Abstract
Low socioeconomic status (SES) has been associated with higher levels of allostatic load (AL). Posited mechanisms for this association include stress, personality, psychosocial variables, coping, social networks, and health behaviors. This study examines whether these variables explain the SES-AL relationship in a population-based sample of 208 51–69 year-old White, Black, and Hispanic adults in the Chicago Health, Aging, and Social Relations Study. AL was based on nine markers of physiological dysregulation. SES was inversely associated with a composite measure of AL; hostility and poor sleep quality helped to explain the association between AL and SES. Factor analyses revealed four AL components corresponding to the bodily systems of interest. SES was significantly associated with two AL components, suggesting that the effects of SES on physiological dysregulation are specific to certain systems in a middle to early-old age population.
Socioeconomic status (education, occupation, and/or income) is consistently found to be a strong, graded predictor of all-cause mortality and morbidity (e.g., Seeman, et al., 2008; Marmot, 2005). The effect of SES is not limited to the very poor who may not be able to meet basic needs, nor does there appear to be a level sufficient to prevent health problems. Rather, differences in health can be found across all levels of SES (Adler et al., 1994). The question of why these differences are found, however, remains unanswered.
One line of research has focused on biological mechanisms by which lower SES may lead to increased morbidity and mortality. Under normal conditions, bodily systems maintain constancy in the internal milieu, in part through negative feedback systems that defend set points or at least relative levels. These set points may not be optimal under all circumstances, however, and may be adaptively adjusted to respond to physiological and environmental challenges (Cacioppo & Berntson, 2007). Higher levels of the neuraxis regulate responses across a broad range of lower systems, altering the set points of individual systems and allowing for maximal flexibility in response to challenge – a process termed allostasis (Berntson & Cacioppo, 2000; Sterling & Eyer, 1988). Responses to repeated or extended challenges are thought to contribute to an accumulation of wear and tear on biological systems, termed allostatic load (AL; McEwen & Stellar, 1993).
Because biological systems are mutually dependent, challenges to one system may alter multiple set points and attempts to return one system to a more ideal set point may lead to compensation in others. Thus, AL is characterized by dysregulation across bodily systems and markers of AL are derived from multiple regulatory dimensions (e.g., Seeman et al., 2004). Typical formulations of AL include measures of cardiovascular, autonomic, sympathetic adrenomedullary, hypothalamic-pituitary-adrenocortical, and metabolic regulation. Specific markers of dysregulation have varied somewhat among studies (reviewed in Szanton, Gill, & Allen, 2005), but this has not compromised the ability to assess how physiological systems as a whole are affected by the burdens of socioeconomic, behavioral, social, and emotional factors (Kubzansky, Kawachi, & Sparrow, 1999).
Recent research has demonstrated that lower SES is associated with increased AL (Seeman et al., 2008) and that AL mediates the relationship between SES and mortality, explaining 35% of the difference in mortality attributable to SES differences (Seeman et al., 2004). To date, however, research on the pathways from SES to AL has been sparse. Many variables associated with increased AL are also associated with SES (Adler, et al., 1994) suggesting potential pathways through which SES may affect AL. These variables tend to fall into five major domains: stress (Clark, Bond, & Hecker, 2007; Glei, Goldman, Chuang, & Weinstein, 2007; Sun, Wang, Zhang, & Li, 2007; Weinstein, Goldman, Hedley, Yu-Hsuan, & Seeman, 2003), personality and psychosocial variables (Glei et al., 2007; Kubzansky et al., 1999; Maselko, Kubzansky, Kawachi, Seeman, & Berkman, 2007; Seeman, Singer, Ryff, Dienberg Love, & Levy-Storms, 2002; Sun et al., 2007; Szanton et al., 2005), coping styles (Glei et al., 2007), social networks (Maselko, Kubzansky, Kawachi, Seeman, & Berkman, 2007; Seeman et al., 2004; Seeman et al., 2002), and health behaviors (Kinnunen, Kaprio, & Pulkkinen, 2005; Schnorpfeil et al., 2003; Sun et al., 2007).
In the stress domain, one study examined whether perceived job demands in the workplace or the stress of recent widowhood mediated the association between SES and AL (Weinstein et al., 2003). Neither type of stress explained the association between SES and AL. Decision latitude in a job setting has also been examined but failed to explain the link between SES and AL (Sun et al., 2007). In the personality and psychosocial domain, Type-A personality, optimism, hopelessness, and hostility have been proposed to mediate the SES-AL association (Kubzansky, et al., 1999; Sun, et al., 2007), but the only psychosocial factor shown to do so has been hostility (Kubzansky et al., 1999). Hostility, in turn, has been proposed to operate through an actual or perceived increase in social stress or through health behaviors (Kubzansky et al., 1999), but no evidence has been provided for these hypotheses. In the coping domain, coping resources (e.g., sense of mastery, perceived control) tend to be more limited in low than high SES individuals and have been shown to contribute to physiological dysregulation (Glei, et al., 2007), but their role in explaining the influence of SES on AL has not been tested.
In the social network domain, previous research suggests that high quality social relationships may buffer the effects of low SES, a moderating role for social relationships that differs from a mediating role in which SES influences AL indirectly through its influence on social relationships quality (Singer & Ryff, 1999). However, many aspects of social networks have not yet been explored, including individual differences in social opportunities. People low in SES may have fewer opportunities to form high quality social relationships and this may explain why low SES individuals are at increased risk for high AL.
In the health behavior domain, SES has been associated with smoking, physical activity, and alcohol consumption (Adler, et al., 1994), as well as subjective sleep quality (Friedman et al., 2007). Interventions targeting health behaviors (diet, exercise, and stress management) associated with low SES have been shown to reduce AL-related coronary risk factors (Govil, Weidner, Merritt-Worden, & Ornish, 2009). Some researchers have called for greater attention to the role of health behaviors in mediating the association between SES and AL (Szanton et al., 2005), but to date, health behaviors have not been examined in this capacity.
We investigate pathways through which SES could potentially lead to physiological dysregulation in a population-based urban sample of 51–69 year-old adults in Cook County, Illinois. We first assess whether the inverse association between SES and AL replicates in a population-based sample. Second, we examine whether stress, personality, psychosocial variables, coping style, social network, and health behaviors are associated with both SES and AL, a prerequisite condition for mediation. Third, we investigate the extent to which each plausible mediating variable explains the relationship between SES and AL. Finally, we decompose the measure of AL to determine the association of SES with individual components of AL and evaluate the premise that a composite measure of allostatic load is a more reliable and informative measure of physiological dysregulation than measures of dysregulation in any one physiological system.
Methods
Participants
Data for this study were collected in 2003, during the second year of the Chicago, Health, Aging and Social Relations Study (CHASRS), a population-based study of 229 older adults in Cook County, Illinois. The sample consists of relatively equal proportions of White, Black, and non-Black Hispanic persons living in Cook County who were born between 1935 and 1952. Details of sample selection can be obtained elsewhere (Hawkley, Masi, Berry, & Cacioppo, 2006). Briefly, the sample was selected using a multistage probability design in which the first stage involved identifying a subset of households estimated to have high probability of containing at least one adult aged 50–65 years (24 percent of the total frame). A quota sampling strategy was used at both the household and individual levels to achieve an approximately equal distribution of men and women in each of the three racial/ethnic groups. The rate at which respondents agreed to participate approached 45% overall, an impressive rate given that individuals agreed to participate knowing that they would have to give up an entire day (typically, 8 am to 4 pm) at the University to undergo physiological testing and complete questionnaires and interviews. The distribution of our sample on a number of characteristics (e.g., education, self-rated health) compares quite closely to that obtained from the national population-based Health and Retirement Survey (HRS). At the time of assessment, 208 adults participated, and their age ranged from 51 to 69 (M =58.4 yrs). Gender and racial/ethnic composition were relatively equally distributed: 37.5% White (54% female), 34.6% Black (54% female), and 27.9% non-Black Hispanic (50% female).
Measures
SES
Education was measured in years of schooling (M=13.4, SD=3.1). Household income was reported in 12 ordinal categories (less than $5,000 to more than $200,000). These categories were converted into dollar values corresponding to the median of each household income category. The median household income category was $50,001-$75,000. To minimize positive skew in the distribution, the natural log-transformed category median was used in analyses. A composite measure of SES was calculated by summing z-scored values for education and household income.
Allostatic Load
AL was computed from 9 markers encompassing cardiovascular system functioning (systolic and diastolic blood pressure), sympathetic nervous and adrenomedullary system functioning (urinary norepinephrine and epinephrine), central obesity (waist circumference), hypothalamic-pituitary-adrenal axis functioning (cortisol), risk factors for development of atherosclerosis (high density lipoprotein and total cholesterol), and glucose metabolism (glycosylated hemoglobin). This combination of markers was chosen to match as closely as possible those markers employed in prior research on AL (e.g., Seeman et al., 1997), and correspondingly, each was measured at rest. Descriptive statistics for each marker are provided in Table 1. No physiological data was obtained for two individuals. Causes of missing data for individual markers are explained under their respective headings below.
Table 1.
Descriptives of individual components of the allostatic load index
| Variable | N | Mean | SD | Range |
|---|---|---|---|---|
| SBP (mmHG) | 202 | 129.7 | 18.4 | 86.7–197.2 |
| DBP (mmHg) | 200 | 71.3 | 11.6 | 46.4–111.5 |
| NE (ng/mg creatinine)* | 189 | 30.0 | 13.0 | 0.3–71.2 |
| EPI (ng/mg creatinine)* | 188 | 1.4 | 1.0 | 0.1–4.6 |
| Waist circumference (cm) Males |
96 | 105.5 | 13.1 | 76.2–142.8 |
| Females | 110 | 96.3 | 15.1 | 67.3–132.1 |
| Cortisol (ng/mg creatinine)* | 186 | 11.8 | 9.4 | 0.4–46.5 |
| TC (mg/dL) | 193 | 216.9 | 45.8 | 110.0–401.0 |
| HDL (mg/dL) | 188 | 52.3 | 15.7 | 19.0–98.0 |
| HbA1c (%) | 180 | 6.0 | 1.4 | 2.10–11.80 |
Values below lower detection limit set to half of detection limit
Values over 8.6 removed
SBP=systolic blood pressure; DBP=diastolic blood pressure; NE=norepinephrine; EPI=epinephrine; TC=total cholesterol; HDL=high-density lipoprotein; HbA1c=glycosylated hemoglobin.
Norepinephrine (NE), epinephine (EPI), cortisol and creatinine in overnight urine samples were assayed using high-performance liquid chromatography. Inter- and intra-assay variability were 3–5% for NEPI, 4–7% for EPI, 6–10% for cortisol, and 2% for creatinine. Non-detectable amounts of NE (<0.3 ng/dl; N=1), EPI (<0.3 ng/dl; N=33) and cortisol (<1.2 ng/dl; N=12) were coded as half the detectable limit (Hornung & Reed, 1990). NE, EPI, and cortisol concentrations are commonly standardized in terms of creatinine concentration to correct for urinary volume differences. Creatinine production is influenced by muscle mass differences, however, so this method tends to underestimate concentrations of these hormones in men and Black individuals. To correct for both muscle mass and urinary volume, we used lean-mass corrected creatinine values to calculate standardized urinary hormone concentrations (Masi, Rickett, Hawkley, & Cacioppo, 2004). Urinary hormone concentrations were missing for 15 individuals who failed to provide a urine sample, for an additional two individuals for whom no lean mass values were available with which to standardize hormone concentrations, and, depending on the hormone in question, for two to four cases whose values were statistical outliers (i.e., <3 SDs from the mean).
Resting systolic (SBP) and diastolic (DBP) blood pressure were measured continuously using an arterial tonometer (Colin Medical Instruments Corp., San Antonio, TX). Blood pressure readings were recorded in a seated posture after a 15-minute quiet resting period during two intervals of two minutes each. SBP and DBP were calculated as the average of 4 minutes of beat-by-beat BP readings. Two cases exhibited mean DBP values that were less than 45 mm Hg, values that were declared missing because they were deemed artifactually low according to criteria established by Marler, Jacob, Lehoczky, & Shapiro (1988). Mean SBP and DBP for four additional cases were declared missing because values were statistical outliers (> 3 SDs from the mean).
Central adiposity was assessed using waist circumference. Body mass index, waist to hip ratio, and waist circumference all measure obesity and/or fat accumulation (Taylor, Jones, Williams, & Goulding, 2000), but waist circumference has been found to be the best predictor of disease risk (Pouliot et al., 1994; Janssen, Katzmarzyk, & Ross, 2002) and mortality, especially in elderly populations (Visscher et al., 2001). Waist circumference was measured at the smallest part of the waist at the navel by two experimenters, and was calculated as the average of the two independent measurements.
Glycosylated hemoglobin (HbA1c), high density lipoprotein (HDL), and total cholesterol (TC) were measured from non-fasting whole blood collected the morning of the lab day. HbA1C was measured using the Cholestech GDX, a system previously shown to produce results within the acceptable limits of the National Glycohemoglobin Standardization Program (Little, 2003). HDL and TC were measured using the Cholestech LDX system (Cholestech Corporation, USA), a system previously shown to have inter- and intra-assay variability of 2–4% for HDL and 2–7% for TC (Santee, 2002). Blood samples for lipids and HbA1c were not obtained for seven cases, and technical difficulties with the blood tests accounted for a further loss of 6 total cholesterol values, 11 HDL values, and 19 HbA1c values.
Z-scores were calculated for participants on each marker. Waist circumference was z-scored separately for males and females. The HDL z-score was reversed so that high values reflected greater dysregulation. Z-scores were then averaged to produce a cumulative measure of AL. AL scores are typically computed as the number of markers for which a participant is in the highest risk quartile. However, prior research has shown that averaging the computed z-scores for each measure predicts health outcomes equally well (Seeman, Singer, Rowe, Horwitz, & McEwen, 1997). Moreover, averaging over continuous z-scores more accurately reflects the continuous nature of cumulative allostatic load.
Health
Prior research has shown an association between health and allostatic load measured as a count of markers in the top quartile of values (Seeman, et al., 2008). To validate our continuous measure of AL, we assessed health in our sample by querying participants about their chronic health conditions. The Charlson comorbidity index (Charlson, Pompei, Ales, & MacKenzie, 1987) was derived as the sum of chronic conditions weighted by severity (Katz, Chang, Sangha, Fossel, & Bates, 1996). For the purposes of analysis, this positively skewed variable was dichotomized to contrast those with any degree of chronic health condition (i.e., scores >0) with those without any conditions (scores = 0).
Explanatory Variables
Table 2 provides sample size, range, and mean (standard deviation) or percentage for each explanatory variable described below. The varying N's for the psychosocial measures are attributable primarily to subjects failing to complete entire surveys. However, we also set scale total scores to missing if subjects failed to respond to ten percent or more of the items. If subjects were missing responses on less than ten percent of the items on any given scale, we replaced missing item values with the mean of responses to the remaining items before calculating a total score.
Table 2.
Descriptive statistics of posited explanatory variables.
| Variable (N) | Range | Mean (SD) or percentage |
|---|---|---|
| Stress | ||
| Perceived Stress (197) | 0–38 | 13.4 (6.5) |
| Chronic Stressors (196) | 0–67 | 19.3 (12.1) |
| Childhood Trauma (207) | 0–6 | 1.4 (1.4) |
| Negative Life Events (202) | 0–224 | 36.2 (28.1) |
| Neighborhood Disorder (205) | 1–2.67 | 1.4 (0.5) |
| Neighborhood Violence (200) | 1–4 | 1.6 (0.7) |
| Personality and Psychosocial Variables | ||
| Hostility (202) | 3–38 | 17.0 (7.9) |
| Loneliness (206) | 20–66 | 35.4 (9.7) |
| Depression (205) | 20–71 | 30.6 (9.3) |
| Social Support (194) | 4–16 | 12.7 (2.1) |
| Emotional Stability (192) | 3–8 | 5.6 (0.9) |
| Agreeableness (194) | 5–9 | 7.0 (0.9) |
| Surgency (189) | 3–9 | 5.8 (1.0) |
| Satisfaction with Life (195) | 5–35 | 23.2 (6.6) |
| Optimism (195) | 8–24 | 16.7 (3.3) |
| Spiritual Well-Being (196) | 44–120 | 98.5 (16.3) |
| Coping Style | ||
| Avoidance Coping (196) | 0–14 | 5.0 (3.3) |
| Task-Oriented Coping (196) | 0–12 | 8.5 (2.5) |
| Emotion-Focused Coping (193) | 0–12 | 5.0 (2.7) |
| Active Coping (207) | 4–8 | 7.4 (1.0) |
| Behavioral Withdrawal (207) | 2–8 | 3.5 (1.4) |
| Seeking Social/Instrumental Support (207) | 2–8 | 6.3 (1.1) |
| Seeking Emotional Support (206) | 2–8 | 6.5 (1.2) |
| Social Network | ||
| Network Roles (208) | 0–7 | 4.7 (1.3) |
| Attend church more than once/month (199) | 60% | |
| Married or living with partner (208) | 52% | |
| Social Network Inventory (199) | 1–4 | 3.0 (0.9) |
| Health Behaviors | ||
| Poor Sleep Quality (175) | 2–14 | 6.7 (2.5) |
| Caloric Intake (173) | 517–3615 | 1524 (625) |
| Any exercise in prior 2 weeks (195) | 87% | |
| Alcohol: drinks/day (203) | 0–10 | 1.3 (1.7) |
| Smokes Now (208) | 14% | |
| Ever Smoked (206) | 59% |
Stress
In order to study stress comprehensively, we measured perceived stress, chronic stressors, childhood traumas, negative life events, neighborhood disorder, and neighborhood violence. The previously validated 10-item Perceived Stress Scale (PSS; Cohen & Williamson, 1988) assessed perceived stress in the past week on a scale of 0 (never) to 4 (very often). Responses were summed across all items. Cronbach's alpha across the 10 items was .88.
A 51-item inventory of chronic stress (Turner, Wheaton, & Lloyd, 1995) assessed the presence of stress in eight domains (e.g., financial, employment, marital/romantic) using a response scale of 0=not true, 1=somewhat true, and 2=very true. Chronic stressors were quantified by summing responses across domains for a possible total score of 102.
An 8-item childhood trauma scale (Turner et al., 1995) asked about potentially traumatic events which could occur during childhood or adolescence (e.g., parental divorce, parental problems with drugs or alcohol, repeating a grade in school). Childhood trauma was calculated by summing responses (yes/no) across events.
Negative life events were measured using a 51-item version of the revised social readjustment rating scale (Hobson et al., 1998). The frequency of each of the lifetime events endorsed (e.g., death of close family member; geographic relocation; life-threatening illness or injury; loss of job) was summed for a total life event score. A natural log transformation was applied to this variable to achieve a normal distribution.
Neighborhood disorder was measured with a validated scale that asks participants whether litter, graffiti, vacant houses, drinking in public, selling drugs, and groups of people hanging out and causing trouble are a problem in their neighborhood (Sampson & Raudenbush, 2004). A disorder score was calculated by averaging responses (1=not a problem, 2=somewhat of a problem, 3=big problem) across the six items. Cronbach's alpha across the 6 items was .88. A natural log transform was applied to this variable to normalize its distribution.
Neighborhood violence was measured with a validated scale that asks participants how often in the past year there were fights with weapons, violent arguments between neighbors, gang fights, sexual assaults, and robberies in their neighborhood (Sampson, Raudenbush, & Earls, 2004). A violence score was calculated by averaging responses (1=never, to 4=often) across the five items. Cronbach's alpha across the 5 items was .89. A natural log transform was applied to this variable to normalize its distribution.
Personality and psychosocial variables
Hostility, loneliness, depression, social support, emotional stability, agreeableness, surgency, satisfaction with life, optimism, and spiritual well-being were assessed using standard validated scales. Hostility was measured using the 50-item Cook-Medley Hostility Scale (CMHo; Cook & Medley, 1954). Responses (0=false, 1=true) were summed across all items. Cronbach's alpha across the 50 items was .87.
Loneliness was measured using the well-validated 20-item revised UCLA loneliness scale (R-UCLA; Russell, Peplau, & Cutrona, 1980). The item response scale ranges from 1 (never) to 4 (often), and after reverse-coding appropriate items, responses were summed across all items. Cronbach's alpha across the 20 items was .91. Depression was measured using the 20-item Center for Epidemiological Studies-Depression Scale (CES-D; Radloff, 1977) which assesses frequency of depressive feelings and behaviors in the past week (1=rarely/none of the time, to 4=most/all of the time). Responses were summed across all items. Cronbach's alpha across the 20 items was .89.
Social support was measured using the 12-item Interpersonal Support Evaluation List (ISEL; Cohen, 2008). Responses to each item range from 1 (definitely false) to 4 (definitely true). Responses were summed to generate the subscale scores (tangible, appraisal, belonging), and the mean of the subscales was taken as our measure of social support. Cronbach's alpha across the 12 items was .87.
Emotional stability, agreeableness, and surgency were measured using the Big 5 personality inventory (Goldberg, 1992). These three traits were assessed using 60 trait words which participants endorsed on a scale from 1 (extremely inaccurate) to 9 (extremely accurate). Scores for each trait were calculated by averaging across the corresponding 20 trait words such that higher values denoted greater emotional stability, agreeableness, and surgency (i.e., extraversion). Cronbach's alphas were .83, .88, and .88, respectively.
Satisfaction with life was measured using the 5-item Satisfaction with Life Scale (SWLS; Diener, Emmons, Larsen, & Griffin, 1985). Responses to each item range from 1 (strongly disagree) to 7 (strongly agree). Scores were calculated as the sum across all items. Cronbach's alpha across the 5 items was .88.
Optimism was measured using the Revised Life Orientation Test (LOT-R; Scheier, Carver, & Bridges, 1994). Participants were asked to rate the degree to which they agreed with ten items related to optimism (0=strongly disagree, to 4=strongly agree). Responses were summed across all items. Cronbach's alpha across the 10 items was .73.
Spiritual well-being was measured using the 20-item Spiritual Well-Being Scale (SWBS) developed by Paloutzian and Ellison (1982). Participants were asked to indicate the extent to which they agreed with each statement using a scale that ranged from 1 (strongly agree) to 6 (strongly disagree). Responses were summed across all items. Cronbach's alpha across the 20 items was .87.
Coping style
We assessed differences in coping style using subscales of the Coping Inventory for Stressful Situations (CISS; Cosway, Endler, Sadler, & Deary, 2000) and the Coping Orientations to Problems Experienced Scale (COPE; Carver, Scheier, & Weintraub, 1989). The CISS identifies avoidance coping (4 items), task-oriented coping (3 items), and emotion-focused coping (3 items) as three independent coping style dimensions. Participants were asked to indicate how much they engage in each of 10 behaviors on a scale of 0 (not at all) to 4 (very much). Responses were summed across items for each coping dimension. Cronbach's alpha were .68, .82, and .70, for avoidance, task-oriented, and emotion-focused coping, respectively.
We used a shortened form of the COPE scale to assess active coping, behavioral withdrawal, seeking social/instrumental support, and seeking emotional support using two items for each type of coping. Participants were asked to indicate, when confronted with difficult or stressful events, how much they usually engage in each strategy on a scale of 1 (never) to 4 (often). Responses were summed across items for each of the four coping styles. Cronbach's alpha were .65, .75, .70, and .73, for active coping, behavioral withdrawal, seeking social/instrumental support, and seeking emotional support, respectively. Active coping exhibited a high degree of negative skew that did not respond well to log-transformation. Values were therefore dichotomized to contrast scores >0 with scores of 0.
Social network
Self-report questionnaires were used to generate four measures of social integration. Network roles (e.g., spouse, relative, friend, neighbor, volunteer, group member, workmate) was calculated as the number of roles the participant reported that involved social interactions at least once every two weeks. Church attendance was coded in five categories, from “not at all” to “more than once a week.” Those who attended more than once a month were contrasted with those who attended less frequently (the reference category). Marital status was dummy-coded to contrast those who were married or living with a partner with all other marital statuses (combined reference category). A simplified social network index (SNI) was computed based on Berkman and Syme (1979) by calculating a weighted composite of social ties that includes friends, relatives, marital status, religious group affiliation, and group membership. Procedures have been described elsewhere (Hughes, Waite, Hawkley, & Cacioppo, 2004). The SNI score is categorical and ranges from 1 (low) to 4 (high).
Health behaviors
Sleep quality, caloric intake, exercise, alcohol consumption, and current and past smoking were assessed. Sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). A composite global sleep quality score was calculated, with higher scores denoting poorer sleep quality.
Caloric intake was assessed by asking participants to complete the Brief Block 2000 Food Frequency Questionnaire (Block Dietary Data Systems, Berkeley, CA) about their eating behavior “during the last year or so.” Completed survey forms were scored by Block Dietary Data Systems, and total daily food and beverage intake was reported in calories.
Exercise data were obtained using a standardized questionnaire developed for an older adult sample, which asks respondents the frequency and duration of their engagement in 14 physical activities (e.g., gardening and horseback riding; McPhillips, Pellettera, Barrett-Connor, Wingard, & Criqui, 1989). Participants who engaged in any of these activities during the last two weeks were contrasted with those who did not report any exercise in the past two weeks (reference category).
Alcohol consumption was recorded as the average number of drinks participants reported consuming per day over the previous three-month period. This variable exhibited a high degree of positive skew that did not respond well to log-transformation. Values were therefore dichotomized to contrast drinkers (>0) with non-drinkers (0). Participants who reported being a current smoker were contrasted with non-smokers (reference category). Current information on smoking status was not available for three subjects, so their smoking status in the prior year was carried forward to the present year (one smoker, two non-smokers). In addition, participants who were presently non-smokers but had smoked over 100 cigarettes during their lifetime (ever smoked) were contrasted with those who had never smoked (reference category).
Procedure
Prior to their lab visit, participants were asked to complete a series of questionnaires and were mailed a urine collection container pre-filled with 25 ml of 50% glacial acetic acid as a catecholamine preservative. Following the protocol introduced by Reuben et al. (Reuben, Talvi, Rowe, & Seeman, 2000), participants were asked to collect any urine excreted if they awoke during the night and upon awakening the morning of the lab day. When participants arrived at the laboratory between 8 and 9 a.m., their overnight urine sample was immediately aliquoted and frozen at –80°C until biochemical analyses were performed. During the lab visit, participants completed approximately 8 hours of testing including survey questionnaires, interviews, a cardiovascular protocol, and blood collection. Blood pressure data were obtained between 9 and 11 AM, and blood samples were collected and tested between 10 am and 12 noon.The research followed APA standards for the ethical treatment of human participants and met with approval by the Institutional Review Board of the University of Chicago.
Data Analytic Strategy
Missing data ranged from zero to 15.8% of cases for any given variable (see Table 2). Cases missing data on at least one mediator did not differ as a function of age, gender, ethnicity, allostatic load, or income, but had fewer years of education (12.6 vs. 14 yrs, p<.05). To maximize statistical power, we imputed values for all missing data using a regression approach. Age, gender, and ethnicity were chosen as predictors because these three variables define the basic demographic composition of our sample, and our goal was to ensure that our results generalized across the whole sample. Health status (presence of a chronic condition or not) was added as a predictor because we wished to generalize across subjects with and without clinically dysregulated physiology. The same procedure was used to impute values for six cases missing education data, and twelve cases missing income data. All reported analyses were conducted on imputed data. Results using only cases with complete data (N = 145) did not differ substantively from results obtained using imputed data. These analyses are available from the first author (LCH) upon request.
The first step in the data analytic process was to calculate the associations of composite SES, income, and education with AL. To evaluate whether the association between SES and AL was affected by age, gender, or ethnicity, we examined the degree to which these demographic variables were associated with SES and AL. Demographic variables associated with SES or AL were held constant in all subsequent analyses and the association between SES and AL was only explored further if the association remained significant.
The next step was to identify variables in the domains of stress, personality and psychosocial variables, coping, social network, and health behavior that were related to both SES and AL and therefore met prerequisite criteria to be potential mediators of the SES-AL relationship. Because potential mediators were selected on a priori theoretical grounds as representative variables for constructs previously hypothesized or shown to explain SES differences in AL, and because potential mediators had to relate significantly to both SES and AL, the alpha level was set at .05. Each variable that met prerequisite criteria for mediation was then tested in a mediational model that employed a bootstrapping procedure (10,000 bootstraps) and indicated a significant indirect effect when the 95% bias-corrected and accelerated (BCa) confidence interval around the unstandardized coefficient did not include zero (Preacher & Hayes, 2004). Bootstrapping minimizes the problem of a non-normal and non-symmetric distribution of the indirect effect that is common with small samples and that results in diminished statistical power. Bootstrapping is accomplished by taking a large number of samples from the data (sampling with replacement) and computing the indirect effect, ab, in each sample. The larger number of effects is used to calculate a bootstrap estimate of the indirect effect ab, its estimated standard error, and the confidence intervals for the population value of ab (Preacher & Hayes, 2004).
Finally, we conducted a principal axis factor analysis with oblique rotation to determine the nature of and interrelationships among latent factors representing the markers in our measure of AL. Correlation analyses were employed to assess the association of SES with factor scores representing each of the latent AL factors.
Results
SES and AL Association
We first validated our continuous measure of AL by showing a significant correlation with the presence of a chronic health condition, r=.19, p<.05. Lower SES was also significantly correlated with the presence of a chronic health condition, r=−.17, p<.05, as were household income and education individually, r's=−.13 and -.14, although only approaching significance, p's < .1. Consistent with prior research, AL was negatively correlated with the composite measure of SES, r=−.14, p<.01, and with years of education, r=−.17, p<.05, but nonsignificantly with household income, r=−.06, p>.4. Age was not significantly associated with AL or SES (all p’s>.1). A two-way ANOVA revealed no racial/ethnic group differences in AL (p’s>.1), but males had higher AL than females (Mmales=.13, SD=0.43; Mfemales=−.11, SD=0.37; p<.01). A second two-way ANOVA revealed no gender differences in SES (p's >.1), but significant racial/ethnic group differences in SES (p<.01). Scores on the standardized SES variable were significantly higher in Whites (M=0.65, SD=1.48) than in Blacks (M=−0.34, SD=1.68) and Hispanics (M=−0.46, SD=1.49), who did not differ from each other. All subsequent analyses were therefore conducted with gender and White race/ethnicity as covariates. Results did not differ substantively if age was also included as a covariate. In partial correlations that adjusted for gender and White race/ethnicity, AL continued to be significantly associated with the composite SES measure, r=−.15, p<.05, with years of education, r = −.14, p = .05, and nonsignificantly with household income, r = −.11, p > .1.
Correlations of Posited Mediators with SES and AL
The next step was to assess whether differences in stress, personality and psychosocial variables, coping styles, social networks, and health behaviors could explain the association between SES and AL. Partial correlations of these variables with SES and AL, controlling for gender and White race/ethnicity are reported in Table 3. Only two variables met the criteria of exhibiting significant partial correlations with both SES and AL. These were hostility and poor sleep quality. Household income and education exhibited comparable associations with hostility and with poor sleep quality (i.e., nonsignificant differences between dependent r's, p's > .1) so we focused on composite SES in our subsequent mediation analyses.
Table 3.
Partial correlations of posited mediators with socioeconomic status and allostatic load controlling for gender and White race/ethnicity.
| Allostatic load | Socioeconomic status |
Household income |
Years education | |
|---|---|---|---|---|
| Stress | ||||
| Perceived Stress | .14* | −.12 | −.07 | −.13 |
| Chronic Stressors | .09 | −.08 | −.09 | −.03 |
| Childhood Trauma | −.05 | −.05 | −.04 | −.05 |
| Negative Life Events | −.07 | .17* | .06 | .21** |
| Neighborhood Disorder | −.05 | −.23** | −.16** | −.21** |
| Neighborhood Violence | .02 | −.03 | −.02 | −.02 |
| Personality and Psychosocial Variables | ||||
| Hostility | .22** | −.18* | −.10 | −.19** |
| Loneliness | .09 | −.19** | −.19** | −.11 |
| Depression | .11 | −.16* | −.12* | −.14 |
| Social Support | −.08 | .23** | .22** | .16* |
| Emotional Stability | .02 | .14 | .06 | .16* |
| Agreeableness | −.02 | .12 | .13 | .05 |
| Surgency | −.08 | .13 | .11 | .11 |
| Satisfaction with Life | −.04 | .13 | .21** | < .01 |
| Optimism | −.08 | .18* | .13 | .15* |
| Spiritual Well-Being | −.02 | .01 | .07 | −.05 |
| Coping Style | ||||
| Avoidance Coping | .13 | .04 | −.04 | .10 |
| Task-Oriented Coping | .07 | .09 | .04 | .10 |
| Emotion-Focused Coping | −.08 | .08 | −.02 | .16* |
| Active Coping | −.03 | .16* | .16* | .10 |
| Behavioral Withdrawal | .07 | −.06 | −.07 | −.02 |
| Seeking | ||||
| Social/Instrumental | −.10 | −.01 | ||
| Support | −.03 | −.07 | ||
| Seeking Emotional | .03 | −.04 | ||
| Support | < .01 | <−.01 | ||
| Social Network | ||||
| Network Roles | −.07 | .21** | .21** | .12 |
| Church Attendance | .02 | < −.01 | .06 | −.06 |
| Marital Status | .05 | .08 | .21** | −.09 |
| Social Network Inventory | −.02 | .07 | .16* | −.06 |
| Health Behaviors | ||||
| Poor Sleep Quality | .14* | −.18** | −.15** | −.13 |
| Caloric Intake | .12 | −.10 | −.10 | −.07 |
| Exercise | −.06 | .18* | .11 | .19** |
| Drinks Alcohol | −.04 | .16* | .12 | .13 |
| Smokes Now | −.06 | −.03 | −.05 | < .01 |
| Ever Smoked | −.01 | −.07 | −.11 | < −.01 |
p<.05, two-tailed;
p<.01, two-tailed
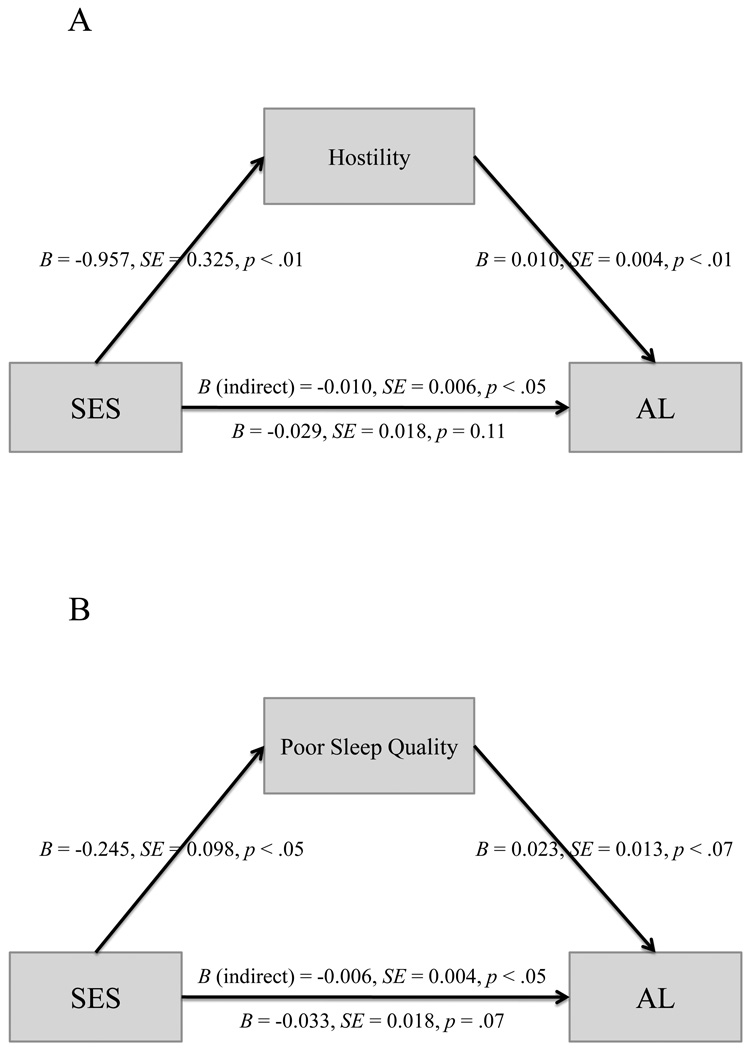
Mediation of Association between SES and AL
Hostility and poor sleep quality were tested in separate mediational models (see Figure 1A & 1B). Adjusting for gender and White race/ethnicity, hostility exhibited a significant indirect effect on AL, B=−0.0099 (BCa 95% CI: −0.0235, −0.0017) and independently of SES explained 3.2% of the variance in AL. In a second model, poor sleep quality exhibited a significant indirect effect on AL, B=−0.0056 (BCa 95% CI: −0.0152, −0.0005), and independently explained 1.4% of the variance in AL. Finally, we combined both mediators in a single model. The multiple mediation model revealed that hostility and poor sleep quality did not have significant independent utility in explaining the association between SES and AL, both p's>.05.
Figure 1.
Ancillary analyses conducted on the subsample of individuals who reported no chronic health conditions (N = 144) revealed that the association between AL and SES remained statistically significant, r=−.21, p < .05, indicating that the inclusion of chronically ill individuals was not responsible for the observed association between AL and SES in the total sample. The indirect effect of SES through hostility in the healthy sample was attenuated relative to the full sample, and did not achieve statistical significance, B=−0.0071, (BCa 95% CI: −0.0228, 0.0011). Similarly, poor sleep quality did not exhibit a significant indirect effect on AL, B=−0.0036, (BCa 95% CI: −0.0151, 0.0029).
Association between SES and components of AL
The measures chosen to constitute AL were subjected to an exploratory principal axis factor analysis with oblique rotation to determine whether the number of variables could be reduced to a smaller number of factors that represented the systems of interest. Table 4 shows that the resulting four factor structure and the pattern of factor loadings corresponded closely to the functional systems of interest described earlier. SBP and DBP loaded on a cardiovascular factor; urinary norepinephrine and epinephrine on a sympathetic adrenomedullary (SAM) factor; and total cholesterol and HDL on a lipid metabolism factor. Waist circumference loaded on the fourth factor with HbA1c contributing a low loading. We labeled this an obesity factor. Urinary cortisol had low loadings on both the SAM and lipid metabolism factors. Correlations among the four factors ranged from .04 to .4, with the higher correlations indicating the potential for redundancy in the measures used to assess allostatic load.
Table 4.
Principal axis factor analysis of the AL measures.
| Factor | ||||
|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 |
| Systolic blood pressure | .858 | −.081 | −.032 | .096 |
| Diastolic blood pressure | .845 | .035 | .029 | −.081 |
| High density lipoprotein1 | .028 | .869 | .003 | .105 |
| Total cholesterol | .079 | −.372 | .020 | .026 |
| Norephinephrine | −.015 | −.082 | .734 | .151 |
| Epinephrine | .003 | .058 | .565 | −.171 |
| Cortisol | .043 | .249 | .287 | −.157 |
| Waist circumference | −.013 | .019 | −.013 | .684 |
| Hemoglobin A1c | .016 | .022 | −.095 | .230 |
| Factor Intercorrelations | ||||
| Factor 1 | 1.000 | |||
| Factor 2 | .064 | 1.000 | ||
| Factor 3 | .035 | .040 | 1.000 | |
| Factor 4 | .303 | .099 | .437 | 1.000 |
HDL was reverse-scored so that higher values reflected greater dysregulation.
Factor scores were calculated using a regression approach, and partial correlations with SES were computed holding constant gender and White race/ethnicity. The cardiovascular and obesity factors exhibited significant negative correlations with SES, r's = −.16 and −.17, respectively (p's < .05), whereas the adrenomedullary and lipid factors exhibited nonsignificant negative correlations with SES, r's = −.11 and -.07, respectively (p's > .1). The cardiovascular and obesity factors seemed particularly effective at capturing physiological dysregulation associated with SES. Partial correlations between the factors and the two variables we found to mediate the SES-AL association revealed that hostility was significantly correlated with the cardiovascular and obesity factors, r's >.15, p's< .05, but not with the SAM and lipid factors, |r|'s<.06, p's >.4. A similar result was observed for PSQI; partial correlations were r's >.15, p's <.05, for the cardiovascular and obesity factors, but |r|'s <.11, p's >.1, for the SAM and lipid factors.
Discussion
In accordance with its definition, allostatic load has been operationalized as the extent of dysregulation aggregated across markers of a variety of allostatic systems (Seeman et al., 1997). Indeed, health risks associated with allostatic load are expected to result not only from extreme dysregulation in one system but from modest dysregulation that occurs in numerous systems. In support of this hypothesis, a study of 70–79 year-old high-functioning adults in the MacArthur Study of Successful Aging showed that the individual components of allostatic load were only modestly and generally nonsignificantly associated with physical and cognitive health outcomes, and that allostatic load, measured as a continuous canonical score, was superior to its individual components in predicting outcomes over a 7-year period (Karlamangla, Singer, McEwen, Rowe, & Seeman, 2002). In contrast, we found that some individual components behaved as well as our composite continuous measure in analyses of AL as an outcome. Cross-sectional data from our study of a population-based sample of 51–69 year-old adults revealed that the components of AL cohered in a meaningful way across the bodily systems they were intended to measure. Among the four components extracted, cardiovascular functioning and obesity (including glucose regulation) were as highly correlated with SES as was the composite measure of AL. The SAM and lipid factors, on the other hand, exhibited very small and nonsignificant correlations with SES. Our results suggest that SES may increase system-specific physiological risk rather than broad-based physiological dysregulation (Adler et al., 1994). A test of this conjecture requires, among other tests, a test of time; with increasing age and/or ongoing exposure to low SES, broad-based physiological dysregulation may be more likely. For instance, in NHANES III data (Crimmins, Johnston, Hayward, & Seeman, 2003), AL (number of markers in the top quartile of the population) continued to increase through age 65, fourteen years older than the current age of our youngest CHASRS participants. On the other hand, some research has suggested that physiological dysregulation across particular combinations of systems may be a sufficient improvement to single system indicators to predict outcomes (e.g., mortality). In other words, broad-based dysregulation may be less informative than the gradual accrual of dysregulation in especially critical systems (Gruenewald, Seeman, Ryff, Karlamangla, & Singer, 2006). Longitudinal research is needed to help answer whether the effects of SES on physiological dysregulation accrue across particularly crucial systems.
AL has been shown to contribute to the SES gradient in health (Kubzansky et al., 1999), but the mechanisms for an SES gradient in AL remain relatively unexplored. Stress, psychological characteristics, coping strategies, social relationships, and health behaviors have been posited as mediators of the association between SES and AL, but to date, only one study has empirically examined and identified a factor that helps to explain the effect of SES on AL. That factor was hostility, and it partially explained the association between SES and AL in a community-dwelling sample of 21–80 year-old men (Kubzansky et al., 1999). In a replication and extension of these results, we found that hostility was a statistically significant mediator that explained 3% of the variance in the association between SES on AL in a population-based sample of 51–69 year-old White, Black, and non-Black Hispanic men and women.
Why and how might hostility play a mediating role between SES and AL? Low SES has frequently been shown to be associated with hostility, not only in adults (Elovainio, Kivimäki, Kortteinen, & Tuomikoski, 2001; Kubzansky et al., 1999), but as early as adolescence (Gump et al., 1999). Hostility, in turn, has been shown to influence physiological functioning across several systems. For example, hostility has been associated with exaggerated blood pressure reactions to stress (Fredrickson et al., 2000; Gump et al., 1999), elevated fasting glucose prospectively (Shen, Countryman, Spiro III, & Niaura, 2008), and higher levels of plasma lipids (Weidner, Sexton, McLellarn, Connor, & Matarazzo, 1987). Interestingly, the reverse direction from lipids to hostility is also plausible: a cholesterol-lowering program in 233 men and women (mean age = 37.7 yrs) resulted not only in a reduction in plasma cholesterol but also a significant reduction in aggressive hostility (Weidner, Connor, & Hollis, 1992). Alternatively, this finding could be interpreted to suggest that the care and attention of physicians helping patients reduce their cholesterol, rather than the reductions in cholesterol per se, were responsible for the reduction in hostility. This interpretation is also consistent with the conjecture that hostility is the result of perceived inattention and peripheralization often accorded individuals of low socioeconomic status. Moreover, because hostile individuals tend to view the world as more hostile, hostility tends to evoke hostility from others (Gallo, Smith, & Cos, 2006) and may also perpetuate low SES (Matthews, Gallo, & Taylor, 2010). Interestingly, among adolescents, lower family SES is associated with greater adolescents' hostility (Gump et al., 1999), and adolescents' hostility predicts metabolic syndrome risk factors, risk factors that bear a strong resemblance to markers of AL (Räikkönen, Matthews, & Salomon, 2003). Whether hostility mediates the SES-AL association in a younger age group remains an open question, but given extant data, hostility is a prime candidate for this role.
In addition to hostility, poor sleep quality helped to explain the effect of SES on AL. Van & Spiegel (1999) proposed that sleep quality may mediate the pathway between SES and health, but did not test or report such an analysis. Our finding that poor sleep quality is associated with AL calls for an expansion of thinking about AL. The foundation of the formulation of AL is that the catabolic actions of the body contribute to cumulative wear and tear. Cacioppo and Berntson (2007) noted that human physiology is characterized by anabolic as well as catabolic processes, and they suggested that maintenance and restorative (anabolic) processes should help reduce the cumulative wear and tear on the body. The present findings for sleep quality are consistent with the notion that our understanding of AL – and the SES-AL association – may require that we not only consider the effects of catabolic processes, or challenges to bodily systems, but also the effects of restorative and anabolic processes. Of course, it is equally plausible that physiological dysregulation leads to poor sleep quality. In support of a causal role for sleep quality, Kumar and colleagues (Kumar et al., 2010) found that physiologically compromised kidney disease patients benefited from good sleep quality by showing fewer symptoms and reduced risk of mortality. Longitudinal research is needed to address the causal direction between sleep quality and AL.
Poor health status (i.e., presence of chronic health conditions) is associated with AL, but chronic health conditions are a consequence, not a cause, of dysregulated physiology (e.g., high blood pressure, elevated blood sugar). Thus, our examination of variables that mediate the association between SES and AL irrespective of health status sheds light on psychosocial and behavioral factors that might contribute to the SES-health gradient. Nevertheless, somewhat attenuated indirect effects in the smaller sub-sample of healthy individuals suggest that health status may moderate their roles. A larger sample will be needed to test this hypothesis.
The link between SES and AL, and mediators of that link, may be moderated by other factors in addition to health status. Adler and Ostrove (1999) highlight race/ethnicity and gender as potentially important moderators of the link between SES and health which suggests that the SES-AL linkage may be similarly affected. Level of SES may itself moderate the link between SES and AL. That is, variability in the low SES range could contribute to AL through different mechanisms than variability at the high SES range. For instance, Merjonen et al. (2008) found that anger was associated with carotid artery intima-media thickness in low SES but not high SES individuals. In addition, differences in AL and health may be as much the consequence of differences in physiological responses to stressful circumstances as to physiological dysregulation at rest. Differences in SES and hostility have each been associated with differences in physiological reactivity that may influence AL and health outcomes (Carroll, Davey, Sheffield, Shipley, & Marmot, 1997; Chen &Matthews, 2001; Gump et al., 1999). It remains for future research to explore whether these and other factors moderate the association of and pathways between SES and AL.
We selected a wide range of variables as potential mediators, all of which were theoretically plausible and empirically shown to be associated with SES and AL. Nevertheless, only hostility and poor sleep quality were significant mediators in our sample, suggesting these variables have the most robust effects. Although other potential mediators did not exhibit statistically significant effects, effect sizes appear consistent with prior research using large samples. For instance, in a study of 851 adults, Glei et al. (2007) reported that a 1-SD increase in number of chronic stressors (i.e., 2.1 additional stressors) was associated with a .08 SD increase in their measure of AL (i.e., 0.17 AL units), and a 1-SD increase in perceived stress was associated with a .12 SD increase in AL. These effect sizes correspond to r's of .08 and .12 respectively, and are within the range of correlations we observed in our data. Table 3 provides effect sizes for all of the variables studied in our sample for those who wish to explore these variables further.
Alternative operationalizations of AL may produce different results. Unlike the MacArthur studies (e.g., Seeman et al., 1997; 2004), our measure of AL did not include DHEA-S, it substituted waist circumference for the waist-hip ratio, and it included total cholesterol instead of the cholesterol/HDL ratio (HDL was included separately in each of these studies, including ours). These measures represent physiological systems that are already included in our AL measure, however. Associations between SES and AL have been observed in studies that vary in the specific markers used to assess AL (Szanton et al., 2005), but an explicit comparison of the specificity of SES effects on various AL systems has yet to be conducted. Our factor analysis results suggest that some systems (i.e., blood pressure, obesity) may be as reliable as a composite measure of physiological dysregulation in revealing associations with SES. Differences in data collection methods for each physiological measure could also have an impact on the composite measure of AL. In our study, for instance, we did not use fasting blood samples for total cholesterol and high-density lipoproteins. Recent food intake has minimal impact on levels of total cholesterol and high density lipoprotein (Langsted, Freiberg, & Nordestgaard, 2008), however, and nonfasting levels of these blood lipids predict risk of cardiovascular events as well if not better than fasting levels (Mora, Rifai, Buring, & Ridker, 2008). It therefore seems unlikely that our non-fasting lipid assays had an undue influence on our measure of AL.
In future research, it may be important to distinguish among types of stress that may mediate the SES-AL association. Weinstein et al. (2003) found somewhat higher correlations of AL with specific types of stress, such as widowhood and financial difficulties, r's = .21-.24. Another possibility is that stress at any one point in time may not be related to allostatic load but that stress accumulates to alter allostatic load. In 13-year old children, Evans & Schamberg (2009) found that proportion of life in poverty accumulated to increase AL. Similar complexity may characterize the role of other correlates of AL. In elderly adults, social factors accumulated to influence AL: number of waves (out of four) at which respondents reported having close ties with friends and relatives was associated with lower AL at the time of the final survey (Seeman et al., 2004). Additional potential mediators to consider include access to and utilization of healthcare, including medications.
Are our findings generalizable? Sample recruitment for The Chicago Health, Aging, and Social Relations Study (CHASRS) was more successful than might have been expected given that recruits were asked to spend an entire day in our lab for each of five years. Impressively, 45 percent of recruits agreed to participate in the face of this response burden, and 91 percent of these returned for the second wave of data collection upon which this study is based. The success of recruitment efforts is also borne out in the fact that CHASRS has a population-based sample that closely resembles the national population-based Health and Retirement Study in terms of education and self-rated health, for example. These factors suggest that our urban Cook County sample represents an urban population reasonably well, and that our findings may generalize to the larger population of middle-age and older adults. In sum, the current study adds to the understanding of the SES-AL relationship in several ways. First, the current study provides support for AL as a cumulative, continuous measure of SES related wear-and-tear. Second, none of the stress measures mediated the relationship between SES and AL, whereas hostility and poor sleep quality each contributed to the association between SES and AL. Although the stresses of low SES may contribute to the emergence of hostility and poor sleep quality, these latter variables are important proximal mediators of the SES-AL association. Third, although low SES is typically thought to lead to AL through increased challenge, we provide the first evidence that the restorative properties associated with sleep quality play a significant role in explaining the relationship between SES and AL.
Acknowledgments
This research was supported by grants from the National Institute on Aging to J. T. Cacioppo (PI), PO1 AG-034052, and L. C. Hawkley (PI), RO1 AG-036433, and by a grant to J. T. Cacioppo from the John Templeton Foundation.
REFERENCES
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health. The challenge of the gradient. American Psychologist. 1994;49(1):15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adler NE, Ostrove JM. Socioeconomic status and health: What we know and what we don't. Annals of the New York Academy of Sciences. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Syme SL. Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda County residents. American Journal of Epidemiology. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT. From homeostasis to allodynamic regulation. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2 ed. New York: Cambridge University Press; 2000. pp. 459–481. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, 3rd, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. The brain, homeostasis, and health: Balancing demands of the internal and external milieu. In: Friedman HS, Silver RC, editors. Foundations of Health Psychology. New York: Oxford University Press; 2007. pp. 73–91. [Google Scholar]
- Carroll D, Davey SG, Sheffield D, Shipley MJ, Marmot MG. The relationship between socioeconomic status, hostility, and blood pressure reactions to mental stress in men: Data from the Whitehall II study. Health Psychology. 1997;16:131–136. doi: 10.1037//0278-6133.16.2.131. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. Journal of Personality and Social Psychology. 1989;56(2):267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA. Cognitive appraisal biases: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Annals of Behavioral Medicine. 2001;23:101–111. doi: 10.1207/S15324796ABM2302_4. [DOI] [PubMed] [Google Scholar]
- Clark MS, Bond MJ, Hecker JR. Environmental stress, psychological stress and allostatic load. Psychology, Health & Medicine. 2007;12(1):18–30. doi: 10.1080/13548500500429338. [DOI] [PubMed] [Google Scholar]
- Cohen S. [Retrieved September 2, 2008];Basic psychometrics for the ISEL 12-item scale. 2008 from http://www.psy.cmu.edu/~scohen/.
- Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and Pharisaic-virtue scales for the MMPI. Applied Psychology. 1954;38(6):414–418. [Google Scholar]
- Cosway R, Endler NS, Sadler AJ, Deary IJ. The coping inventory for stressful situations: Factorial structure and associations with personality traits and psychological health. Journal of Applied Behavioural Research. 2000;5(2):121–143. [Google Scholar]
- Crimmins EM, Johnston M, Hayward M, Seeman T. Age differences in allostatic load: An index of physiological dysregulation. Experimental Gerontology. 2003;38:731–734. doi: 10.1016/s0531-5565(03)00099-8. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction with Life scale. Journal of Personality Assessment. 1985;49(1):71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Kivimäki M, Kortteinen M, Tuomikoski H. Socioeconomic status, hostility and health. Personality and Individual Differences. 2001;31:303–315. [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Maynard KE, Helms MJ, Haney TL, Siegler IC, Barefoot JC. Hostility Predicts Magnitude and Duration of Blood Pressure Response to Anger. Journal of Behavioral Medicine. 2000;23:229–243. doi: 10.1023/a:1005596208324. [DOI] [PubMed] [Google Scholar]
- Friedman EMP, Love GDP, Rosenkranz MA, Urry HLP, Davidson RJP, Singer BHP, et al. Socioeconomic status predicts objective and subjective sleep quality in aging women. Psychosomatic Medicine. 2007;69(7):682–691. doi: 10.1097/PSY.0b013e31814ceada. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Smith TW, Cox CM. Socioeconomic status, psychosocial processes, and perceived health: An interpersonal perspective. Annals of Behavioral Medicine. 2006;31:109–119. doi: 10.1207/s15324796abm3102_2. [DOI] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Chuang YL, Weinstein M. Do chronic stressors lead to physiological dysregulation? Testing the theory of allostatic load. Psychosomatic Medicine. 2007;69(8):769–776. doi: 10.1097/PSY.0b013e318157cba6. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. The development of markers for the big-five factor structure. Psychological Assessment. 1992;4(1):26–42. [Google Scholar]
- Govil SR, Weidner G, Merritt-Worden T, Ornish D. Socioeconomic status and improvements in lifestyle, coronary risk factors, and quality of life: the Multisite Cardiac Lifestyle Intervention Program. American Journal of Public Health. 2009;99(7):1263–1270. doi: 10.2105/AJPH.2007.132852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. PNAS. 2006;103:14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Matthews KA, Räikkönen K. Modeling relationships among socioeconomic status, hostility, cardiovascular reactivity, and left ventricular mass in African American and White children. Health Psychology. 1999;18:140–150. doi: 10.1037//0278-6133.18.2.140. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychology and Aging. 2006;21(1):152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- Hobson CJ, Kamen J, Szostek J, Nethercut CM, Tiedmann JW, Wojnarowicz S. Stressful life events: A revision and update of the social readjustment rating scale. International Journal of Stress Management. 1998;5(1):1–23. [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied Occupational & Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys - Results from two population-based studies. Research on Aging. 2004;26(6):655–672. doi: 10.1177/0164027504268574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: Evidence in support of current National Institutes of Health Guidelines. Archives of Internal Medicine. 2002;162(18):2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline MacArthur studies of successful aging. Journal of Clinical Epidemiology. 2002;55:696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Medical Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Kinnunen M-L, Kaprio J, Pulkkinen L. Allostatic load of men and women in early middle age. Journal of Individual Differences. 2005;26(1):20–28. [Google Scholar]
- Kubzansky LD, Kawachi I, Sparrow D. Socioeconomic status, hostility, and risk factor clustering in the Normative Aging Study: any help from the concept of allostatic load? Annals of Behavioral Medicine. 1999;21(4):330–338. doi: 10.1007/BF02895966. [DOI] [PubMed] [Google Scholar]
- Kumar B, Tilea A, Gillespie BW, Zhang X, Kiser M, Eisele G, et al. Significance of self-reported sleep quality (SQ) in chronic kidney disease (CKD): The Renal Research Institute (RRI)-CKD study. Clinical Nephrology. 2010;73(2):104. doi: 10.5414/cnp73104. [DOI] [PubMed] [Google Scholar]
- Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: Influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047–2056. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- Little RR. Glycated hemoglobin standardization--National Glycohemoglobin Standardization Program (NGSP) perspective. Clinical Chemistry and Laboratory Medicine. 2003;41(9):1191–1198. doi: 10.1515/CCLM.2003.183. [DOI] [PubMed] [Google Scholar]
- Marler MR, Jacob RG, Lehoczky JP, Shapiro AP. The statistical analysis of treatment effects in 24-hour ambulatory blood pressure recordings. Statistics in Medicine. 1988;7:697–716. doi: 10.1002/sim.4780070608. [DOI] [PubMed] [Google Scholar]
- Marmot M. Social determinants of health inequalities. Lancet. 2005;365(9464):1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- Maselko JS, Kubzansky LP, Kawachi IMDP, Seeman TP, Berkman LP. Religious service attendance and allostatic load among high-functioning elderly. Psychosomatic Medicine. 2007;69(5):464–472. doi: 10.1097/PSY.0b013e31806c7c57. [DOI] [PubMed] [Google Scholar]
- Masi CM, Rickett EM, Hawkley LC, Cacioppo JT. Gender and ethnic differences in urinary stress hormones: the population-based Chicago Health, Aging, and Social Relations Study. Journal of Applied Physiology. 2004;97(3):941–947. doi: 10.1152/japplphysiol.00256.2004. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC, Taylor SE. Are psychosocial factors mediators of socioeconomic status and health connections?: A progress report and blueprint for the future. Annals of the New York Academy of Sciences. 2010;1186:146–173. doi: 10.1111/j.1749-6632.2009.05332.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. American Journal of Preventive Medicine. 1989;5(2):65–72. [PubMed] [Google Scholar]
- Merjonen P, Pulkki-Rêaback L, Puttonen S, Keskivaara P, Juonala M, Telama R, et al. Anger is associated with subclinical atherosclerosis in low SES but not in higher SES men and women. The Cardiovascular Risk in Young Finns Study. Journal of Behavioral Medicine. 2008;31(1):35–44. doi: 10.1007/s10865-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloutzian RF, Ellison CW. Loneliness, spiritual wellbeing and quality of life. In: Peplau LA PD, editor. Loneliness: A Sourcebook of Current Theory, Research, and Therapy. New York: Wiley; 1982. pp. 224–237. [Google Scholar]
- Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. American Journal of Cardiology. 1994;73(7):460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Räikkönen K, Matthews KA, Salomon K. Hostility predicts metabolic syndrome risk factors in children and adolescents. Health Psychology. 2003;22:279–286. doi: 10.1037/0278-6133.22.3.279. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Talvi SLA, Rowe JW, Seeman TE. High urinary catecholamine excretion predicts mortality and functional decline in high-functioning community-dwelling older persons: MacArthur Studies of Successful Aging. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55:M618–M624. doi: 10.1093/gerona/55.10.m618. [DOI] [PubMed] [Google Scholar]
- Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: concurrent and discriminant validity evidence. Journal of Personality and Social Psychology. 1980;39(3):472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW. Seeing disorder: Neighborhood stigma and the social construction of "broken windows.". Social Psychology Quarterly. 2004;67:319–342. [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science. 2004;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Santee J. Accuracy and precision of the Cholestech LDX System in monitoring blood lipid levels. American Journal of Health-System Pharmacy. 2002;59(18):1774–1779. doi: 10.1093/ajhp/59.18.1774. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67(6):1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Schnorpfeil P, Noll A, Schulze R, Ehlert U, Frey K, Fischer JE. Allostatic load and work conditions. Social Science & Medicine. 2003;57(4):647–656. doi: 10.1016/s0277-9536(02)00407-0. [DOI] [PubMed] [Google Scholar]
- Seeman T, Merkin SS, Crimmins E, Koretz B, Charette S, Karlamangla A. Education, income and ethnic differences in cumulative biological risk profiles in a national sample of U.S. adults: NHANES III (1988–1994) Social Science and Medicine. 2008;66(1):72–87. doi: 10.1016/j.socscimed.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science and Medicine. 2004;58(10):1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation--allostatic load and its health consequences. MacArthur Studies of Successful Aging. Archives of Internal Medicine. 1997;157(19):2259–2268. [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Ryff CD, Dienberg Love G, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosomatic Medicine. 2002;64(3):395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Shen B-J, Countryman AJ, Spiro A, III, Niaura R. The prospective contribution of hostility characteristics to high fasting glucose levels. The moderating role of marital status. Diabetes Care. 2008;31:1293–1298. doi: 10.2337/dc07-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B, Ryff CD. Hierarchies of life histories and associated health risks. Annals of the New York Academy of Sciences. 1999;896:96–115. doi: 10.1111/j.1749-6632.1999.tb08108.x. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. Chichester: John Wiley; 1988. pp. 629–649. [Google Scholar]
- Sun J, Wang S, Zhang J-Q, Li W. Assessing the cumulative effects of stress: The association between job stress and allostatic load in a large sample of Chinese employees. Work & Stress. 2007;21(4):333–347. [Google Scholar]
- Szanton S, Gill J, Allen J. Allostatic load: A mechanism of socioeconomic health disparities? Biological Research for Nursing. 2005;7(1):7–15. doi: 10.1177/1099800405278216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 y. American Journal of Clinical Nutrition. 2000;72(2):490–495. doi: 10.1093/ajcn/72.2.490. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Wheaton B, Lloyd DA. The epidemiology of social stress. American Sociological Review. 1995;60(1):104–125. [Google Scholar]
- Van CE, Spiegel K. Sleep as a mediator of the relationship between socioeconomic status and health: a hypothesis. Annals of the New York Academy of Sciences. 1999;896:254–261. doi: 10.1111/j.1749-6632.1999.tb08120.x. [DOI] [PubMed] [Google Scholar]
- Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: The Rotterdam Study. International Journal of Obesity and Related Metabolic Disorders. 2001;25(11):1730–1735. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- Weidner G, Connor SL, Hollis JF. Improvements in hostility and depression in relation to dietary change and cholesterol lowering: The Family Heart Study. Annals of Internal Medicine. 1992;117:820–823. doi: 10.7326/0003-4819-117-10-820. [DOI] [PubMed] [Google Scholar]
- Weidner J, Sexton G, McLellarn R, Connor SL, Matarazzo JD. The role of type A behavior and hostility in an elevation of plasma lipids in adult women and men. Psychosomatic Medicine. 1987;49:136–145. doi: 10.1097/00006842-198703000-00004. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Goldman N, Hedley A, Yu-Hsuan L, Seeman T. Social linkages to biological markers of health among the elderly. Journal of Biosocial Science. 2003;35(3):433–453. doi: 10.1017/s0021932003004334. [DOI] [PubMed] [Google Scholar]