Abstract
Back pain is commonly classified based on duration. There is currently limited information regarding differences in the clinical features of back pain between these duration-based groupings. Here, we compared the pain characteristics of patients with subacute (SBP; pain 6–16 weeks, n = 40) and chronic back pain (CBP; pain ≥ 1year, n = 37) recruited from the general population. CBP patients reported significantly higher pain intensity on the Visual Analogue Scale (VAS) compared to SBP patients. Based on this finding, we investigated group differences and their dependence on VAS for the Beck Depression Inventory (BDI), sensory and affective dimensions of the McGill Pain Questionnaire (MPQ-S and MPQ-A), Neuropathic Pain Scale (NPS) and the variability of spontaneous pain. Correction for VAS abolished significant group differences on the MPQ-S, MPQ-A and NPS. Only a significant difference in the variability of spontaneous pain was independent of VAS. Finally, whereas SBP patients displayed a higher incidence of unilateral pain radiating down the legs/buttocks, there was a shift towards more bilateral pain in CBP patients. In summary, SBP and CBP groups differ on three independent parameters: VAS ratings, pain location and temporal dynamics of spontaneous pain.
Keywords: Low Back Pain, Chronic Back Pain (CBP), Subacute Back Pain (SBP), Pain Intensity, Visual Analogue Scale (VAS), McGill Pain Questionnaire (MPQ), Fractal Dimension
Introduction
Low back pain has been hailed a modern “health care enigma”, 49, affecting an estimated 60–85% of the population. 27. Chronic back pain that is non-specific, or idiopathic, accounts for the vast majority of cases in primary care. 50. Thus far, no dominant factors have been able to substantively explain the occurrence of low back pain, or predict the transition from acute to chronic low back pain. Current treatments for low back pain provide only small, short-term benefits relative to no treatment. 48. Furthermore, no single treatment can be considered superior to others for alleviating chronic back pain symptoms. 16.
A major obstacle in the management of low back pain is that it remains poorly characterized and defined. The natural course of back pain continues to be a topic of controversy. Although the common view is that 5–10% of patients go on to develop chronic pain and disability,1, 44, much higher estimates have also been reported for chronic back pain (42–75%) and recurrence of back pain episodes (24–84%). 8, 24, 33, 45. The estimated 5–10% prevalence of chronic back pain is largely based on those patients seeking medical consultation, while an estimated 50% or more cases may go unreported. 52. Furthermore, current estimates of the recovery rate from acute back pain (80–90%) are often based on measures other than pain itself, including a return to the work-place, 1, or cessation of medical consultation, 13. However, these outcomes are not necessarily correlated with pain resolution. 12.
Back pain classification systems currently differentiate between pain that is secondary to a well-characterized spinal disorder and nerve root pain, while the majority of cases are labelled ‘non-specific low back pain’ 43. The apparent heterogeneity of non-specific back pain has proven to be a major challenge in clinical trials; no consensus has been reached regarding the appropriate sub-grouping of this population. 50. A commonly used duration-based classification system for back pain defines pain lasting less that 6 weeks as acute, 7 to 12 weeks as subacute and pain ≥ 3 months as chronic. 11. However, there is no consensus regarding the optimal definition of symptom duration, 11, 15, as other experts have placed the transition from acute to chronic back pain at 6 months and 12 months after the onset of an episode. 11, 32, 47. Furthermore, there is currently limited information regarding differences in clinical features between duration-based groupings. Pain characteristic differences as a function of back pain duration clearly warrant further investigation.
We investigated differences in pain characteristics between subacute back pain (SBP) and chronic back pain (CBP), and their dependence on pain intensity. Our questionnaire-based outcomes included the visual analogue scale, the sensory and affective components of the McGill Pain Questionnaire, the Neuropathic Pain Scale and Beck Depression Inventory. Based on patients’ continuous ratings of their pain, we investigated variability of spontaneous pain in SBP and CBP. Finally, we compared pain location in SBP and CBP, including pain radiating down the legs and/or buttocks.
2. Methods
2.1. Patients and screening procedures
We recruited 40 SBP patients and 37 CBP patients to participate in the study (See Table 1). All participants were recruited from the general population through flyers, newspaper advertisements and the internet (i.e. Craig’s List). Our participants consisted of 43 males and 34 females of a mean age of 43.9 years (range: 21–74). Patients were included if they fulfilled the IASP criteria for either SBP or CBP, 36, and were diagnosed in accordance with current guidelines. 10. CBP patients reported low back pain duration ≥1 year, while SBP patients reported a history of low back pain lasting a minimum of 6 weeks and a maximum of 16 weeks. All patients qualifying for the study reported a pain greater than 4 on a 0–10 VAS scale (where 0 equals no pain and 10 is the worst imaginable pain). Pain radiating down the leg and/or buttock was an inclusion criterion for SBP patients, but not for CBP. For the analysis of depression scores, we included data from a group 15 healthy control participants that had been recruited as part of another study. For SBP, CBP and control groups, a depression index score ≥19 was considered an exclusion criterion. Participants signed written informed consent to the experimental procedure, which was approved by the Northwestern University Institutional Review Board.
Table 1.
Demographic characteristics of patients.
| Outcomes | SBP | CBP |
|---|---|---|
| Gender : | ||
| Males (n) | 22 | 22 |
| Females (n) | 18 | 15 |
| Patient Characteristics: | ||
| Mean Age | 38.5 yrs | 49.6 yrs |
| Mean Pain Duration | 11.9 wks | 12.8 yrs |
2.2. Experimental design and pain rating
2.2.1. Spontaneous pain
Prior to testing, patients were trained to rate their spontaneous back pain (in the absence of an external stimulus) using a finger-span logging device designed to measure voltage changes corresponding to the opening and closing of their fingers. 2. During testing, patients were instructed to use the finger-span device to continuously rate fluctuations in their pain on a scale from 0–100 by opening and closing their fingers, with fingers open to their comfortable maximum indicating the worst pain imaginable (VAS=100) and fingers closed all the way indicating no pain at all (VAS=0). Patients received feedback on their pain rating in real-time represented as a visual bar projected onto a screen that moved up and down on a scale from 0–100.
We characterized patients’ continuous ratings of their spontaneous pain using the time-series for their pain ratings. We examined the time-dependent variability of fluctuations in spontaneous pain by computing the fractal dimension (D) of each time series. Spontaneous pain fluctuations are described as “persistent” when periods of increasing or decreasing pain have a higher probability of being followed by a trajectory in the same direction and “anti-persistent” when they are more likely to be followed by a trajectory in the opposite direction. 18. The fractal dimension of chronic pain is a value from 1–2, which indicates whether the spontaneous fluctuations follow a pattern of persistence (D=1.0–1.5), anti-persistence (D=1.5–2.0) or randomness (D=1.5). 17, 34.
2.2.2. Self-reported questionnaire-based outcomes
Pain characteristics and depression were determined using validated questionnaires administered on the day of testing, immediately prior to the spontaneous pain-rating task. Patients completed the Beck Depression Inventory (BDI). 5, the short form of the McGill Pain Questionnaire (MPQ). 35 and Neuropathic Pain Scale (NPS). 19. Patients’ total scores were obtained for the BDI and NPS. We separately analyzed sections of the MPQ, including the intensity (VAS), sensory dimension (MPQ-S) and affective dimension (MPQ-A) of pain. Based on patients’ reported medication use (see Table 2), we calculated their scores on the Medication Quantification Scale (MQS) in accordance with currently recommended guidelines. 22.
Table 2.
Percentage of patients taking medications at the time of the study.
| Medication Type | SBP (% of total) | CBP (% of total) |
|---|---|---|
| Opiates | 10 | 8 |
| Non-steroidal anti-inflammatory | 27 | 49 |
| Antidepressants | 2 | 0 |
| Acetominophen | 5 | 38 |
| Anticonvulsants | 2 | 0 |
| Muscle relaxants | 5 | 8 |
| Combinations | 12 | 0 |
2.2.3. Pain location
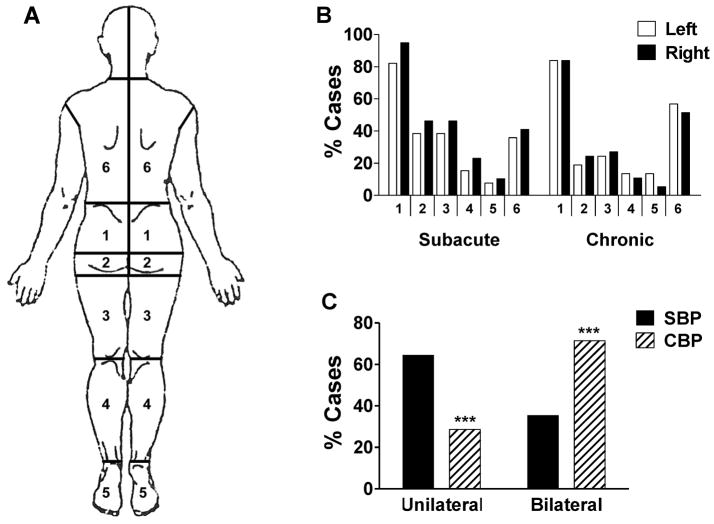
On the day of testing, we administered the MPQ form, which contains a diagram of the the human body (fronta and back view). Patients freely shaded in the region of their pain on the body diagram using a pencil. We then conducted a post hoc analysis of the prevalence of pain within specific body sites and the laterality of pain radiating down the legs/buttocks. After the completed forms had been returned to us, we divided the body into six sections on the left and right side. For each section, we used a binary coding system to indicate the presence or absence of pain based on whether there was shading within that region. We identified patients with pain radiating down the legs and/or buttocks based on regions 2–5; radiating pain was indicated by the presence of shading within ≥ 1 of these regions either unilaterally or bilaterally. Radiating pain was classified as unilateral if the shading within these regions was limited to one side of the body and bilateral if there was shading on both sides of the body.
We further distinguished between patients reporting a spatial distribution consistent with radicular pain and those with non-radicular pain. Leg/buttock pain of lumbar origin is typically classified as radicular pain or somatic referred pain, based on the location and sensory qualities of the pain. Radicular pain is characterized by a sharp or lancinating sensation that travels down the entire length of the leg in a narrow band. 6. In contrast, somatic referred pain is a dull, aching sensation of fixed location that is typically confined to the buttocks and upper thigh region. 6, 21. Based on these criteria, we classified radicular patients as those reporting the presence of pain that extended down the entire length of the leg (i.e. regions 2–5, inclusively). The remainder of patients reporting pain within some, but not all of these regions, were categorized as non-radicular.
3. Statistical analysis
We first analyzed differences in VAS ratings between SBP and CBP using an unpaired t- test for the effect of group. We subsequently investigated the contribution of VAS to differences in depression and pain characteristics using a regression analysis for the interaction effect of group by VAS rating on BDI, NPS, MPQ-S, MPQ-A and fractal dimension. For those measures that were independent of VAS (and for VAS itself), we performed multiple regression analyses testing the interaction of group with the joint effects of age, gender, depression, and radiating pain score (bilateral, unilateral or none). To determine the depression levels of patients compared to the general population, we performed a one-way ANOVA comparing the BDI scores of SBP, CBP and a group of healthy control participants; post-hoc t-test were used when appropriate. The aforementioned analyses were based on a sample of 40 SBP patients and 37 CBP patients. Statistical outliers were identified using Grubbs Test and removed from the analyses. Sample sizes for the fractal dimension analysis were smaller (SBP = 32, CBP = 36) due to the fact that 8 SBP and 1 CBP patient did not complete the spontaneous pain rating task.
For VAS and fractal dimension, we investigated the contribution of medication use to outcomes both within and between-groups. We first performed a regression analysis for the interaction effect of group by MQS score, and then performed separate regression analyses for the effect of MQS score in SBP and CBP groups. These analyses were based on 32 SBP and 26 CBP patients that had provided sufficient information for the calculation of the MQS score (i.e. name of medication, dosage, frequency of use).
Pain location was investigated with separate between-groups comparisons of (1) pain at individual body sites (2) bilateral and unilateral pain radiating pain and (3) radicular pain. For each of the twelve body regions, we conducted separate unpaired t-tests comparing the incidence of reported pain between SBP and CBP groups. Among those patients with pain radiating down the legs/buttocks, we performed two separate Fisher’s exact tests. We compared the incidence of unilateral and bilateral radiating pain in SBP and CBP groups, and then the incidence of radicular versus non-radicular pain in both groups. We then compared the sensory and affective pain characteristics of radicular and non-radicular patients using an unpaired t-test. Based on the inclusion criteria described in Section 2.1., radiating pain was present in 31/40 SBP and 14/37 CBP patients. The group incidence of unilateral and bilateral pain and radicular and non-radicular pain were therefore expressed as a percentage of these radiating pain cases. For all statistical tests in the present study, the level of significance was set to p=0.05.
4. Results
4.1. The contribution of VAS to depression and pain characteristic differences between SBP and CBP
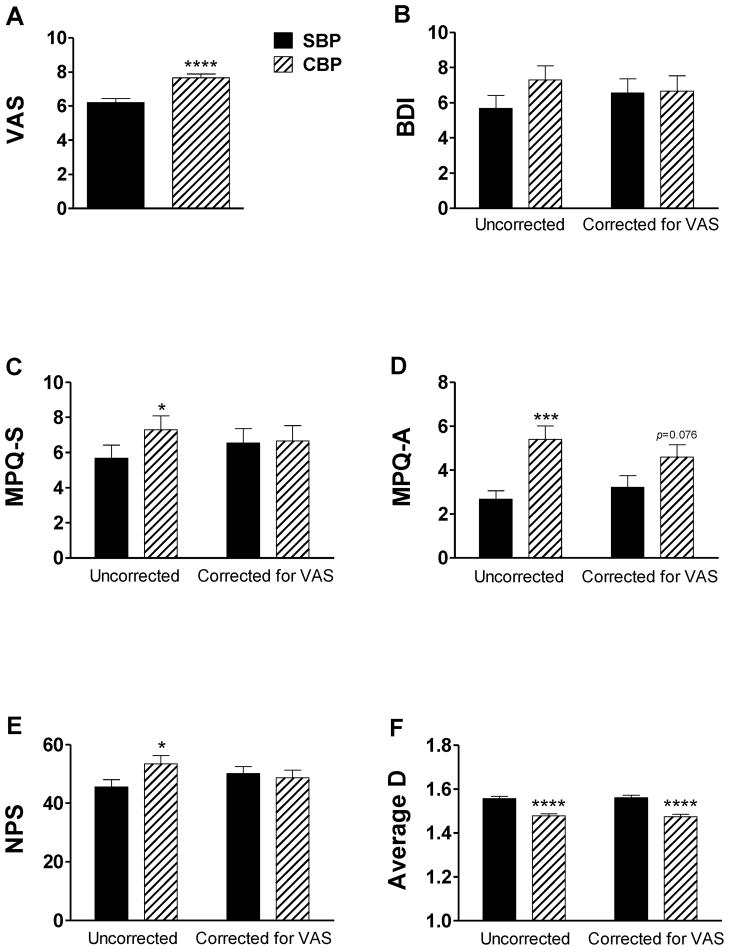
We initially found that VAS ratings differed between patients with SBP and CBP (Mean±SEM: 6.2±0.2 and 7.7±0.2, respectively). As shown in Figure 1a, CBP patients displayed a highly significant increase in VAS ratings compared to SBP patients (t1, 75=4.6, p=2×10−5). Based on this robust effect, we further investigated the contribution of pain intensity to group differences in depression, sensory and affective pain ratings and the variability of spontaneous pain. Before correction for VAS (Figure 1b–f, left side), patients with CBP showed a significant increase relative to SBP patients on the NPS (t1, 75=2.1, p=0.04), MPQ-S (t1, 74=2.6, p=0.01) and MPQ-A (t1, 74=3.8, p=3×10−4), as well as a highly significant increase in the fractal dimension of spontaneous pain (t1, 66=−5.5, p=10−7).
Figure 1. Differences in sensory and affective dimensions between SBP and CBP failed to survive correction for pain intensity.
(a) CBP patients displayed a robust increase in VAS ratings compared to SBP patients. In graphs b–f, we present the mean total scores for the (b) BDI (c) MPQ-S and (d) MPQ-A, (e) NPS and (f) fractal dimension both uncorrected (left) and corrected for VAS rating (right). Before correction for VAS, patients with CBP had significantly higher ratings for MPQ-S, MPQ-A, NPS and lower fractal dimension. Only the significant increase in fractal dimension survived correction, indicating that variability of spontaneous pain is the sole parameter independent of VAS. Bars represent mean ± S.E.M. (n=32–40). *p<0.05; ***p<0.001; ****p<0.0001.
No significant differences between SBP and CBP patients in BDI score were observed (Figure 1b), although there was a trend towards higher depression levels among CBP patients (t1, 75 =1.5, p=0.14). However, when we compared the BDI scores of SBP patients (Mean±SEM: 5.7±0.7), CBP patients (Mean±SEM: 7.3±0.8) and healthy controls (Mean±SEM: 1.5±0.5), we found a significant main effect of group (F2, 88=8.5, p=0.0004). Here, control participants reported significantly lower BDI scores compared to both SBP patients (t1, 53=3.3, p=0.002) and CBP patients (t1, 50=4.5, p=5×10−5).
Most of the differences between SBP and CBP did not survive correction for VAS (Figure 1b–f, right side). In the regression analysis, VAS was a factor contributing to BDI (F1, 60 =8.9, p=0.0049), MPQ-S (F1, 58=13.1, p=0.0006), MPQ-A (F1, 58=4. 9, p=0.03) and NPS (F1, 59=30.2, p=10−6), and correction abolished the significant group differences on all these dependent measures. Only group differences in the fractal dimension of spontaneous pain remained statistically significant after correction for VAS (F1, 65=27.56, p=2×10−6), indicating that these two parameters are independent of each other.
Because radiating pain was an inclusion criterion for SBP and not CBP patients, we investigated whether this factor may have accounted for the observed group differences in pain characteristics. We re-tested the group differences in depression and pain characteristics, but only including those SBP and CBP patients that had reported radiating pain. Overall, group differences remained strikingly similar even after omission of those patients without radiating pain. Compared to SBP patients, those with CBP had higher ratings for VAS (t1,42= 2.6, p=0.014), fractal dimension (t1,38= 5.9, p=1×10−6), MPQ-A (t1,41= 2.0, p=0.052), MPQ-S (t1,41= 1.6, p=0.11) and NPS (t1,42= 1.7, p=0.094). Not all group differences retained statistical significance, which is to be expected, given the loss of statistical power. More importantly, the effect of VAS correction on these group differences remained robust. Again, pain intensity was a significant factor contributing to variability on all the above dependent measures except for fractal dimension, which remained significantly different between SBP and CBP after VAS correction (F1,37=29.0, p=4×10−6). These findings suggest that the difference in inclusion criteria between SBP and CBP groups does not account for the observed group differences in pain characteristics. Next, we investigated whether any additional variables contributed to the group differences in VAS and fractal dimension. We performed multiple regression analyses for the interaction effect of group with the joint effects of age, gender, and radiating pain score on these dependent measures. Even after correction for multiple variables, group differences remained highly significant for both VAS ratings (F1, 71=10.7, p=1×10−4) and fractal dimension (F1, 62= 30.4, p=1×10−6). However, we note that age was a near-significant regressor for fractal dimension (F1, 62= 3.4, p=0.09). In separate set of regression analyses, we found that the MQS score for medication use did not significantly correlate with VAS or fractal dimension either within or between-groups (data not shown).
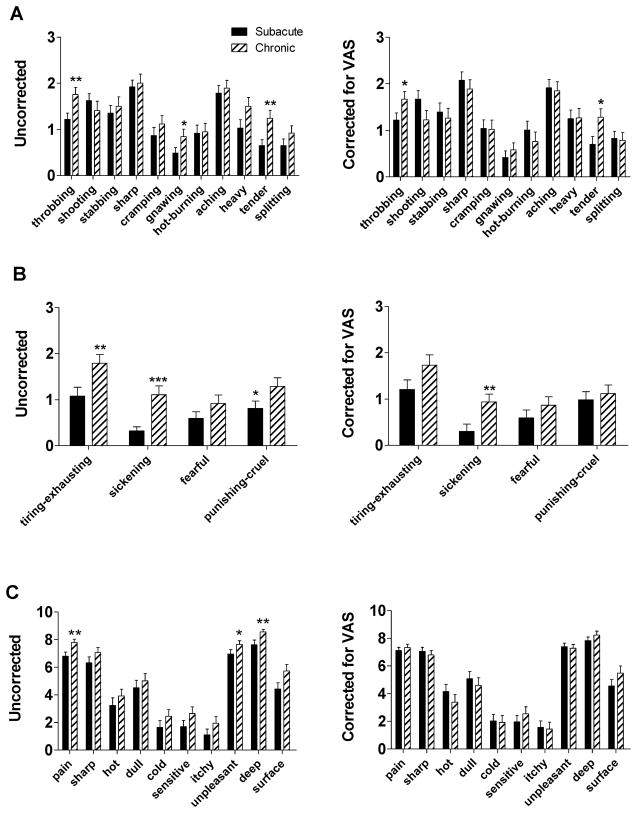
As shown in Figure 2, VAS also contributed to group differences in scores on individual questionnaire items of the MPQ-S, MPQ-A and NPS. Before correction for VAS (Figure 2a–c, left side), CBP patients had significantly higher scores on nine questionnaire items, including the MPQ-S items throbbing (p=0.01), gnawing (p=0.04) and tender (p=0.01), the MPQ-A items tiring-exhausting (p=0.01), sickening (p=10−6) and punishing-cruel (p=0.03) and the NPS items pain (p=0.002), unpleasant (p=0.04) and deep (p=0.001). Correction for VAS abolished most, but not all of the significant group differences on individual questionnaire items scores (Figure 2a–c, right side). When taking VAS into account, the only three MPQ items remained significantly higher among CBP patients, including throbbing (p=0.046), tender (p=0.025) and sickening (p=0.01).
Figure 2. Group differences in scores for sensory and affective pain questionnaire items were abolished by correction for pain intensity.
We investigated differences between SBP and CBP in the individual questionnaire items related to the sensory and affective dimensions of pain. Here, we present the mean scores for each questionnaire item of the (a) MPQ- S (b), MPQ-A and (c) NPS, both uncorrected (left) and corrected for VAS ratings (right). Patients with CBP had higher uncorrected scores on nine questionnaire items (six MPQ-S, three MPQ-A, three NPS items). After correction for VAS, the majority of these significant group differences were abolished. Bars represent mean ± S.E.M. (n=37–40). *p<0.05; **p<0.01; ***p<0.001.
4.3. Pain location
We conducted separate between-groups analyses of each of the twelve body sites we had delineated (Figure 3a), and found no significant group differences in the incidence of pain (Figure 3b). However, the incidence of unilateral and bilateral radiating pain differed significantly between the SBP and CBP based on a Fisher’s Exact Test (p=0.0000). As shown in Figure 3c, there is a shift from a greater prevalence of unilateral radiating pain among SBP patients to predominantly bilateral radiating pain among CBP patients. Across both groups, we found that status of radiating pain (bilateral, unilateral, none) did not covary significantly with either VAS (p = 0.27) or fractal dimension (p = 0.46), indicating that all three parameters are independent of one another.
Figure 3. Evidence of a shift in the laterality of pain during the transition from SBP to CBP.
We characterized pain location based on the body regions that patients had shaded in with pencil on the MPQ form. (a) The body was divided into six sections on the left and right side. (b) We conducted separate between-groups comparisons at each of the twelve body sites, and found that the incidence of reported pain did not differ between SBP and CBP groups for any of the regions. (c) Pain radiating down the legs and/or buttocks (i.e. pain in ≥1 of regions 2–5) was more frequently unilateral in SBP patients, and more bilateral among CBP patients. Bars represent mean ± S.E.M. (n=37–40). *** p<0.001
Among those patients with pain extending down the legs and/or buttocks, we investigated the incidence of radicular pain. In both the SBP and CBP group, roughly 15% of patients displayed a spatial distribution consistent with the clinical definition of radicular pain, in that it extended down the entire length of the leg. Patients displaying a radicular spatial distribution reported similar pain characteristics to those that did not (data not shown). An exception was the MPQ-S item, hot-burning, which was significantly higher among radicular patients (t1, 40=2.8, p=0.007).
5. Discussion
We investigated differences in the characteristics of back pain as a function of pain duration. Based on our analyses, SBP and CBP patients differed on three statistically independent dimensions: (1) pain intensity (2) pain location and (3) the temporal dynamics of spontaneous pain. We first investigated VAS ratings, and found that CBP patients reported significantly greater pain intensity relative to SBP patients. The magnitude of the increase in VAS rating among our CBP patients (14.5mm) is above the minimum change in acute pain considered clinically significant (13mm). 20. With respect to pain location, we observed a shift from a higher incidence of unilateral radiating pain among SBP patients to predominantly bilateral pain among CBP patients. Surprisingly, scores on the sensory and affective dimensions of pain were largely dependent of pain intensity, and correction for this factor abolished most of the significant group differences on these measures. Depression scores only showed a modest increase with greater pain duration, and this effect was also largely a function of the differences in pain intensity between the groups.
A small number of previous studies comparing SBP and CBP patients obtained results largely in agreement with ours. The Olmsted County Health Study, 51, a large cross-sectional study, found that chronic pain sufferers reported greater pain frequency and intensity than those with pain of a shorter duration. Chronic pain sufferers also had a higher incidence of chronic pain at multiple body sites compared to subacute and acute sufferers.
A recent study attempted to differentiate between SBP and CBP patients based on a battery of clinical tests. 38. SBP patients differed from CBP patients based on functional tests (e.g. walking), presence of sensation in the feet and pain provocation tests. The latter category involved the measurement of pain in response to specific test-movements (e.g. heel drop, transverse process pressure). On pain provocation tests, CBP patients showed greater sensitivity relative to SBP patients with significant odds ratios (3.69–5.91).
The results of these two previous studies are in agreement with our main finding, namely that CBP patients report increased pain sensation compared to SBP. A shift towards a higher percentage of bilateral radiating pain among CBP in our study implies the recruitment of a greater number of painful sites outside the lumbar region compared to SBP. This latter finding is consistent with these earlier reports. 38, 51.
On the one hand, differences between SBP and CBP may reflect ongoing symptomatic changes accompanying the transition from acute to persistent back pain. For instance, a shift from unilateral to bilateral radiating pain among CBP patients may indicate the development of contralateral or “mirror pain” – a symptom which has been reported in several different chronic pain conditions and in some rodent models of neuropathic pain. 25. Alternatively, patients displaying high intensity, bilateral pain at the subacute phase might simply be more likely to go on to develop persistent pain; this would suggest that pain intensity and location are unchanging parameters that nonetheless predict CBP. Future longitudinal investigations would be needed to clarify the relationship between pain characteristics and pain duration.
In agreement with the latter hypothesis, there is some evidence that location of pain in SBP patients may serve as predictor of CBP. Early assessment of pain location in response to specific test movements has been recommended as a prognostic tool, particularly for back pain that is non-specific. 14, 29. According to several reports, SBP patients experiencing movement-evoked pain at distal body sites have a poorer prognosis than those in which pain is more localized to the lumbar region. 14, 28, 29, 53.
We observed a robust difference in the fractal dimension of the spontaneous pain of SBP and CBP. Our lab has previously demonstrated that the fractal dimension values of spontaneously-occurring pain differs from that of pain evoked by an acute noxious stimulus. 18. Fractal properties may also be useful in distinguishing between different types of chronic pain states. In the present study, a higher fractal dimension among SBP patients indicates that pain is more anti-persistent, that is, periods of increasing pain are more likely to be followed by a decrease and vice versa. Group differences proved to be independent of VAS, suggesting that fractal dimension may serve as a useful additional marker of the transition from SBP to CBP. Furthermore, changes in fractal dimension may indicate a shift in the neurophysiological mechanisms underlying the pain.
A compelling finding in the present study was correcting for VAS abolished the majority of significant group differences in the sensory and affective characteristics of pain. These results are consistent with another study in our lab examining the effects of a Lidocaine patch therapy for CBP, in which significant effects on pain characteristics were dependent on VAS, with the exception of fractal dimension. 7. Pain affect and intensity are commonly viewed as dissociable dimensions. 40, 41. Furthermore, higher affective pain scores are thought to be a key a feature distinguishing chronic pain from acute pain. 40, 54. The predominance of VAS in the present study suggests that ratings of pain affect and intensity are more interdependent in back pain.
We investigated whether our patients displayed radiating leg/buttock pain symptoms that conformed to existing classifications. We found that in both SBP and CBP groups, roughly 15% of patients showed a spatial distribution consistent with radicular pain/radiculopathy, in that it extended down the entire length of the limb. According to Bogduk, 6, pain traveling down the length of the leg can only qualify as radicular if it has a sharp or lancinating quality, because this is the “only type of pain that has been produced by stimulating nerve roots”. In contrast, somatic referred pain is primarily associated with a dull or aching sensation. 3, 6, 21. Contrary to our expectations, patients displaying a radicular pattern had nearly identical pain characteristics to those who did not. An exception was the significant group difference in the MPQ-S item ‘hot-burning’, a symptom that is commonly associated with nerve compression or inflammation. 6. This dissociation between pain location and sensory characteristics requires further investigation.
Depression is ranked as one of the strongest predictors of low back pain. 37, 42. Here, we observed a trend towards higher depression scores in CBP patients compared to SBP patients, and this difference was also entirely a function of pain intensity ratings. Depression scores were still high among SBP and CBP groups relative to our healthy control group, as well as previously reported estimates of the population mean BDI score for healthy participants (males=2.2, females=2.8). 26. A pertinent question is whether our sample is truly representative of the back pain population, given that we excluded severely depressed patients (BDI score ≥ 19). Yet, only a small number of participants screened for the present study were excluded due to high depression scores (3.2 % of CBP participants, 0.8% of SBP participants).
This small percentage of excluded participants was surprising given the reportedly high prevalence of major depressive disorder among chronic pain patients (30–71%). 4, 23, 39, 46. One possible reason for this discrepancy is that the present study recruited participants from the general population, as opposed to the clinical population. Population-based studies of chronic pain, which have fewer selection biases, do tend to report lower prevalence of depression (16–20%). 9, 30, 31. Our depression rates are even lower than these previous population-based estimates and may partly reflect differences in recruitment methods. For instance, previous population-based studies contacted participants through telephone or mail survey, 9, 30, 31, whereas our study required participants to take a more active role by responding to newspaper or internet advertisements.
A potential concern of the current study regards the clinical evaluation of the two groups of patients. Whereas those evaluated with CBP have had secondary etiologies investigated and largely ruled out, those evaluated as having SBP have not been subject to the same level of health care. It is altogether possible that there may be some individuals in the SBP group who would have an etiology defined for their low back pain; however, the epidemiologic data supports the fact that this number would be expected to be very low and not of a magnitude that would impact our conclusions. Still, ideally the SBP patients should have undergone radiological evaluation to rule out potential secondary etiologies.
In conclusion, we observed significant differences in the properties of pain based on duration-based groupings. On average, SBP and CBP patients differed in their pain intensity ratings, pain location and the time-dependent variability of their spontaneous pain. The fact that these three parameters are independent of one another suggests that they provide novel, non-overlapping information about the status of back pain. Future longitudinal studies will be required to characterize the temporal changes in these pain characteristics that may accompany the transition from SBP to CBP.
Acknowledgments
This research was funded by a National Institute for Health Research Grant NINDS NS35115. We report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersson GB, Svensson HO, Odén A. The intensity of work recovery in low back pain. Spine. 1983;8:880–884. doi: 10.1097/00007632-198311000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Krauss BR, Fredrickson BE, Szeverenyi NM. Imaging the pain of low back pain: Functional magnetic resonance imaging in combination with monitoring subjective pain perception allows the study of clinical pain states. Neurosci Lett. 2001;299:57–60. doi: 10.1016/s0304-3940(01)01504-x. [DOI] [PubMed] [Google Scholar]
- 3.Audette JF, Emenike E, Meleger AL. Neuropathic low back pain. Curr Pain Headache Rep. 2005;9:168–177. doi: 10.1007/s11916-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 4.Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: A diathesis-stress framework. Psychol Bull. 1996;119:95–110. [Google Scholar]
- 5.Beck AT, Rial WY, Rickels K. Psychological Reports. 1974. Short form of depression inventory: Cross-validation. [PubMed] [Google Scholar]
- 6.Bogduk N. On the definitions and physiology of back pain, referred pain, and radicular pain. Pain. 2009;147:17–19. doi: 10.1016/j.pain.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Chanda ML, Parks EL, Baria AT, Geha PY, Baliki MN, Schnitzer TJ, Apkarian AV. A double-blind, placebo-controlled study of the effects of lidocaine patch therapy on brain activity for spontaneous pain. 39th Annual Meeting of the Society for Neuroscience; 2009. Abstract 358. [Google Scholar]
- 8.Costa Lda C, Maher CG, McAuley JH, Hancock MJ, Herbert RD, Refshauge KM, Henschke N. Prognosis for patients with chronic low back pain: Inception cohort study. BMJ. 2009 Oct 6;339:b3829. doi: 10.1136/bmj.b3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie SR, Wang J. Chronic back pain and major depression in the general canadian population. Pain. 2004;107:54–60. doi: 10.1016/j.pain.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363–70. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 11.Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, Wyatt M, Cassidy JD, Rossignol M, Leboeuf-Yde C, Hartvigsen J, Leino-Arjas P, Latza U, Reis S, Gil Del Real MT, Kovacs FM, Oberg B, Cedraschi C, Bouter LM, Koes BW, Picavet HS, van Tulder MW, Burton K, Foster NE, Macfarlane GJ, Thomas E, Underwood M, Waddell G, Shekelle P, Volinn E, Von Korff M. A Consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine. 2008;33:95–103. doi: 10.1097/BRS.0b013e31815e7f94. [DOI] [PubMed] [Google Scholar]
- 12.Dionne CE, Von Korff M, Koepsell TD, Deyo RA, Barlow WE, Checkoway H. A comparison of pain, functional limitations, and work status indices as outcome measures in back pain research. Spine. 1999;24:2339–2345. doi: 10.1097/00007632-199911150-00009. [DOI] [PubMed] [Google Scholar]
- 13.Dixon ASJ. Progress and problems in back pain research. Rheumatol Rehabil. 1973;12:165–175. [Google Scholar]
- 14.Donelson R, Murphy K, Silva G. Centralization phenomenon: Its usefulness in evaluating and treating referred pain. Spine. 1990;15:211–213. [PubMed] [Google Scholar]
- 15.Dunn KM, Croft PR. The importance of symptom duration in determining prognosis. Pain. 2006;121:126–32. doi: 10.1016/j.pain.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Ehlrlich GE. Low back pain. Bull World Health Organ. 2003;81:671–676. [PMC free article] [PubMed] [Google Scholar]
- 17.Feder J. Fractals, Physics of Solids and Liquids. 1988. [Google Scholar]
- 18.Foss JM, Apkarian AV, Chialvo DR. Dynamics of pain: Fractal dimension of temporal variability of spontaneous pain differentiates between pain states. J Neurophysiol. 2006;95:730–736. doi: 10.1152/jn.00768.2005. [DOI] [PubMed] [Google Scholar]
- 19.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: The neuropathic pain scale. Neurology. 1997;48:332–8. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38:633–638. doi: 10.1067/mem.2001.118863. [DOI] [PubMed] [Google Scholar]
- 21.Govind J. Lumbar radicular pain. Aust Fam Physician. 2004;33:409–412. [PubMed] [Google Scholar]
- 22.Harden RN, Weinland SR, Remble TA, Houle TT, Colio S, Steedman S, Kee WG and Physicians. APS. Medication quantification scale version III: Update in medication classes and revised detriment weights by survey of american pain society physicians. J Pain. 2005;6:364–71. doi: 10.1016/j.jpain.2005.01.350. [DOI] [PubMed] [Google Scholar]
- 23.Harris CA, D’Eon JL. Psychometric properties of the beck depression inventory--second edition (bdi-ii) in individuals with chronic pain. Pain. 2008;137:609–22. doi: 10.1016/j.pain.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Hestbaek L, Leboeuf-Yde C, Manniche C. Low back pain: what is the long-term course? a review of studies of general patient populations. Eur Spine J. 2003;12:149–65. doi: 10.1007/s00586-002-0508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang D, Yu B. The mirror-image pain: an unclered phenomenon and its possible mechanism. Neurosci Biobehav Rev. 2010;34:528–532. doi: 10.1016/j.neubiorev.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Knight RG. Some General population norms for the short form of the beck depression inventory. J Clin Psychol. 1984;40:751–3. doi: 10.1002/1097-4679(198405)40:3<751::aid-jclp2270400320>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 27.Krismer M, van Tulder M. Low back pain (non-specific) Best Pract Res Cl Rh. 2007;21:77–91. doi: 10.1016/j.berh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Long A. The centralization phenomenon: its usefulness as a predictor of outcome in conservative treatment of chronic low back pain. Spine. 1995;20:2513–2521. [PubMed] [Google Scholar]
- 29.Long A, May S, Fung T. The comparative prognostic value of directional preference and centralization: a useful tool for front-line clinicians? J Man Manip Ther. 2008;16:248–54. doi: 10.1179/106698108790818332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magni G, Caldieron C, Rigatti-Luchini S, Merskey H. Chronic musculoskeletal pain and depressive symptoms in the general population. an analysis of the 1st national health and nutrition examination survey data. Pain. 1990;43:299–307. doi: 10.1016/0304-3959(90)90027-B. [DOI] [PubMed] [Google Scholar]
- 31.Magni G, Marchetti M, Moreschi C, Merskey H, Luchini SR. Chronic musculoskeletal pain and depressive symptoms in the national health and nutrition examination. i. epidemiologic follow-up study. Pain. 1993;53:163–168. doi: 10.1016/0304-3959(93)90076-2. [DOI] [PubMed] [Google Scholar]
- 32.Main CJ, Spanswick CC. Pain management: An interdisciplinary approach. Elsevier; 2001. [Google Scholar]
- 33.Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA and Physicians. ASoIP: Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12:E35–70. [PubMed] [Google Scholar]
- 34.Mandelbrot BB. The fractal geometry of nature. 1982. [Google Scholar]
- 35.Melzack R. The short-form mcgill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 36.Merskey H, Bogduk N. Classification of chronic pain; 1994. [Google Scholar]
- 37.Meyer T, Cooper J, Raspe H. Disabling low back pain and depressive symptoms in the community-dwelling elderly: A prospective study. Spine. 2007;32:2380–2386. doi: 10.1097/BRS.0b013e3181557955. [DOI] [PubMed] [Google Scholar]
- 38.Paatelma M, Karvonen E, Heiskanen J. Clinical perspective: How do clinical test results differentiate chronic and subacute low back pain patients from “non-patients”? J Man Manip Ther. 2009;17:11–19. doi: 10.1179/106698109790818197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole H, Bramwell R, Murphy P. Factor structure of the beck depression inventory-ii in patients with chronic pain. Clin J Pain. 2006;22:790–798. doi: 10.1097/01.ajp.0000210930.20322.93. [DOI] [PubMed] [Google Scholar]
- 40.Price DD, Harkins SW, Baker C. Sensory-affective relationships among different types of clinical and experimental pain. Pain. 1987;28:297–307. doi: 10.1016/0304-3959(87)90065-0. [DOI] [PubMed] [Google Scholar]
- 41.Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain. 1999;82:159–71. doi: 10.1016/S0304-3959(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 42.Reid MC, Williams CS, Gill TM. The relationship between psychological factors and disabling musculoskeletal pain in community-dwelling older persons. J Am Geriatr Soc. 2003;51:1092–1098. doi: 10.1046/j.1532-5415.2003.51357.x. [DOI] [PubMed] [Google Scholar]
- 43.Robinson PJ, Apkarian AV. Low back pain. In: Mayer EA, Bushnell MC, editors. Functional Pain Syndromes: presentation and pathophysiology. IASP Press; Seattle, WA: 2009. pp. 23–53. [Google Scholar]
- 44.Shekelle PG, Markovich M, Louie R. An epidemiologic study of episodes of back pain care. Spine. 1995;20:1668–73. doi: 10.1097/00007632-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Stanton TR, Latimer J, Maher CG, Hancock M. Definitions of recurrence of an episode of low back pain: A systematic review. Spine. 2009;34:E316–22. doi: 10.1097/BRS.0b013e318198d073. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan MJL, Reesor K, Mikail SF, Fisher R. The treatment of depression in chronic low back pain: Review and recommendations. Pain Med. 1992;50:5–13. doi: 10.1016/0304-3959(92)90107-M. [DOI] [PubMed] [Google Scholar]
- 47.Turk DC, Okifuji A. Pain terms and taxonomies of pain. In: Loeser JB, JJ, editors. Bonica’s Management of Pain. 3. Lippincott Williams & Wilkins; Philadelphia: 2001. [Google Scholar]
- 48.van Tulder MW, Koes B, Malmivaara A. Outcome of non-invasive treatment modalities on back pain: an evidence-based review. Eur Spine J. 2006;15:S64–81. doi: 10.1007/s00586-005-1048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waddell G. Low back pain: A twentieth century health care enigma. Spine. 1996;21:2820–2825. doi: 10.1097/00007632-199612150-00002. [DOI] [PubMed] [Google Scholar]
- 50.Wand BM, O’Connell NE. Chronic non-specific low back pain - sub-groups or a single mechanism? BMC Musculoskelet Disord. 2008;9:11. doi: 10.1186/1471-2474-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watkins EA, Wollan PC, Melton L, Jr, Yawn BP. A population in pain: Report from the olmsted county health study. Pain Med. 2008;9:166–174. doi: 10.1111/j.1526-4637.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 52.Waxman R, Tennant A, Helliwell P. Community survey of factors associated with consultation for low back pain. BMJ. 1998;317:1564–1567. doi: 10.1136/bmj.317.7172.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Werneke M, Hart DL. Centralization phenomenon as a prognostic factor for chronic low back pain and disability. Spine. 2001;26:758–765. doi: 10.1097/00007632-200104010-00012. [DOI] [PubMed] [Google Scholar]
- 54.Wilkie DJ, Savedra MC, Holzemer WL, Tesler MD, Paul SM. Use of the mcgill pain questionnaire to measure pain: A meta-analysis. Nurs Res. 1990;39:36–41. [PubMed] [Google Scholar]