Abstract
Uncontrolled proliferation is a defining feature of the malignant phenotype. Nevertheless, the supportive network provided by the stroma is indispensable for further invasion, progression and metastasis of cancer cells. In addition, the role of inflammation in tumorigenesis is now generally accepted, and it has become evident that an inflammatory microenvironment is an essential component of tumor progression. Since skin tumors are common and easily assessable lesions with features at various stages of tumorigenesis, they provide a wide scope for research in this field to further our understanding of fundamental and clinical carcinogenesis. Some of the basic aspects of epithelial tumorigenesis, invasion and stromal reaction are reviewed in this paper.
Keywords: Fibroblast, Invasion, Stroma, Tumor
INTRODUCTION
Tumor invasion consists of a series of discrete biological processes that move tumor cells from the primary neoplasm to underlying stroma. Invasion involves changes in tumor cell adherence to other cells and to the extracellular matrix (ECM), proteolytic degradation of surrounding stroma and motility to physically propel a tumor cell through the stroma. Tumor cell adherence to the ECM is mediated by integrins and other cell receptors for ECM proteins1. Skin tumors are a common health problem and comprise a wide range of benign and malignant features such as seborrheic keratosis, keratoacanthoma, actinic keratosis, Bowen's disease and squamous cell carcinoma. Therefore, morphological and immunobiological analysis of skin tumors can provide an important model for understanding the multi-step progression of epithelial tumorigenesis.
LOSS OF CELL-CELL ADHESION AND EPITHELIAL-MESENCHYMAL TRANSITION
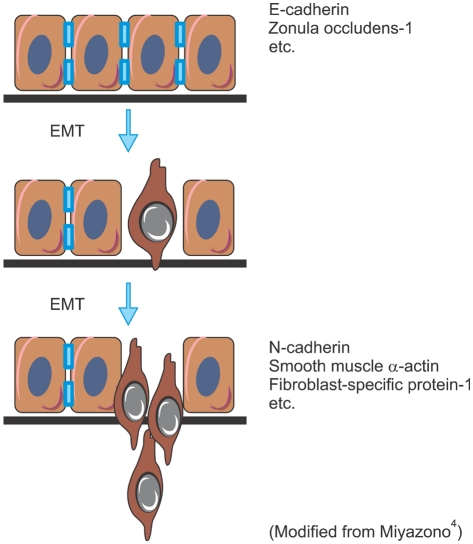
Epithelial cells establish close contact with neighboring cells and an apical-basal axis of polarity through the sequential arrangement of adherens junctions, desmosomes, and tight junctions. Epithelial cell-cell adhesion is mediated by cadherins, which bind cells through homophilic protein-protein interactions of their extracellular domains; intracellularly, cadherins interact with catenins and the actin cytoskeleton. Invasion is accompanied by a 'switch' in tumor cell cadherin expression, for instance from E-cadherin, which promotes tumor cell-tumor cell adhesion (and blocks invasion), to N-cadherin, which is normally expressed on mesenchymal cells and facilitates tumor cell binding to the stroma during invasion (Fig. 1). In this process, certain tumor cells undergo an epithelial-mesenchymal transition (EMT)2. Three types of EMT are recognized depending on the phenotype of the output cells3. Type 1 EMT is seen when primitive epithelial cells transit into mesenchymal cells that form the diaspora of the basic body plan following gastrulation or neural crest migration. Type 2 is seen when secondary epithelial cells or endothelial cells populate interstitial spaces with resident or inflammation-induced fibroblasts, the latter during persistent injury. Type 3 is part of the metastatic process, whereby epithelial tumor cells leave a primary tumor nodule, migrate to a new tissue site, and reform as a secondary tumor nodule3. Type 3 EMT occurs in an orchestrated fashion; one of the earliest events involves the disruption of tight junctions that connect epithelial cells, as well as delocalization of tight junction proteins, including zonula occludens-1, claudin-1, and occludin. Adherens junction complexes, which contain E-cadherin and β-catenin, are also disrupted, and reorganization of the actin cytoskeleton from a cortical location into actin stress fibers anchored to the focal adhesion complexes is observed (Figs. 1 and 2)3,4.
Fig. 1.
Process of epithelial-mesenchymal transition (EMT).
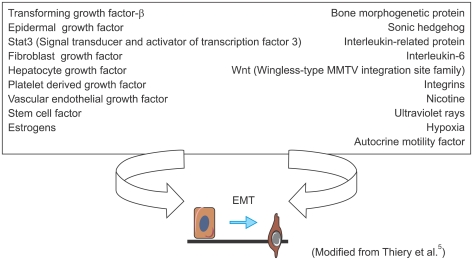
Fig. 2.
Inducers of type 3 epithelial-mesenchymal transition (EMT), carcinoma-metastatic transition.
Upon EMT, apicobasal polarity is lost, and the cells exhibit a spindle-shaped morphology and express mesenchymal markers, including N-cadherin, vimentin, fibronectin, α-smooth muscle actin, and fibroblast-specific protein 14. Cancer metastasis is accelerated by EMT through stimulation of cell migration and invasion, cell-substrate adhesion, intravasation, and extravasation, as well as cell survival. Indeed, cell survival is important for the transit of tumor cells in the blood and lymph systems, and reestablishment at sites of metastasis4.
INDUCERS AND ACTORS IN TYPE 3 EMT
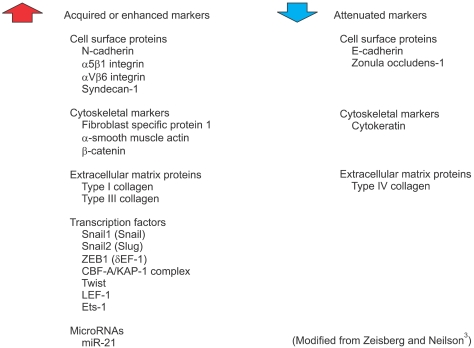
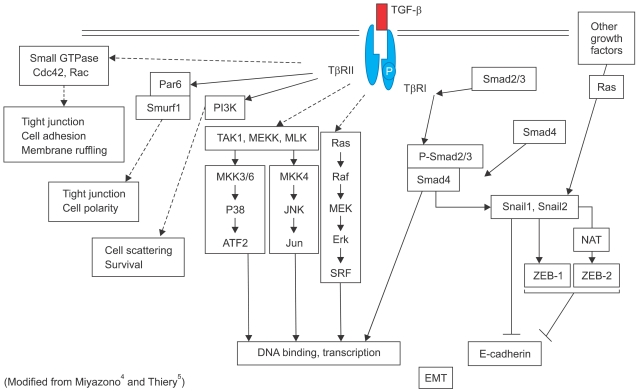
In Type 3 EMT, epithelial carcinoma cells undergo carcinoma-metastatic transitions forming migratory metastatic tumor cells3. Various kinds of stimuli are known to induce Type 3 EMT, including transforming growth factor-β (TGF-β), bone morphogenetic protein, fibroblast growth factor and epidermal growth factor (Fig. 2). In response to these stimuli, tumor cells with EMT acquire or enhance various markers including N-cadherin, α5β1 integrins, nuclear β-catenin as well as Snail-1, an indispensable transcription factor for EMT (Fig. 3). In general, tumor cells lose their expression of E-cadherin and Type IV collagen during EMT (Fig. 3)3. For example, TGF-β binds to Type II and Type I serine-threonine kinase receptors, termed TβRII and TβRI, respectively. TβRII transphosphorylates TβRI, which in turn activates Smad2/3. Once activated, Smad2/3 forms a complex with Smad4 and translocates into the nucleus to regulate the transcription of target genes (Fig. 4)4. Meanwhile, TGF-β induces the expression of several transcription factors and regulators involved in EMT including ZEB-1 and Snail-1/2. Snail suppresses E-cadherin expression by direct binding to an E2-box sequence of the E-cadherin promoter (Fig. 4)4-6. The loss of E-cadherin is considered a crucial step in the progression of papilloma to invasive carcinoma7, and it is also a fundamental event in EMT5.
Fig. 3.
Markers of epithelial-mesenchymal transition (EMT).
Fig. 4.
TGF-β signaling and epithelial-mesenchymal transition (EMT).
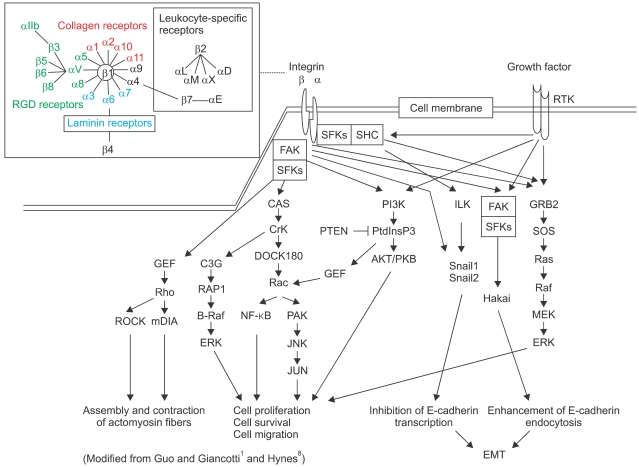
During migration and invasion of cancer cells through ECM, integrins transmit both mechanical and chemical signals. Each integrin consists of two Type-I transmembrane subunits: α and β. In mammals, 18 and 8 subunits associate with various combinations to bind to collagen, laminin or the RGD motif in fibronectin (Fig. 5). Most integrins activate focal adhesion kinase (FAK) and also Src-family kinases, which induces the phosphorylation of CAS and paxillin. Various growth factors and their receptor tyrosine kinases (RTK) activate Ras/ERK signaling together with PI3K and FAK activation. Excessive joint integrin/RTK signaling disrupts cell-cell adhesion by inhibiting E-cadherin transcription through Snail cascade and by enhancing E-cadherin endocytosis via Hakai (Cbl-like E3 ubiquitin protein ligase) recognition, leading to EMT (Fig. 5)1,8.
Fig. 5.
Integrin signaling.
TUMOR PROLIFERATION AND INTRACELLULAR SIGNALING
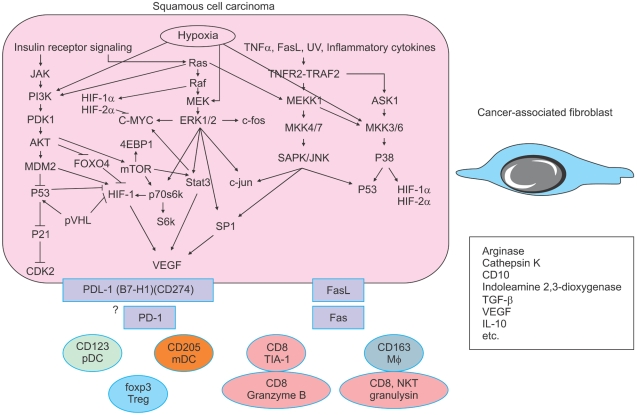
All cancers are thought to share a common pathogenesis. DNA damage and genomic instability are underlying features of human cancer from the earliest stages of tumorigenesis. Damage to genomic DNA is evident even in apparently normal cells and becomes amplified as tumors emerge9. The inappropriate proliferation of cells harboring oncogenic lesions is challenged by multiple layers of mechanisms that suppress tumor formation. Several of these barriers are cell intrinsic, such as the genotoxic stress induced by oncogenes, the expression of growth inhibitory, apoptotic and senescence pathways, and telomere attrition. Evasion of these tumor-suppressive pathways is a hallmark of primary tumors9. However, an entirely distinct class of pressures comes from sources that are extrinsic to the cancerous cells. Factors in the tumor microenvironment that limit tumor progression include extracellular matrix components, basement membranes, reactive oxygen species, limited availability of nutrients and oxygen, and attack by the immune system (Fig. 6)9.
Fig. 6.
Intracellular signaling by hypoxia and stromal reaction.
In tumors, hypoxia is a strong selective pressure that promotes the outgrowth of malignant cells with a diminished susceptibility to apoptosis. The cellular response to low oxygen tension involves the stabilization of a hypoxia-inducible factor-1 (HIF-1) transcriptional complex that activates genes that promote angiogenesis, anaerobic metabolism, cell survival, and invasion9,10. Tumors that exhibit abundant HIF-1 stabilization have a greater likelihood of developing metastatic relapse and correlate with a shorter survival time9,11. Accordingly, by analyzing global transcript levels, an "epithelial cell hypoxia signature" has been established as an independent predictor of metastatic risk for breast and ovarian carcinomas9,12. A hypoxia-induced signaling pathway is, at least in part, depicted in Fig. 6. Overexpression of HIF-1 and downstream signal molecules such as p-STAT3, p-AKT and p-ERK was detected in malignant keratinocytic tumors13-15. A subset of HIF-1 target genes may act as a mediator of metastatic progression. HIF-1 induces the expression of the chemokine receptor CXCR4 in renal cell carcinoma cells, which may promote organ-specific metastatic dissemination9,16.
INVASION, ECM AND CANCER-ASSOCIATED FIBROBLASTS
Cancer cells acquire cell-autonomous capacity for limitless proliferation and survival through the activation of oncogenes and inactivation of tumor suppressor genes. Nevertheless, the formation of a clinically relevant tumor requires support from the surrounding normal stroma, also referred to as the tumor microenvironment. Carcinoma-associated fibroblasts, bone marrow derived cells, and vascular/lymphatic endothelial cells present within the tumor microenvironment contribute to tumor progression17,18. Tumor-associated macrophages and other immune cells participate in the regulation of tumor growth and apoptosis by expressing PD-1/PDL-1 and Fas/FasL (Fig. 6)19,20. Growth, invasion and metastatic potential of tumor cells are influenced through tumor-stroma interaction in which tumor cells and mesenchymal cells including inflammatory cells surrounding the tumor cells interact with each other2.
The engagement of integrins and other attachment molecules is accompanied by the recruitment of proteases to degrade the ECM, providing a pathway for invasion21. Matrix metalloproteinases (MMPs), plasmins, urokinase-type plasminogen activators, cathepsins and heparanases, when transfected into a tumor cell line, augment invasion. Most tumor cell movement in invasion is dynamic, involving the formation of adhesions to the ECM at the leading edge of the cell, detachment from the ECM at the trailing edge and ratcheting of the cell in the forward direction. Virtually every 'growth factor' stimulates tumor cell motility in vitro. Chemokines, chemotactic cytokines that bind G-protein-coupled receptors, represent another important class of motility-inducing proteins2,22. Chemokines also contribute to tumor cell invasion by inducing the infiltration of tumors by macrophages and lymphocytes, which release proteases and other inflammatory stimuli. Growth factors can stimulate motility and invasion by mechanisms distinct from those involved in mitogenesis. In the case of RTK activation, proliferation is mediated by the phosphatidylinositol-3 kinase and ERK pathways, whereas invasion can occur by RTK interaction with integrins to stimulate formation of an FAK-Src complex2,23. Sequential binding of protein cascades to FAK triggers many of the downstream cellular changes in invasion. FAK binding of GEF leads to Rho activation, the formation of cytoplasmic actin stress fibers and mature focal adhesions, and stabilization of the nascent adhesion. Adhesion at the leading edge must be coupled with de-adhesion at other sites so that the cell can be pulled forward by cytoskeletal contraction (Fig. 5)2,23,24.
Cancer-associated fibroblasts are essential for tumor progression providing both a functional and structural supportive environment, and the presence of fibroblast populations within human tumors is usually associated with poor outcome and an increase in metastatic potential by enhancing matrix degradation and angiogenesis through the production of MMPs and various cytokines or growth factors25-27. At the invasion front, the cancer-associated fibroblasts are known to produce large amounts of MMPs and cathepsin K28,29. Cathepsin K, a cysteine protease with strong collagenolytic and elastolytic properties involved in extracellular matrix turnover, plays an essential role in osteoclast-mediated bone resorption30. Cathepsin K is expressed in the stroma of basal cell and squamous cell carcinomas, especially in invasive cases, but not in normal fibroblasts31. A high expression level of cathepsin K together with cathepsin B is significantly correlated with shorter recurrence-free interval and overall survival time in pulmonary adenocarcinomas32.
CD10 is also an interesting molecule produced from cancer-associated fibroblasts. CD10 is a 90- to 110-kDa cell-surface zinc-dependent metalloproteinase also referred to as a neutral endopeptidase (EC 3.4.24.11), enkephalinase, neprilysin and common acute lymphoblastic leukemia antigen, which cleaves various peptide substrates including substance P33. CD10 expression has been detected in peritumoral fibroblasts within the invasive area of various cancers such as prostate, breast, colorectal, and skin carcinomas, suggesting that CD10 expression in peritumoral cells may contribute or correlate to malignant tumor progression34-38. The fact that CD10+ fibroblasts are frequently seen at the invasive front suggests that tumor-stromal interaction exists between cancer cells and CD10+ fibroblasts. CD10 is a potent degrading enzyme for substance P, which is known to promote motility of epithelial cells39. Therefore, CD10-bearing fibroblasts may play a protective role in tumor invasion. The tumor-stroma cellular interaction includes direct and/or indirect cross-talk because the active interplay results in the secretion of autocrine and/or paracrine soluble factors. However, the nature and regulatory mechanisms of cancer-associated fibroblasts still remain largely unknown.
CONCLUSION
Uncontrolled proliferation is a defining feature of the malignant phenotype. Nevertheless, the supportive network provided by the stroma is indispensable for further invasion, progression and metastasis. In addition, the role of inflammation in tumorigenesis is now generally accepted, and it has become evident that an inflammatory microenvironment is an essential component of all tumors40. For example, inflammatory bowel disease greatly increases the risk of colorectal cancer41. However, not all chronic inflammatory diseases increase cancer risk, and some of them, such as psoriasis, may even reduce it42. It is not clear what kinds of external and internal stimuli drive or suppress tumorigenesis.
Footnotes
This work was partly supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare, Japan.
References
- 1.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 2.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 3.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:314–323. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 7.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 9.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 12.Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lining X, Uchi H, Hayashida S, Tsuji G, Kido M, Nakahara T, et al. Expression of hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha with progression of keratinocytic neoplasms. J Dermatol Sci. 2009;56:135–136. doi: 10.1016/j.jdermsci.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Chen SY, Takeuchi S, Moroi Y, Hayashida S, Kido M, Chen SJ, et al. Overexpression of phosphorylated-ATF2 and STAT3 in cutaneous squamous cell carcinoma, Bowen's disease and basal cell carcinoma. J Dermatol Sci. 2008;51:210–215. doi: 10.1016/j.jdermsci.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Lin N, Moroi Y, Uchi H, Fukiwake N, Dainichi T, Takeuchi S, et al. Significance of the expression of phosphorylated-STAT3, -Akt, and -ERK1/2 in several tumors of the epidermis. J Dermatol Sci. 2007;48:71–73. doi: 10.1016/j.jdermsci.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 17.Lorusso G, Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol. 2008;130:1091–1103. doi: 10.1007/s00418-008-0530-8. [DOI] [PubMed] [Google Scholar]
- 18.Hanna E, Quick J, Libutti SK. The tumour microenvironment: a novel target for cancer therapy. Oral Dis. 2009;15:8–17. doi: 10.1111/j.1601-0825.2008.01471.x. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 22.Balkwill F. Chemokine biology in cancer. Semin Immunol. 2003;15:49–55. doi: 10.1016/s1044-5323(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 23.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 24.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 25.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eck SM, Côté AL, Winkelman WD, Brinckerhoff CE. CXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Mol Cancer Res. 2009;7:1033–1044. doi: 10.1158/1541-7786.MCR-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleer CG, Bloushtain-Qimron N, Chen YH, Carrasco D, Hu M, Yao J, et al. Epithelial and stromal cathepsin K and CXCL14 expression in breast tumor progression. Clin Cancer Res. 2008;14:5357–5367. doi: 10.1158/1078-0432.CCR-08-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271:12511–12516. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- 31.Quintanilla-Dieck MJ, Codriansky K, Keady M, Bhawan J, Runger TM. Cathepsin K in melanoma invasion. J Invest Dermatol. 2008;128:2281–2288. doi: 10.1038/jid.2008.63. [DOI] [PubMed] [Google Scholar]
- 32.Cordes C, Bartling B, Simm A, Afar D, Lautenschlager C, Hansen G, et al. Simultaneous expression of Cathepsins B and K in pulmonary adenocarcinomas and squamous cell carcinomas predicts poor recurrence-free and overall survival. Lung Cancer. 2009;64:79–85. doi: 10.1016/j.lungcan.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Stimler-Gerard NP. Neutral endopeptidase-like enzyme controls the contractile activity of substance P in guinea pig lung. J Clin Invest. 1987;79:1819–1825. doi: 10.1172/JCI113023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albrecht M, Gillen S, Wilhelm B, Doroszewicz J, Aumüller G. Expression, localization and activity of neutral endopeptidase in cultured cells of benign prostatic hyperplasia and prostate cancer. J Urol. 2002;168:336–342. [PubMed] [Google Scholar]
- 35.Kesse-Adu R, Shousha S. Myoepithelial markers are expressed in at least 29% of oestrogen receptor negative invasive breast carcinoma. Mod Pathol. 2004;17:646–652. doi: 10.1038/modpathol.3800103. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa H, Iwaya K, Izumi M, Kuroda M, Serizawa H, Koyanagi Y, et al. Expression of CD10 by stromal cells during colorectal tumor development. Hum Pathol. 2002;33:806–811. doi: 10.1053/hupa.2002.125773. [DOI] [PubMed] [Google Scholar]
- 37.Xie LN, Uchi H, Hayashida S, Kido M, Takeuchi S, Takahara M, et al. Stromal CD10 expression is correlated with invasiveness and proliferation of extramammary Paget disease. Br J Dermatol. 2008;158:1389–1391. doi: 10.1111/j.1365-2133.2008.08542.x. [DOI] [PubMed] [Google Scholar]
- 38.Takahara M, Chen S, Kido M, Takeuchi S, Uchi H, Tu Y, et al. Stromal CD10 expression, as well as increased dermal macrophages and decreased Langerhans cells, are associated with malignant transformation of keratinocytes. J Cutan Pathol. 2009;36:668–674. doi: 10.1111/j.1600-0560.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 39.Muangman P, Tamura RN, Muffley LA, Isik FF, Scott JR, Xie C, et al. Substance P enhances wound closure in nitric oxide synthase knockout mice. J Surg Res. 2009;153:201–209. doi: 10.1016/j.jss.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 41.Waldner MJ, Neurath MF. Colitis-associated cancer: the role of T cells in tumor development. Semin Immunopathol. 2009;31:249–256. doi: 10.1007/s00281-009-0161-8. [DOI] [PubMed] [Google Scholar]
- 42.Nickoloff BJ, Ben-Neriah Y, Pikarsky E. Inflammation and cancer: is the link as simple as we think? J Invest Dermatol. 2005;124:x–xiv. doi: 10.1111/j.0022-202X.2005.23724.x. [DOI] [PubMed] [Google Scholar]