Abstract
Background
Despite numerous treatments available for deteriorated cutaneous wound healing such as a diabetic foot, there is still the need for more effective therapy. Adipose-derived stem cells (ASCs) are mesenchymal stem cells, which are self-renewing and multipotent. Mesenchymal stem cells have the potential for tissue repair and regeneration.
Objective
To investigate the effects of human ASCs on the healing of cutaneous wounds in nude mice.
Methods
15-mm round full-thickness skin defects were generated on the back of BALB/c nude mice. The mice were divided into three groups for wound coverage: (i) human ASCs-populated collagen gel, (ii) human dermal fibroblasts-populated collagen gel, and (iii) collagen gel alone. Wound contraction was prevented with a splint method. Wound size was measured 10 days after injury. At 28 days histological analysis was performed.
Results
Both ASCs and dermal fibroblasts accelerated wound closure, but dermal fibroblasts were more effective than ASCs. At 28 days, the dermal portion of ASCs or dermal fibroblasts wound scars were thicker than collagen gel wound scars.
Conclusion
ASCs and dermal fibroblasts stimulate cutaneous wound healing and improve scar thickness.
Keywords: Adipose tissue, Fibroblast, Mesenchymal stem cell, Mouse, Wound healing
INTRODUCTION
Wound healing involves a complex chain of events with interactions among different cells and tissues. The progression of this wound healing process is impaired in a number of medical conditions, including diabetes1. Although new therapeutic methods have been developed, the treatment of many chronic wounds remains unsatisfactory, and more effective treatment strategies are needed.
Bone marrow-derived mesenchymal stem cells (BMSCs) are multipotent stem cells capable of differentiating into numerous cell types, including osteoblasts, chondrocytes, and adipocytes2-4. BMSCs can accelerate wound healing5,6, but BMSC transplantation requires harvesting a large amount of bone marrow under general anesthesia, which may lead to severe complications.
Adipose-derived stem cells (ASCs) represent an alternative source of multipotent cells with similar characteristics to BMSCs7,8. ASCs are easier to isolate and relatively abundant, which may make them a potential source for wound repair and regeneration. The effects of ASCs on cutaneous wound healing have not been clearly demonstrated. Autologous ASCs improved wound healing in healing-impaired db/db mice9, and human ASCs accelerated wound closure in nude mice10. However, wound contraction was not prevented in both animal experiments, so it is uncertain whether ASCs enhanced the wound healing process including re-epithelialization and granulation tissue formation. In this study, we examined the benefit of human ASCs in wound healing on a nude mice wound model with a contraction-preventing splint method. Healing was measured after excisional wounds were covered with ASCs-populated collagen gels (CG), human dermal fibroblasts (DFs)-populated CG, or CG alone.
MATERIALS AND METHODS
All the experimental protocols were approved by the Institutional Review Board and Institutional Animal Care and Use Committee of the Seoul National University Hospital.
Isolation and culture of ASCs, DFs and HaCaT cells
Human subcutaneous adipose tissues were acquired from elective liposuction from healthy female donors with informed consent. The suctioned fat was digested with 0.075% collagenase type I (Sigma-Aldrich, St. Louis, MO, USA) in phosphate-buffered saline under gentle agitation for 30 min at 37℃, and centrifuged at 800 × g for 10 min to obtain the stromal cell fraction. The pellets were resuspended, passed through a 100 mm mesh filter (Millipore, Billerica, MA, USA) to remove connective tissues, and cultured at 37℃ in 5% CO2 in Dulbecco's modified Eagle media (DMEM) supplemented with 10% fetal bovine serum, 100 mg/ml streptomycin, and 100 U/ml penicillin. After 3 days, culture medium was changed to MesenPRO RS™ Medium (Invitrogen, Carlsbad, CA, USA), and was replaced every 3 days. The primary cells were cultured until 80% confluence and were defined as "Passage 1". ASCs were used for the experiment at passage 4.
For harvesting human DFs, foreskin was obtained from donors under informed consent, and DFs were cultured as previously described11. DFs at passages 4~6 were used for the experiment. HaCaT cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 mg/ml streptomycin, and 100 U/ml penicillin at 37℃ in 5% CO2.
Preparing cell-populated collagen gels
Before preparing the gels, 1 ml of 0.1% bovine serum albumin (Sigma-Aldrich) solution was added into each well of a 48-well plate and incubated at 37℃ for 1 h. A collagen solution mixture was prepared by quickly mixing eight volumes of collagen type I solution (Nitta Gelatin, Tokyo, Japan) with one volume of 10-fold concentrated DMEM and one volume of sodium bicarbonate (22 mg/ml). ASCs or DFs (1×106) were added to 300µl of collagen solution mixture, and gently mixed. Collagen solution mixture with ASCs, DFs or alone was poured into a 48-well plate after bovine serum albumin solution was removed, and allowed to gel for 30 minutes in a 5% CO2 atmosphere at 37℃. After 30 minutes, culture media was poured into each well, and the CGs were detached from the surface of the well by rimming the gels with a sterile needle and gently swirling the plate.
Wound healing model and cell transplantation
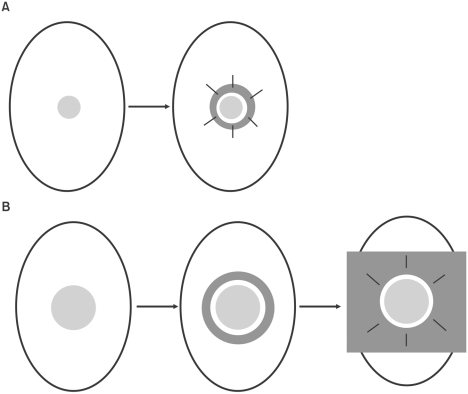
Six to eight-week-old male BALB/c nude mice (Orient Bio, Sungnam, Korea) were used. Mice were anesthetized using an intraperitoneal injection of ketamine (75 mg/kg) and xylazine (15 mg/kg). The wound model which was suggested by Galiano et al.12 was used with some modifications. A 15 mm round full-thickness excisional wound was made on the back of mice using an Iris scissor. A donut-shaped flexible plastic splint with a 16 mm inner and 18 mm outer diameter was fixed to the skin around the wound with an adhesive film (3M Ioban™ 2, 3M, St. Paul, MN, USA) and interrupted 6-0 nylon sutures (Fig. 1). The mice were divided into three groups depending on what covered the wound: (i) ASCs-populated CG (n=4), (ii) DFs-populated CG (n=4), and (iii) CG alone (n=4). Each CG was applied on the wound, and the occlusive dressing (Tegaderm, EM, St. Paul, MN, USA) was placed to cover the wounds. Each animal was then housed in its own cage.
Fig. 1.
Schematic representation of wound model. (A) Galiano's model. 6 mm round wounds were created on the mouse dorsum. The donut-shaped silicone sheet splint was centered on the wound, fixed with an immediate-bonding adhesive and interrupted nylon sutures. (B) Modified model used in our experiment. 15 mm wounds were made on the dorsal skin of mouse. A flexible plastic splint was fixed to the skin around the wound with an adhesive film and interrupted 6-0 nylon sutures.
Wound size measurements
Digital photographs of wounds were taken on day 10 after surgery. Wound photographs were shown on the computer monitor with an image analysis program (ImageJ, National Institutes of Health, Bethesda, MD, USA), and the border of unhealed area that was not re-epithelialized was traced manually. The size of the traced area was calculated automatically by the software.
Histological analysis
For histological analysis of wound scars, the mice were euthanized at 28 days, and the whitish scars were excised, bisected, and fixed in 10% formalin. The samples underwent routine histological processing with hematoxylin and eosin.
Statistical analysis
Results were expressed as means±SEM. A Mann-Whitney U test was used for statistical analysis, and a value of p<0.05 was considered statistically significant.
RESULTS
Wound size measurements
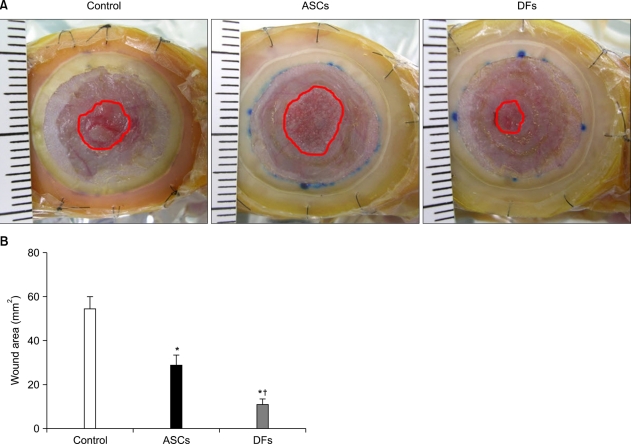
The size of unhealed wounds not re-epithelialized was measured. At 6 days after the injury, it was difficult to determine the boundary of re-epithelialization. The difference in size of the unhealed wound between groups was most obvious on day 10 (Fig. 2A). The size of the wound after ASCs treatment was significantly smaller than CG alone (28.63±5.05 mm2, 54.63±5.69 mm2, p<0.05). DFs treatment healed the wounds significantly faster than both ASCs and CG alone (11.09±2.71 mm2, p<0.05) (Fig. 2B).
Fig. 2.
Both adipose-derived stem cells (ASCs) and dermal fibroblasts (DFs) accelerated wound closure, but DFs were more effective than ASCs. (A) BALB/c nude mice were wounded and treated with collagen gel alone (control) or ASCs or DFs in collagen gel. After 10 days, photomicrographs were taken. The red line is the margin of unhealed wound that was not re-epithelialized. (B) The size of unhealed wound area was calculated with an image analysis program. The values shown are the means±SEM of 4 mice per group. *p<0.05 vs. collagen gel group; †p<0.05 vs. ASCs group.
Histological analysis
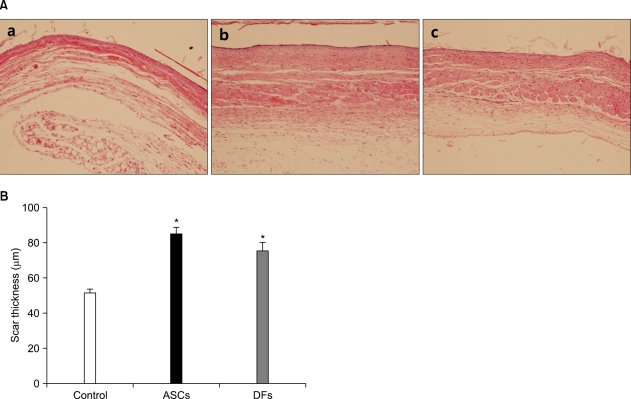
At 28 days after surgery, the wounds that became a whitish scar were excised for histological examination (Fig. 3A). The dermal portion of ASCs or DFs wound scars was thicker than CG wound scars (84.50±4.39µm, 75.78±4.52µm, 51.61±2.31µm, p<0.05) (Fig. 3B).
Fig. 3.
Adipose-derived stem cells (ASCs) or dermal fibroblasts (DFs) wound scars were thicker than collagen gel alone. (A) At 28 days after surgery, whitish scars of collagen gel (a), ASCs in collagen gel (b) or DFs in collagen gel (c) wounds were harvested and processed for histological examination. (B) The thickness of dermal portion of wound scars was measured with an image analysis program. The values shown are the means±SEM of 4 mice per group (H&E, ×200). *p<0.05 vs. collagen gel group.
DISCUSSION
Mice are commonly used in wound healing models, but with some limitations. Because wound contraction makes the defect close rapidly, it is difficult to measure re-epithelialization and granulation tissue formation. Galiano et al.12 suggested preventing contraction by fixing donut-shaped silicone splint around the wound. We used Galiano's model with some modifications. To make it easier to distinguish differences in wound closure, we made a larger wound of 15 mm diameter compared with the 6 mm wound used in Galiano's model. We were able to prevent wound contraction successfully and accurately measure wound closure.
We showed that ASCs promoted healing of full thickness wounds in nude mice, and, unexpectedly, DFs closed the wounds more rapidly than ASCs. ASCs combined with the atelocollagen matrix improved wound healing in healing-impaired db/db mice, but only had minor effects in normal (db/+) mice, perhaps because the closure assay was not sensitive enough to detect a difference in normal mice9. Preventing wound contract can improve assay sensitivity, as we showed here in healing-competent nude mice.
There have been several reports suggesting that DFs may be able to accelerate cutaneous wound healing. DFs seeding in conjunction with a collagen sponge matrix facilitated dermal and epidermal wound healing compared with collagen sponge only13. Skin substitutes with dermal components containing DFs induced the proliferation and differentiation of keratinocytes and the formation of basement membrane, accelerating re-epithelialization14-16. These reports support our result of positive effects of DFs on wound healing. However, Wu et al.5 reported that DFs did not improve wound healing in Galiano's wound model. To avoid immunorejection, we used BALB/c nude mice that have defective T cell immunity. Wu et al.5 used allogeneic cells, DFs derived from C57BL/6 mice on immunocompetent BALB/c mice, which may elicit an immunogenic response. In a porcine wound model, allogeneic DFs induced more inflammatory reactions and scar formation than autologous ones17, indicating that transplanted DFs can be influenced by the immune response of the host, which may lead to different healing outcomes.
Wound healing depends on re-epithelialization, granulation tissue formation, and angiogenesis mediated by keratinocytes, DFs, and endothelial cells. It has been shown that ASCs affect DFs and endothelial cells in a paracrine fashion. Conditioned medium of ASCs promotes different functions of DFs including proliferation, migration, and production of extracellular matrix10. Proangiogenic factors such as vascular endothelial growth factor are secreted at high levels from ASCs, so conditioned medium of ASCs accelerates proliferation of endothelial cells in vitro18,19. This paracrine effect of ASCs on endothelial cells may contribute to wound healing by promoting angiogenesis in vivo.
The dermal portion of ASCs- or DFs-populated CG wound scars was thicker than CG wound scars 28 days after surgery. Therefore, when scar thickness is an issue, particularly problematic atrophic scars, ASCs or DFs in dermal skin substitution may be useful. Although DFs showed greater effects in wound healing than ASCs, DFs have some clinical limitations. Autologous DFs require self skin for isolation. Although allogeneic DFs are relatively easy to obtain, their immunogenicity could reduce their effects on wound healing. Autologous ASCs are easily obtained by liposuction. BMSC have low immunogenicity and immunoregulatory action by suppressing the functions of immune cells, including T cells20,21. Because ASCs have similar characteristics to BMSC, allogeneic ASCs might not be affected by the host immune response, and might be able to accelerate wound healing like autologous ones.
In conclusion, ASCs and DFs accelerated wound healing, and DFs showed a greater effect. ASCs and DFs application formed a thicker dermal portion of scars than control.
Footnotes
This study was supported by Grant No. 04-2006-1050 from the Seoul National University Hospital Research Fund.
References
- 1.Steeper R. A critical review of the aetiology of diabetic neuropathic ulcers. J Wound Care. 2005;14:101–103. doi: 10.12968/jowc.2005.14.3.26746. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282:148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–1732. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nambu M, Kishimoto S, Nakamura S, Mizuno H, Yanagibayashi S, Yamamoto N, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg. 2009;62:317–321. doi: 10.1097/SAP.0b013e31817f01b6. [DOI] [PubMed] [Google Scholar]
- 10.Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Park JS, Park WY, Cho KA, Kim DI, Jhun BH, Kim SR, et al. Down-regulation of amphiphysin-1 is responsible for reduced receptor-mediated endocytosis in the senescent cells. FASEB J. 2001;15:1625–1627. doi: 10.1096/fj.00-0723fje. [DOI] [PubMed] [Google Scholar]
- 12.Galiano RD, Michaels J, 5th, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 13.Marks MG, Doillon C, Silver FH. Effects of fibroblasts and basic fibroblast growth factor on facilitation of dermal wound healing by type I collagen matrices. J Biomed Mater Res. 1991;25:683–696. doi: 10.1002/jbm.820250510. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto E, Kitano Y. Expression of basement membrane components in skin equivalents--influence of dermal fibroblasts. J Dermatol Sci. 1993;5:81–88. doi: 10.1016/0923-1811(93)90074-y. [DOI] [PubMed] [Google Scholar]
- 15.Maruguchi T, Maruguchi Y, Suzuki S, Matsuda K, Toda K, Isshiki N. A new skin equivalent: keratinocytes proliferated and differentiated on collagen sponge containing fibroblasts. Plast Reconstr Surg. 1994;93:537–546. [PubMed] [Google Scholar]
- 16.Medalie DA, Eming SA, Collins ME, Tompkins RG, Yarmush ML, Morgan JR. Differences in dermal analogs influence subsequent pigmentation, epidermal differentiation, basement membrane, and rete ridge formation of transplanted composite skin grafts. Transplantation. 1997;64:454–465. doi: 10.1097/00007890-199708150-00015. [DOI] [PubMed] [Google Scholar]
- 17.Lamme EN, van Leeuwen RT, Mekkes JR, Middelkoop E. Allogeneic fibroblasts in dermal substitutes induce inflammation and scar formation. Wound Repair Regen. 2002;10:152–160. doi: 10.1046/j.1524-475x.2002.10901.x. [DOI] [PubMed] [Google Scholar]
- 18.Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279–290. doi: 10.1159/000094140. [DOI] [PubMed] [Google Scholar]
- 19.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 20.Morandi F, Raffaghello L, Bianchi G, Meloni F, Salis A, Millo E, et al. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells. 2008;26:1275–1287. doi: 10.1634/stemcells.2007-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]