Abstract
We explored the relation between individual differences in working memory (WM) and color constancy, the phenomenon of color perception that allows us to perceive the color of an object as relatively stable under changes in illumination. Successive color constancy (measured by first viewing a colored surface under a particular illumination and later recalling it under a new illumination) was better for higher-WM individuals than for lower-WM individuals. Moreover, the magnitude of this WM difference depended on how much contextual information was available in the scene, which typically improves color constancy. By contrast, simple color memory, measured by viewing and recalling a colored surface under the same illumination, showed no significant relation to WM. This study reveals a relation between WM and a low-level perceptual process not previously thought to operate within the confines of attentional control, and provides a first account of the individual differences in color constancy known about for decades.
Keywords: working memory, color memory, individual differences, color constancy
Color constancy reflects our ability to maintain a (nearly) stable perceived color of a surface despite changes in the light illuminating the surface, which in turn changes the light that reaches the eye from the surface (Helmholtz, 1866/1962). If we did not have color constancy, the light reaching the eye from a surface would be the only factor that determines its perceived color. Thus, we would be unable to identify an object based on its color, because its color would appear to shift with every lighting change. For example, a shirt that appears blue when viewed under sunlight would appear a washed-out brown under a typical light bulb at home. Color constancy is an essential component of human vision because it enables us to see consistent colors of objects irrespective of the illuminating light.
Some people have better color constancy than others (e.g., Kraft, Maloney, & Brainard, 2002). Although previous research identifies many factors that contribute to color constancy, such as the number of different surfaces in the scene (Linnell & Foster, 2002) and whether the scene is viewed in 3D (Yang & Shevell, 2002), little is known about why people vary in this ability. Here, we reveal a clear relation between color constancy and working memory (WM) – a general cognitive construct related to complex cognitive abilities ranging from mathematical problem solving (Beilock & Carr, 2005) to emotion regulation (Ochsner, Bunge, Gross, & Gabrieli, 2002). We provide the first evidence of a link between individual differences in WM and color constancy, while at the same time demonstrating that WM is related to a perceptual process previously thought to be outside the influence of complex cognitive processes.
Color Constancy
To achieve color constancy, one needs cues to the illuminating light. These cues include neural responses to the spectral distribution of light from different colored surfaces (Land, 1959), shadows (D’Zmura, 1992) and specular highlights reflected from glossy surfaces (Yang & Maloney, 2001). Information from these cues is used by the visual system to discount changes in color appearance that otherwise would accompany a change of illuminating light. In this way, one can establish an (approximately) illuminant-independent representation of the color of a surface – exactly what is needed for color constancy.
At first glance, it might seem as if color-constancy ability should be independent of higher-level cognitive constructs, such as WM. Indeed, color constancy is often considered to be automatic, implying that it happens without the type of attentional control that is one hallmark of WM (Engle, 2002). In particular, simultaneous color constancy, which occurs across illumination changes over space within a single scene, may seem to operate without attentional control (though this idea is challenged in the Discussion). However, when illumination changes take place across different time periods, one needs an illuminant-independent representation of the surface seen previously to compare it to the percept of the surface under the new illumination (Jin & Shevell, 1996). As a result, successive color constancy may be related to one’s ability to hold information in mind in the face of interfering information – precisely what WM has been proposed to involve.
Note that successive color constancy is not the same as simple color memory, though both require the ability to remember and compare the color of a surface across time. The key difference is that simple color memory requires maintaining a representation of a color with no change in illumination, so it can be achieved by recalling a neural representation of the spectral distribution of the light entering the eye. Successive color constancy, on the other hand, requires establishing and maintaining an illuminant-independent representation of an object’s color under different illuminants. For a discussion of how to best define and measure color constancy, see Foster (2003).
Working Memory
Individual differences in WM are linked to performance on a wide array of complex tasks, ranging from reasoning (Kyllonen & Christal, 1990), to reading comprehension (Daneman & Carpenter, 1980), to mathematical problem solving (Beilock & Carr, 2005). The component of WM that makes the most significant contribution to these individual differences is thought to be the domain-general ability to control attention. Thus, when successful task performance requires attentional control, correlations with WM capacity are often found (Engle, 2002).
Although individual differences in WM are linked to a wide array of complex cognitive tasks, significant relations between WM and lower-level perceptual tasks such as subitizing (Tuholski, Engle, & Baylis, 2001) or visual search for “pop out” targets (Kane, Poole, Tuholski, & Engle, 2006) have not been found. Performance on these lower-level perceptual tasks is thought to occur relatively automatically, not requiring domain-general attentional control (Engle, 2002). Thus, one might assume that WM would not be linked to color constancy – another low-level perceptual task. However, there are individual differences in color constancy that are in need of an explanation. Moreover, given that successive color constancy may require some degree of attentional control (being less automatic in nature than previously thought), we sought to examine whether a complex cognitive construct such as WM could predict color constancy ability.
Current Study
We measured both successive color constancy and simple color memory in lower-working-memory (LowWM) and higher-working-memory (HighWM) individuals. We found that WM was related to better successive color constancy, but not simple color memory. Moreover, this relation between WM and color constancy (the higher one’s WM, the better one’s color-constancy ability) was stronger when there was little context in view as opposed to when there was considerably more context. This finding suggests that LowWMs have difficulty creating an illuminant-independent representation when they do not have an abundance of contextual illuminant cues.
Method
Participants
Our aim was to test for a relation between WM and successive color constancy, rather than estimate the magnitude of this relation. Thus, we employed an extreme-WM-group design in this study (Conway et al., 2005). LowWM and HighWM groups were derived from the distribution of scores on two common WM measures (presented counterbalanced in order across participants): Reading Span (RSPAN; Daneman & Carpenter, 1980) and Operation Span (OSPAN; Unsworth, Heitz, Schrock, & Engle, 2005).
In the RSPAN, participants are presented with a series of sentences on a computer (e.g., “The ranger told the hikers to look out for snakes.”). Participants indicate whether the sentence makes sense by clicking “TRUE” or “FALSE” on the screen. A letter is then presented for participants to hold in memory. These sentence-letter trials are presented in sets, with 3–7 trials per set, for a total of 75 letters in 15 sets. At the end of each set, a screen with twelve letters appears, and participants use the mouse to select the letters they remember in the correct order. The procedure for the OSPAN is identical to that of the RSPAN, except participants view math equations (e.g., “(3*2) + 4 = ?”) instead of sentences and judge whether or not the answer provided for the equation is correct.
Scores for both the OSPAN and RSPAN were calculated using the partial-credit unit scoring method (Conway et al., 2005) and then averaged together. Participants with an average score of at least 60 (out of a possible 75) were classified as HighWM; participants with a score of 40 or less were classified as LowWM. These criterial scores were similar to those used in a previous WM study of nearly 300 individuals to differentiate lower and higher WM (Unsworth et al., 2005), and were adopted because criterial scores based on the top and bottom quartiles of a sample of University of Chicago students may be biased towards higher scores than are typically used in the literature. Only University of Chicago students whose score fell within one of these categories were invited to participate in the study.
Twelve HighWM (three male, Mage = 21.3, SD = 2.70) and 12 LowWM (three male, Mage = 20.7, SD = 2.19) individuals participated in the study. The HighWM [LowWM] participants had working memory scores ranging from 60.5–74.5, Mscore = 67.0 [6.5–38.0, Mscore = 25.7]. All reported normal or corrected-to-normal visual acuity, and had normal color vision as determined by Rayleigh matching (Rayleigh, 1881).
Materials and Procedure
Apparatus
Stimuli were generated using a Macintosh computer, on a precisely calibrated NEC AccuSync 120 high-resolution CRT color monitor (Jenness & Shevell, 1995). A color lookup table generated stimuli according to their Judd (1951) tristimulus values. Participants viewed the CRT screen binocularly without head restraint from a distance of about 1m. The participants controlled the chromaticity (hue and saturation) of a central test patch using three pairs of buttons on a Gravis game controller: one pair adjusted the hue (hues were ordered as if in a circle, and each button scrolled through the hues in a different direction around this circle), one pair adjusted the saturation in large steps, and one pair adjusted the saturation in small steps. Luminance (the overall light intensity of the stimulus) was fixed at 8 cd/m2. The response was recorded when the participant pressed another button on the controller.
Stimuli
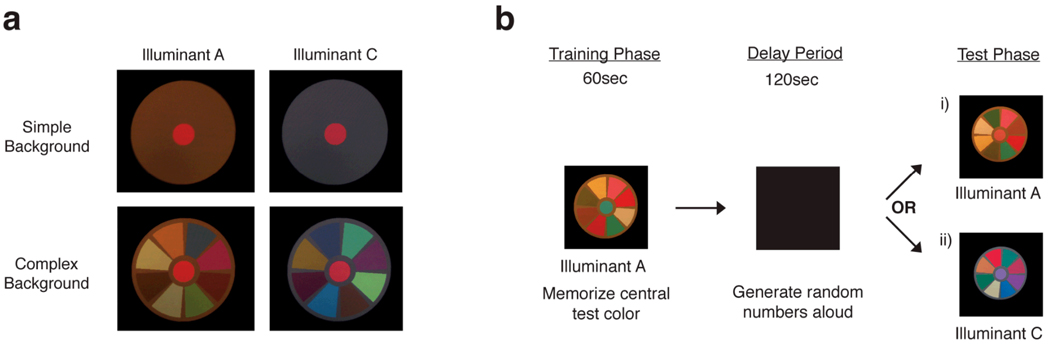
The uniform-background stimulus consisted of a circular central test patch of 1.2° diameter visual angle within a contiguous, uniform annular surround of outer diameter 5.9° (Figure 1a). The complex-background stimulus was identical except eight colored sectors, separated by 6.5°, were embedded within the surround (sector inner/outer diameter of 1.8°/5.4°; Figure 1a). In a practice session, only the central test patch was presented. In all cases, the area beyond the stimulus was dark.
Figure 1.
(a) Examples of the four combinations of illuminant and background using the “red” central test color. Note that in the complex-background condition, the colored sectors embedded within the surround are randomly generated every time a new stimulus is displayed, and thus differ in the examples shown here. Also note that the color appearance of the printed stimuli in the figure may be different from their appearance on the fully calibrated computer monitor used in the study. (b) A schematic of the procedure for one trial using the complex background, training illuminant A and “blue” central test color. Each trial was comprised of a training phase and a test phase. Participants first memorized the central test color in the training phase for 60sec. Next, the screen became dark for 120sec and participants generated random numbers aloud. Finally, a new stimulus was displayed with the central test patch set to a random chromaticity. Participants set the central test patch to look like the color they saw during the training phase. i) The test illuminant is the same as the training illuminant; this is a test of simple color memory. ii) The test illuminant is different from the training illuminant; this is a test of successive color constancy.
All stimulus chromaticities were (simulated) papers from the Munsell Book of Color (Munsell Color Corporation, Baltimore MD) under one of two CIE standard illuminants: illuminant A, which approximates the spectral emission of a typical tungsten (screw-in) light bulb, and illuminant C, which approximates average daylight. To simulate a color paper under an illuminant, its surface reflectance (Kelly, Gibson, & Nickerson, 1943; Nickerson, 1957) was multiplied, wavelength-by-wavelength, by the spectral power distribution of the illuminant, giving the spectral power distribution of the light reflected from the paper. From this, the Judd (1951) tristimulus values were calculated and the computer reproduced these values on the CRT after applying a luminance normalization. The CRT had a limited color gamut, and thus could not reproduce all of the possible combinations of color papers and illuminants. Therefore, only 165 of 462 possible color papers could be used. These colors were divided into 10 groups according to the perceptual spacing of the Munsell hue circle (Munsell, 1905): (1) {5R, 10R}, 11 papers; (2) {5YR, 10YR}, 5 papers; (3) {5Y, 10Y}, 5 papers; (4) {5GY, 10GY}, 20 papers; (5) {5G, 10G}, 19 papers; (6) {5BG, 10BG}, 18 papers; (7) {5B, 10B}, 21 papers; (8) {5PB, 10PB}, 27 papers; (9) {5P, 10P}, 23 papers; (10) {5RP, 10RP}, 16 papers.
The Munsell papers used for the test colors were 5R 4/6 (“red”), 10GY 4/6 (“green”), and 5B 4/6 (“blue”). In the practice session, 10PB 4/8 (“purple”) was used as the only test color. The surround always was based on paper N 4/0 (“gray”), which essentially reflected all wavelengths nonselectively.
For the complex-background condition, the central test color was chosen and then the colors for the sectors embedded within the surround were selected according to a pseudo-random process (Jin & Shevell, 1996). A new randomization was used every time a new training or test stimulus was displayed. The purpose of this was twofold: it prevented any systematic influence of a sector color on the test color (e.g., chromatic induction), and it prevented participants from using the appearance of a sector color to help them remember the test color (e.g., by remembering that the test color appeared more bluish than a particular sector color).
Procedure
After giving informed consent, participants completed a practice session followed by two experimental sessions counterbalanced in order: one with the uniform-background stimulus and one with the complex-background stimulus. In all sessions, participants were instructed to think of the colored patches that made up the stimuli as papers on a table (see Reeves, Amano, & Foster, 2008).
Prior to a session, participants dark-adapted for five minutes. Participants then completed a series of 12 trials (6 trials in the practice session). Each trial consisted of a training phase, a delay interval, and a test phase. A schematic of the procedure for one trial is presented in Figure 1b.
In the training phase, the stimulus was presented for 60sec; participants were instructed to memorize the central test color. During this training phase, half of the participants always saw the stimulus under (simulated) illuminant A, and the other half always under (simulated) illuminant C. After the training phase, the 120sec-long delay interval began, during which the screen was black and a beep was emitted from the computer every second. At each beep, the participant was required to say out loud a number from 0–9 in “random” order (Hegarty, Shah, & Miyake, 2000). This distracting task was used to prevent verbal rehearsal of the test color, and to ensure that all participants were engaged in the same activity during the delay interval. An audio monitor allowed the experimenter to listen to the participant to ensure that the participant performed the distracting task.
Next, in the test phase, the stimulus appeared with the central test patch set to a random chromaticity. Participants then used the controls on the game pad to set the test patch to look like the test color they remembered seeing previously, an instruction used in other studies of successive color constancy (e.g., Jin & Shevell, 1996). After the desired color was set, there was a 10sec break between trials, during which the screen was dark.
Importantly, on half the trials, the test-phase stimulus was presented under (simulated) illuminant A, and on the other half under (simulated) illuminant C. This allowed measurement of both simple color memory (no illuminant change between study and test phases) and successive color constancy (different illuminants in study and test phases). Thus, it is important to note that for both types of backgrounds, both simple color memory and successive color constancy were tested, simply by varying whether the illuminant stayed the same or changed between the study and test phases.
All color settings made by participants in the test phase (the dependent measure) were recorded as values in the chromaticity coordinate system of MacLeod and Boynton (1979). This coordinate system is commonly used to quantify measurements of color, and represents the precise stimulation of each type of cone photoreceptor (up to a scalar for overall light level, which is not important here). MacLeod-Boynton space contains two orthogonal axes, l and s, which indicate the color’s relative stimulation of the long- versus middle-wavelength-sensitive cones and of the short-wavelength-sensitive cones, respectively. Results of statistical analyses are reported separately for the l and s measurements.
Results
Before performing any analyses, outliers, defined as measurements falling beyond the “outer fence” (Tukey, 1977), were transformed to the value of the nearest outer fence. Eleven of 1152 measurements were outliers.
Perfect successive color constancy requires discounting the contribution of the training illuminant to the percept held in memory, creating an illuminant-independent representation. Thus, perfect successive color constancy implies no effect of the training illuminant on the color settings made in the test phase, regardless of the test illuminant. In other words, whether the training and test illuminants are the same or different is irrelevant; what is important is the extent to which the color settings made by participants in the test phase show an effect of the training illuminant, with a larger training-illuminant effect corresponding to poorer color constancy.
To facilitate visual presentation of the results, the values of the color settings (the dependent measure) are plotted in the form of color constancy error – calculated for each test illuminant as the absolute value of the difference between the color settings made when the training illuminant was the same as the test illuminant and when it was different, and then averaged across test illuminants (Figure 2). The larger the color constancy error, the more the color settings were affected by the training illuminant, and thus the worse the color constancy.
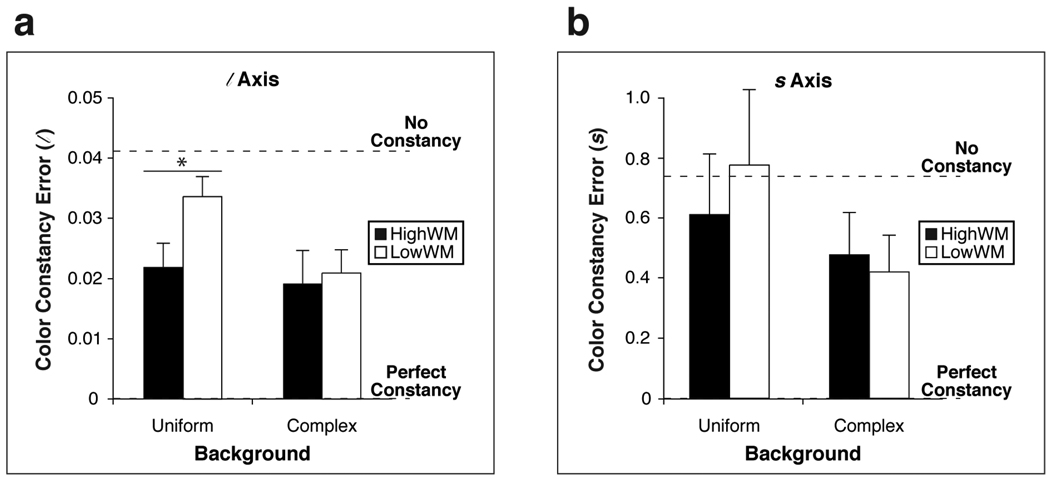
Figure 2.
Color constancy errors averaged across test colors for (a) the l axis and (b) the s axis. Results are separated by background condition (uniform and complex). Black [white] bars indicate values for HWM [LWM] participants. The vertical axis indicates the color constancy error, calculated for each test illuminant as the absolute value of the difference between the color settings made when the training illuminant was the same as the test illuminant, and when it was different (and then averaged across test illuminants). A smaller value indicates a smaller effect of the training illuminant, and thus better color constancy. “Perfect constancy” refers to a color constancy error of 0. “No constancy” refers to a color constancy error equal in magnitude to the shift in chromaticity of the (averaged) test color under a change in illuminants. A value larger than this indicates a color constancy error that is even greater in magnitude than this shift in chromaticity of the test color. Error bars indicate standard errors of the mean. An asterisk indicates significance at the p < 0.05 level.
To test for an effect of WM on color constancy, we submitted color settings made in the test phase to a 2 × 2 × 3 × 2 × 2 (Background [uniform, complex] × Test Illuminant [A, C] × Test Color [red, green, blue] × WM [high, low] × Training Illuminant [A, C]) analysis of variance (ANOVA) with the last two factors between-subjects. Any interactions not discussed below were not significant.
The critical test for a WM-based difference in color constancy is a WM × Training Illuminant interaction, which would indicate that the WM groups show a difference in the ability to establish, maintain, and/or retrieve an illuminant-independent representation of a test color. In other words, the WM × Training Illuminant interaction reveals whether there is a larger deviation from perfect color constancy for one of the WM groups. This interaction was significant for the l axis of color specification, F(1, 20) = 4.52, p = .046, ηp2 = .22, with a stronger effect of training illuminant (and thus poorer color constancy) for LowWMs (MLowWM = .027 l ) than for HighWMs (MHighWM = .020 l ) (Figure 2a).
The interaction was in the same direction but did not reach significance for the s axis, F(1, 20) = 0.300, p = .590, ηp2 = .02 (Figure 2b). It is important to note that measurements on the s axis exhibit far greater variability than measurements on the l axis, because a large difference in the s chromaticity coordinate can correspond to a small change in perceived color. This reduces the power to detect a true WM × Training Illuminant interaction for the s axis, if one exists.
In addition to a significant WM × Training Illuminant effect for the l axis, the Background × Training Illuminant interaction also reached significance for this axis, F(1, 20) = 5.91, p = .025, ηp2 = .22, corroborating previous findings (Jin & Shevell, 1996). To further investigate the effect of Background on color constancy for the two WM groups, planned tests of the WM × Training Illuminant interaction for each Background were performed, and revealed that the interaction was significant for the uniform background, F(1, 20) = 6.30, p = .021, ηp2 = .22, but not for the complex background, F(1, 20) = .120, p = .732, ηp2 = .01 (Figure 2a). LowWMs had worse color constancy than HighWMs in the uniform-background condition (MLowWM = .034 l ; MHighWM = .022 l). However, color constancy was similar across WM groups in the complex-background condition (MLowWM = .021 l ; MHighWM = .019 l). This is because LowWMs’ color constancy improved considerably from the uniform- to the complex-background condition while HighWMs’ color constancy did not change substantially.
These results suggest that LowWMs are able to make use of the many cues to the illuminant when they are available in the complex-background condition, but that LowWMs require more cues than HighWM participants to achieve a particular level of color constancy; thus, LowWMs show poorer color constancy in the uniform-background condition. The current findings may help to explain previous measurements of successive color constancy, where some individuals showed substantial improvement in their color constancy when scene complexity was increased, while others did not (Kraft, Maloney, & Brainard, 2002). Additionally, the current results are consistent with work showing, across all participants (who were not categorized according to low or high WM), better color constancy for a complex than a uniform background (Jin & Shevell 1996). While the magnitude of these WM-based differences found here may not be identical across all color constancy tasks, performance across different types of color constancy tasks typically is correlated (Reeves, Amano, & Foster, 2008).
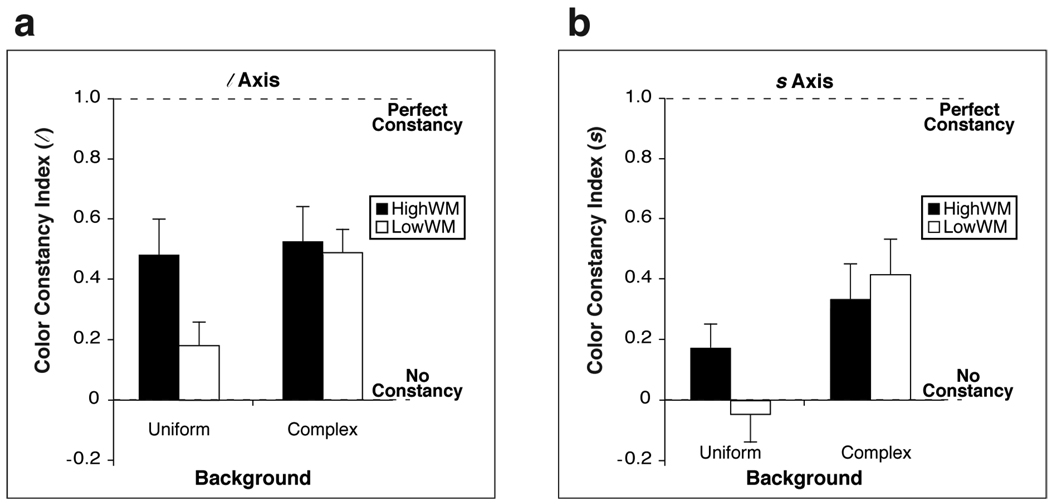
A color constancy index (CCI; Yang & Shevell, 2002) is commonly used to compare the degree of color constancy across studies, as it scales the color constancy error by the true (physical) chromaticity difference of the test color under the two illuminants (Figure 3). Thus, we calculated a CCI here as well (for a discussion of alternative calculations for the CCI, see Reeves, Amano & Foster, 2008). The CCI is calculated here for a given test illuminant and test color as 1 – (a/b), where a is the difference (in chromaticity coordinates) between the average color setting made for the group of observers trained under illuminant A and illuminant C, and b is the actual difference in chromaticity of the test color under illuminant A and illuminant C. Thus, a CCI value of 0 indicates no color constancy (difference in color settings for the two training illuminant conditions is as large as the actual difference in chromaticity of the test color under the two illuminants; a/b = 1), and a value of 1 indicates perfect color constancy (no difference in color settings for the two training illuminants; a/b approaches 0). Overall, CCI values for HighWMs were 44% higher than for LowWMs (averaging across backgrounds and MacLeod-Boynton axes). However, as seen in Figure 3, there were greater differences in CCI values between LowWM and HighWM participants for the uniform-background than for the complex-background. This was true for both the l axis (Figure 3a) and for the s axis (Figure 3b). Statistics were not performed on the CCI because its sampling distribution is unknown.
Figure 3.
Color constancy index values averaged across test colors for (a) the l axis and (b) the s axis. Results are separated by background condition (uniform and complex). Black [white] bars indicate values for HWM [LWM] participants. The vertical axis indicates the value of the color constancy index. A larger value indicates better color constancy. “Perfect constancy” refers to a color constancy index of 1. “No constancy” refers to a color constancy index of 0. A negative value is possible if the corresponding color constancy error is greater in magnitude than the shift in chromaticity of the test color under a change in illuminants (see Figure 2). Error bars indicate standard errors of the mean.
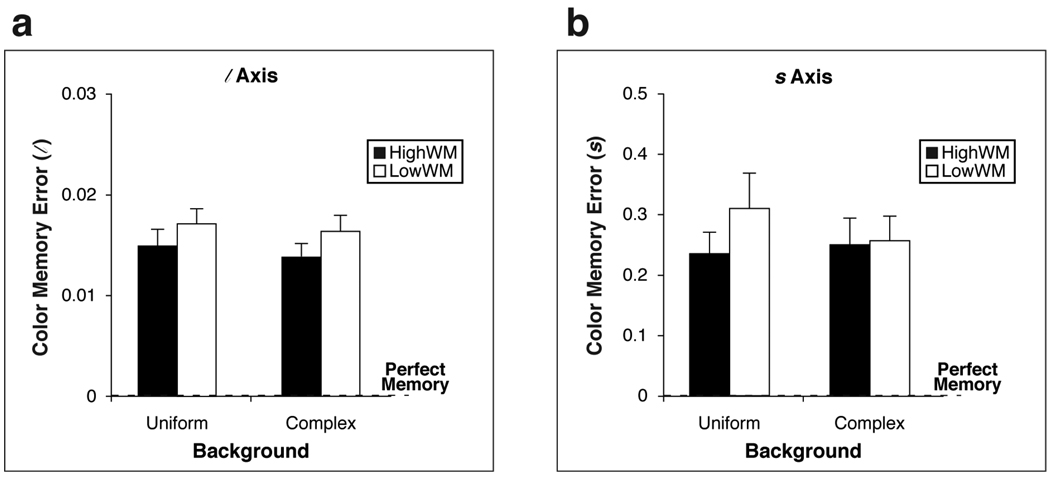
Simple color memory, as opposed to color constancy, also was assessed for the settings made when the training and test illuminants were the same. Color memory error was quantified by the absolute value of the difference (in the l and s coordinate system) between the participant’s setting and the true test color chromaticity (Figure 4). We submitted color memory errors to a 2 × 3 × 2 × 2 (Background [uniform, complex] × Test Color [red, green, blue] × WM [high, low] × Training/Test Illuminant [A, C]) ANOVA with the last two factors between-subjects. The main effect of WM was not significant for either the l axis, F(1, 20) = 1.83, p = .191, ηp2 = .05, or s axis, F(1, 20) = .890, p = .357, ηp2 = .04. HighWMs and LowWMs did not differ significantly in simple color memory.
Figure 4.
Color memory measurements (for trials where the training and test illuminants were the same) averaged across test colors for (a) the l axis and (b) the s axis. Results are separated by background condition (uniform and complex). Black [white] bars indicate values for HWM [LWM] participants. The vertical axis indicates the color memory error, calculated as the absolute value of the difference between the participant’s setting and the true test color chromaticity. A smaller value indicates better color memory. “Perfect memory” refers to a color memory error of 0. Error bars indicate standard errors of the mean. No difference between HWM and LWM participants was significant.
Participants had unlimited time to make their color settings, so the total time taken to complete each experimental session differed among participants. LowWM and HighWM participants did not differ significantly in the time taken to complete an experimental session, t(46) = 1.19, p = .239 (two-tailed).
Discussion
HighWMs showed significantly better successive color constancy than LowWMs, but HighWMs and LowWMs did not differ in simple color memory. These results represent the first relation found between individual differences in color constancy and WM.
An influential idea in the WM literature is that the main factor driving variation in WM ability is differences in the ability to control attention (Engle, 2002). Below, we outline some mechanisms by which attentional control might play a role in color constancy. Importantly, some of these mechanisms could play a role not only in successive color constancy tasks like the one used here, but also in simultaneous color constancy tasks where an illuminant-independent representation must be established but not maintained in WM.
First, attentional control may be involved in establishing the illuminant-independent representation needed for color constancy (both successive and simultaneous). Reduced attentional control can lead individuals to integrate irrelevant information with relevant information at encoding (DeCaro, Wieth, & Beilock, 2007), so LowWMs may have more difficulty than HighWMs in establishing a representation that discounts information that depends on the illuminant, thus leading to poorer color constancy. This provides a possible explanation as to why HighWMs are able to achieve better color constancy than LowWMs when very few cues to the illuminant are available (uniform-background condition). Additionally, it suggests that LowWMs may need more cues than HighWMs to achieve a particular level of color constancy, thus explaining why LowWMs perform approximately as well as HighWMs in the complex-background condition. That LowWMs would be able to maintain in WM the additional cues contained in the complex-background condition is suggested by work showing that a fixed number of objects can be maintained in WM regardless of their complexity (Awh, Barton & Vogel, 2007).
An additional mechanism suggested by past research may also provide insight as to why LowWMs perform reasonably well in the complex-background condition. A viewing strategy where more time is spent scanning different colored patches surrounding a test color improves color constancy, as the observer is better able to adapt to and then discount the training illuminant (Cornelissen & Brenner, 1995). Thus, if the gaze of LowWMs tends to wander away from the central test color during the study phase due to their reduced ability to maintain attention, it may actually benefit their color constancy in the complex-background condition. HighWMs, who may be better able to maintain attention on the central test color during the study phase, do not show a benefit from the complex-background condition as do LowWMs. This hypothesis is consistent with work showing that, during search tasks, distractibility by color singletons increases with increased WM load (Lavie & de Fockert, 2005).
Finally, the superior successive color constancy of HighWMs overall may also be related to their ability to inhibit irrelevant memory representations. Because a representation of the spectral distribution of the light entering the eye is encoded in addition to an illuminant-independent representation, conflict may occur at retrieval between these different representations. If there is an automatic tendency to recall the representation of the light entering the eye, which must be inhibited, then the performance of LowWMs will suffer, as the inhibition of automatic responses likely requires attentional control (e.g., Kane & Engle, 2003).
Importantly, we found no significant difference in simple color memory for HighWMs and LowWMs. This is consistent with work showing that individual differences in WM are unrelated to WM accuracy for shapes (Awh, Barton & Vogel, 2007). A plausible explanation for this finding is that the ability to maintain an accurate representation of the spectral distribution of the light that entered the eye from the test color (i.e., a simple representation that is not illuminant-independent) does not require attentional control.
One possible criticism of the methods used in this study is that the instruction to “set the test patch to look like the test color you remember seeing previously” might be interpreted differently among participants. However, there is no theoretical reason to predict that the two groups would systematically interpret this instruction in different ways. Moreover, another instruction that was used (to think of the colored patches composing the stimuli as “papers on a table”) is similar to one shown in other tasks to guide participants to adopt a particular interpretation that typically leads to good color constancy (e.g., Reeves, Amano, & Foster, 2008). Thus, we believe that by using this instruction, we induced an interpretation of the task that would be common across the two WM groups.
In sum, the results of this study reveal a relation between WM and color constancy, a component of color perception that we use continuously to navigate our environment. We speculate that this relation may be driven by a requirement for attentional control for successive color constancy, an idea that has not been considered previously. Whether this relation will also be found in simultaneous color constancy tasks is an interesting question for further research. In addition, these results provide the first account for the well-known individual differences in color constancy, which have been reported frequently but not investigated. More generally, this study reveals that WM capacity is related not only to complex cognitive functions but also to low-level perceptual processes.
Acknowledgments
This research was supported by a National Science Foundation CAREER award (DRL-0746970) to Sian L. Beilock and National Institutes of Health Grant EY-04802 awarded to Steven K. Shevell.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/xlm
Contributor Information
Elizabeth C. Allen, Department of Psychology and Institute for Mind & Biology, University of Chicago
Sian L. Beilock, Department of Psychology, University of Chicago
Steven K. Shevell, Department of Psychology, Institute for Mind & Biology, and Ophthalmology & Visual Science, University of Chicago
References
- Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychological Science. 2007;18(7):622–628. doi: 10.1111/j.1467-9280.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- Beilock SL, Carr TH. When high-powered people fail: Working memory and “choking under pressure” in math. Psychological Science. 2005;16(2):101–105. doi: 10.1111/j.0956-7976.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12(5):769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Cornelissen FW, Brenner E. Simultaneous color constancy revisited: An analysis of viewing strategies. Vision Research. 2003;35(17):2431–2448. [PubMed] [Google Scholar]
- Daneman M, Carpenter P. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- DeCaro MS, Wieth M, Beilock SL. Methodologies for examining problem solving success and failure. Methods. 2007;42:58–67. doi: 10.1016/j.ymeth.2006.12.006. [DOI] [PubMed] [Google Scholar]
- D’Zmura M. Color constancy: Surface color from changing illumination. Journal of the Optical Society of America A. 1992;9(3):490–493. [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11(1):19–23. [Google Scholar]
- Foster DH. Does color constancy exist? Trends in Cognitive Sciences. 2003;7(10):439–443. doi: 10.1016/j.tics.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Hegarty M, Shah P, Miyake A. Constraints on using the dual-task methodology to specify the degree of central executive involvement in cognitive tasks. Memory & Cognition. 2000;28(3):376–385. doi: 10.3758/bf03198553. [DOI] [PubMed] [Google Scholar]
- Helmholtz H. In: Helmholtz’s treatise on physiological optics. 3rd ed. Southall JPC, translator. New York: Dover; 1962. (Original work published 1866) [Google Scholar]
- Jenness JW, Shevell SK. Color appearance with sparse chromatic context. Vision Research. 1995;35(6):797–805. doi: 10.1016/0042-6989(94)00169-m. [DOI] [PubMed] [Google Scholar]
- Jin EW, Shevell SK. Color memory and color constancy. Journal of the Optical Society of America A. 1996;13(10):1981–1991. doi: 10.1364/josaa.13.001981. [DOI] [PubMed] [Google Scholar]
- Judd DB. Colorimetry and artificial daylight. Technical Committee No. 7 Report of Secretariat United States Commission, International Commission on Illumination, Twelfth Session; Stockholm. 1951. pp. 1–60. [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Poole BJ, Tuholski SW, Engle RW. Working memory capacity and the top-down control of visual search: Exploring the boundaries of “Executive attention”. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:749–777. doi: 10.1037/0278-7393.32.4.749. [DOI] [PubMed] [Google Scholar]
- Kelly KL, Gibson KS, Nickerson D. Tristimulus specification of the Munsell Book of Color from spectrophotometric measurements. Journal of the Optical Society of America. 1943;33:355–376. [Google Scholar]
- Kraft JM, Maloney SI, Brainard DH. Surface-illuminant ambiguity and color constancy: Effects of scene complexity and depth cues. Perception. 2002;31(2):247–263. doi: 10.1068/p08sp. [DOI] [PubMed] [Google Scholar]
- Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity? ntelligence. 1990;14:389–433. [Google Scholar]
- Land EH. Color vision and the natural image. Proceedings of the National Academy of Sciences of the United States of America. 1959;45:115–129. doi: 10.1073/pnas.45.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N, de Fockert J. The role of working memory in attentional capture. Psychonomic Bulletin & Review. 2005;12(4):669–674. doi: 10.3758/bf03196756. [DOI] [PubMed] [Google Scholar]
- Linnell KJ, Foster DH. Scene articulation: Dependence of illuminant estimates on number of surfaces. Perception. 2002;31:151–159. doi: 10.1068/p03sp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod DIA, Boynton RM. Chromaticity diagram showing cone excitation by stimuli of equal luminance. Journal of the Optical Society of America A. 1979;69(8):1183–1186. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- Munsell AH. A color notation. Boston: G. H. Ellis; 1905. [Google Scholar]
- Nickerson D. Spectrophotometric data for a collection of Munsell samples. Washington: U.S. Department of Agriculture; 1957. [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Rayleigh L. Experiments on colour. Nature. 1881;25:64–66. [Google Scholar]
- Reeves AJ, Amano K, Foster DH. Color constancy: Phenomenal or projective? Perception & Psychophysics. 2008;70(2):219–228. doi: 10.3758/pp.70.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuholski SW, Engle RW, Baylis GC. Individual differences in working memory capacity and enumeration. Memory & Cognition. 2001;29(3):484–492. doi: 10.3758/bf03196399. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- Unsworth N, Heitz RP, Schrock JC, Engle RW. An automated version of the operation span task. Behavioral Research Methods. 2005;37(3):498–505. doi: 10.3758/bf03192720. Research, 42, 1979–1989. [DOI] [PubMed] [Google Scholar]
- Yang JN, Maloney LT. Illuminant cues in surface color perception: Tests of three candidate theories. Vision Research. 2001;41:2581–2600. doi: 10.1016/s0042-6989(01)00143-2. [DOI] [PubMed] [Google Scholar]
- Yang JN, Shevell SK. Stereo disparity improves color constancy. Vision Research. 2002;42:1979–1989. doi: 10.1016/s0042-6989(02)00098-6. [DOI] [PubMed] [Google Scholar]