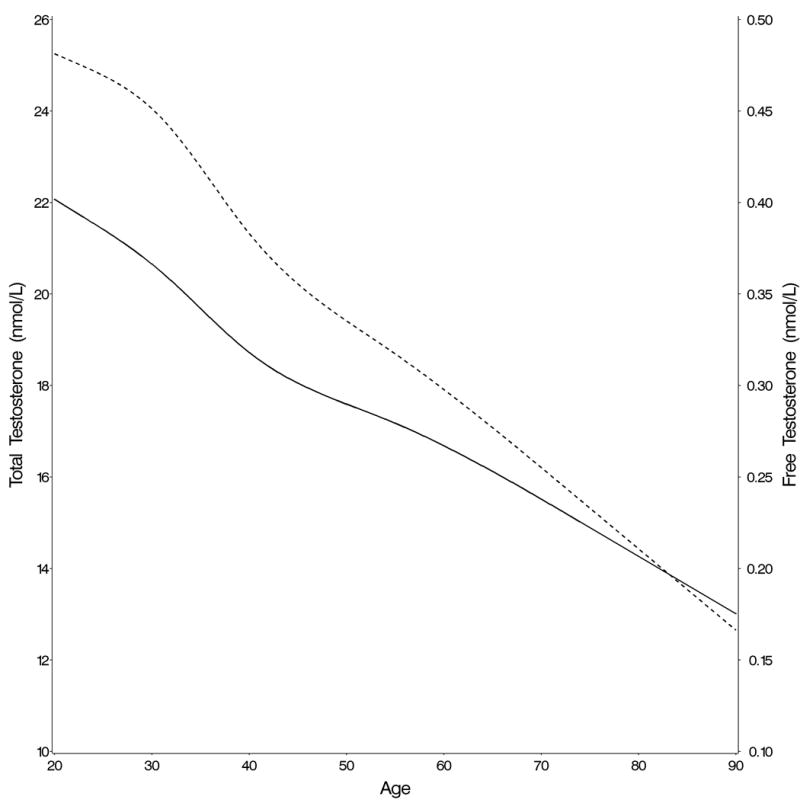
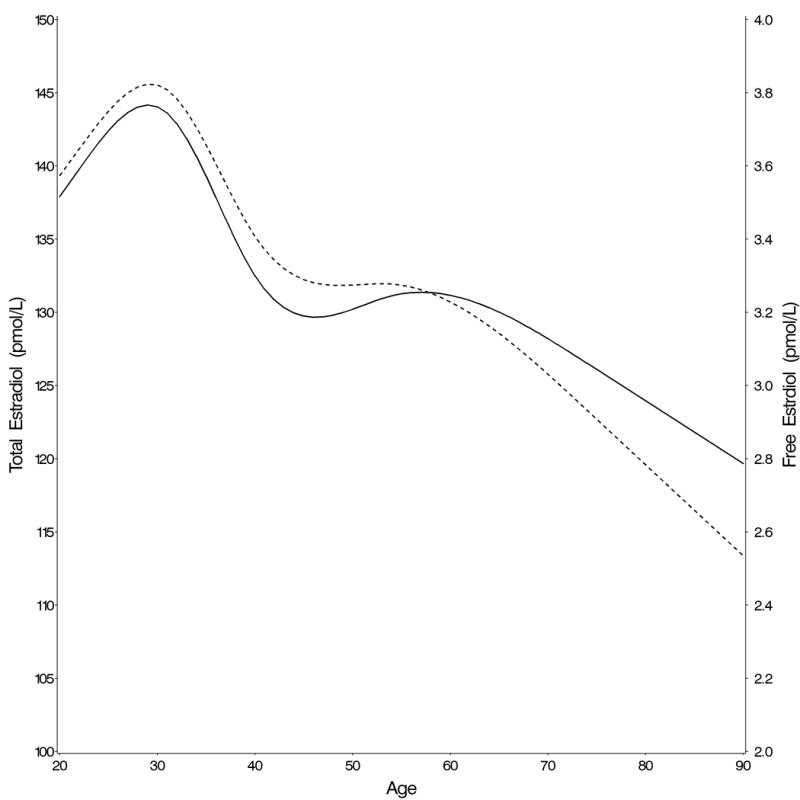
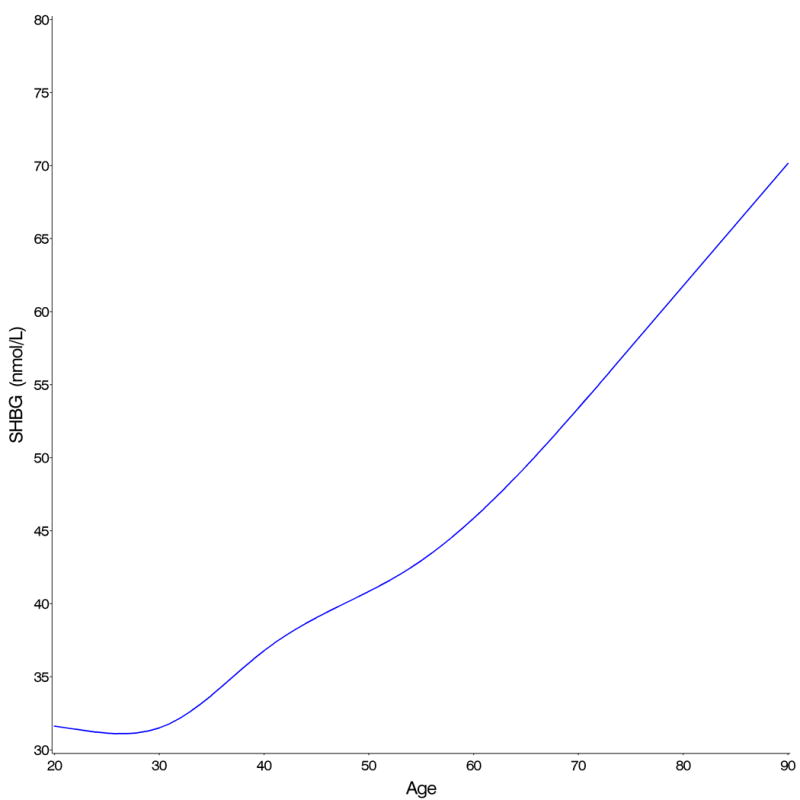
Change in (A) total (full line) and free (dotted line) testosterone, (B) total (full line) and free (dotted line) estradiol, and (C) SHBG concentrations with age
The prevalence of low total testosterone concentration (<10.4 nmol/L) increased with age from 2% in men 20-29 to 25% in men 70+ years old (Table 3). A larger increase with age was observed for low free testosterone concentration (<0.17 nmol/L), from essentially 0% in men 20-29 years of age to 24.9% in men 70+ years old. Among men 40+ years old, we estimated that 8.4 (95% confidence interval [CI] 4.7-12.2) million may have low total testosterone and 6.2 (95% CI 3.1-9.2) million may have low free testosterone concentrations. Among men 70+ years old, we estimated the number who may have low total and free testosterone levels to be 2.6 and 3.1 million, respectively. After excluding men with prevalent chronic diseases, we estimated the number of men 40+ years old who may have low total and free testosterone concentrations to be 7.9 (95% CI 11.9-20.2) and 5.5 (95% CI 2.3-8.8) million, respectively. The prevalence of low total estradiol (<73.4 pmol/L) was below 5% until the age of 60, but reached 24% in men 70+ years old. We estimated that 6.0 (95% CI 3.1-9.0) million US men 40+ years old may have low free estradiol concentrations. The prevalence of an SHBG concentrations >66 nmol/L (i.e., >90th percentile of this group of men) was below 10% until the age of 70. In men 70+ years old, the prevalence (32%) was triple that at younger ages.
Table 3. Prevalence of low hormone and high SHBG concentration by age, 1,351 male participants, NHANES III, 1988-1991.
| Age group (years) |
Prevalence | Population size** | ||
|---|---|---|---|---|
| % | 95% CI | population | 95% CI | |
| Total testosterone (<10.41 nmol/L) | ||||
| 20-29 | 2.3 | * | 450,313 | * |
| 30-39 | 4.8 | (1.8 -7.8) | 938,276 | (330,431 -1,546,121) |
| 40-49 | 8.7 | (4.1 -13.2) | 1,901,808 | (898,901 -2,904,716) |
| 50-59 | 12.8 | (7.4 -18.3) | 2,316,136 | (1,328,953 -3,303,319) |
| 60-69 | 15 | (7.4 -22.6) | 1,645,256 | (813,351 -2,477,160) |
| 70+ | 24.9 | (16.3 -33.5) | 2,581,414 | (1,689,385 -3,473,443) |
| 40+ | 8,444,613 | (4,730,590 -12,158,637) | ||
| Free testosterone (<0.173 nmol/L) | ||||
| 20-29 | 0 | * | 0 | * |
| 30-39 | 1.3 | * | 264,693 | * |
| 40-49 | 4.5 | (1.0 -7.9) | 977,257 | (211,088 -1,743,427) |
| 50-59 | 6.8 | (0.9 -12.7) | 1,227,570 | (162,545 -2,292,595) |
| 60-69 | 7.6 | (3.8 -11.4) | 829,191 | (413,239 -1,245,143) |
| 70+ | 30.2 | (22.2 -38.2) | 3,125,688 | (2,296,650 -3,954,726) |
| 40+ | 6,159,706 | (3,083,521 -9,235,892) | ||
| Total estradiol (<73.42 pmol/L) | ||||
| 20-29 | 0.3 | * | 54,038 | * |
| 30-39 | 3.4 | * | 669,633 | * |
| 40-49 | 4.2 | * | 922,355 | * |
| 50-59 | 0.9 | * | 153,446 | * |
| 60-69 | 5.1 | (0.8 -9.5) | 561,181 | (87,339 -1,035,023) |
| 70+ | 8.3 | (2.2 -14.4) | 860,471 | (230,565 -1,490,378) |
| 40+ | 2,497,454 | * | ||
| Free estradiol (<2.17 pmol/L) | ||||
| 20-29 | 4.8 | * | 960,667 | * |
| 30-39 | 8.1 | (3.0 -13.2) | 1,600,008 | (601,129 -2,598,887) |
| 40-49 | 5.8 | (1.2 -10.3) | 1,262,748 | (272,754 -2,252,742) |
| 50-59 | 5.3 | (1.7 -8.8) | 951,367 | (307,398 -1,595,336) |
| 60-69 | 12.2 | (5.0 -19.4) | 1,335,676 | (544,510 -2,126,843) |
| 70+ | 23.9 | (18.8 -28.9) | 2,472,559 | (1,952,378 -2,992,740) |
| 40+ | 6.022.350 | (3.077.039 -8.967.661) | ||
| SHBG (>66 nmol/L) | ||||
| 20-29 | 0.4 | * | 72,050 | * |
| 30-39 | 2 | (0.4 -3.5) | 385,187 | (71,586 -698,789) |
| 40-49 | 6.2 | (3.2 -9.2) | 1,354,984 | (696,422 -2,013,545) |
| 50-59 | 9.6 | (3.9 -15.4) | 1,740,261 | (703,542 -2,776,980) |
| 60-69 | 8.8 | (5.1 -12.4) | 961,556 | (562,756 -1,360,355) |
| 70+ | 32.1 | (23.4 -40.7) | 3,325,773 | (2,429,681 -4,221,866) |
| 40+ | 7,382,573 | (4,392,401 -10,372,746) | ||
95% CIs were not estimated because relative standard error is >30% and, thus, unreliable
Projected population size between the 2000 and 2010 US Censuses.
Discussion
To our knowledge, this is the first large, nationally representative cross-sectional study to estimate the prevalence and number of men with low concentrations of total and free testosterone and total and free estradiol concentrations, and high SHBG concentrations, and to characterize the association of age with serum sex steroid hormone concentrations in US adult men overall and in men without prevalent chronic diseases. We observed a strong age-associated decline in circulating free testosterone concentration, which was attributable to decreasing total testosterone and increasing SHBG concentrations with age. However, the decline was continuous without an ‘andropause’ threshold. Less steep declines with age were also present for estradiol and free estradiol. Using one set of cutpoints for low hormones, we estimate that millions of men may have low hormone levels. At this time, no consensus exists for the definition of low testosterone in men as they age.22 For example, Wang et al state that there is general agreement that the total testosterone level above 12 nmol/L (350 ng/dL) does not require substitution and that there is consensus that patients with serum total testosterone levels below 8 nmol/L (230 ng/dL) will usually benefit from testosterone treatment.22 The estimated number of affected men would be lower or higher using different definitions of low hormone levels. Based on our prior work in NHANES III, these men may be at elevated risk for morbidities and premature death.1 Because the US population continues to grow and the number of older individuals has increased, these estimates may increase substantially over time (assuming no change in the prevalence of low androgens over time).
Although the extent to which total testosterone concentration declines with age has been previously reported in longitudinal studies,23-25 our study adds to the literature because of the wide age range (20 to greater than 90 years old) because we were able to take into account factors that influence hormone levels and the prevalences of which differ by age, and because the data are from a nationally representative sample of Americans. A concurrent increase in the concentration of SHBG, the major determinant of bioavailable testosterone and estradiol concentrations, leads to a greater decrease in circulating free testosterone than in total testosterone concentration. Consistent with previous studies,24-26 we observed an increase in serum SHBG concentration with age even after taking into account percent body fat, which is strongly correlated with SHBG. Wu et al. have shown that SHBG increases in men with normal BMI and in overweight and obese men.26 The reasons for the increase in SHBG concentration with age are unclear but some have speculated that the age-associated decreases in growth hormone and insulin-like growth factor-1 might be contributory.27
Related to lower total and free testosterone concentrations at higher age was a notable increase in the prevalence of low circulating levels of total and free testosterone with age. In a longitudinal US study, an increase in the prevalence of low total testosterone concentration (<11.3 nmol/L) from 5% (20-29 years old) to 50% (80+ years old) and from 0% to 95% for the free testosterone index was noted across age.23 Differences in the prevalence estimates across studies might be due to different age structures, the use of different cut points for defining low concentrations, and because these estimates do not account for differences in the prevalence of obesity and other factors that influence hormone levels. Our figures for the number of men with low total and free testosterone might underestimate the prevalence in US men because the prevalence of obesity, which is related to lower circulating levels of testosterone, increased in the US during the last 20 years,28 a trend that we did not capture with our cross-sectional data. Secondly, a US study29 has reported secular declines in male testosterone concentrations, the reasons of which are yet unclear.
We did not measure dihydrotestosterone, the major and most potent androgen in the prostate. Instead, we measured androstanediol glucuronide as a surrogate marker of 5α-reductase activity and, thus, the conversion of testosterone to dihydrotestosterone. We observed a statistically significant decrease in androstanediol glucuronide levels with increasing age, similar to other studies24, 30 in which a decline was observed, but not always found to be statistically significant. Despite a decline in androstanediol glucuronide levels, another study did not report decreases in blood dihydrotestosterone concentrations.31
The observed decline in circulating total and free estradiol concentrations in our study is consistent with the results of some studies32, 33 but not others.30 To our knowledge, no other study has yet reported on the change of the molar ratio of estradiol to testosterone with age. We observed a higher ratio of estradiol to testosterone in elderly men compared with younger men. On the one hand, this might be due to a stronger decrease of circulating testosterone than estradiol with age. On the other hand this ratio can also be considered an indirect marker of aromatase activity, i.e. the conversion of testosterone to estradiol especially in fat tissue and, thus, the higher ratio may reflect higher conversion of testosterone to estradiol at older age. Our observed differences in this ratio by age were, however, independent of estimated percentage of body fat.
Mazur has previously analyzed the same dataset we used in our current analysis to examine the association between changes in age and hormone concentration by race/ethnicity.34 The main aim of our analysis was to estimate the prevalence of low hormone concentrations independent of race/ethnicity. In addition, we also examined the changes in circulating hormone levels by age. In contrast to Mazur34, we used – as indicated by National Center for Health Statistics (NCHS)12 – sampling weights to account for oversampling of specific population groups such as elderly, African-Americans, and Mexican-Americans.
Circulating sex steroid hormone and SHBG concentrations in humans are influenced by a number of factors. To overcome differences in serum concentrations between age due to body composition or lifestyle factors we adjusted in the regression models for percentage of body fat, cigarette smoking, alcohol consumption, and frequency of moderate and vigorous physical activity. Twelve men reported a previous diagnosis of prostate cancer a treatment for which is androgen deprivation therapy if the disease is metastatic. Excluding these men from the analysis did not change the results. Due to the cross-sectional design of the study, we are unable to evaluate secular trends that might partially account for observed differences in hormone concentrations across age. Finally, among the men who had low testosterone, we could not discern what proportion had pathological hypogonadism from those who merely experienced a decline in androgens with age because we did not measure luteinizing hormone, follicle stimulating hormone, and prolactin nor did we have information on specific androgens deficiency symptoms.35 Taking low testosterone concentrations as well as symptoms into account, Araujo et al.35 estimated that for the year 2000 that there are 4.7 million American men 30–79 yr old with symptomatic androgen deficiency. This is lower than our estimate of 8.4 (95% confidence interval [CI] 4.7-12.2) million men who may have low total testosterone and 6.2 (95% CI 3.1-9.2) million who may have low free testosterone concentrations, which are based on hormone measurements only.
For relevance to the question of how many apparently well men are at risk for morbidities and premature death, we also estimated the prevalence of low hormones or high SHBG and also assessed whether the change in circulating levels differed in these men from the prevalence when including all men. Thus, it is interesting to note that the estimates of men with low hormone and high SHBG concentrations were only modestly lower after excluding men with prevalent chronic diseases.
In conclusion, substantial numbers of US men, especially men 70+ years old, may have low levels of testosterone and estradiol, possibly putting them at risk for adverse health consequences and pre-mature death. At this time, the risk to benefit ratio of treating low testosterone levels in older men is unclear.19, 20 The FDA approved testosterone therapy for treatment of male hypogonadism, which has been found effective in improving a number of symptoms in hypogonadal men. However, only a few studies have been conducted in middle-aged or older men who may have testosterone levels in the low range and show one or more symptoms that are common to both aging and hypogonadism, but who do not meet all the criteria for being hypogonadal.20 Therefore, as recommended by the Institute of Medicine report on testosterone therapy,20 further research is warranted on potential benefits and adverse effects of testosterone therapy in middle-aged and older men. Determining whether losing weight, controlling diabetes, and/or increasing physical activity would increase total and free testosterone and estradiol and decrease SHBG should be of priority, as other health benefits of these behaviors are well established.
Acknowledgments
We thank Gary Bradwin in Dr. Rifai's laboratory. This is the fourth study from the Hormone Demonstration Program, which is supported by the Maryland Cigarette Restitution Fund Research Grant Program at Johns Hopkins. Dr. Selvin was supported by NIH/NIDDK grant (K01 DK076595). Drs. Shiels and Joshu were supported by the NIH/NCI National Research Service Award (T32 CA009314).
Role of the Funding Source: The funding source supported the analyses of blood specimens and data but did not influence the interpretation of the data or the decision to submit the work for publication.
Footnotes
Conflicts of Interest Statement: We declare that we have no conflict of interest.
References
- 1.Menke A, Guallar E, Rohrmann S, Nelson WG, Rifai N, Kanarek N, Feinleib M, Michos ED, Dobs A, Platz EA. Sex steroid hormone concentrations and risk of death in US men. Am J Epidemiol. 171:583–592. doi: 10.1093/aje/kwp415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 3.Vikan T, Schirmer H, Njolstad I, Svartberg J. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromso Study. Eur J Endocrinol. 2009;161:435–442. doi: 10.1530/EJE-09-0284. [DOI] [PubMed] [Google Scholar]
- 4.Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, McKinlay JB. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med. 2007;167:1252–1260. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- 5.Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation. 2005;112:332–340. doi: 10.1161/CIRCULATIONAHA.104.489088. [DOI] [PubMed] [Google Scholar]
- 6.Neaves WB, Johnson L, Porter JC, Parker CR, Jr, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59:756–763. doi: 10.1210/jcem-59-4-756. [DOI] [PubMed] [Google Scholar]
- 7.Veldhuis JD, Veldhuis NJD, Keenan DM, Iranmanesh A. Age diminishes the testicular steroidogenic response to repeated intravenous pulses of recombinant human LH during acute GnRH-receptor blockade in healthy men. Am J Physiol Endocrinol Metab. 2005;288:E775–781. doi: 10.1152/ajpendo.00410.2004. [DOI] [PubMed] [Google Scholar]
- 8.Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55:663–672. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- 9.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–638. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- 10.Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67:460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 11.Hobbs F, Stoops N. US Census Bureau, Census 2000 Special Reports. Washington: 2002. Demographic Trends in the 20th Century. (CENSR-4). [Google Scholar]
- 12.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat. 1994;1:1–407. [PubMed] [Google Scholar]
- 13.Comstock GW, Burke AE, Norkus EP, Gordon GB, Hoffman SC, Helzlsouer KJ. Effects of Repeated Freeze-Thaw Cycles on Concentrations of Cholesterol, Micronutrients, and Hormones in Human Plasma and Serum. Clin Chem. 2001;47:139–142. [PubMed] [Google Scholar]
- 14.Södergard R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17[beta] to human plasma proteins at body temperature. Journal Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen A, Verdonck L, Kaufman JM. A Critical Evaluation of Simple Methods for the Estimation of Free Testosterone in Serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 16.Rinaldi S, Geay A, Dechaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002;11:1065–1071. [PubMed] [Google Scholar]
- 17.Shah BV, Barnwell BG, Bieler GS. SUDAAN User's Manual: Software for Analysis of Correlated Data. Release 6.0. Research Triangle Institute; Research Triangle Park, NC: 1995. [Google Scholar]
- 18.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 20.Liverman C, Blazer D. Testosterone and Aging: Clinical Research Directions. Institute of Medicine of the National Academies; Washington, DC: 2003. [PubMed] [Google Scholar]
- 21.Greenspan F. Basic and Clinical Endocrinology. 4. McGraw Hill; East Norwalk, Conneticut: 1994. [Google Scholar]
- 22.Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FCW. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159:507–514. doi: 10.1530/EJE-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 24.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 25.Gapstur SM, Kopp P, Gann PH, Chiu BC, Colangelo LA, Liu K. Changes in BMI modulate age-associated changes in sex hormone binding globulin and total testosterone, but not bioavailable testosterone in young adult men: the CARDIA Male Hormone Study. Int J Obes (Lond) 2006 doi: 10.1038/sj.ijo.0803465. [DOI] [PubMed] [Google Scholar]
- 26.Wu FCW, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D, The European Male Aging Study Group Hypothalamic-Pituitary-Testicular Axis Disruptions in Older Men Are Differentially Linked to Age and Modifiable Risk Factors: The European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 27.Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab. 1996;81:1821–1826. doi: 10.1210/jcem.81.5.8626841. [DOI] [PubMed] [Google Scholar]
- 28.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 29.Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92:196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- 30.Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked Decline in Serum Concentrations of Adrenal C19 Sex Steroid Precursors and Conjugated Androgen Metabolites During Aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 31.Barrett-Connor E, von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older Men: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 32.Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 33.Leifke E, Gorenoi V, Wichers C, von zur Muhlen A, von Buren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clinical Endocrinology. 2000;53:689–695. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- 34.Mazur A. The age-testosterone relationship in black, white, and Mexican-American men, and reasons for ethnic differences. Aging Male. 2009;12:66–76. doi: 10.1080/13685530903071802. [DOI] [PubMed] [Google Scholar]
- 35.Araujo AB, Esche GR, Kupelian V, O'Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]


