Abstract
Heparan sulfate proteoglycans (HSPGs) are found in the basement membrane and at the cell-surface where they modulate the binding and activity of a variety of growth factors and other molecules. Most of the functions of HSPGs are mediated by the variable sulfated glycosaminoglycan (GAG) chains attached to a core protein. Sulfation of the GAG chain is key as evidenced by the renal agenesis phenotype in mice deficient in the HS biosynthetic enzyme, heparan sulfate 2-O sulfotransferase (Hs2st; an enzyme which catalyzes the 2-O-sulfation of uronic acids in heparan sulfate). We have recently demonstrated that this phenotype is likely due to a defect in induction of the metanephric mesenchyme (MM), which along with the ureteric bud (UB), is responsible for the mutually inductive interactions in the developing kidney (Shah et al., 2010). Here, we sought to elucidate the role of variable HS sulfation in UB branching morphogenesis, particularly the role of 6-O sulfation. Endogenous HS was localized along the length of the UB suggesting a role in limiting growth factors and other molecules to specific regions of the UB. Treatment of cultures of whole embryonic kidney with variably desulfated heparin compounds indicated a requirement of 6O-sulfation in the growth and branching of the UB. In support of this notion, branching morphogenesis of the isolated UB was found to be more sensitive to the HS 6-O sulfation modification when compared to the 2-O sulfation modification. In addition, a variety of known UB branching morphogens (i.e., pleiotrophin, heregulin, FGF1 and GDNF) were found to have a higher affinity for 6-O sulfated heparin providing additional support for the notion that this HS modification is important for robust UB branching morphogenesis. Taken together with earlier studies, these findings suggest a general mechanism for spatio-temporal HS regulation of growth factor activity along the branching UB and in the developing MM.
Keywords: kidney development, ureteric bud, branching morphogenesis, heparan sulfate, growth factor
INTRODUCTION
Kidney development is initiated when the ureteric bud (UB) forms as an outpouching of the Wolffian duct (WD) in response to signals emanating from the adjacent metanephric mesenchyme (MM). The UB then invades the MM where it undergoes multiple iterative rounds of dichotomous branching morphogenesis to form the tree-like collecting system of the urinary tract. Since nephrons, derived from the MM, are induced at UB tips, UB branching morphogenesis is not only a primary determinant of final nephron number (Nigam, 1995), but also the scaffolding for final kidney architecture (al-Awqati and Goldberg, 1998; Meyer et al., 2004). Since the functions of the adult kidney are critically dependent upon the number and arrangement of nephrons, factors that control UB branching morphogenesis can ultimately viewed as modulators of susceptibility to diseases such as hypertension (Brenner et al., 1988) and chronic kidney disease (Brenner and Milford, 1993), as well as congenital kidney diseases such as multicystic dysplastic kidney or congenital hydronephrosis.
Multiple growth factors have been identified as modulators of UB branching and architecture. Of these, some of the most prominent members have belonged to the FGF and TGFβ families (Bush et al., 2004; Qiao et al., 2001; Qiao et al., 1999b; Sakurai et al., 1997; Santos et al., 1993). FGFs have been shown to affect the formation of UB tips and stalks, and signaling through a variety of FGF receptors has been shown to play a role in UB branching and stromal mesenchymal patterning (Poladia et al., 2006; Qiao et al., 2001; Zhao et al., 2004). Similarly, members of the TGFβ superfamily have been shown to play a role in UB patterning, limit the extent of UB branching and may function as a stop signal at a variety of major developmental stages (Bush et al., 2004). While it is clear that these factors play a role in these various morphogenetic processes (e.g., initiation of branching, rapid branching, patterning, and the cessation of branching), how they exert these spatiotemporal effects remains undefined.
Nevertheless, heparan sulfate proteoglycans (HSPGs) are believed to play a key role in modulating the effects of growth factors based on their ability to bind to and regulate their actions either by 1) presenting the growth factor to its receptor, 2) acting as a co-receptor, 3) concentrating or sequestering the growth factors, and/or 4) negatively modulating growth factor signaling. HSPGs are composed of a core protein to which are attached heparan sulfate (HS) side-chains comprised of highly complex glycosaminoglycan (GAG) chains with a variable number of disaccharide repeats sulfated at a variety of positions (Esko and Lindahl, 2001). Sulfation of the disaccharide, which is catalyzed by a number of enzymes (i.e., members of the N-deacetylase-N-sulfotransferase (Ndst) family, the uronyl C5-epimerase (Hsglce) and 2-O-sulfotransferase (Hs2st), the glucosaminyl 6-O-sulfotransferases (Hs6st) and 3-O-sulfotransferases (Hs3st) (reviewed in (Esko and Lindahl, 2001)), generally occurs sequentially (i.e., Ndst→ HsGlce →Hs2st→Hs6st→Hs3st). However since some reactions do not go to completion, unique patterns of modified sulfate residues are created in sections of the chains. It believed that both the density and pattern of sulfation are important determinants of the HS-growth factor interactions (Deakin et al., 2009; Kreuger et al., 2006; Shah et al., 2010; Sugaya et al., 2008). For example, in the kidney, knockout of the HS biosynthetic enzyme, heparan sulfate 2-O sulfotransferase (Hs2st) results in a severe kidney phenotype (Bullock et al., 1998). Despite compensation in sulfation at other positions, specifically the 6-O position (Wilson et al., 2002), that preserves overall charge, knockout of Hs2st results in renal agenesis (Bullock et al., 1998) suggesting that sulfation at this position is critical in the modulation of the activity of one or more key growth factors involved in kidney development (Merry et al., 2001). We have recently shown that HS modifications by Hs2st, previously thought to be important for binding growth factors involved in UB branching, actually influences the binding of factors more likely involved in MM induction and differentiation (Shah et al., 2010). While there is ample evidence that UB branching is modulated by growth factors that bind HS, this raises the following question: Are these UB branch modulating growth factors similarly dependent upon a specific HS sulfation pattern? Here we sought to further examine this question and to define the role that HS plays in growth factor-mediated UB branching morphogenesis. Since knockouts of Hs3st1 and Hs3st2 do not have any apparent kidney defects (HajMohammadi et al., 2003) and an increase in 6-O sulfation has been reported in the Hs2st knockout that has defective nephrogenesis (Wilson et al., 2002), we decided to investigate the role of 6-O sulfation in kidney development. We found that endogenous HS is found all along the UB with the potential to localize exogenous growth factors to specific regions of the UB (tip vs. stalk) and that isolated UB branching is more sensitive to the HS 6-O sulfation modification. We also show that a variety of known UB branching morphogens demonstrate a higher affinity to heparin 6-O sulfation suggesting that this modification may be necessary for robust UB branching. These studies outline a general mechanism for spatio-temporal HS regulation of growth factor activity along the branching UB.
MATERIALS AND METHODS
Materials
Heparin and desulfated heparin compounds (2-O-desulfated heparin and 6-Odesulfated heparin) were obtained from Neoparin (Alameda, CA). Tissue culture media were obtained from GIBCO-BRL (Grand Island, NY) and fetal calf serum obtained from Biowhittaker (East Rutherford, NJ). Transwell filters (0.4-μm pore size) were obtained from Costar (Corning, NY). Growth factor-reduced Matrigel was obtained from Becton Dickinson (Franklin Lakes, NJ). Recombinant rat glial-cell-line-derived neurotrophic factor (GDNF), FGF1, FGF2 and recombinant mouse FGFR2IIIb-human Fc chimera were from R&D systems (Minneapolis, MN). FITC-conjugated Dolichos biflorus lectin (DB) was obtained from Vector Laboratories (Burlingame, CA). The primary antibody against E-cadherin [mouse monoclonal, 1:100] was from BD Transduction Laboratories (San Jose, CA); secondary antibodies were from Jackson Immunoresearch Laboratories (West Grove, PA). All other reagents and chemicals, unless otherwise indicated, were from Sigma (St. Louis, MO). Antibodies against pleiotrophin and heregulin were purchased from R&D systems (Minneapolis, MN), antibodies against FGF1 and GDNF were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Culture of isolated embryonic kidneys
Embryonic kidneys were isolated from gestational day 13.5 (E13.5) Sprague-Dawley rat embryos and cultured on top of Transwell filters as previously described (Barasch et al., 1997; Bush et al., 2004; Karihaloo et al., 2001; Meyer et al., 2006; Qiao et al., 2001; Qiao et al., 1999a; Sakurai et al., 2001; Sakurai et al., 2005; Shah et al., 2010; Shah et al., 2009; Zent et al., 2001).
Ligand and carbohydarate engagement (LACE) assay
A modification of the LACE assay (Allen and Rapraeger, 2003) was performed as previously described (Patel et al., 2007). Briefly, to probe the ability of endogenous HS to bind growth factor (Fig. 1), embryonic kidneys isolated at e13.5 and cultured for 6-7 days in normal growth media were treated for 3 hours with 0.005 U/ml heparinase III (Sigma) at 37°C and then washed with PBST. The kidneys were then blocked overnight at 4°C followed by incubation with 50nM recombinant mouse FGFR2IIIb-human Fc chimera with or without 50nM FGF1. The FGFR-FGF-HS high affinity complex was detected using an anti-human Fc (Invitrogen; Carlsbad, CA).
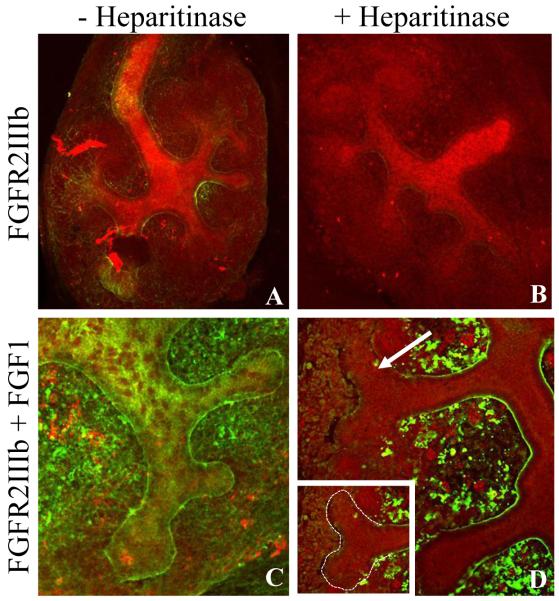
Figure 1.
Localization of endogenous heparan sulfate in the embryonic kidney utilizing a modified in situ ligand and carbohydrate engagement (LACE) assay. Embryonic kidneys isolated at e13.5 and cultured for 6-7 days were probed for the presence of endogenous HS using FGF1-recombinant huFGFR2IIIb binding as a probe. Recombinant huFGFR2IIIb binding occurs at minimal levels in the presence (A) and absence (B; treatment with heparitinase) of endogenous HS. Binding of recombinant huFGFR2IIIb is significantly increased in the presence of FGF1 in the presence of endogenous HS (C) but is specifically diminished at UB tips in the absence of endogenous HS (D; treatment with heparitinase). (FGFR (green) and D. biflorus (red); (A,B 10X; C,D 40X)
Growth of whole embryonic kidneys in presence of variably sulfated heparin
E13.5 isolated kidneys were cultured for 4-6 days in the absence or presence of varying concentrations of heparin, de2OS-heparin or de6OS-heparin as previously described (Shah et al., 2010).
Isolation and culture of the UB in the presence of variably sulfated heparin
UBs were isolated from E13.5 embryonic rat kidneys and cultured as previously described (Bush et al., 2004; Karihaloo et al., 2001; Qiao et al., 2001; Qiao et al., 1999a; Sakurai et al., 2001; Sakurai et al., 2005). UBs were cultured for 6-7 days in the absence or presence of varying concentrations of either heparin or the desulfated heparin compounds as previously described (Shah et al., 2010).
Heparin and desulfated heparin column chromatography and analysis of fractions
6OS-depleted heparin and 2OS-depleted heparin were conjugated to Sepharose beads as per instructions for EHS-Sepharose column (GE Healthcare). The efficiency of heparinoid conjugation was assessed via the carbazole method of Dische (Dische, 1947), with D-glucuronic acid as standard, and found to be similar for both 6OS-depleted and 2OS-depleted heparin. Hi-trap heparin (1ml) column (GE healthcare) was used as unmodified heparin column. An EHS Sepharose column without ligand conjugation was used as a negative control. A metanephric mesenchyme conditioned media, BSN (Barros-Sakurai-Nigam)-CM was fractionated across the columns as previously described (Bush et al., 2004; Sakurai et al., 2001; Sakurai et al., 2005). The buffer of the BSN-CM was changed to 50 mM HEPES pH 7.2 and an equal amount was applied to each heparinoid column. Bound proteins were eluted by increasing NaCl (0-2 M) concentration. 1 ml fractions were collected followed by buffer change to DMEM/F12 by Ultra-Free centrifugal filter with molecular weight cutoff of 5000 dalton (Millipore).
Isolation and culture of the UB in the presence of eluted purified fractions of BSN-CM
The morphogenetic activity of each purified fraction of BSN-CM was assayed using the isolated UB culture. Isolated E13.5 UBs were suspended within an extracellular matrix gel and cultured on top of Transwell filters placed within the individual wells of a 24-well tissue culture dish as previously described (Bush et al., 2004; Karihaloo et al., 2001; Qiao et al., 2001; Qiao et al., 1999a; Sakurai et al., 2001; Sakurai et al., 2005). 400 μl of either whole BSN-CM, purified fractions of BSN-CM, or DMEM/F12 supplemented with fibroblast growth factor 1 (FGF1; 250 ng/ml; R&D Systems), rat recombinant GDNF (125 ng/ml; R&D Systems) and 10% FCS was applied to the bottom of each well and the UB was cultured for 4-6 days.
Immunoblot analysis of purified fractions
15 microliters of each purified fraction of BSN-CM was also subjected to SDS-PAGE followed byWestern blot analysis for the presence of various factors previously found to be important in UB growth and branching (Sakurai et al., 2001; Sakurai et al., 2005). Assessment of binding affinity for purified recombinant growth factors (GDNF and FGF1) was carried out by dissolving growth factors in 50mM HEPES, pH 7.2 buffer to a concentration of 200 ng/ml and 200 μl of the mixture was applied to the heparin and desulfated heparin columns. Elution and fractionation were performed as in the case with BSN conditioned media.
Immunocytochemistry and confocal analysis
Isolated UB grown in extracellular matrix gels or cultured embryonic kidneys were fixed in 4% paraformaldehyde (EM Sciences, Fort Washington, PA) for 30 min at room temperature and then washed with PBS. UBs were dissected from the Transwell insert and excess extracellular matrix gel was removed. Immunocytochemistry was performed as previously described (Meyer et al., 2004). Briefly, the UBs were equilibrated in quenching solution (20mM glycine, 75mM NH4Cl, 0.1% Triton X-100, in PBS) for 30 minutes at room temperature followed by incubation in blocking buffer (0.05% Triton X-100, 0.75% Fish gelatin in PBS) for 1 hour at 4°C before antibody staining. Whole cultured kidneys were incubated in blocking buffer for 1 hour at room temperature after fixation. Staining was then performed. Specimens were examined by scanning laser confocal microscopy (Zeiss LSM-510). Images were processed with Photoshop software (Adobe, San Jose, CA).
Statistics
Embryonic kidneys and isolated UBs were grown as described in the presence of heparin, 2OS-depleted heparin, or 6OS-depleted heparin. Embryonic kidneys and isolated UBs were fixed at day 3 and day 7, respectively, and stained with Dolichos biflorus lectin to delineate UB tips. Each assay was performed in at least triplicate and data are presented as mean values + S.D. The Anova single factor test was applied to data from the experiments. A p value of <0.05 was accepted to indicate statistical significance.
RESULTS
We have previously hypothesized that HS acts as a “switch” to modulate growth factor activity in the kidney and perhaps regulate branching morphogenesis of the epithelial ureteric bud (UB) (Bush et al., 2004; Nigam and Shah, 2009; Shah et al., 2004; Steer et al., 2004). In other words, specifically sulfated HS expressed in distinct areas of the UB potentially control/modulate growth factor activity (either positively or negatively) and thus may act as a regulatory mechanism for specific morphogenetic events.
Heparan sulfate along the branching UB acts to localize growth factor binding
Taking advantage of the requirement for HS in the formation of high affinity fibroblast growth factor (FGF)/FGF receptor complexes (Ornitiz, 2000), the endogenous HS in the developing rodent kidney was evaluated utilizing a modified in situ whole mount ligand and carbohydrate engagement (LACE) assay (Allen and Rapraeger, 2003; Pan et al., 2008; Pan et al., 2006; Patel et al., 2007; Qu et al., 2011). In these experiments the binding of the FGF-FGFR complex with HS was examined using FGF1 (although for the purposes of this experiment any FGF would have sufficed; FGF1 is often utilized to the promiscuous nature of its binding to FGFRs), a human FGFR-FC chimera (FGFR2IIIb-human Fc chimera) and a FITC-conjugated anti-human Fc IgG antibody. The FGF1-FGFR-Fc chimera serves as the HS binding ligand which, when probed with the FITC-conjugated anti-human Fc antibody, allows direct visualization of the distribution of endogenous HS in the embryonic rodent kidney (Fig. 1). As a control for non-specific binding, cultured kidneys were incubated with the FGFR-Fc chimera in the absence of the FGF. The FGFR-Fc chimera displayed some binding to the kidney which was completely eliminated in the presence of heparitinase (an enzyme which cleaves heparan sulfate chains) demonstrating the HS dependence of the observed binding (Fig. 1A-1B). Incubation with both FGF1 and the FGFr-Fc chimera revealed the presence of endogenous HS throughout the embryonic kidney particularly along the UB basement membrane where it appeared to be localized to epithelial cell membranes (Fig 1C). Following treatment with heparitinase, the HS-FGF-FGFR signal was significantly diminished overall with an almost complete loss around the UB tips (Fig 1D). Thus there appears to be a close association of HS and the epithelial UB, which taken together with the fact that HS plays a crucial role in cell signaling and tissue development through its interactions with a wide variety of growth factors and morphogens (Thompson et al., 2010), provides support for the notion that HS plays a role in kidney development perhaps by participating in the localization of growth factor activity to distinct regions of the branching UB.
Growth factor stimulated UB branching in organ culture demonstrates greater sensitivity to HS 6-O sulfation over HS 2-O sulfation
Recently, we showed that the 2-O sulfation of HS 2-O is critical for mesenchymal induction, a key step in early kidney development (Shah et al., 2010). In addition, we found that growth factors that modulated UB branching did not appear as sensitive to the 2-O modification as those modulating mesenchymal induction, raising the likelihood sulfation modifications other than 2-O sulfation might be important in UB branching morphogenesis. Since a number of lines of evidence implicated 6-O sulfation as a likely candidate, including 1) the apparently normal development of mice bearing null mutations in 3-O sulfotransferases (HajMohammadi et al., 2003), 2) the observed increase in 6-O sulfation displayed in 2-O sulfotranferase mutant mice (Wilson et al., 2002) and, 3) many lines of biochemical data which suggest that growth factors that are involved in UB branching are sensitive to 6-O sulfation modification (Kamimura et al., 2001; Kamimura et al., 2006; Sugaya et al., 2008), the prospect that 6-O sulfation plays a role in UB branching morphogenesis was investigated. Since three isoforms of glucosaminyl 6-O sulfotransferases exist (Hs6st 1-3) and, to date, knockouts of these genes have shown no kidney defects (Habuchi and Kimata, 2010), we examined the role of 6-O-desulfated (de6OS) heparin on UB branching morphogenesis in vitro using cultured embryonic kidneys and cultured isolated UB.
Embryonic kidneys were isolated at e13 and were cultured in the absence or presence of varying concentrations of 6OS-depleted heparin added to standard media (Fig. 2). As previously described (Shah et al., 2010), heparin or desulfated heparin compounds added to the growth media will act as a sponge “absorbing” heparin-binding growth factors that may be important to specific morphogenetic processes. For example, heparin, a highly sulfated form of HS which binds a large number of growth factors with high affinity, would be expected to “soak up” multiple growth factors interacting with 2-O sulfated, 6-O sulfated and other moieties in the media thus limiting their access to the developing kidney. Thus as expected, addition of heparin to cultures of the whole embryonic kidneys severely impaired their growth and development (Fig. 2A, 2D). On the other hand, if a specifically desulfated heparin compound (i.e., 6OS-depleted heparin) is added to the media, and growth factors are present in the media which require this particular sulfation modification for interaction with heparin, then these growth factors will not bind to this de-sulfated heparin and thus will still be free in the media to modulate growth and development of the cultured kidney. Therefore, if the growth and development of the cultured kidney is not be as perturbed in the presence of a de-sulfated heparin (compared to “normal” heparin), then this particular sulfation modification of HS would be implicated, albeit indirectly, as potentially important in the binding and/or modification of the activity of growth factor(s) and/or morphogenetic molecules involved in kidney development.
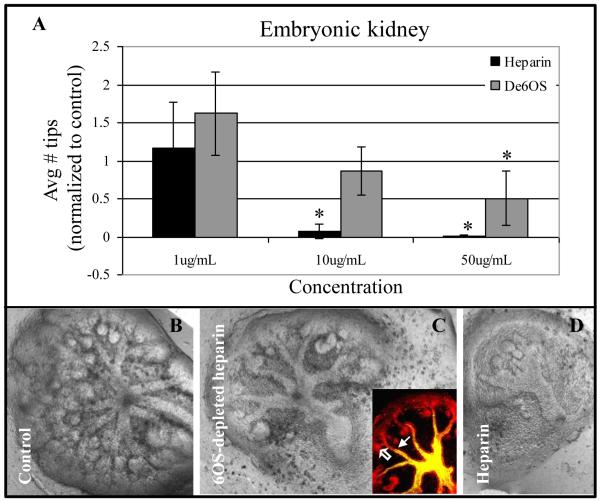
Figure 2.
Effect of 6OS-depleted heparin on embryonic kidney branching morphogenesis. A: Graphical analysis of the average number of UB tips as a percentage of control when cultured in the presence of varying concentrations of heparin or 6OS-depleted heparin. Mean ± SD, n>3, *p<0.05 compared to control. B-D: Phase contrast photomicrographs of whole embryonic kidneys cultured for 4-6 days in the absence (B) or presence of 100 μg/ml of either de6OS-heparin (C) or heparin (D). Even at higher concentrations, compared to heparin-treated kidneys (C), UB architecture is largely preserved despite further reduction in UB tip number in the presence of de6OS (B; inset is a confocal image of the embryonic kidney; open arrow points to UB tip and closed arrow points to UB stalk (D. biflorus lectin (green) and E-cadherin (red)). Photomicrographs taken at 10X magnification.
We and others have previously shown that the addition of heparin to the media of cultured embryonic kidneys inhibits branching morphogenesis at a concentration of 10 ug/mL and higher (Davies et al., 2003; Shah et al., 2010). In our recent study, it was found that addition of 2OS-depleted heparin (heparin in which sulfates have been removed from the 2-O position, but sulfations at other positions such as 3-O and 6-O are still present) did not significantly impair embryonic kidney growth at the same concentration (Shah et al., 2010). Thus growth factors that require HS to be sulfated at the 2-O position for binding to heparin were still free in media and were able to stimulate growth of the cultured kidney (while those that require 3-O or 6-O sulfation would be “soaked up” by the 2OS-depeleted heparin). These results demonstrated the importance of 2OS HS modifications in the binding and/or modulation of growth factors that stimulate kidney development (Shah et al., 2010). In the studies presented here, titration with 6OS-depleted heparin revealed that a concentration of 10 μg/mL of 6OS-depleted heparin (no 6-O sulfation, but other sulfations still present) did not significantly perturb embryonic kidney growth (MM induction and UB branching; Fig 2A). Unexpectedly, even at a de6OS concentration of 100 μg/mL, overall UB branch architecture (the formation of clearly discernible tips and stalks), while perturbed, was largely similar to control (Fig 2B, middle panel), although tip number was reduced. This is in contrast to both heparin, which at a 10-fold lower concentration (10 ug/ml) almost completely inhibited branching morphogenesis and kidney development (Fig. 2A) and 2OS-depleted heparin which at 100 μg/mL resulted in severely malformed kidneys (data not shown; (Shah et al., 2010)). Since heparin and 2OS-depleted heparin both possess 6-O sulfated moieties (and thus deplete the organ culture of growth factors which bind to 6-O sulfated HS, but not those which bind to 2-O sulfated HS), and de6O heparin still possesses 2-O sulfated moieties (and thus would deplete the organ culture of growth factors which bind to 2-O sulfated HS, but not those which bind to 6-O sulfated HS), these results indicate that HS 6-O sulfation is relatively more important than is HS 2-O sulfation in the binding and/or modulating the activity of growth factors specifically involved in UB branching. This is because in the presence of 6OS-depleted heparin those growth factors which require HS 6-O sulfation for binding will not bind the exogenous 6OS-depleted heparin and remain free to stimulate kidney development. In other words, if 6-O sulfation was unimportant, one would expect to see greater perturbation of UB architecture due to the binding of growth factors in the media by de6OS as seen with heparin.
Isolated UB branching also depends largely on 6-O sulfation
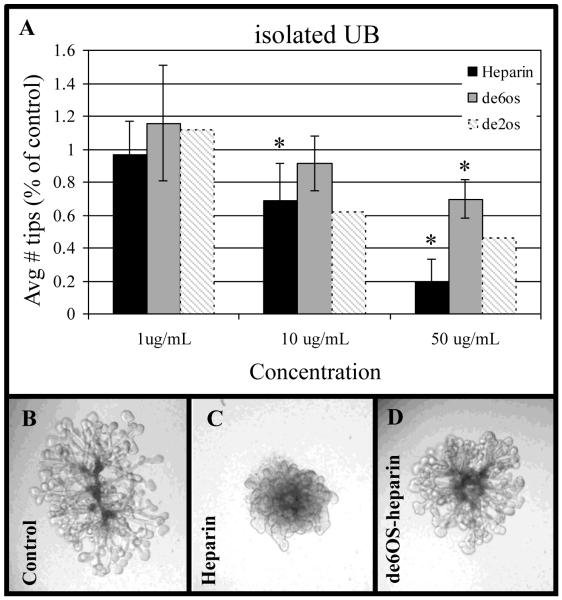
To test the notion that HS 6-O sulfation was important in the binding of growth factors involved in UB branching, we performed similar titration studies on cultured isolated UBs. Similar to the whole organ culture studies above, isolated UBs were cultured in the absence or presence of either heparin or 6OS-depleted heparin. Heparin clearly inhibited isolated UB branching in a dose-dependent fashion (Fig 3A). In fact, in the presence of heparin, the cultured UB lost the distinction between stalks and tips (Fig 3B, far left panel) and became more amorphous (Fig 3B, middle panel) and generally proliferative (vs. the control isolated UB that mainly proliferates at the tips; results not shown). Consistent with our recent study (Shah et al., 2010) and similar to heparin, 2OS-depleted heparin (which is still sulfated at the 6-O position and thus will bind growth factors in the media which require 6-O sulfated HS, but not those which require 2-O sulfated HS) also inhibited UB branching in a dose dependent fashion (Shah et al., 2010) (Fig 3A, shaded bars); however, addition of exogenous 6OS-depleted heparin (which is still sulfated at the 2-O and 3-O positions but not at the 6-O position and thus will not bind growth factors which require 6-O sulfated HS leaving them free in the media) had significantly less inhibitory effect on the growth and branching of the isolated UB at 10 μg/mL, the dose at which both heparin (which is sulfated at the 2-O, 3-O and 6-O positions) and 2OS-depleted heparin (which is sulfated at the 3-O and 6-O positions, but not the 2-O position) significantly inhibited these processes (Fig 3A). In fact, even at 50 μg/mL 6OS-depleted heparin reduced branching by only ~25% compared to a greater than 50% reduction induced by 2OS-depleted heparin and an 80% reduction in the presence of heparin compare to the 1 mg/ml condition (Fig. 3A). This result supports the notion that the pattern of HS sulfation is critical to UB branching morphogenesis and that, overall, 6-O sulfation likely plays a key role in regulating UB branching morphogenesis via growth factor binding.
Figure 3.
Effects of 6OS-depleted heparin on isolated ureteric bud (UB) branching. A: Graphical analysis of the average number of UB tips as a percentage of control when cultured in the presence of varying concentrations of heparin or 6OS-depleted heparin (hatched bars indicate results for isolated UBs cultured with 2OS-depleted heparin; historical data from (Shah et al., 2010) shown for comparison). Mean ± SD, n>3, *p<0.05 compared to control. B-D: Phase-contrast photomicrographs of isolated UBs cultured in the absence (control) or presence of 50 μg/ml heparin (middle panel) or 6OS-depleted heparin (far right panel) demonstrating the loss of distinct UB tips and stalks that occurs in with heparin.
Growth factors that stimulate UB branching require HS 6-O sulfate moieties for high affinity binding
Taken together with our recent study (Shah et al., 2010), these findings seem to indicate that for those growth factors modulating UB branching morphogenesis 6-O sulfated HS are more critical than those interacting with 2-O sulfated HS. To further assess this, MM conditioned media (BSN-CM), media that stimulates isolated UB branching (Qiao et al., 2001; Qiao et al., 1999a; Sakurai et al., 2001; Sakurai et al., 2005), was fractionated over either a heparin, a de2OS or a 6OS-depleted heparin column. The bound factors were eluted using increasing NaCl concentrations and the fractions were assayed for morphogenetic activity using the isolated UB branching assay (Bush et al., 2004; Sakurai et al., 2001; Sakurai et al., 2005). Consistent with previous fractionation studies, the peak morphogenetic activity was found to elute from the heparin column starting at a NaCl concentration of 0.94M (Fig 4g,h) (Bush et al., 2004; Sakurai et al., 2001; Sakurai et al., 2005). However, when BSN-CM was fractionated over a 2OS-depleted heparin column (i.e., 6OS sulfation still present), this peak stimulatory activity eluted from the column at a NaCl concentration of 0.62M, a significant downward shift in mobility (Fig 4k,l). When fractionated over a 6OS-depleted heparin column (i.e., 2OS sulfation still present) the elution fraction was shifted even further downward to 0.27M (Fig 4q). Since lower salt concentrations were needed to elute the bound proteins from the de6OS column (which still possess 2-O sulfated moieties) than from the de2OS or heparin columns (which still possess 6-O sulfated moieties), this indicates that the branch-stimulating factors present in BSN-CM bind with higher affinity to the 6-O sulfate moiety of HS than they do to the 2-O moiety. In support of this notion, significant branching activity was also seen in the flow-through fraction from the de6OS column which was not seen with the other columns (Fig 4o). This suggests that one or more of the branch stimulating factors present in BSN-CM actually have lower affinity for heparin lacking 6-O sulfation modifications leading to decrease or no binding to the column.
Figure 4.
Morphogenetic activity of heparin and de2OS and de6OS column fractions. Phase contrast photomicrographs of isolated ureteric buds (UB) cultured in either whole BSN-CM (a), DMEM/F12 (b) or purified fractions eluted from the columns (c-t). Isolated UBs cultured in whole BSN-CM (a, positive control) are large and have distinct stalks and tips while UBs cultured in DMEM/F12 alone (b, negative control) display little growth or branching. Isolated UBs cultured in purified fractions of BSN-CM run over the heparin column (c-h), 2OS-depleted heparin column (i-n) or 6OS-depleted heparin column (o-t) demonstrate the presence of branch and growth promoting factors. The elution profiles demonstrate a progressive downward shift of binding affinity of stimulatory activity suggesting that HS 6-O sulfation is necessary for high affinity binding. Of note, robust branch stimulatory activity is detected in the flow-through fraction of the de6OS column (o), but not in the heparin (c) or de2OS (i) columns, indicating a lack of binding to the de6OS column despite the presence of other sulfation modifications.
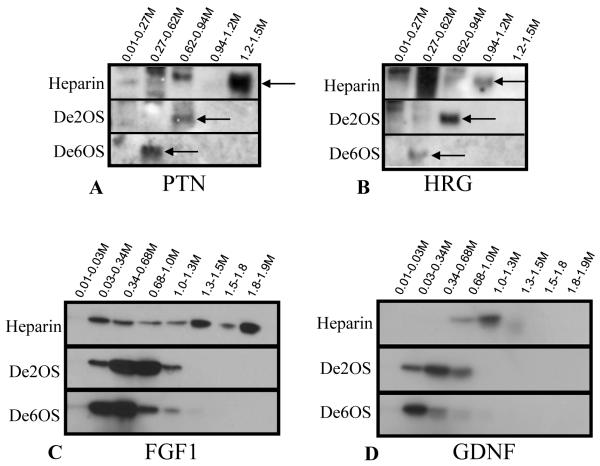
We then sought to identify the factor that might rely upon the 6-O sulfation modification by probing for the known branching morphogens present in BSN-CM in the various fractions. Pleiotropin (PTN) and heregulin (HRG) have both been isolated from BSN-CM as potent stimulators of isolated UB branching (Sakurai et al., 2001; Sakurai et al., 2005). PTN and HRG were found in the fractions that correlated to peak branching activity in all three columns (Fig 5A,B). Similarly, we found that purified GDNF and FGF1, necessary branching factors that are added to BSN-CM in the isolated UB assay, also elute at similar salt concentrations (Fig 5C,D). These same growth factors were also found in the flow-through fraction collected from the de6O heparin column (data not shown), indicating decreased binding affinity of the known branch-stimulating factors in the absence of 6-O sulfation. It is also possible that, yet to be identified, factors which strictly require 6-O sulfation are also present in the flow-through as demonstrated by the significant activity in the flow through fraction. Nevertheless, taken together, there clearly is a greater affinity for 6-O sulfation over 2-O sulfation for many of the branching-stimulating factors,.
Figure 5.
Known branching morphogens have stronger binding affinity to 6-O sulfate moieties over 2-O sulfate moieties. Western blots of the various fractions from the columns demonstrate that the stimulatory activity corresponds to presence of pleiotrophin (A) and heregulin (B), known potent branching morphogens (Sakurai et al., 2001; Sakurai et al., 2005). When purified growth factors, FGF1 (C) and GDNF (D) are run over the columns, similar to PTN and HRG, there is a progressive downward shift in binding affinity from the heparin column to the 2OS-depleted heparin column to the de6OS column, demonstrating that 6-O sulfate residues are required for high affinity binding.
DISCUSSION
It is believed that spatio-temporally controlled microenvironments of HSPGs modulate a variety of growth factor-mediated morphogenetic events during UB branching morphogenesis from branch point formation to stalk elongation (Bush et al., 2004; Sampogna and Nigam, 2004; Shah et al., 2010; Shah et al., 2004; Steer et al., 2004). These events likely occur through regulated growth factor-growth factor receptor binding, the specificity of which is dictated by the HS moieties. The roles for HSPGs in the control of morphogen distribution and signaling during Drosophila development have been well established, and a number of genetic and in vitro analyses have demonstrated the critical importance of HS moieties for HSPG function during developmental patterning (Han et al., 2004; Nakato and Kimata, 2002; Takei et al., 2004). The involvement of HS in the creation of morphogen gradients is not without precedent as HSPGs have been shown to be involved in both Dpp and Wingless morphogen gradient formation in Drosophila (Akiyama et al., 2008; Kleinschmit et al., 2010). This is supported by biochemical data demonstrating that HS plays a critical role in the assembly of the FGF-FGFR complex such that certain FGF-FGFR interactions are dependent upon the level of HS O-sulfation (Jastrebova et al., 2006), while others appear to be dependent upon the specific sulfation pattern (Guimond et al., 1993; Ostrovsky et al., 2002). However, evidence that heparan sulfates are similarly involved in mammalian organ patterning has been difficult to obtain. In an effort to better understand the general role of HS in mammalian organogenesis and specifically in kidney branching morphogenesis, we employed a variety of experimental techniques to elucidate how UB-derived HS might function in the binding of growth factors important to UB branching.
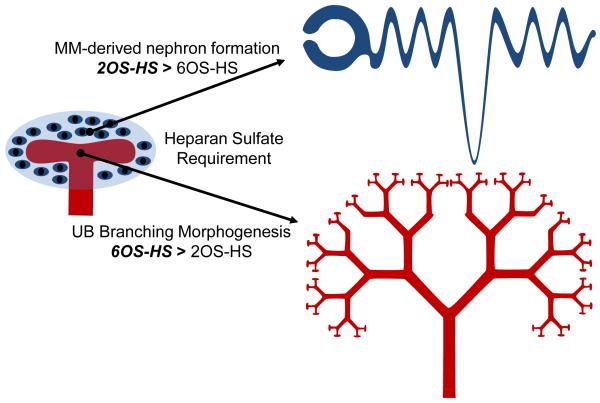
In this study, it was found that 1) while a modified in situ LACE assay demonstrated the presence of endogenous HS along the length of the UB, depletion of endogenous HS with heparitinase suggested a role for HS in limiting growth factors to specific regions of the UB (i.e., tip vs. stalk), 2) culture of whole embryonic kidneys in the presence of variably sulfated heparins (i.e., heparin, de2OS-heparin and de6OS-heparin) suggested a specific role for 6-O sulfated HS in the growth and development of the embryonic kidney, particularly in UB branching morphogenesis, 3) treatment of the isolated UB with the same variably sulfated heparinoids demonstrated a dependence of branching morphogenesis on the presence of 6-O sulfation, providing further evidence of a role for this HS modification in branching, and 4) fractionation of the BSN-conditioned medium (a demonstrated UB branch promoting growth media) indicated that known UB branching morphogens (i.e., pleiotrophin, heregulin, FGF1 and GDNF) have a higher affinity for 6-O sulfated heparin. Taken together with our recent finding in the Hs2st knockout mouse (Shah et al., 2010), variable sulfation of HS clearly plays a role in the regulation/modulation of kidney development. In particular, the development and differentiation of the MM into the tubular nephron appears to be more dependent upon 2OS modified HS, while growth and branching of the UB requires 6OS modified HS (Fig. 6).
Figure 6.
Diagram illustrating the relative importance of 2O-sulfated HS versus 6O-sulfated HS in the major morphogenetic processes critical to kidney development. Together with our earlier study (Shah et al., 2010), data suggest that 2O-sulfated HS have greater importance in MM-derived nephron formation, while 6O-sulfated HS appear to have greater importance in UB branching morphogenesis.
We have previously shown that the defect underlying the renal agenesis phenotype in mice with an Hs2st null genotype is a failure of MM induction (Shah et al., 2010). Given the dependency of MM induction on a specific HS sulfation pattern, and the existing biochemical data, it seems likely that growth factors which stimulate UB branching may also demonstrate a strong dependency upon a specific HS sulfation pattern. Our finding that factors that stimulate UB branching are particularly dependent upon HS 6-O sulfation for high affinity binding is consistent with this hypothesis. However, unlike mesenchymal induction, there appears to be more flexibility in the HS sulfation requirements for UB branching factors such that these factors, which include GDNF, PTN and HRG, still show significant binding affinity to 2OS-depleted heparin. This flexibility in HS requirement may be a mechanism through which morphogen gradients form: the strongest concentration of morphogen is in those areas in which the HS-growth factor binding is most avid.
The finding that many UB branching factors avidly bind 6-O sulfated HS may also be a mechanism for resiliency in the branching program. We have previously hypothesized that UB branching itself is buffered by many redundant growth factor-growth factor receptor combinations as evidenced by the lack of intermediate UB branching phenotypes (Nigam and Shah, 2009; Sampogna and Nigam, 2004). In other words, the predominant renal phenotypes are fairly dichotomous-- either the kidney forms in totality or kidney agenesis occurs; among clinical case reports and knockout studies, to our knowledge, there are no reports of kidneys forming with under 100 nephrons. This lack of intermediate phenotype may be due to the ability of HS to retain a number of redundant branch stimulatory factors in the vicinity of the UB.
It seems plausible that the HS-growth factor interaction also determines the morphogenetic outcome of certain growth factors such that a growth factor can induce a variety of different morphogenetic events. Studies in submandibular gland morphogenesis have shown that depending on the HS sulfation pattern, FGF10 was able to induce either end bud expansion or duct elongation (Patel et al., 2008). In addition, a recent study demonstrated a clear requirement for 6-O sulfated HS in the Fgf10-Fgfr2b signaling critical for lacrimal gland branching morphogenesis and development (Qu et al., 2011), while double mutants of Hs2st/Hs6st displayed a complete lack of lacrimal gland development and a loss of FGF-mediated Erk signaling (Qu et al., 2011). Here, the finding that heparin can change the phenotype of an isolated UB stimulated by FGF1 from a branching structure with clear tips and stalks to an amorphous UB (Fig. 3) with a phenotype that resembles an isolated UB stimulated by FGF7 (Qiao et al., 2001) (perhaps through changes in FGFR2IIIb activity (LaRochelle et al., 1999; Reich-Slotky et al., 1994)), suggests similar functional redundancy in UB branching morphogenesis. Thus, one can envision that depending on the sulfation status of endogenous HS, a variety of factors could result in the formation of stalks vs. tips, adding functional redundancy to the branching program above and beyond growth factor redundancy.
MM induction, which appears to be dependent upon very specific factors, has a strong dependence upon 2-O sulfation (Shah et al., 2010), and it may be that in sites of reciprocal induction, critical areas for nephron formation, a more stringent requirement of HS sulfation exists to ensure proper spatiotemporal activity of certain growth factors or other molecules. It is conceivable then, that the fine-tuning of growth factor binding along various points of the branching UB may occur through the type of mechanism described here. For a variety of stimulatory factors that induce growth and branching, there is flexibility in the HS requirement in order to diffusely retain such factors near the branching UB but there may be a stringent requirement for specific sulfation modifications in areas that are critical for growth factor localization/activation (i.e. tip).
In total, these results outline a very dynamic role for variably sulfated HS in modulating UB branching morphogenesis: it acts to localize growth factor-growth factor receptor activity to certain regions; varying binding affinities of the same growth factor to 2-O vs 6-O sulfated HS may assist in gradient formation; 6-O sulfated HS avidly binds a variety of stimulatory growth factors that can buffer growth factor mutations; depending on the HS sulfation pattern, a single growth factor can have a variety of morphogenetic outcomes therefore adding another layer of redundancy to the branching program. Although the complexity of patterning an organ such as the kidney is seemingly vast, these studies demonstrate how HSPGs are central to the modulation of growth factor activity along the branching UB. While the exact growth factor-HS interactions are yet to be elucidated, and there is likely to be significant overlapping and redundant interactions, it is becoming clear that HSPG-mediated control of morphogen activity provides the “fine tuning” necessary to instruct the complex interactions which result in the development of a specific organ.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health: DK57286 (S.K.N), DK65831 (S.K.N.), DK79784 (S.K.N), DK069324 (M.M.S), and GM33063 (J.D.E.); and the American Heart Association: Scientist Development Award (H.S.). H. Sakurai is also supported by an American Heart Association Scientist Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akiyama T, Kamimura K, Firkus C, Takeo S, Shimmi O, Nakato H. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev Biol. 2008;313:408–19. doi: 10.1016/j.ydbio.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Awqati Q, Goldberg MR. Architectural patterns in branching morphogenesis in the kidney. Kidney Int. 1998;54:1832–42. doi: 10.1046/j.1523-1755.1998.00196.x. [DOI] [PubMed] [Google Scholar]
- Allen BL, Rapraeger AC. Spatial and temporal expression of heparan sulfate in mouse development regulates FGF and FGF receptor assembly. J Cell Biol. 2003;163:637–48. doi: 10.1083/jcb.200307053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D. Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol. 1997;273:F757–67. doi: 10.1152/ajprenal.1997.273.5.F757. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–47. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Milford EL. Nephron underdosing: a programmed cause of chronic renal allograft failure. Am J Kidney Dis. 1993;21:66–72. doi: 10.1016/0272-6386(93)70097-i. [DOI] [PubMed] [Google Scholar]
- Bullock SL, Fletcher JM, Beddington RS, Wilson VA. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 1998;12:1894–906. doi: 10.1101/gad.12.12.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, Schwesinger C, Qiao J, Nigam SK. TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266:285–98. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Davies JA, Yates EA, Turnbull JE. Structural determinants of heparan sulphate modulation of GDNF signalling. Growth Factors. 2003;21:109–19. doi: 10.1080/08977190310001621005. [DOI] [PubMed] [Google Scholar]
- Deakin JA, Blaum BS, Gallagher JT, Uhrin D, Lyon M. The binding properties of minimal oligosaccharides reveal a common heparan sulfate/dermatan sulfate-binding site in hepatocyte growth factor/scatter factor that can accommodate a wide variety of sulfation patterns. J Biol Chem. 2009;284:6311–21. doi: 10.1074/jbc.M807671200. [DOI] [PubMed] [Google Scholar]
- Dische Z. A new specific color reaction of hexuronic acids. J Biol Chem. 1947;167:189–98. [PubMed] [Google Scholar]
- Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001;108:169–73. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond S, Maccarana M, Olwin BB, Lindahl U, Rapraeger AC. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J Biol Chem. 1993;268:23906–14. [PubMed] [Google Scholar]
- Habuchi H, Kimata K. Mice deficient in heparan sulfate 6-O-sulfotransferase-1. Prog Mol Biol Transl Sci. 2010;93:79–111. doi: 10.1016/S1877-1173(10)93005-6. [DOI] [PubMed] [Google Scholar]
- HajMohammadi S, Enjyoji K, Princivalle M, Christi P, Lech M, Beeler D, Rayburn H, Schwartz JJ, Barzegar S, de Agostini AI, Post MJ, Rosenberg RD, Shworak NW. Normal levels of anticoagulant heparan sulfate are not essential for normal hemostasis. J Clin Invest. 2003;111:989–99. doi: 10.1172/JCI15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004;131:1563–75. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Jastrebova N, Vanwildemeersch M, Rapraeger AC, Gimenez-Gallego G, Lindahl U, Spillmann D. Heparan sulfate-related oligosaccharides in ternary complex formation with fibroblast growth factors 1 and 2 and their receptors. J Biol Chem. 2006;281:26884–92. doi: 10.1074/jbc.M600806200. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Fujise M, Villa F, Izumi S, Habuchi H, Kimata K, Nakato H. Drosophila heparan sulfate 6-O-sulfotransferase (dHS6ST) gene. Structure, expression, and function in the formation of the tracheal system. J Biol Chem. 2001;276:17014–21. doi: 10.1074/jbc.M011354200. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Koyama T, Habuchi H, Ueda R, Masu M, Kimata K, Nakato H. Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. J Cell Biol. 2006;174:773–8. doi: 10.1083/jcb.200603129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karihaloo A, Karumanchi SA, Barasch J, Jha V, Nickel CH, Yang J, Grisaru S, Bush KT, Nigam S, Rosenblum ND, Sukhatme VP, Cantley LG. Endostatin regulates branching morphogenesis of renal epithelial cells and ureteric bud. Proc Natl Acad Sci U S A. 2001;98:12509–14. doi: 10.1073/pnas.221205198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmit A, Koyama T, Dejima K, Hayashi Y, Kamimura K, Nakato H. Drosophila heparan sulfate 6-O endosulfatase regulates Wingless morphogen gradient formation. Dev Biol. 2010;345:204–14. doi: 10.1016/j.ydbio.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J, Spillmann D, Li JP, Lindahl U. Interactions between heparan sulfate and proteins: the concept of specificity. J Cell Biol. 2006;174:323–7. doi: 10.1083/jcb.200604035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRochelle WJ, Sakaguchi K, Atabey N, Cheon HG, Takagi Y, Kinaia T, Day RM, Miki T, Burgess WH, Bottaro DP. Heparan sulfate proteoglycan modulates keratinocyte growth factor signaling through interaction with both ligand and receptor. Biochemistry. 1999;38:1765–71. doi: 10.1021/bi982092z. [DOI] [PubMed] [Google Scholar]
- Merry CL, Bullock SL, Swan DC, Backen AC, Lyon M, Beddington RS, Wilson VA, Gallagher JT. The molecular phenotype of heparan sulfate in the Hs2st−/− mutant mouse. J Biol Chem. 2001;276:35429–34. doi: 10.1074/jbc.M100379200. [DOI] [PubMed] [Google Scholar]
- Meyer TN, Schwesinger C, Bush KT, Stuart RO, Rose DW, Shah MM, Vaughn DA, Steer DL, Nigam SK. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev Biol. 2004;275:44–67. doi: 10.1016/j.ydbio.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Meyer TN, Schwesinger C, Sampogna RV, Vaughn DA, Stuart RO, Steer DL, Bush KT, Nigam SK. Rho kinase acts at separate steps in ureteric bud and metanephric mesenchyme morphogenesis during kidney development. Differentiation. 2006;74:638–47. doi: 10.1111/j.1432-0436.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- Nakato H, Kimata K. Heparan sulfate fine structure and specificity of proteoglycan functions. Biochim Biophys Acta. 2002;1573:312–8. doi: 10.1016/s0304-4165(02)00398-7. [DOI] [PubMed] [Google Scholar]
- Nigam SK. Determinants of branching tubulogenesis. Curr Opin Nephrol Hypertens. 1995;4:209–14. doi: 10.1097/00041552-199505000-00001. [DOI] [PubMed] [Google Scholar]
- Nigam SK, Shah MM. How does the ureteric bud branch? J Am Soc Nephrol. 2009;20:1465–9. doi: 10.1681/ASN.2008020132. [DOI] [PubMed] [Google Scholar]
- Ostrovsky O, Berman B, Gallagher J, Mulloy B, Fernig DG, Delehedde M, Ron D. Differential effects of heparin saccharides on the formation of specific fibroblast growth factor (FGF) and FGF receptor complexes. J Biol Chem. 2002;277:2444–53. doi: 10.1074/jbc.M108540200. [DOI] [PubMed] [Google Scholar]
- Pan Y, Carbe C, Powers A, Zhang EE, Esko JD, Grobe K, Feng GS, Zhang X. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development. 2008;135:301–10. doi: 10.1242/dev.014829. [DOI] [PubMed] [Google Scholar]
- Pan Y, Woodbury A, Esko JD, Grobe K, Zhang X. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development. 2006;133:4933–44. doi: 10.1242/dev.02679. [DOI] [PubMed] [Google Scholar]
- Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, Eftekhari S, Whitelock JM, Elkin M, Vlodavsky I, Hoffman MP. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development. 2007;134:4177–86. doi: 10.1242/dev.011171. [DOI] [PubMed] [Google Scholar]
- Patel VN, Likar KM, Zisman-Rozen S, Cowherd SN, Lassiter KS, Sher I, Yates EA, Turnbull JE, Ron D, Hoffman MP. Specific heparan sulfate structures modulate FGF10-mediated submandibular gland epithelial morphogenesis and differentiation. J Biol Chem. 2008;283:9308–17. doi: 10.1074/jbc.M709995200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol. 2006;291:325–39. doi: 10.1016/j.ydbio.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Qiao J, Bush KT, Steer DL, Stuart RO, Sakurai H, Wachsman W, Nigam SK. Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech Dev. 2001;109:123–35. doi: 10.1016/s0925-4773(01)00592-5. [DOI] [PubMed] [Google Scholar]
- Qiao J, Sakurai H, Nigam SK. Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney. Proc Natl Acad Sci U S A. 1999a;96:7330–5. doi: 10.1073/pnas.96.13.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development. 1999b;126:547–54. doi: 10.1242/dev.126.3.547. [DOI] [PubMed] [Google Scholar]
- Qu X, Carbe C, Tao C, Powers A, Lawrence R, van Kuppevelt TH, Cardoso WV, Grobe K, Esko JD, Zhang X. Lacrimal Gland Development and Fgf10-Fgfr2b Signaling Are Controlled by 2-O- and 6-O-sulfated Heparan Sulfate. J Biol Chem. 2011;286:14435–44. doi: 10.1074/jbc.M111.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich-Slotky R, Bonneh-Barkay D, Shaoul E, Bluma B, Svahn CM, Ron D. Differential effect of cell-associated heparan sulfates on the binding of keratinocyte growth factor (KGF) and acidic fibroblast growth factor to the KGF receptor. J Biol Chem. 1994;269:32279–85. [PubMed] [Google Scholar]
- Sakurai H, Bush KT, Nigam SK. Identification of pleiotrophin as a mesenchymal factor involved in ureteric bud branching morphogenesis. Development. 2001;128:3283–93. doi: 10.1242/dev.128.17.3283. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Bush KT, Nigam SK. Heregulin induces glial cell line-derived neurotrophic growth factor-independent, non-branching growth and differentiation of ureteric bud epithelia. J Biol Chem. 2005;280:42181–7. doi: 10.1074/jbc.M507962200. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Tsukamoto T, Kjelsberg CA, Cantley LG, Nigam SK. EGF receptor ligands are a large fraction of in vitro branching morphogens secreted by embryonic kidney. Am J Physiol. 1997;273:F463–72. doi: 10.1152/ajprenal.1997.273.3.F463. [DOI] [PubMed] [Google Scholar]
- Sampogna RV, Nigam SK. Implications of gene networks for understanding resilience and vulnerability in the kidney branching program. Physiology (Bethesda) 2004;19:339–47. doi: 10.1152/physiol.00025.2004. [DOI] [PubMed] [Google Scholar]
- Santos OF, Moura LA, Rosen EM, Nigam SK. Modulation of HGF-induced tubulogenesis and branching by multiple phosphorylation mechanisms. Dev Biol. 1993;159:535–48. doi: 10.1006/dbio.1993.1262. [DOI] [PubMed] [Google Scholar]
- Shah MM, Sakurai H, Sweeney DE, Gallegos TF, Bush KT, Esko JD, Nigam SK. Hs2st mediated kidney mesenchyme induction regulates early ureteric bud branching. Dev Biol. 2010;339:354–65. doi: 10.1016/j.ydbio.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Sampogna RV, Sakurai H, Bush KT, Nigam SK. Branching morphogenesis and kidney disease. Development. 2004;131:1449–62. doi: 10.1242/dev.01089. [DOI] [PubMed] [Google Scholar]
- Shah MM, Tee JB, Meyer T, Meyer-Schwesinger C, Choi Y, Sweeney DE, Gallegos TF, Johkura K, Rosines E, Kouznetsova V, Rose DW, Bush KT, Sakurai H, Nigam SK. The instructive role of metanephric mesenchyme in ureteric bud patterning, sculpting, and maturation and its potential ability to buffer ureteric bud branching defects. Am J Physiol Renal Physiol. 2009;297:F1330–41. doi: 10.1152/ajprenal.00125.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer DL, Shah MM, Bush KT, Stuart RO, Sampogna RV, Meyer TN, Schwesinger C, Bai X, Esko JD, Nigam SK. Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney. Dev Biol. 2004;272:310–27. doi: 10.1016/j.ydbio.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Sugaya N, Habuchi H, Nagai N, Ashikari-Hada S, Kimata K. 6-O-sulfation of heparan sulfate differentially regulates various fibroblast growth factor-dependent signalings in culture. J Biol Chem. 2008;283:10366–76. doi: 10.1074/jbc.M705948200. [DOI] [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Jesudason EC, Turnbull JE, Fernig DG. Heparan sulfate in lung morphogenesis: The elephant in the room. Birth Defects Res C Embryo Today. 2010;90:32–44. doi: 10.1002/bdrc.20169. [DOI] [PubMed] [Google Scholar]
- Wilson VA, Gallagher JT, Merry CL. Heparan sulfate 2-O-sulfotransferase (Hs2st) and mouse development. Glycoconj J. 2002;19:347–54. doi: 10.1023/A:1025325222530. [DOI] [PubMed] [Google Scholar]
- Zent R, Bush KT, Pohl ML, Quaranta V, Koshikawa N, Wang Z, Kreidberg JA, Sakurai H, Stuart RO, Nigam SK. Involvement of laminin binding integrins and laminin-5 in branching morphogenesis of the ureteric bud during kidney development. Dev Biol. 2001;238:289–302. doi: 10.1006/dbio.2001.0391. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276:403–15. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]