Abstract
The bipolar spiral ganglion neurons predominantly delaminate from the growing cochlear duct and migrate to Rosenthal’s canal. They project radial fibers to innervate the organ of Corti (type I neurons to inner hair cells, type II neurons to outer hair cells) and also project tonotopically to the cochlear nuclei. The early differentiation of these neurons requires transcription factors to regulate migration, pathfinding and survival. Neurog1 null mice lack formation of neurons. Neurod1 null mice show massive cell death combined with aberrant central and peripheral projections. Prox1 protein is necessary for proper type II neuron process navigation, which is also affected by the neurotrophins Bdnf and Ntf3. Neurotrophin null mutants show specific patterns of neuronal loss along the cochlea but remaining neurons compensate by expanding their target area. All neurotrophin mutants have reduced radial fiber growth proportional to the degree of loss of neurotrophin alleles. This suggests a simple dose response effect of neurotrophin concentration. Keeping overall concentration constant, but misexpressing one neurotrophin under regulatory control of another one results in exuberant fiber growth not only of vestibular fibers to the cochlea but also of spiral ganglion neurons to outer hair cells suggesting different effectiveness of neurotrophins for spiral ganglion neurite growth. Finally, we report here for the first time that losing all neurons in double null mutants affects extension of the cochlear duct and leads to formation of extra rows of outer hair cells in the apex, possibly by disrupting the interaction of the spiral ganglion with the elongating cochlea.
Keywords: ear, spiral ganglion, development, neuronal survival
Introduction
The function of the mammalian auditory system depends on two neurosensory tissues adjacent to each other: A) The organ of Corti with two types of sensory hair cells and associated supporting cells that allow sound of a specific frequency to generate localized hair cell activity; B) The two types of sensory neurons of the spiral ganglion that transmit this localized electrical activity from hair cells to the second order sensory neurons in the cochlear nucleus for sound processing. Hearing requires that sound mediated activity from either functional hair cells or a cochlear implant is faithfully transmitted to the cochlear nucleus in a tonotopic fashion. In this paper we review first the molecular basis of sensory neuron formation, including migration of spiral ganglion neurons and initial fiber growth to the organ of Corti, followed by a review combined with new data, on the role of neurotrophins in pathfinding, neuronal survival and maintenance of connections. Excellent reviews were recently published and the reader is referred to those for a more complete coverage of aspects only touched upon here (Fekete and Campero, 2007; Rubel and Fritzsch, 2002; Webber and Raz, 2006)
A. Topological origin and migration of spiral ganglion neurons
Embryonic studies in chicken (D’Amico-Martel and Noden, 1983) and the expression of a unique combination of neurotrophin receptors (as compared to neurons derived from neural crest, the other previously suggested source) clarified that all inner ear sensory neurons derive from the otocyst (Fritzsch et al., 2002; Rubel and Fritzsch, 2002). In the vestibular system, neurons delaminate from or adjacent to sensory epithelia (Farinas et al., 2001; Fritzsch, 2003; Kim et al., 2001; Raft et al., 2007). In the developing cochlea, sensory neurons do not delaminate uniformly throughout the growing cochlear duct (Fig. 1). Rather neurons seem to delaminate most abundantly from the area of the future ductus reuniens and the middle turn and later from the apex of the cochlear duct (Farinas et al., 2001). Whether the neurons that delaminate from the future ductus reuniens develop into spiral ganglion neurons (SGNs) or inferior vestibular ganglion neurons is unknown. However, recent data in mice in which the saccule is confluent with the basal turn of the cochlea suggest that some basal turn neurons project to the saccule (Kopecky et al., 2011). SGNs exit the cell cycle between embryonic day (E) 10.5 and E12.5 (Matei et al., 2005; Ruben, 1967) in a base to apex progression. During this time the cochlear duct grows from a barely discernable anlage to a half turn. As the cochlear duct of mice extends to the full two or more turns within about three days, it appears that the spiral ganglion aggregate and elongate along with the growing cochlear duct. The elongation of the cochlear duct is driven by convergent extension movements, including those of the organ of Corti cells (Kelly and Chen, 2009). The elongation process of the spiral ganglion may be passive and be driven by the convergent extension movement of the cochlear duct. Alternatively, the spiral ganglion elongation may consist in part of active movements of SGNs and may be an extension of the initial migration away from the growing cochlear duct which, in turn, enhances the cochlear duct extension. Irrespective to these unresolved issues of SGN movements, at the end of the elongation of the cochlear duct, the spiral ganglion is visible as a discrete entity in Rosenthal’s canal, connected by radial fibers to the developing organ of Corti (Fig. 1, 2). However, the spiral ganglion shows an enlargement at the base and the apex, is shorter than the cochlea and has many spiral ganglion neurons that project not in all cases through the nearest radial bundle to the cochlea.
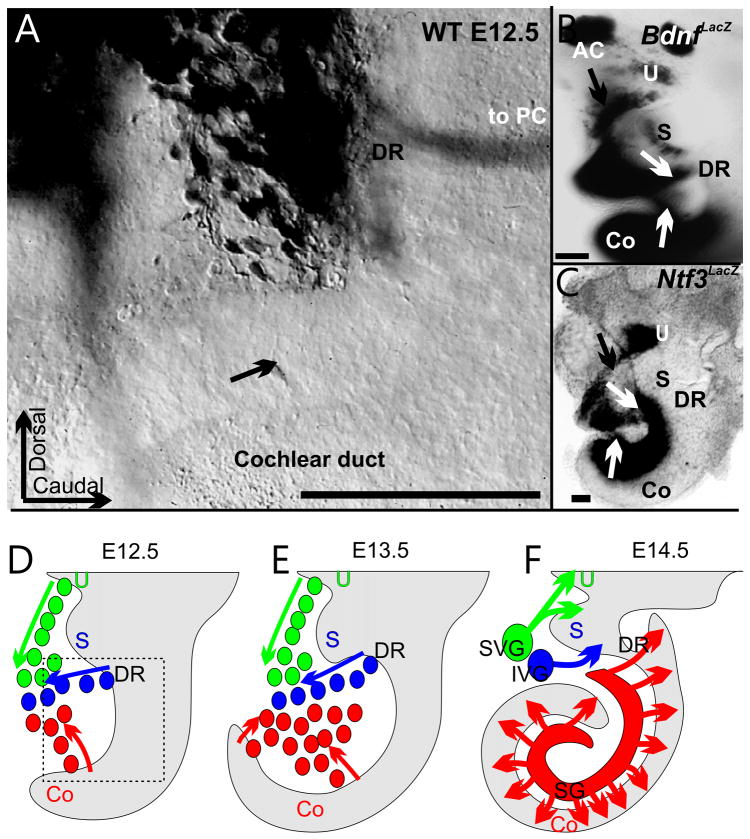
Fig. 1. Spiral ganglion neurons seem to delaminate from specific positions along the cochlea.
The distribution of delaminating neurons can be revealed by retrograde dye tracing (A) and through labeling with neurotrophin markers (B,C). Note that the original spiral ganglion neuron accumulation is adjacent to the inferior vestibular ganglion neurons that delaminate out of the future region of the ductus reuniens (DR). Bdnf-LacZ (B) and Ntf3-LacZ positive ganglion cell precursors (C) delaminate form the middle turn around E12.5 (B,C,D) from areas that express Bdnf and Ntf3 in the otocyst wall (B, C). At E13.5, the apex shows many delaminating cells (E, arrows indicate delaminating cells). As the neurons aggregate the cochlear duct elongates and the spiral ganglion extends seemingly along with the cochlear duct elongation (F). What type of neuron (s) delaminate from the ductus reuniens is unclear and it is unclear how the most basal spiral ganglion neurons become molecularly distinct from vestibular ganglion neurons. Green color indicates cells of the superior vestibular ganglion, blue color of the inferior vestibular ganglion and red color of the spiral ganglion. Arrows indicate single spiral ganglion neuron in the cochlear duct (A) or delaminating neurons (B,C). AC, anterior canal crista; Co, cochlear duct; DR, ductus reuniens; IVG, inferior vestibular ganglion; PC, posterior canal crista; S, saccule; SVG, superior vestibular ganglion; U, utricle. Modified after (Fritzsch, 2003; Matei et al., 2005). Bar indicates 100 um.
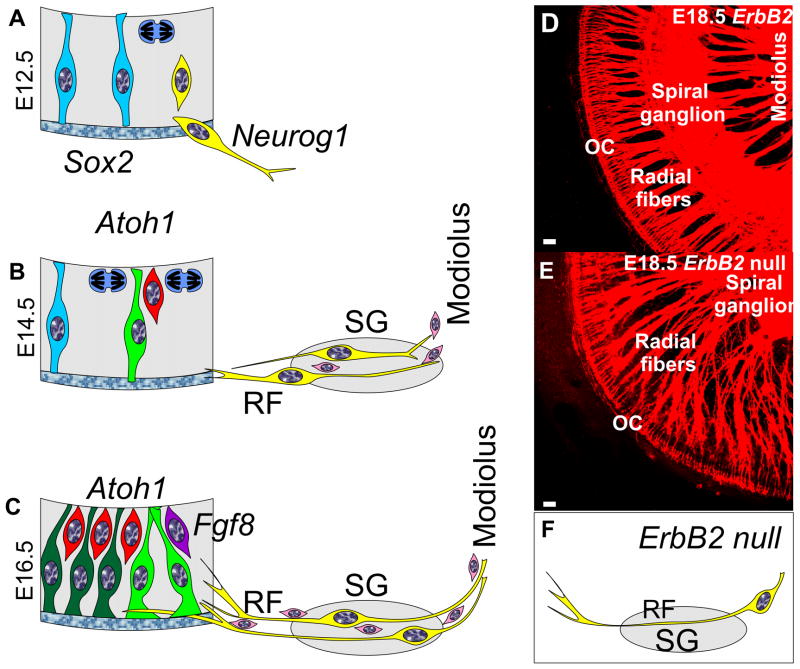
Fig. 2. Spiral ganglion cell development as compared to cochlear duct is shown.
Spiral ganglion neurons become postmitotic in the cochlear duct between E11.5–12.5 (A), migrate to the spiral ganglion location by E14.5 (B) and send fibers as radial fibers (RF) to the organ of Corti to innervate outer and inner hair cells by E16.5 (C). Neurosensory precursor cells (blue) are organized like radial glial cells in the brain and undergo mitosis (blue cell with mitotic spindle) near the future endolymphatic space. Specification of spiral ganglion neuroblasts (yellow cell) and possibly regulation of delamination of neuroblats (A) is mediated by Neurog1 protein. As spiral ganglion neurons aggregate in the spiral ganglion (SG) they send processes to the organ of Corti which form radial fibers (RF) mostly before hair cells (red) become postmitotic (B,C) and upregulate Atoh1 for differentiation. As neurites extend into the cochlear duct (C) they segregate to inner hair cells (lilac, with Fgf8 expression) and outer hair cells (red) across the tunnel of Corti between the two Pillar cells (green). Pillar cells, Deiters cells and spiral ganglion neurons express Prox1 a gene implicated in type II fiber segregation. Schwann cells (pink) migrate into the ear through the modiolus and may be responsible for a stop signal of migrating spiral ganglion neurons in Rosenthal’s canal to form the spiral ganglion. Schwann cells may also help guide axons in the modiolus to the brain and along radial fibers to the organ of Corti. ErbB2 null mice have no Schwann cells (F) and display an overshooting migration of spiral ganglion neurons past Rosenthal’s canal (E,F) into the modiolus. As a consequence of this overshooting migration radial fibers become longer and extent from the modiolus to the organ of Corti (compared D,E). It is possible that Schwann cells provide a stop signal for spiral ganglion migration. SG, spiral ganglion; OC, organ of Corti; RF, radial fibers. Modified after (Fritzsch et al., 2010b; Morris et al., 2006). Bars in D,E indicate 10 um.
Consistent with this idea that migration and possibly convergent extension movements of the spiral ganglion neurons add to this elongation process are data on mutants that also disrupt cochlear duct growth. In these mutants the spiral ganglion forms a single lump of sensory neurons (Bouchard et al., 2010; Pauley et al., 2006) or extends exactly the same length as the shortened cochlear duct (Nichols et al., 2008). However, aberrant migration of neurons beyond the typical location of the spiral ganglion into the modiolus as in ErbB2 null mice (Morris et al., 2006) results in the formation of a near normal cochlear duct, suggesting a more complex influence of spiral ganglion migration on the coiling of the cochlear duct. Interestingly, conditional deletion of Rac1 leads to shortening of the cochlear duct with normal rows of hair cells (Grimsley-Myers et al., 2009). Unfortunately no data were presented on the sensory neurons in this mutant which could shed light on the involvement of sensory neurons in cochlear duct elongation as Rac1 has been shown to be necessary for collective migration of cells in other developing systems (Migeotte et al., 2010). Other mutants with a a shortened cochlear duct like Foxg1 null mice have a shorter spiral ganglion and severely disorganized projections (Pauley et al., 2006).
The data on delaminating neurons that suggest them coming only from specific areas and not uniformly along the entire cochlear duct implies that the topological relationship of sensory neurons and the organ of Corti is mostly secondary to the elongation of the spiral ganglion and cochlear duct after spiral ganglion neurons have delaminated. It appears that the upper middle turn is unique in having some sensory neurons delaminate after differentiation, likely sprouting a dendrite toward the organ of Corti area as early as E12.5 (Fritzsch, 2003). However, in most spiral ganglion neurons, dendritic growth and formation of radial fiber tracts occurs prior to differentiation of the organ of Corti. The area of the future organ of Corti already expresses neurotrophic factors prior to hair cell differentiation (Fig. 1B,C) and those factors attract growing nerve fibers before the onset of hair cell differentiation (Farinas et al., 2001; Tessarollo et al., 2004) and even in the absence of hair cell differentiation (Pan et al., 2010a). This inability to reach the remaining cells of the organ of Corti might relate to the approximation of Claudius cells to the habenula perforate and thus of factors expressed in those cells that could act repulsive on nerve fibers, such as Bmp4 (Pan et al., 2010a). More detailed work on the early growth of dendrites is needed in E12.5 – 13.5 mice, before the onset of hair cell differentiation. Genetic markers and tracing techniques are now available to experimentally label small sets of neurons to reveal decision-making processes during this crucial period of development in more detail (Fritzsch et al., 2010b; Koundakjian et al., 2007).
Migration of neurons from the cochlea to form and possibly elongate the spiral ganglion likely depends on protein interactions such as those already well characterized in other developing systems, for example the developing cerebral cortex (Heng et al., 2008), the migrating facial motoneurons of the hindbrain (Muller et al., 2003; Qu et al., 2010) or delaminating epidermal cancer cells (Kern et al., 2010). Cellular interactions via specific surface molecules such as integrins (Davies, 2007) and cadherins (Schreiner and Weiner, 2010) can mediate signals between glial cells and neurons to induce rearrangements of the cytoskeleton for elongation and migration of the cell bodies. Some of the cytoskeletal rearrangement seems to relate to the regulation of actin polymerization regulating G-protein RhoA-GTP by the atypical Rho-related GTP-binding proteins Rnd2 and Rnd3. These proteins are important regulators of migration of neuroblasts, tumor cells and cortical neurons (Pacary et al., 2011), also in vitro (Lie et al., 2010). B-Raf, a RAF kinase that can regulate the MEK-ERK signaling pathway, but also cellular migration of tumor cells via Rock2 interaction (Galabova-Kovacs and Baccarini, 2010; Grabocka and Bar-Sagi, 2009) is expressed in the developing ear and is necessary for sensory neuron process growth (Magarinos et al., 2010; Markus et al., 2002; Zhong et al., 2007). Given that the basic Helix-Loop-Helix (bHLH) transcription factors regulate the Rnd gene expression in other developing neurons, it is possible that the bHLH gene Neurog1, which is essential for inner ear sensory neuron formation (Ma et al., 1998), may also regulate Rnd expression and thereby neuronal migration and influence other Rho, Rac and Raf mediated migration and fiber elongation processes. Clearly, neurons never delaminate in Neurog1 null mice (Matei et al., 2005), which could indicate an involvement of Neurog1 protein in delamination and/or migration. Similar to the glial effect on guidance of neurons in the CNS (Pacary et al., 2011), the Schwann cells in the ear may play a role in guiding the migrating spiral ganglion neurons and provide a stop signal (Fig. 2) to form a spiral ganglion. ErbB2 null mice lack Schwann cells and have spiral ganglion neurons that migrate beyond their normal position and develop massively disorganized radial fibers (Morris et al., 2006) consistent with PNS guidance defects in other mouse models lacking Schwann cells (Finzsch et al., 2010). Based on these apparent similarities it is conceivable that comparable cellular and molecular interactions are responsible for migration of spiral ganglion neurons and cortical neurons. Recent evidence suggests a correlation of spiral ganglion length with cochlear coiling in mammalian fossils (Luo et al., 2011). Exactly how the migrating spiral ganglion neurons can affect cochlear elongation mechanistically or if the two processes evolved independently and are not mechanistically connected requires further analysis.
B. The Molecular Basis of Neurogenesis
Over 70 years of research in fly development has demonstrated that basic Helix-Loop-Helix (bHLH) proteins are used for proneural cell fate decision making whereas the Delta/Notch system activates neurogenic bHLH factors (Hes, Hey) that suppress neuronal differentiation in neighboring cells (Garcia-Bellido and de Celis, 2009). Mechanisms that specify neuronal formation involve cellular convergence of several diffusible factors of the Wnt, Fgf, Bmp and Shh families (Gaspard and Vanderhaeghen, 2010) to induce neuronal progenitors that express proneural genes and allows these precursor populations to switch to glia cell formation using the COUP-TFI/II transcription factor (Naka et al., 2008). Ear development follows that general developmental scheme and requires multiple diffusible factors such as Fgfs, Wnts, Bmps, Shh and retinoic acid to regulate local expression of transcription factors (Fritzsch et al., 2006a; Ohyama et al., 2010; Romand et al., 2008) such as bHLH proteins to define and differentiate all neurosensory cells (Fritzsch et al., 2010b). As with the CNS, the transcription factor COUP-TFI/II seems to switch neurosensory precursors to supporting cell formation via enhancing the Delta/Notch signaling (Tang et al., 2006). These factors, in combination with other known (see below) and unknown factors, have the ability to activate directly or through intermediates the expression of bHLH genes. Among those factors that define areas of bHLH gene expression are Sox2 (Puligilla et al., 2010), Gata3 (Karis et al., 2001), Eya1 (Zou et al., 2004; Zou et al., 2008) and Tbx1 (Raft et al., 2004). The co-expression of multiple bHLH genes in fly sensory development (Garcia-Bellido and de Celis, 2009) and retina development (Ohsawa and Kageyama, 2008) in combination with other local transcription factors as well as diffusible factors define subtypes of neurons in the CNS and PNS (Peljto and Wichterle, 2010). A similar co-expression of several bHLH genes exists in sensory neurons of the ear (Fritzsch et al., 2010a). Analogous to fly and retina development, this may indicate a function of these transcription factors to define, in a combinatorial code, sensory neuron subtypes such as Type I and Type II spiral ganglion neurons. Below we will highlight some effects of bHLH and other transcription factors as revealed studying mutant mice.
Neurogenin 1 (Neurog1)
Cell fate determination and differentiation of spiral sensory neurons requires the proneural bHLH gene Neurog1 (Ma et al., 1998). Ganglion neuron precursors express Neurog1 earlier than the genes of the Delta/Notch signaling pathway to initiate sensory neuron differentiation (Brooker et al., 2006; Ma et al., 1998; Pan et al., 2010b). In the absence of Neurog1, no afferent, efferent, or autonomic nerve fibers to the ear are ever detectable, leading to an ear devoid of any innervation. Absence of innervation is nevertheless compatible with a near normal hair cell development in a shortened cochlea (Ma et al., 2000). Neurog1 is thus necessary for all inner ear sensory neuron development and may play a role in the delamination of neuroblasts that is completely abolished in Neurog1 null mice (Matei et al., 2005). Obviously, this single transcription factor cannot define alone the three vestibular and two spiral ganglion neuronal types. Absence of Neurog1 protein also affects hair cell development through a variable reduction in all sensory epithelia and through formation of ‘ectopic’ hair cells in ‘non-sensory’ areas (Ma et al., 2000). This seems to relate in part to the cross-regulation of the Atoh1 gene expression, at least in the utricle and saccule (Pan et al., 2010a; Raft et al., 2007). In the cochlear duct, cells of the greater epithelial ridge (GER) in the middle turn can differentiate into hair cells in the absence of Neurog1 [see Fig 1 in (Matei et al., 2005)]. How the loss of Neurog1 protein affects cochlear growth and leads to the generation of extra hair cells in the GER, the ductus reuniens, and the cruciate eminence of the canal cristae remains unclear, but fate switching from neurons to hair cells is one possibility (Ma et al., 2000; Matei et al., 2005). Exactly how neurons delaminate under the guidance of Neurog1 protein to modulate their cytoskeleton in analogy to cortical neuron migration (Heng et al., 2008; Pacary et al., 2011) and whether most of the delaminating neurons are indeed proliferative neuronal precursors as suggested (Fritzsch et al., 2002) remains to be shown.
Neuronal differentiation 1 (Neurod1)
The expression of Neurod1 in inner ear sensory neurons is dependent on Neurog1 (Ma et al., 1998). Following the expression of Neurog1, Neurod1 and other genes related to the Delta/Notch signaling pathway are up-regulated in and around the delaminating ganglion neurons (Adam et al., 1998; Ma et al., 1998) and Neurog1 expression is suppressed (Jahan et al., 2010b). Neurod1 null mutants display a near complete loss of spiral ganglion neurons, probably due to defective neurotrophin receptor expression and a concomitantly increased cell death (Kim et al., 2001; Liu et al., 2000). In contrast to Neurog1, some neurons (mostly vestibular ganglion neurons) survive in Neurod1 null mice (Jahan et al., 2010a; Kim et al., 2001). The remaining spiral and vestibular ganglion neurons appear to be retained by the expression of at least one (Kruger et al., 2006) and possibly more bHLH genes (Fritzsch et al., 2010a). Some of the surviving ganglion cells apparently differentiate into hair cells in these Neurod1 null mice, apparently due to lack of Atoh1 suppression (Jahan et al., 2010b).
The emerging complexity of bHLH factor interaction in the developing ear is indeed nowhere more obvious than in the conditional knockout of Neurod1 that leads to ectopic hair cell formation in the sensory ganglia, a shortened cochlea and alterations in the rows of disorganized hair cells in the apical cochlea (Jahan et al., 2010b). In addition to these effects, attributed to cross-inhibition of Atoh1 and Neurog1 gene expression by Neurod1 protein, it is needed for proper central projection of sensory neurons (Jahan et al., 2010a).
Nhlh1, Nhlh2 and Bhlhb5
Other bHLH genes that are expressed in differentiating neurons are Nhlh1 and Nhlh2 [formerly Nscl-1 and Nscl-2 (Ma et al., 1998)]. Nhlh1 protein seems to cooperate with Neurod1 protein to maintain and differentiate vestibular sensory neurons and rescue many vestibular and some spiral ganglion neurons in Neurod1 null mice (Jahan et al., 2010a). Consistent with this interpretation, a double null mutant for both Neurod1 and Nhlh1 shows near complete loss of all sensory neurons (Kruger et al., 2006), suggesting some redundancy and perhaps involvement of Nhlh1 protein in neuronal subtype development. How these four bHLH transcription factors expressed in the developing sensory neurons interact with the fifth uncharacterized bHLH gene, Bhlhb5 (Brunelli et al., 2003; Ross et al., 2010), remains unclear.
Beyond the multiple bHLH genes now identified in the developing sensory neurons (Fritzsch et al., 2010a), several additional transcription factors have been found in developing sensory neurons. For example, loss-of-function of Tbx1 increases the number of neurons whereas gain-of-function of Tbx1 shows a more profound loss of neurons (Raft et al., 2004), suggesting that Tbx1 protein is a negative regulator of neurosensory development in the ear. The zinc-finger protein Gata3 is expressed in delaminating spiral sensory neurons (Lawoko-Kerali et al., 2002) and may play a function in specifying spiral ganglion neurons in analogy to the function of the Gata factor pannier in flies (Garcia-Bellido and de Celis, 2009; Karis et al., 2001). Gata3 protein may also play a later role in pathfinding in central projection (Fritzsch et al., 2006b) in collaboration with Neurod1 (Jahan et al., 2010a). Conditional deletion only in the developing neurons of the available floxed Gata3 gene is needed to assess this speculation independently because those more specific effects might be masked by the massive reduction in ear development in a systemic Gata3 null mouse (Karis et al., 2001). Similar to hair cell development, where Pou4f3 (formerly Brn3c) is expressed and necessary for hair cell survival, Pou4f1 (formerly Brn3a) is expressed in the developing sensory neurons. Knockout mice of this gene show some pathfinding errors and reduced innervation consistent with the regulation of neurotrophin receptors (Huang et al., 2001).
Several other genes are expressed in developing sensory neurons and have been implicated in various aspects of differentiation. However, those alleged functions have not been completely verified in deletion models and are thus tentative. For example, Tlx3 plays a role in the CNS glutamatergic specification and is expressed very early in ganglion development (Qian et al., 2001). In tissue culture, Tlx3 protein specifies glutamatergic differentiation (Kondo et al., 2008) and may do so also in vivo.
These examples show that the progress in neuronal development during the last 10 years has been remarkable. However, it is equally clear that a systematic analysis of all transcription factors expressed in the developing sensory neurons using deep sequencing, validation of the obtained data, and characterization of the function of validated genes in targeted deletions is needed before we can begin to synthesize the possibly far more complex interplay of these factors (Roy et al., 2010) in spiral neuron development.
C. Neurite pathfinding
As the SGNs migrate away from the cochlear duct and the future ductus reuniens and differentiate (Fig. 2), they extend axons toward the brain to innervate the cochlear nuclei and dendrites toward the ear to innervate the hair cells in the cochlea (Ginzberg and Morest, 1983; Rubel and Fritzsch, 2002). The dendrites have to be sorted to innervate their target: they mix longitudinally from SGNs at different longitudinal positions but segregate radially to innervate either inner hair cells (approximately 90–95% Type I spiral ganglion neurons) or outer hair cells (approximately 5–10% of Type II neurons). The longitudinal segregation from base to apex corresponds to the tonotopic projection that connects a given longitudinal position of the organ of Corti to a specific central projection to the cochlear nuclei (Leake et al., 2008; Rubel and Fritzsch, 2002).
Past literature has generated the idea that the target epithelium releases chemo-attractants to guide the growth of SGNs to reach hair cells (Bianchi et al., 2005). Clearly, ectopic hair cells forming in the GER (Zheng and Gao, 2000) or within the sensory ganglia (Jahan et al., 2010a) can redirect the growth of SGNs toward the topologically “wrong” targets. However, in Pou4f3 and Atoh1 mutants whose hair cells fail to differentiate or degenerate, or in Fgf10 mutants that do not have a posterior canal crista, the initial growth of nerve fibers is relatively normal (Pan et al., 2010a; Pauley et al., 2003; Xiang et al., 2003). These results suggest that hair cells are not necessary for the initial innervation guidance (Fritzsch et al., 2005b). Supporting this idea, in the Atoh1 null cochlea that lacks hair cells, there is directed growth of the radial fibers to the habenula perforata that does not require hair cells or supporting cells (Fritzsch et al., 2005c; Pan et al., 2010a). However, once fibers have reached the habenula perforata they appear to require diffusible factors released from the differentiated organ of Corti to branch around the GER and inner hair cell and to grow to outer hair cells (Fig. 2).
Beyond these attractive interactions, other possible guidance mechanisms are related to repulsive signals. Semaphorin3/Npn1, Eph/Ephrins, and Slit/Robo are typical chemorepellent genes expressed in the inner ear (Fekete and Campero, 2007; Webber and Raz, 2006). A mutation in the Sema3 receptor Neuropilin1 (Npn1), which disrupts the binding between Sema3 and Npn1 proteins, causes some axons to pass their target sensory epithelia and grow dorsally to the skin (Gu et al., 2003). EphA4 is a gene expressed by fibroblasts along the trajectory of SGN dendrite extension. Genes encoding for its transmembrane ligands, ephrinB2 and ephrinB3, are expressed in SGNs and the modiolus, respectively (van Heumen et al., 2000). EphA4 protein has a repulsive effect on postnatal 3–5 day old SGNs in vitro (Brors et al., 2003) and may play a role in radial fiber fasciculation. Taken together, it is possible that Eph/Ephrin signaling can define a path along which SGN dendrites navigate to the target (Fekete and Campero, 2007). In chicken, Robo and its ligand Slit have been shown by in situ hybridization to be expressed in migrating neuroblasts, the SGNs, and later in the otic epithelium (Battisti and Fekete, 2008). This expression might suggest a role of Slit/Robo proteins in the early stage of neuron migration and axon outgrowth (Battisti and Fekete, 2008). Recently it was shown that another factor, Slitrk6, is apparently interacting with neurotrophins to affect growth and maintenance of the pattern of innervation, in particular the formation of radial fiber bundles (Katayama et al., 2009), but the molecular details how Slitrk6 can achieve fiber fasciculation and what role Schwann cells play in that process or what interaction xists between the EphA4 positive fibroblasts along the radial fibers is not yet worked out.
Morphogens such as Wnts and Shhs form a dorsal-ventral gradient in the otic vesicle, and the gradient is necessary for the specification and morphogenesis of the dorsal vestibule and ventral cochlea (Riccomagno et al., 2005) and may also play a role in specification of the cochlear basal-to-apical topology. Wnts and Shh play an important role in axon guidance in the spinal cord (Parra and Zou, 2010). The dorsal-ventral gradient of Wnt and Shh, and the presence of Fgfs and Bmps in sensory primordia, suggest their potential roles as guidance cues for the growth of SGN neurites (Fekete and Campero, 2007). How these factors influence the formation of guidance molecules for radial fiber growth of type I and type II SGNs remains unclear. However, in vitro data suggest that Bmp4 may exert a repulsive force and thus possibly help in the sorting of type I from type II fibers, with the latter possibly being less refractory (Whitlon et al., 2011; Whitlon et al., 2007).
Radial segregation of type I and type II fibers
Adult inner hair cells receive radial fibers of type I SGN and outer hair cells receive type II SGN afferents that spiral along outer hair cells for half a turn or more. During development, when type I and type II neurons are committed to develop into the inner and outer hair cell specific innervation is unclear. However, as early as E16.5, type II fibers can be identified as growth cones directed to outer hair cells (OHCs) that turn toward the base (Bruce et al., 1997; Koundakjian et al., 2007). Previous work had proposed that both types of SGN neurons innervate both types of hair cells transiently in early development but then undergo refinement leading to the final innervation pattern (Rontal and Echteler, 2003). It has been speculated that this sorting mechanism is driven by expression of the protein Peripherin in type II SGNs in early postnatal stage (Barclay et al., 2010; Huang et al., 2007; Lallemend et al., 2007). However, type II fiber growth is normal in Peripherin knockout mice, suggesting Peripherin protein is not required for the innervation pattern in vivo. Nevertheless, absence of Peripherin protein seems to affect elongation of type II fibers in vitro (Barclay et al., 2010). Furthermore, with the technique to selectively label subpopulations of SGNs, it was shown that no fibers innervating both inner and outer hair cells could be found even at E16.5 (Koundakjian et al., 2007) before Peripherin protein has been detected in spiral ganglion neurons. When exactly in development Peripherin message first appears requires investigation using appropriate in situ hybridization to correlate onset of expression with neurite outgrowth.
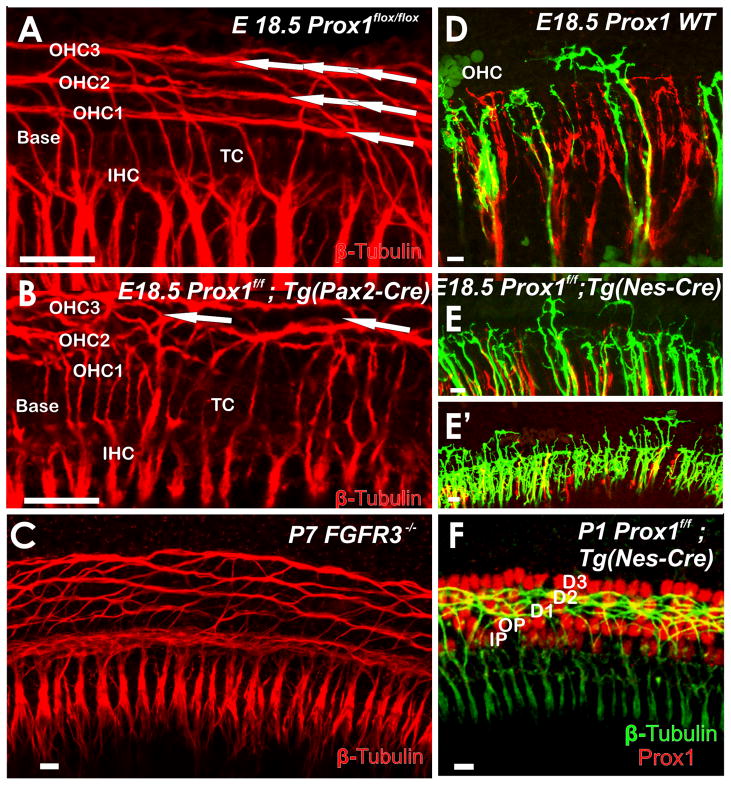
Consistent with this interpretation of a much earlier and genetic base of decision making process for fiber type sorting, recent data show that coordinated growth of type II fibers toward the base requires Prox1 protein to be present in the spiral ganglion neurons and possibly also in the supporting cells along which the type II fibers project (Fritzsch et al., 2010b). In conditional Prox1 null mice type II fibers grow out to the third row of Deiter’s cells (Fig. 3) and are randomly oriented to the base or the apex. Alterations in the growth of type II fibers are also obvious in Fgfr3 mutants that have modification in pillar cell development (Puligilla et al., 2007). Interaction of growing type II afferents with supporting cells is therefore important for directed type II fiber growth among Deiter’s cells. It is possible that identical molecules, such as protocadherins (Schreiner and Weiner, 2010), could be driven by the simultaneous expression of Prox1 protein in type II neurons and supporting cells and that those adhesion molecules are needed for this navigation process.
Fig. 3. Coordinated growth of type II fibers requires proper guidance by supporting cells and the presence of Prox1 protein.
Eliminating either Prox1 (A,B) or Fgfr3 (C) results in disruption of the regular arrangement of type II afferents. The initial fiber growth shows disorientation in the mutants in contrast to the stereotyped directional growth toward the base (to the left in all images) in wild type mice (D). In Prox1 mutants, afferents turn randomly towards the apex or base, or may branch in both directions (E, E′). Later stages of mutants in which Prox1 was eliminated only in neurons with Nestin-Cre show profound outgrowth of type II fibers to the third row of Deiter’s cells (F), bypassing other Prox1 positive supporting cells. Arrows in A show the three parallel rows of fibers extending toward the base, but only one or two disorganized bundles in Prox1 mutants (B). D1–3; Deiter’s cells 1–3; IHC, inner hair cell; IP, inner pillar cell; OHC, outer hair cell; OP, outer pillar cell; TC, tunnel of Corti. Modified after (Fritzsch et al., 2010b). Bar indicates 10 um.
D. The neurotrophins, their expression and their role in survival and guidance of spiral ganglion neurons
The mammalian inner ear expresses two neurotrophins, brain derived neurotrophic factor (Bdnf) and neurotrophin 3 (Ntf3, formerly NT3), and the matching high affinity neurotrophin receptors, Ntrk2 and Ntrk3 [formerly TrkB and TrkC (Farinas et al., 2001; Pirvola et al., 1992)]. Below we first present an overview on the molecular mechanisms of neurotrophin mediated cell death and fiber guidance, arguably one of the best understood processes in the development and maintenance of the innervation pattern in the developing mammalian ear (Fritzsch et al., 2004). We will present data as they appear in the literature supplemented by a more detailed analysis indicating that the topographical innervation is directly affected by the neurotrophin type whereas radial fiber growth is primarily dependent on neurotrophin concentration. For easier reading we have combined all set of data into a single narrative.
D. 1. Longitudinal projection development requires neurotrophins
Two neurotrophins, Bdnf and Ntf3, play a role in the longitudinal topographic innervation process. During development they form an apical-to-basal Bdnf-Ntf3 gradient of expression in the organ of Corti (Pirvola et al., 1992). Bdnf is first weakly expressed at embryonic day 12.5 at the apex (Fig. 1B) and then progresses over time to the basal turn. Ntf3 is first extensively expressed in the base and middle turn (Fig. 1C) and progresses toward the apex in older animals (Farinas et al., 2001). In neonates this gradient of neurotrophin expression seems to change into a base-to apex gradient for Bdnf and a apex-to-base gradient for Ntf3, apparently underlying neonatal fiber reorganization (Schimmang et al., 2003) and spiral neuron differentiation (Zhou et al., 2005). At the cellular level, Bdnf is expressed in the hair cells and supporting cells in the apex, but later it is restricted to hair cells in the base. Ntf3 expression in the cochlea is restricted to supporting cells but later it expands into the inner hair cells (Farinas et al., 2001; Pirvola et al., 1992).
Data on null mutants suggest that Bdnf is more important for apex innervation whereas Ntf3 is more important for base innervation (Farinas et al., 2001). At birth, mice lacking Bdnf (Bdnf−/−) have reduced fiber density in the apex, whereas it remains near normal in middle and basal regions (Bianchi et al., 1996).
In contrast to the fairly normal basal cochlea innervation in the absence of Bdnf, the absence of Ntf3 (Ntf3−/−) leads to the absence of innervation at the base and greatly reduced fiber density in the middle turn (Fig. 4D). The middle turn fibers expand along the organ of Corti to innervate the inner hair cells in the basal turn (Coppola et al., 2001; Farinas et al., 2001; Fritzsch et al., 1997). Absence of Ntf3 leads to over 80% loss of sensory neurons, mostly in the base, whereas loss of Bdnf leads only to about 7% spiral neuron loss, apparently preferentially in the apex. Reduction of fibers seems to reflect the loss of spiral ganglion neurons and this loss is therefore a clear indication of the topographically restricted action of neurotrophins that closely match the basal and apical non-overlapping early expression (Fig. 1).
Fig 4. Absence of Neurotrophins has longitudinal and radial effects on the growth of cochlear afferents in newborn mutant mice.
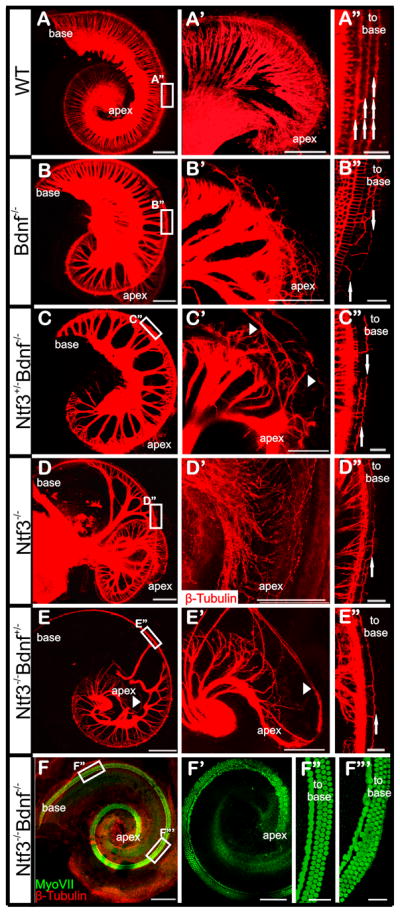
(A–F) Shows overview of afferents to the whole cochlea (A–F) and a higher magnification of the apex (A′–F′). Loss of neurotrophins results in radial fiber density decreases. Compared with wild type (A–A′), in Bdnf −/− cochlea, the fiber density is wild type-like, with slightly reduced density in middle and apex, and disorganized fibers in the apex (B–B′). The fiber density is further reduced in Ntf3+/− Bdnf−/−, and some fibers are overshooting the apex (arrowhead, C′). In Ntf3−/− null mice, the basal turn has no radial fibers (D). However, middle turn fibers extend along the organ of Corti towards the basal turn. In the apex, the afferents are disorganized (D–D′). In Ntf3−/− Bdnf+/−, the basal turn lacks radial fibers while the middle turn has reduced density (E). Some fibers grow to the lateral wall in the apex (arrowhead in E–E′). This demonstrates that haploinsufficiency of Bdnf exagegerates the Ntf3 null phenotype by expanding spiral neuron loss toward the apex (E′). In Ntf3 and Bdnf double null mice, no afferent or efferent fibers are detected in the cochlea (F). Hair cells form a shortened organ of Corti and are disorganized in the upper middle turn and the apex (F′–F″′). There are three rows of OHCs at the base (F″) but multiple rows of disorganized OHCs are present in the apex and middle turn. (A″–E″) shows that the outgrowth of fibers to the OHCs is impaired somewhat proportional to the reduction of neurotrophins. (A″) In wild type, the fibers are organized into three rows to innervate three rows of OHCs (arrows). (B″ and D″) The fiber outgrowth to the third row of OHCs is greatly impaired comparing with the outgrowth to the first and second rows of OHCs in the absence of one neurotrophin factor. The outgrowth to the first and second rows of OHCs is further impaired in Ntf3+/− Bdnf −/− (A″) and Ntf3+/−/Bdnf −/− (E″) where loss of one neurotrophin factor is combined with haploinsufficiency of the other neurotrophin factor. Note the directional growth towards the base is disorganized to a variable degree in all mutants (arrows pointing in different directions in the middle turns of A″–E″). The images are from dye filling in the brainstem unless indicated otherwise. Scale bar is 200 μm in A–F, 100 μm in A′–F′ and 25 μm in A″–F″ and F″′.
Taken together, these data suggest a topographic effect of neurotrophins on the outgrowth of type I fibers to the IHCs. Surprisingly, the absence of three alleles of neurotrophins (either two Bdnf and one Ntf3 or two Ntf3 and one Bdnf allele) causes overshooting and disorganized fiber growth to the lateral wall in the apical tip, suggesting a more complex regional effect (arrowheads in Fig. 4C′, E′). It has been reported that Prox1 null mice (Fritzsch et al., 2010b) have more growth of type II fibers mostly to the third row of outer hair cells (Fig. 3), paralleling somewhat the effects found here in composite neurotrophin null mice. It would therefore be important to find out how the absence of Prox1 protein combined with absence of Bdnf or Ntf3 protein affects the innervation of the third row of outer hair cells. This could help to elucidate the relative importance of diffusible factors compared to guidance along supporting cells for type II fiber growth. Other factors such as fibroblast growth factors may interact with this process (Hossain et al., 2008). The complete loss of all neurons (Fig. 4F) in mice lacking either both neurotrophins [Bdnf and Ntf3 (Ernfors et al., 1995)] or both neurotrophins receptors [Ntrk2 and Ntrk3 (Silos-Santiago et al., 1997)] supports that they are the dominant player for this process in vivo during embryogenesis, relegating other suggested neurotrophic factors to bystanders for this prenatal developmental process.
D.2. Radial projection development requires neurotrophins
In addition to their role in longitudinal fiber growth, the two neurotrophins are also necessary for the radial outgrowth of type II fibers to OHCs. The radial outgrowth of type II fibers to the OHCs was proposed to be driven by Bdnf since Bdnf mutant mice have disrupted type II fiber growth while the type I fiber to IHCs are maintained.
We investigated this possibility in various mutants. In Bdnf null, the type II SGNs outgrowth to the outer hair cells is disrupted throughout the cochlea: fewer fibers grow to the OHCs, and instead of turning toward the base as in the wild type mice, they turn either to the base or the apex, or bifurcate to both directions (Fig. 4B″). However Ntf3 null mice also show loss of innervation of outer hair cells (Fig. 4D′), implying that other factors such as concentration of neurotrophins might be important. Furthermore, deletion of one allele of Ntf3 in Bdnf null mice, or haploinsufficiency of Bdnf combined with Ntf3 null, leads to further reduction of type II outgrowth to OHCs (Fig. 4C″, E″).
Replacing Bdnf with Ntf3 was claimed to rescue the outgrowth of type II fibers (Agerman et al., 2003). Work on adult mice lacking Bdnf indicates that the later embryonic pattern of reduced innervation in the apex is changing, resulting in a reduced innervation in the base instead (Schimmang et al., 2003), complicating the interpretation of the effects of Bdnf on fiber growth to outer hair cells in neonates. While there is a trend toward a more profound effect of fiber growth to outer hair cells in Bdnf null mice compared to overall neuronal loss, Ntf3 null mice clearly have even fewer fibers growing to outer hair cells, suggesting that the outgrowth is not simply under the Bdnf guidance
D.3. Swapping neurotrophins distinguishes topological from signal specific survival effects
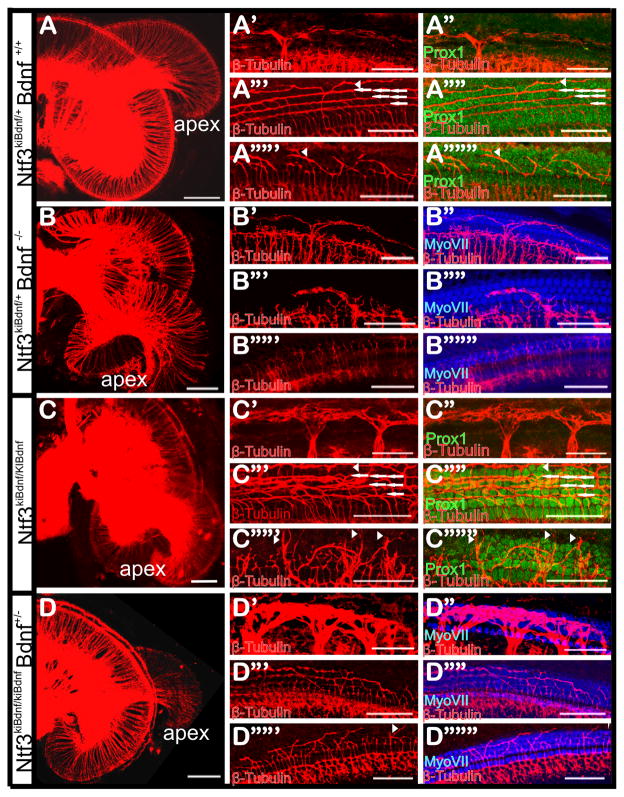
Whether this guidance effect is due to a specific neurotrophin or its expression time and place was tested by replacing Ntf3 with Bdnf (Ntf3kiBdnf) or Bdnf with Ntf3 (BdnfkiNtf3). Mice with these genetic modifications were both tested with respect to the effects on ear innervation (Agerman et al., 2003; Coppola et al., 2001; Tessarollo et al., 1994). For example, in the Ntf3kiBdnf mice, the apical-basal gradient of Bdnf-Ntf3 is eliminated and Bdnf is expressed in the supporting cells at the base in early embryos instead of late in embryonic development. In these mutants, the expression of Bdnf protein rescues basal turn spiral ganglion neurons and they show no reduction in radial fiber density as in Ntf3 null mutants (Fig. 4). In addition to the rescue of basal turn neurons and radial fibers to the organ of Corti, fibers from the vestibular ganglion are routed to the basal turn of the cochlea (Tessarollo et al., 2004). The rerouted vestibular fibers do not enter the organ of Corti but instead stay underneath the basilar membrane. Focal stacks of confocal images below and above the basilar membrane demonstrate the distinct pattern of innervation of spiral ganglion neurons around supporting cells and vestibular fibers below the basilar membrane (Fig. 5). A comparison of Ntf3kiBdnf/kiBdnf with Ntf3kiBdnf/+ mice suggests that there is a higher density of spiral afferents to the OHCs (Fig. 5). Unusual in these mutants is that some fibers grow past the third row of Deiter’s cells and appear to interact with Hensen’s cells (Fig. 5). Combining mice with a replacement of Ntf3 with Bdnf and with a deletion of native Bdnf (Bdnf−/−; Ntf3kiBdnf) shows that more fibers reroute from the vestibule that reach the cochlea, resulting in an even denser aggregation of nerve fibers in the scala vestibuli (Fig. 5). These data suggest that Bdnf and Ntf3 proteins are for the most part functionally equivalent in the embryonic development of the organ of Corti innervation, but exert different effects only through their graded differential distribution and their concentration.
Fig 5. The Ntf3 promoter-driven Bdnf expression rescues the fiber outgrowth to OHCs and leads to overshooting fibers in addition to vestibular fiber growth to the organ of Corti in newborn mice.
Images represent Z-stacks from the entire cochlea (left column) or from specific focal depth (right two columns). (A–A″″″) Ntf3kiBdnf/+ mice have a normal radial fiber density (B) but abnormal fibers beneath the basilar membrane (A′–A″). Note the absence of Prox1 labeling in A″, which shows that the fibers are not in the same focal plane as the supporting cells. In contrast, the afferents interacting with the Prox1 labeled supporting cells form three bundles of fibers but occasionally there are fibers growing out to form a fourth bundle (arrowhead, A″′–A″″). In the apex, the fibers grow mostly to the third row of Deiter cells (arrowhead, A″″′–A″″″). (B–B″″″) Ntf3kiBdnf/+ Bdnf−/− composite mutant mice have greatly reduced afferents to OHCs. The radial fiber density is slightly reduced (B) but there are few fibers growing to the OHCs (B′–B″″). In the apex, there are more fibers growing to OHCs but they are overshooting the organ of Corti (B″″′–B″″″). (C–C″″″) The complete replacement of Ntf3 by Bdnf (Ntf3kiBdnf/kiBdnf) leads to normal radial fiber density but more rerouting of vestibular fibers to the basal turn (C) resulting in thick fiber bundles beneath the basilar membrane (C′–C″). Fibers in the organ of Corti form four bundles (arrow and arrowhead) in the basal and lower middle turn, with somewhat increased contribution of fibers to the second and third rows (C″′–C″″). In the apex, the fibers are overshooting to pass the third row of Deiter’s cells (arrowhead, C″″′–C″″″). (D–D″″″) The Ntf3kiBdnf/kiBdnf Bdnf+/− has normal radial fiber density and very substantial bundles of rerouted vestibular fibers below the basilar membrane (D–D″′). In the middle turn, there are occasionally fibers growing out to innervate the second and third row of hair cells, but the growth to the first row is disrupted (D″′–D″″). In the apex, the row of fibers the first row of outer hair cells is reduced but there are also overshooting fibers (D″″′–D″″″). Images are from dye tracing of afferents from the brainstem and the auto-fluorescence of hair cells, or immunocytochemistry as labeled. Scale bars indicate 200 μm in A–D, and 50 μm in other images.
This conclusion on the function of Ntf3kiBdnf is supported through matching data on mice in which Bdnf is replaced with Ntf3 (BdnfkiNtf3). In agreement with the assumption of functional equivalence of both neurotrophins in the cochlea, the fiber density and innervation of OHCs could be rescued compared with Bdnf−/− (Agerman et al., 2003). In contrast, the vestibular system showed only partial rescues of neurons and a gradual loss after birth (Agerman et al., 2003) consistent with multiple suggestions of alterations of neurotrophin function and expression in postnatal mice (Davis, 2003; Flores-Otero et al., 2007; Schimmang et al., 2003). This functional equivalence in the cochlea seems related to the fact that most, if not all, SGNs express both Ntrk2 and Ntrk3, receptors for Bdnf and Ntf3, respectively (Farinas et al., 2001). Beyond the topological effects, the possible concentration effects remain to be revealed, as it is unlikely that the promoters of both neurotrophins are driving a concentration-matching expression of neurotrophins. A logical next test of this assumption is to wipe out the expression concentration gradient. This could be done by generating a complex mouse in which either neurotrophin is expressed at all times under control of either promoter. This can simply be achieved by generating a mouse double heterozygous for the two above outlined constructs: BdnfkiNtf3/+; Ntf3kiBdnf/+. Such mice should allow testing all assumptions on effects of rapid expression changes of individual neurotrophins. Lastly, the final test would be to replace either neurotrophin by the other, generating a mouse with the following genotype BdnfkiNtf3/kiNtf3; Ntf3kiBdnf/kiBdnf. Such mice would express Bdnf under Ntf3 promoter control and Ntf3 under Bdnf promoter control. These mice should show little to no effect on fiber growth in the cochlea, thus validating the functional equivalence concept developed with simpler mouse models. However, the vestibular part should show both loss of vestibular fibers and rerouting of others to the cochlea, indicating that neurotrophin receptors on vestibular ganglion neurons show differential rather than overlapping distribution.
In conclusion, as is obvious from previous and our new data, the complexity of neurotrophin interactions in cochlear innervation pattern development, both longitudinally and radially, is still underrated despite over 15 years of research on this simple system: only two ligands and two receptors play a role in the ear innervation development. Beyond the underrated complexity in signaling (Koppel et al., 2010) lies a complexity of receptor signaling intracellularly. For example, Ntrk2 activates intracellular signals via two routes, one activated by the Shc and the other by the Plc-γ activated pathways (Postigo et al., 2002; Sciarretta et al., 2010). While the Shc pathway affects fiber growth and size of neurons, the Plc-γ pathway affects pathfinding inside the vestibular sensory epithelia (Sciarretta et al., 2010). Combined loss of both docking sites through point mutations of their respective amino acids shows that only these two docking sites are relevant for the Ntrk2 signaling. Only the combined loss of both pathways causes neuronal loss comparable to the simple Ntrk2 loss (Sciarretta et al., 2010)., indicating a cellular convergence of both pathways. Somewhat similar data exist for Ntrk3 that has a small intracellular domain apparently able to mediate some of the pathfinding signaling (Esteban et al., 2006). In addition, neurotrophin receptors seem to form multiple splice forms with possibly different function (Luberg et al., 2010), increasing further the inherent complexity of this superficially simple system.
Finally, the effect of loss of neurotrophins is not exclusive to the sensory neurons. The cochlear duct in double Bdnf/Ntf3 null mice is much shorter (about 3 mm compared approximately 5 mm in wild type). In addition, the hair cells in the upper middle turn and apex form multiple disorganized rows of outer hair cells (Fig. 4F″′) not unlike the situation found in Neurog1 null mice (Matei et al., 2005) and Neurod1 null mice (Jahan et al., 2010b). In contrast to Neurog1 null mice, where neurons never delaminate, spiral ganglion neurons form in neurotrophin null mutants but degenerate after two or more days due to lack of neurotrophic support. Most likely the effect on shortening the organ of Corti relates to an interaction of the spiral ganglion with the convergent-extension movements of the cochlear duct. It remains to be shown whether spiral ganglion neurons play an active or passive role in the overall elongation of the cochlear duct and organ of Corti that seems to occur even in the absence of hair cell differentiation and delayed progressive loss of spiral ganglion neurons (Pan et al., 2010a).
As is obvious, the results presented here are restricted to embryonic mice. Most data presented in the literature on neonates have not been verified in mutant mice and are thus only correlative. The existence of viable conditional mutant mice will soon show how many of the claims concerning molecular and activity mediated neuronal refinement will be validated once tested in conditional mutant mice.
Materials and Methods
Mice and genotyping
Ntf3+/−, Bdnf+/− and Ntf3kiBdnf/+ mice were bred to generate single and compound knockout and knockin mice as previously described (Farinas et al., 2001; Tessarollo et al., 2004). Offspring were perfusion fixed and genotyped using already characterized protocols and primers. All animal work was approved by the University of Iowa Institutional Animal Care and Use Committee (IACUC) for research on animals (ACURF #0804066).
Immunofluorescence
For immunofluorescence staining, the ears were dehydrated in ethanol for one hour and then rehydrated. Samples were blocked with 0.5% normal donkey serum in PBS containing 0.01% Triton-X-100 for 1 hour and then were incubated with the primary antibodies for Myosin 7a (Myo7a, 1/200, Proteus Biosciences), β-tubulin (1/800, Sigma), and Prox1 (1/500; Covance) for 48 hours at 4°C. After three washes with PBS, corresponding secondary antibodies (Alexa fluor molecular probe 647, 532 or 488, 1/500, Invitrogen) were added and incubated overnight at 4°C. The samples were washed with PBS and mounted in glycerol and images were taken with a Leica TCS SP5 confocal microscope.
Dye tracing
Embryos were perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4) and their heads were isolated, postfixed in 4% PFA for at least 24 hours. The heads were then split into two halves through the mid-sagittal plane. Double labeling was used in these experiments. One dye-loaded filter strip was implanted in the rhombomere 4, and the second dye implanted in the cerebellum. The preparation was stored in 0.4% PFA, and incubated at 60°C for 2 or more days depending on the size of the mice. After the appropriate diffusion time, the inner ears were removed and their sensory epithelia were dissected and mounted flat on slides. Images were taken with a Leica TCS SP5 confocal microscope. The excitation laser and emission filter settings for both tracers were used as described (Fritzsch et al., 2005a).
Acknowledgments
This work was supported by a NIH grant (R01 DC 005590) to B.F. We express our thanks to Drs. Ma, Tessarollo, Farinas, Reichardt and Ernfors for sharing mouse lines and reagents. We thank the Roy J Carver Foundation for the support of the Confocal Imaging Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–1491. doi: 10.1242/dev.00378. [DOI] [PubMed] [Google Scholar]
- Barclay M, Julien JP, Ryan AF, Housley GD. Type III intermediate filament peripherin inhibits neuritogenesis in type II spiral ganglion neurons in vitro. Neurosci Lett. 2010;478:51–5. doi: 10.1016/j.neulet.2010.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti AC, Fekete DM. Slits and robos in the developing chicken inner ear. Developmental Dynamics. 2008;237:476–484. doi: 10.1002/dvdy.21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi LM, Conover JC, Fritzsch B, DeChiara T, Lindsay RM, Yancopoulos GD. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development. 1996;122:1965–1973. doi: 10.1242/dev.122.6.1965. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Daruwalla Z, Roth TM, Attia NP, Lukacs NW, Richards AL, White IO, Allen SJ, Barald KF. Immortalized mouse inner ear cell lines demonstrate a role for chemokines in promoting the growth of developing statoacoustic ganglion neurons. J Assoc Res Otolaryngol. 2005;6:355–67. doi: 10.1007/s10162-005-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Busslinger M, Xu P, De Caprona D, Fritzsch B. PAX2 and PAX8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev Biol. 2010 doi: 10.1186/1471-213X-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Brors D, Bodmer D, Pak K, Aletsee C, Schäfers M, Dazert S, Ryan AF. EphA4 provides repulsive signals to developing cochlear ganglion neurites mediated through ephrin-B2 and -B3. The Journal of Comparative Neurology. 2003;462:90–100. doi: 10.1002/cne.10707. [DOI] [PubMed] [Google Scholar]
- Bruce LL, Kingsley J, Nichols DH, Fritzsch B. The development of vestibulocochlear efferents and cochlear afferents in mice. Int J Dev Neurosci. 1997;15:671–92. doi: 10.1016/s0736-5748(96)00120-7. [DOI] [PubMed] [Google Scholar]
- Brunelli S, Innocenzi A, Cossu G. Bhlhb5 is expressed in the CNS and sensory organs during mouse embryonic development. Gene Expr Patterns. 2003;3:755–9. doi: 10.1016/s1567-133x(03)00135-2. [DOI] [PubMed] [Google Scholar]
- Coppola V, Kucera J, Palko ME, Martinez-De Velasco J, Lyons WE, Fritzsch B, Tessarollo L. Dissection of NT3 functions in vivo by gene replacement strategy. Development. 2001;128:4315–4327. doi: 10.1242/dev.128.21.4315. [DOI] [PubMed] [Google Scholar]
- D’Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–68. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Davies D. Temporal and spatial regulation of alpha6 integrin expression during the development of the cochlear-vestibular ganglion. J Comp Neurol. 2007;502:673–82. doi: 10.1002/cne.21302. [DOI] [PubMed] [Google Scholar]
- Davis RL. Gradients of neurotrophins, ion channels, and tuning in the cochlea. Neuroscientist. 2003;9:311–6. doi: 10.1177/1073858403251986. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–64. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Esteban PF, Yoon HY, Becker J, Dorsey SG, Caprari P, Palko ME, Coppola V, Saragovi HU, Randazzo PA, Tessarollo L. A kinase-deficient TrkC receptor isoform activates Arf6-Rac1 signaling through the scaffold protein tamalin. J Cell Biol. 2006;173:291–9. doi: 10.1083/jcb.200512013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–80. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Campero AM. Axon guidance in the inner ear. Int J Dev Biol. 2007;51:549–56. doi: 10.1387/ijdb.072341df. [DOI] [PubMed] [Google Scholar]
- Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, Bosl MR, Meijer D, Wegner M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189:701–12. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Otero J, Xue HZ, Davis RL. Reciprocal regulation of presynaptic and postsynaptic proteins in bipolar spiral ganglion neurons by neurotrophins. J Neurosci. 2007;27:14023–34. doi: 10.1523/JNEUROSCI.3219-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B. Development of inner ear afferent connections: forming primary neurons and connecting them to the developing sensory epithelia. Brain Res Bull. 2003;60:423–33. doi: 10.1016/s0361-9230(03)00048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of Neurotrophin 3 Causes Losses of Both Classes of Spiral Ganglion Neurons in the Cochlea in a Region-Specific Fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? Bioessays. 2006a;28:1181–93. doi: 10.1002/bies.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Eberl D, Beisel K. The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cellular and Molecular Life Sciences. 2010a;67:3089–3099. doi: 10.1007/s00018-010-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–78. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Muirhead KA, Feng F, Gray BD, Ohlsson-Wilhelm BM. Diffusion and imaging properties of three new lipophilic tracers, NeuroVue(TM) Maroon, NeuroVue(TM) Red and NeuroVue(TM) Green and their use for double and triple labeling of neuronal profile. Brain Research Bulletin. 2005a;66:249–258. doi: 10.1016/j.brainresbull.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Feng F, Matei V, Nichols DH. The evolution of the vertebrate auditory system: transformations of vestibular mechanosensory cells for sound processing is combined with newly generated central processing neurons. International Journal of Comparative Psychology. 2006b;19:1–24. [Google Scholar]
- Fritzsch B, Dillard M, Lavado A, Harvey NL, Jahan I. Canal cristae growth and fiber extension to the outer hair cells of the mouse ear require Prox1 activity. PLoS One. 2010b;5:e9377. doi: 10.1371/journal.pone.0009377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain. Hear Res. 2005b;206:52–63. doi: 10.1016/j.heares.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–56. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn. 2005c;233:570–83. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galabova-Kovacs G, Baccarini M. Deciphering signaling pathways in vivo: the Ras/Raf/MEK/ERK cascade. Methods Mol Biol. 2010;661:421–31. doi: 10.1007/978-1-60761-795-2_26. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A, de Celis JF. The complex tale of the achaete-scute complex: a paradigmatic case in the analysis of gene organization and function during development. Genetics. 2009;182:631–9. doi: 10.1534/genetics.109.104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N, Vanderhaeghen P. Mechanisms of neural specification from embryonic stem cells. Curr Opin Neurobiol. 2010;20:37–43. doi: 10.1016/j.conb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Ginzberg RD, Morest DK. A study of cochlear innervation in the young cat with the Golgi method. Hear Res. 1983;10:227–46. doi: 10.1016/0378-5955(83)90056-4. [DOI] [PubMed] [Google Scholar]
- Grabocka E, Bar-Sagi D. Raf-1 and squamous cell carcinoma: Rok-ing the boat. Cancer Cell. 2009;16:85–6. doi: 10.1016/j.ccr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Grimsley-Myers CM, Sipe CW, Geleoc GS, Lu X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J Neurosci. 2009;29:15859–69. doi: 10.1523/JNEUROSCI.3998-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 Conveys Semaphorin and VEGF Signaling during Neural and Cardiovascular Development. Developmental Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng JI, Nguyen L, Castro DS, Zimmer C, Wildner H, Armant O, Skowronska-Krawczyk D, Bedogni F, Matter JM, Hevner R, Guillemot F. Neurogenin 2 controls cortical neuron migration through regulation of Rnd2. Nature. 2008;455:114–8. doi: 10.1038/nature07198. [DOI] [PubMed] [Google Scholar]
- Hossain WA, D’Sa C, Morest DK. Interactive roles of fibroblast growth factor 2 and neurotrophin 3 in the sequence of migration, process outgrowth, and axonal differentiation of mouse cochlear ganglion cells. J Neurosci Res. 2008;86:2376–91. doi: 10.1002/jnr.21685. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–32. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Thorne PR, Housley GD, Montgomery JM. Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development. 2007;134:2925–2933. doi: 10.1242/dev.001925. [DOI] [PubMed] [Google Scholar]
- Jahan I, Kersigo J, Pan N, Fritzsch B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010a;341:95–110. doi: 10.1007/s00441-010-0984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One. 2010b;5:e11661. doi: 10.1371/journal.pone.0011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–30. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Katayama K, Zine A, Ota M, Matsumoto Y, Inoue T, Fritzsch B, Aruga J. Disorganized innervation and neuronal loss in the inner ear of Slitrk6-deficient mice. PLoS One. 2009;4:e7786. doi: 10.1371/journal.pone.0007786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MC, Chen P. Development of form and function in the mammalian cochlea. Curr Opin Neurobiol. 2009;19:395–401. doi: 10.1016/j.conb.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern F, Niault T, Baccarini M. Ras and Raf pathways in epidermis development and carcinogenesis. Br J Cancer. 2010 doi: 10.1038/sj.bjc.6606009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–26. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Sheets PL, Zopf DA, Aloor HL, Cummins TR, Chan RJ, Hashino E. Tlx3 exerts context-dependent transcriptional regulation and promotes neuronal differentiation from embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:5780–5. doi: 10.1073/pnas.0708704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional Deletion of N-Myc Disrupts Neurosensory and Non-sensory Development of the Ear. Dev Dyn. 2011 doi: 10.1002/dvdy.22620. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel I, Aid-Pavlidis T, Jaanson K, Sepp M, Palm K, Timmusk T. BAC transgenic mice reveal distal cis-regulatory elements governing BDNF gene expression. Genesis. 2010;48:214–9. doi: 10.1002/dvg.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundakjian EJ, Appler JL, Goodrich LV. Auditory neurons make stereotyped wiring decisions before maturation of their targets. J Neurosci. 2007;27:14078–88. doi: 10.1523/JNEUROSCI.3765-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger M, Schmid T, Kruger S, Bober E, Braun T. Functional redundancy of NSCL-1 and NeuroD during development of the petrosal and vestibulocochlear ganglia. Eur J Neurosci. 2006;24:1581–90. doi: 10.1111/j.1460-9568.2006.05051.x. [DOI] [PubMed] [Google Scholar]
- Lallemend F, Vandenbosch R, Hadjab S, Bodson M, Breuskin I, Moonen G, Lefebvre PP, Malgrange B. New insights into peripherin expression in cochlear neurons. Neuroscience. 2007;150:212–22. doi: 10.1016/j.neuroscience.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Lawoko-Kerali G, Rivolta MN, Holley M. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J Comp Neurol. 2002;442:378–91. doi: 10.1002/cne.10088. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Bonham BH, Snyder RL. Topography of auditory nerve projections to the cochlear nucleus in cats after neonatal deafness and electrical stimulation by a cochlear implant. J Assoc Res Otolaryngol. 2008;9:349–72. doi: 10.1007/s10162-008-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie M, Grover M, Whitlon DS. Accelerated neurite growth from spiral ganglion neurons exposed to the Rho kinase inhibitor H-1152. Neuroscience. 2010;169:855–62. doi: 10.1016/j.neuroscience.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–54. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luberg K, Wong J, Weickert CS, Timmusk T. Human TrkB gene: novel alternative transcripts, protein isoforms and expression pattern in the prefrontal cerebral cortex during postnatal development. J Neurochem. 2010;113:952–64. doi: 10.1111/j.1471-4159.2010.06662.x. [DOI] [PubMed] [Google Scholar]
- Luo ZX, Ruf I, Schultz JA, Martin T. Fossil evidence on evolution of inner ear cochlea in Jurassic mammals. Proc Biol Sci. 2011;278:28–34. doi: 10.1098/rspb.2010.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–43. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, Barrantes IdB, Luis de la Pompa J, Anderson DJ. neurogenin 1 Is Essential for the Determination of Neuronal Precursors for Proximal Cranial Sensory Ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Magarinos M, Aburto MR, Sanchez-Calderon H, Munoz-Agudo C, Rapp UR, Varela-Nieto I. RAF Kinase Activity Regulates Neuroepithelial Cell Proliferation and Neuronal Progenitor Cell Differentiation during Early Inner Ear Development. PLoS One. 2010;5:e14435. doi: 10.1371/journal.pone.0014435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–50. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeotte I, Omelchenko T, Hall A, Anderson KV. Rac1-dependent collective cell migration is required for specification of the anterior-posterior body axis of the mouse. PLoS Biol. 2010;8:e1000442. doi: 10.1371/journal.pbio.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Maklad A, Hansen LA, Feng F, Sorensen C, Lee KF, Macklin WB, Fritzsch B. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006;1091:186–99. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Jabs N, Lorke DE, Fritzsch B, Sander M. Nkx6.1 controls migration and axon pathfinding of cranial branchio-motoneurons. Development. 2003;130:5815–26. doi: 10.1242/dev.00815. [DOI] [PubMed] [Google Scholar]
- Naka H, Nakamura S, Shimazaki T, Okano H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008;11:1014–23. doi: 10.1038/nn.2168. [DOI] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334:339–58. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 2008;1192:90–8. doi: 10.1016/j.brainres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2010;30:15044–51. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacary E, Heng JI, Azzarelli A, Riou P, Castro D, Lebel-Potter M, Parras C, Bell DM, Ridley AJ, Parsons M, Guillemot F. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-meidated inhibition of RhoA signaling. Neuron. 2011 doi: 10.1016/j.neuron.2011.02.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2010a doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci U S A. 2010b;107:15798–803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci. 2010;13:29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235:2470–82. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–15. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peljto M, Wichterle H. Programming embryonic stem cells to neuronal subtypes. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci U S A. 1992;89:9915–9. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postigo A, Calella AM, Fritzsch B, Knipper M, Katz D, Eilers A, Schimmang T, Lewin GR, Klein R, Minichiello L. Distinct requirements for TrkB and TrkC signaling in target innervation by sensory neurons. Genes Dev. 2002;16:633–45. doi: 10.1101/gad.217902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW. Sox2 induces neuronal formation in the developing mammalian cochlea. J Neurosci. 2010;30:714–22. doi: 10.1523/JNEUROSCI.3852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236:1905–17. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen CL, Choi Y, Ma Q. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–45. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Glasco DM, Zhou L, Sawant A, Ravni A, Fritzsch B, Damrau C, Murdoch JN, Evans S, Pfaff SL, Formstone C, Goffinet AM, Chandrasekhar A, Tissir F. Atypical cadherins Celsr1–3 differentially regulate migration of facial branchiomotor neurons in mice. J Neurosci. 2010;30:9392–401. doi: 10.1523/JNEUROSCI.0124-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Nowotschin S, Liao J, Morrow BE. Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development. 2004;131:1801–12. doi: 10.1242/dev.01067. [DOI] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–15. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes & Development. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romand R, Kondo T, Cammas L, Hashino E, Dolle P. Dynamic expression of the retinoic acid-synthesizing enzyme retinol dehydrogenase 10 (rdh10) in the developing mouse brain and sensory organs. J Comp Neurol. 2008;508:879–92. doi: 10.1002/cne.21707. [DOI] [PubMed] [Google Scholar]
- Rontal DA, Echteler SM. Developmental segregation in the efferent projections to auditory hair cells in the gerbil. J Comp Neurol. 2003;467:509–20. doi: 10.1002/cne.10931. [DOI] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–98. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Ernst J, Kharchenko PV, Kheradpour P, Negre N, Eaton ML, Landolin JM, Bristow CA, Ma L, Lin MF, Washietl S, Arshinoff BI, Ay F, Meyer PE, Robine N, Washington NL, Di Stefano L, Berezikov E, Brown CD, Candeias R, Carlson JW, Carr A, Jungreis I, Marbach D, Sealfon R, Tolstorukov MY, Will S, Alekseyenko AA, Artieri C, Booth BW, Brooks AN, Dai Q, Davis CA, Duff MO, Feng X, Gorchakov AA, Gu T, Henikoff JG, Kapranov P, Li R, Macalpine HK, Malone J, Minoda A, Nordman J, Okamura K, Perry M, Powell SK, Riddle NC, Sakai A, Samsonova A, Sandler JE, Schwartz YB, Sher N, Spokony R, Sturgill D, van Baren M, Wan KH, Yang L, Yu C, Feingold E, Good P, Guyer M, Lowdon R, Ahmad K, Andrews J, Berger B, Brenner SE, Brent MR, Cherbas L, Elgin SC, Gingeras TR, Grossman R, Hoskins RA, Kaufman TC, Kent W, Kuroda MI, Orr-Weaver T, Perrimon N, Pirrotta V, Posakony JW, Ren B, Russell S, Cherbas P, Graveley BR, Lewis S, Micklem G, Oliver B, Park PJ, Celniker SE, Henikoff S, Karpen GH, Lai EC, Macalpine DM, Stein LD, White KP, Kellis M. Identification of Functional Elements and Regulatory Circuits by Drosophila modENCODE. Science. 2010 doi: 10.1126/science.1198374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol, Suppl. 1967;220:1–44. [PubMed] [Google Scholar]
- Schimmang T, Tan J, Muller M, Zimmermann U, Rohbock K, Kopschall I, Limberger A, Minichiello L, Knipper M. Lack of Bdnf and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development. 2003;130:4741–50. doi: 10.1242/dev.00676. [DOI] [PubMed] [Google Scholar]
- Schreiner D, Weiner JA. Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc Natl Acad Sci U S A. 2010;107:14893–8. doi: 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciarretta C, Fritzsch B, Beisel K, Rocha-Sanchez SM, Buniello A, Horn JM, Minichiello L. PLCgamma-activated signalling is essential for TrkB mediated sensory neuron structural plasticity. BMC Dev Biol. 2010;10:103. doi: 10.1186/1471-213X-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silos-Santiago I, Fagan AM, Garber M, Fritzsch B, Barbacid M. Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors. Eur J Neurosci. 1997;9:2045–56. doi: 10.1111/j.1460-9568.1997.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Tang LS, Alger HM, Pereira FA. COUP-TFI controls Notch regulation of hair cell and support cell differentiation. Development. 2006;133:3683–93. doi: 10.1242/dev.02536. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–84. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Vogel KS, Palko ME, Reid SW, Parada LF. Targeted mutation in the neurotrophin-3 gene results in loss of muscle sensory neurons. Proc Natl Acad Sci U S A. 1994;91:11844–8. doi: 10.1073/pnas.91.25.11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heumen WRA, Claxton C, Pickles JO. Expression of EphA4 in developing inner ears of the mouse and guinea pig. Hearing Research. 2000;139:42–50. doi: 10.1016/s0378-5955(99)00158-6. [DOI] [PubMed] [Google Scholar]
- Webber A, Raz Y. Axon guidance cues in auditory development. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:390–6. doi: 10.1002/ar.a.20299. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Grover M, Lie M. High content analysis of neurite length’s in cultures of dissociated spiral ganglia. ARO Midwinter meeting #421.2011. [Google Scholar]
- Whitlon DS, Grover M, Tristano J, Williams T, Coulson MT. Culture conditions determine the prevalence of bipolar and monopolar neurons in cultures of dissociated spiral ganglion. Neuroscience. 2007;146:833–40. doi: 10.1016/j.neuroscience.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Maklad A, Pirvola U, Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neuroscience. 2003;4:2. doi: 10.1186/1471-2202-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Li X, McNamee C, Chen AP, Baccarini M, Snider WD. Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci. 2007;10:598–607. doi: 10.1038/nn1898. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu Q, Davis RL. Complex regulation of spiral ganglion neuron firing patterns by neurotrophin-3. J Neurosci. 2005;25:7558–66. doi: 10.1523/JNEUROSCI.1735-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Fritzsch B, Xu PX. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004 doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Erickson C, Kim EH, Jin D, Fritzsch B, Xu PX. Eya1 gene dosage critically affects the development of sensory epithelia in the mammalian inner ear. Hum Mol Genet. 2008;17:3340–56. doi: 10.1093/hmg/ddn229. [DOI] [PMC free article] [PubMed] [Google Scholar]