Abstract
LEOPARD multiple congenital anomaly syndrome inherited in an autosomal dominant manner. LEOPARD is an acronym for Lentigines, Eletrocardiographic conduction defects, Ocular hypertelorism, Pulmonary valve stenosis, Abnormalities of the genitalia, Retardation of growth, and Deafness. Clinical diagnosis is primarily based on multiple lentigines, typical facial features, and the presence of hypertrophic cardiomyopathy and/or café-au-lait macules. We report a typical case of LEOPARD syndrome with PTPN11 gene mutation associated with lentigines, electrocardiograph abnormality, ocular hypertelorism, pulmonary valve stenosis, growth retardation, and sensorineural hearing loss.
Keywords: LEOPARD syndrome, Multiple lentigines, PTPN11 gene
INTRODUCTION
LEOPARD syndrome (LS) is a multiple congenital anomaly syndrome inherited in an autosomal dominant manner. It has high penetrance and markedly variable phenotypic expression. Diagnostic clues to LS include multiple lentigines, café-au-lait macules, hypertrophic cardiomyopathy, and deafness. To date, about 200 LS patients have been reported globally. It has recently been reported that LS is related to PTPN11 gene mutation1, which is located on chromosome 12q24.1. Herein, we report a case of typical LS associated with PTPN11 gene mutation.
CASE REPORT
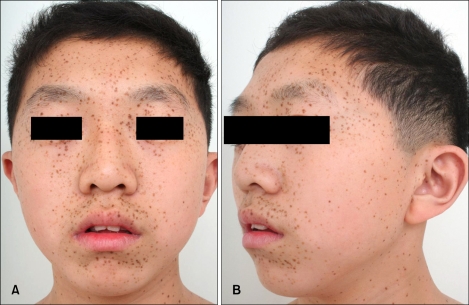
A 16 year-old male visited our clinic with multiple dark macules of varying size over his whole body from the age of 2 years associated with pigmented macules on the face, trunk, and upper extremities (Fig. 1). He had been diagnosed as hypertrophic cardiomyopathy (HCMP) at the age of 1 and for which he has been managed with a beta blocker. In addition, he developed severe bilateral neuronal deafness and underwent a cochlear implant in his right ear at the age of 5. His development suffers delay: he sat at 1 year, walked at 2 years, and experienced speech delay. His height and weight were below the 25th percentile. However, no other family members are similarly affected.
Fig. 1.
(A) Numerous brownish macules on the face with ocular hypertelorism. (B) Low set, posteriorly rotated ears and mandibular prognathism.
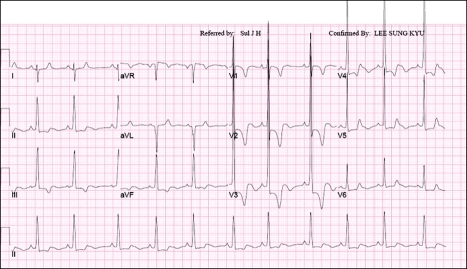
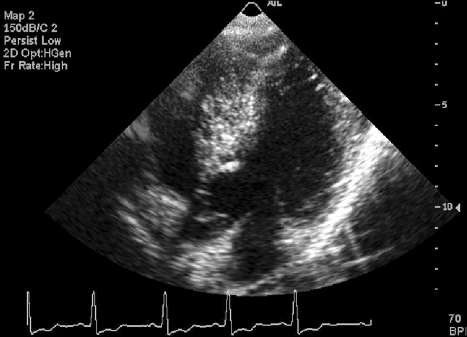
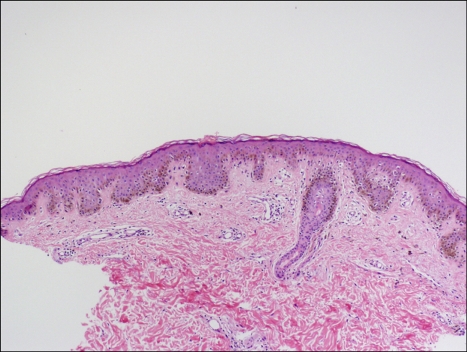
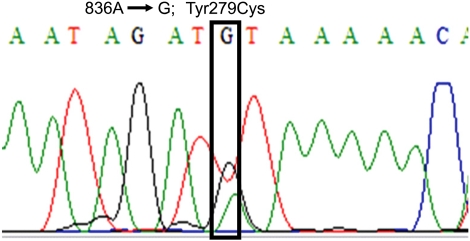
Physical examination revealed ocular hypertelorism (interpupillary distance 5.6 cm, >97th percentile), mandibular prognathism, and posteriorly rotated ears. His genitalia was normal on physical examination. Twelve-lead electrocardiograph (EKG) (Fig. 2) showed right-axis deviation, ST-segment abnormalities, and T-wave inversion. Echocardiogram (Fig. 3) showed hypertrophic obstructive cardiomyopathy with thickened interventricular septum and systolic anterior motion of the mitral valve leaflets. However, there were no abnormalities on chromosomal study. Skin biopsies were performed on the face and trunk. Histopathologically, the lesion from the trunk showed a slight elongation of the rete ridges and increase in melanocyte concentration in the basal layer (Fig. 4). The pathologic diagnosis was consistent with lentigo simplex. Based upon the clinical, pathologic, and echocardiographic findings, a diagnosis of LS was made. After obtaining the patient's consent, mutation analysis of PTPN11 was performed. Genomic DNA for germ-line mutation analyses was prepared from peripheral blood leukocytes. We performed polymerase chain reaction amplification of all 15 exons and introns of PTPN11, and sequenced all of exon and intron junctions bilaterally. We found missense mutation (836A3G; Tyr279Cys) in exon 7 (Fig. 5).
Fig. 2.
Twelve-lead EKG shows right-axis deviation, ST-segment abnormalities and T-wave inversion.
Fig. 3.
Echocardiographic analysis showed hypertrophic obstructive cardiomyopathy with thickened interventricular septum and systolic anterior motion of the mitral valve leaflets.
Fig. 4.
Histopathologic findings of pigmented macules revealed slight elongation of the rete ridges with increased melanocytes in the basal layer (H&E stain, ×100).
Fig. 5.
Mutation analysis of PTPN11 gene revealed a missense mutation 836A→G in exon 7.
We treated the lentigines on face with a triple combination cream of 0.05% tretinoin, 4.0% hydroquinone, and 0.01% fluocinolone acetonide (Tri-Luma®, Hill Dermaceutical, Inc., Stanford, CA, USA) and the affected lesions showed slight improvement after 2 months of therapy.
DISCUSSION
The LS is coined by Gorlin et al.2 in 1969 based on an acronym of multiple lentigines, EKG conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormal genitalia, growth retardation, and sensorineural deafness. This condition is also known as multiple lentigines syndrome, cardio-cutaneous syndrome, Moynahan syndrome, lentiginosis profusa, and progressive cardiomyopathic lentiginosis. About 200 patients have been reported in the English literature, but only seven cases of LS have been reported in the Korean dermatologic literature3-9.
The most plausible explanation for the pathogenesis of LS is neural crest cell abnormality associated with PTPN11 gene mutation, which codes for non-receptor protein tyrosine phosphatase, SHP21,10, which plays important roles in cell proliferation, differentiation, migration and adhesion. To the best of our knowledge, only 11 different missense PTPN11 mutations in exon 7, 12 and 13 have been reported. Recently, missense mutations in the RAF1 gene have been found in two out of six PTPN11-negative LS patients11. However, all of 7 reported LS cases in the Korean dermatologic literature did not include mutation analysis of the PTPN11 gene. In our patient, mutation analysis of the PTPN11 gene revealed a missense mutation (836A→G; Tyr279Cys) in exon 7, which is one of two frequent mutations that constitute more than 60% of cases. We did not, however, investigate for RAF1 gene mutation.
The presence of lentigines remains an aesthetic problem, since malignant transformation of the lentigines has not been reported. Kontoes et al.12 reported successful use of intense pulsed light for the treatment of lentigines in a patient with LS. Lee et al.9 reported that alexandrite laser treatment was effective for the treatment of lentigines in a 11-year-old patient with LS. Our patient was treated by triple combination cream containing 0.05% tretinoin, 4.0% hydroquinone, and 0.01% fluocinolone acetonide (Tri-Luma®) for 6 weeks and slight improvement was noted.
In general, the long-term prognosis of LS patients varies with the type of cardiac anomalies. Among patients with PTPN11 mutation, an association between exon 7 and exon 12 mutations with HCMP has been established13. Our patient experienced cyanosis and dyspnea after delivery secondary to HCMP with pulmonary valve stenosis for which he is undergoes periodic cardiology assessment. In addition, Seishima et al.14 suggested that PTPN11 mutation have specific tumorigenic effect recently. Due to the rarity of malignant cases associated with LS, the association is inconclusive. However, acute lymphoblastic leukemia15, acute myeloid leukemia16, and multiple granular cell tumor17 associated with LS with mutation in PTPN11 gene have been reported. Although predisposition of malignancy in LS remains debatable, LS patients with PTPN11 mutations should be closely monitored for malignancy, particularly during their childhood. Identification of PTPN11 gene mutation in LS would be a good marker to estimate prognosis. Our patient receives regular dermatological review to monitor for the development of malignancy.
References
- 1.Sarkozy A, Conti E, Digilio MC, Marino B, Morini E, Pacileo G, et al. Clinical and molecular analysis of 30 patients with multiple lentigines LEOPARD syndrome. J Med Genet. 2004;41:e68. doi: 10.1136/jmg.2003.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorlin RJ, Anderson RC, Blaw M. Multiple lentigenes syndrome. Am J Dis Child. 1969;117:652–662. doi: 10.1001/archpedi.1969.02100030654006. [DOI] [PubMed] [Google Scholar]
- 3.Paik SA, Kook HI. A case of multiple lentigines syndrome. Korean J Dermatol. 1978;16:131–136. [Google Scholar]
- 4.Park JH, Lee CY, Kim DH, Kim KH. A case of multiple lentigines syndrome. Korean J Dermatol. 1985;23:100–104. [Google Scholar]
- 5.Shin DY, Koo DW, Roh JY. A case of multiple lentigines syndrome. Ann Dermatol. 1997;9:219–223. [Google Scholar]
- 6.Kim SJ, Seo PS, Yoon NH, Park SD. A case of multiple lentigines sydrome with a family history of multiple lentigines. Korean J Dermatol. 2004;42:1581–1584. [Google Scholar]
- 7.Lee ES, Ko SH, Chi JS, Hur M, Park HM. A case of leopard syndrome associated with pure gonadal dysgenesis. Korean J Obstet Gynecol. 2002;45:1273–1276. [Google Scholar]
- 8.Lee SG, Lee SY, Im SH, Yoo KD, Baek SH, Kim CM, et al. A case of LEOPARD syndrome with cor triatriatum. Korean J Med. 2003;65:99–103. [Google Scholar]
- 9.Lee HJ, Chung HJ, Cho YH, Chung KY. A case of LEOPARD syndrome. Korean J Dermatol. 2005;43:949–952. [Google Scholar]
- 10.Digilio MC, Conti E, Sarkozy A, Mingarelli R, Dottorini T, Marino B, et al. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002;71:389–394. doi: 10.1086/341528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 12.Kontoes PP, Vlachos SP, Marayiannis KV. Intense pulsed light for the treatment of lentigines in LEOPARD syndrome. Br J Plast Surg. 2003;56:607–610. doi: 10.1016/s0007-1226(03)00218-2. [DOI] [PubMed] [Google Scholar]
- 13.Sarkozy A, Conti E, Seripa D, Digilio MC, Grifone N, Tandoi C, et al. Correlation between PTPN11 gene mutations and congenital heart defects in Noonan and LEOPARD syndromes. J Med Genet. 2003;40:704–708. doi: 10.1136/jmg.40.9.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seishima M, Mizutani Y, Shibuya Y, Arakawa C, Yoshida R, Ogata T. Malignant melanoma in a woman with LEOPARD syndrome: identification of a germline PTPN11 mutation and a somatic BRAF mutation. Br J Dermatol. 2007;157:1297–1299. doi: 10.1111/j.1365-2133.2007.08229.x. [DOI] [PubMed] [Google Scholar]
- 15.Laux D, Kratz C, Sauerbrey A. Common acute lymphoblastic leukemia in a girl with genetically confirmed LEOPARD syndrome. J Pediatr Hematol Oncol. 2008;30:602–604. doi: 10.1097/MPH.0b013e31817588fb. [DOI] [PubMed] [Google Scholar]
- 16.Uçar C, Calýskan U, Martini S, Heinritz W. Acute myelomonocytic leukemia in a boy with LEOPARD syndrome (PTPN11 gene mutation positive) J Pediatr Hematol Oncol. 2006;28:123–125. doi: 10.1097/01.mph.0000199590.21797.0b. [DOI] [PubMed] [Google Scholar]
- 17.Schrader KA, Nelson TN, De Luca A, Huntsman DG, McGillivray BC. Multiple granular cell tumors are an associated feature of LEOPARD syndrome caused by mutation in PTPN11. Clin Genet. 2009;75:185–189. doi: 10.1111/j.1399-0004.2008.01100.x. [DOI] [PubMed] [Google Scholar]