Abstract
In mammals and insects, paracellular blood barriers isolate the nervous system from the rest of the animal. Glia and accessory cells of the nervous system use pumps, channels, cotransporters, and exchangers collectively to maintain the extracellular ion environment and osmotic balance in the nervous system. At present the molecular mechanisms that regulate this process remain unclear. In humans, loss of extracellular ion and volume regulation in the nervous system poses serious health threats. Drosophila is a model genetic organism with a proven track record for uncovering molecular mechanisms relevant to human health and disease. Here, we review what is known about extracellular ion and volume regulation in larval abdominal nerves, present some new data about the impact of neural activity on the extracellular environment, and relate the findings to mammalian systems. Homologies have been found at the level of morphology, physiology, molecular mechanisms, and mutant phenotypes. The Fray-Ncc69 module regulates extracellular volume in larval nerves. Genetic rescue experiments with the mammalian orthologs prove this module has a direct correlate in humans. This and other molecular homologies, together with the similar physiological needs, suggest that uncovering the molecular mechanisms of ion and volume regulation in larval nerves will likely provide significant insights into this process in mammalian systems.
Keywords: blood-nerve barrier, blood-brain barrier, extracellular fluid, osmoregulation
Introduction
In many insects the hemolymph has an ionic composition that is incompatible with neural function (Bishop et al. 1925; Bone 1944; Brecher 1929; Drilhon 1934; Tobias 1948). For example, the potassium (K) concentration of hemolymph often can range from 35 to 60 mM, levels that would depolarize a neuron and effectively block action potential firing. In a classic experiment nearly 60 years ago, Graham Hoyle found that while bathing locust nerves in 70 mM K saline had little effect on evoked action potential propagation, the injection of a small volume of 70 mM K solution into the nerve blocked firing within seconds (Hoyle 1952). Significantly, within minutes after the injection, action potential activity recovered. This result demonstrated both the presence of an effective barrier isolating the nervous system from the blood, as well as a homeostatic mechanism within the nervous system to maintain low extracellular K concentration around neurons.
In both mammals and insects, blood-brain barriers play several roles, including: 1) maintaining an extracellular ionic environment within the nervous system that is compatible with electrical activity; 2) controlling the access and levels of neurally active molecules, metabolites, and immune cells; and 3) blocking the entry of harmful substances, such as neurotoxins.
Given the universal need to isolate the nervous system from its environment, it is not surprising that key molecular mechanisms are evolutionarily conserved. Many of the effector molecules responsible for maintaining the extracellular ionic balance have been identified, including proteins that mediate the movement of ions, such as the Na/K ATPase, cotransporters, exchangers, and channels, as well as the proteins that form the cellular junctions that restrict ionic movement. The transfer of solutes across an otherwise impermeable membrane introduces the additional challenge of controlling osmotic pressure and edema, a problem common to all organisms with a blood-nervous system barrier. We do not yet have a full understanding of the physiological and signaling mechanisms that determine how extracellular ion and volume regulation are jointly regulated.
The nervous system of Drosophila is emerging as a valuable model for studying the in vivo mechanisms governing ionic homeostasis and extracellular volume regulation. Important insights have emerged from studies of the peripheral nerves of Drosophila larvae. The larval abdominal nerves have a relatively simple structure, composed of four cells types. The critical paracellular blood-barrier is established by a glial ensheathment located near the surface of the nerve. This arrangement is simpler and experimentally more accessible than that of vertebrates, where the principal paracellular barrier is located at the interface of capillaries that ramify throughout the nervous system (see Table 1). Many of the key molecules are conserved from Drosophila to mammals, making it likely that at least some of the physiological mechanisms are also the same.
Table 1. Properties of fly and human paracellular barriers of the nervous system.
| Property | Drosophila | Human |
|---|---|---|
| location of blood barriers |
|
|
| paracellular junction |
|
|
| cells that regulate extracellular volume |
|
|
Here we review what is known about ion and volume regulation in the larval nerves of Drosophila. Beginning with a morphological description of the nerves and of the cells that form the blood barrier, we turn to the molecular properties of the paracellular junctions, and examine a molecular signaling cassette that regulates extracellular volume, along with the corresponding mutant phenotypes. We next relate the findings in insects to what is known in mammalian systems, and close by describing a path for future studies that will lead to a more complete description of this physiological mechanism.
Morphology of larval nerves
The larval abdomen consists of 8 bilaterally symmetric segments. The first seven, termed A1-A7, have nearly identical arrangements of muscle fibers, sensory cells, and neural connectivity (for review, see Ruiz-Canada and Budnik 2006). The main innervation of each hemisegment is provided by a single abdominal nerve that originates from the lateral edge of each ventral ganglion segment (Figure 1A). There is also a smaller transverse nerve that emerges from the dorsal midline. In the periphery, the abdominal nerves divide into five major branches: the intersegmental nerve branch (ISN), and the four segmental nerve branches (SNa-d). Between the ventral ganglion and the periphery, the nerves float freely in hemolymph. Like the hemolymph of other phytophagous insects, larvae have higher levels of K (circa 40 mM; Begg and Cruickshank 1963; Croghan and Lockwood 1960; Stewart et al. 1994) than is compatible with neural function (Auld et al. 1995; Baumgartner et al. 1996; Schwabe et al. 2005).
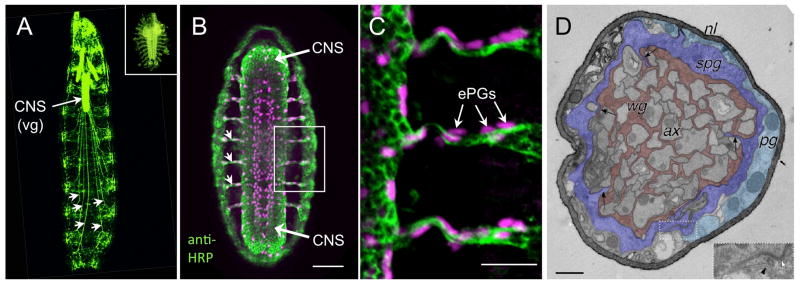
Figure 1.
Anatomy of the larval nervous system. A. An intact 2nd instar larva expressing GFP in the nervous system. The abdominal nerves (some of which are marked with arrowheads) are hundreds of microns long, linking the ventral ganglion (vg) to the periphery. Inset: Nervous system of an embryo (fillet prep) to the same scale as the larva, stained with the neuronal marker anti-HRP. B. Whole embryo stained with anti-HRP (green) and anti-repo (magenta). At this stage the precursors of the subperineurial glia, the embryonic peripheral glia (ePG, magenta) are migrating along the nerves (green), which are less than 20 μm long. Bar: 50 microns. C. Enlarged view of the boxed area in B shows the close association of the ePG (some of which are indicated by arrows) with the nerves Bar: 20 microns. D. TEM of a 3rd instar larval abdominal nerve, showing the four cellular components: axons (ax), wrapping glia (wg), subperineurial glia (spg), and perineurial glia (pg). The inset shows an example of an autocellular septate junction between two processes of a SPG (with permission from Stork et al. 2008). Bar: 1 micron.
The abdominal nerves of segments A1-A7 each contain about 90 axons, surrounded by three layers of glial cells (Figure 1D). The inner-most layer of glia, termed wrapping glia by Stork et al. (2008), extend processes around both single axons and axon fascicles. The middle layer consists of subperineurial glia that form the blood-nerve barrier. The perineurial glia form the outermost layer, which underlies the neural lamella, an extracellular matrix.
The three glial cell types are distinguished by their patterns of gene expression and can be identified in enhancer trap lines. The innermost wrapping glia strongly express Nervana2 (Nrv2), a beta subunit of the Na/K ATPase; there are corresponding enhancer trap and gene trap lines. The subperineurial glia form “autocellular” pleated septate junctions—the junctions form between membrane extensions from the same cell—which are the basis for the paracellular blood barrier in the larval CNS and nerves (Stork et al. 2008). They also express proteins required for septate junction formation, including NeurexinIV (NrxIV), Gliotactin (Gli), and Moody (Auld et al. 1995; Bainton et al. 2005; Banerjee et al. 2006a; Baumgartner et al. 1996; Schwabe et al. 2005; Stork et al. 2008). There are GAL4 lines available that use gli and moody enhancer sequences to drive expression in these cells. Unfortunately, no molecular marker has been found that is expressed exclusively in perineurial glia, although the c527 enhancer trap line reportedly labels these cells preferentially (Hummel et al. 2002; Stork et al. 2008). The availability of GAL4 lines that express in subsets of glia allows researchers to test gene function specifically in these cells, in a “mosaic” fashion.
The development of the nerve-associated glia has been the subject of several studies (Edenfeld et al. 2006; Fredieu and Mahowald 1989; Schmid et al. 1999; Schmidt et al. 1997; Sepp et al. 2000; Silies et al. 2007; von Hilchen et al. 2008) and reviews (Klambt 2009; Parker and Auld 2006). For convenience, we provide a brief summary because many of the molecular markers and genetic tools that are used to analyze the larval glia were first characterized in the embryo, when the nerves are formed.
The abdominal nerves are prefigured by motor and sensory axon projections, beginning at about 10 hours post-fertilization (PF) at 25°C (stage 13; Campos-Ortega and Hartenstein 1997). The sensory neuron cell bodies are located in the periphery and the motoneuron cell bodies are located in the developing ventral ganglion. By 16 hours PF (stage 16), most motoneurons have formed synapses with their targets, and the abdominal and transverse nerves (and associated branches) are established. At this stage, twelve glial cells, termed embryonic peripheral glia (ePG), associate with these nerves (Figure 1B and 1C; von Hilchen et al. 2008). Seven of these cells originate in the CNS, having migrated along the nerves. The five others originate from sensory organ precursors in the periphery. The nomenclature for the peripheral glia has led to inconsistencies and confusion. Recently, von Hilchen et al. (2008) proposed a standardized nomenclature, ePG1-12 and provide a table of equivalencies. The correspondence between ePG and larval nerve glia has not been unequivocally established. Nevertheless, several molecular markers and genetic tests provide strong evidence for some of the relationships.
One major difficulty for following peripheral glial development is the tremendous growth of larval abdominal nerves, increasing from a few hundred micrometers at hatching to up to 4 mm in a mature 3rd instar larva (compare inset embryo to 2nd instar larva in Figure 1A). Remarkably, the larval wrapping and subperineurial glia do not divide in larvae (Sepp et al. 2000). Most of their prospective progenitors, the ePG, are located in the periphery, leaving only three to five ePG (#1-5) in a position along the nerve to differentiate into the larval wrapping and subperineurial glia. Stork et al. (2008) report there are two to three wrapping glia per abdominal nerve, which leaves perhaps only three ePG to account for all the subperineurial glia. This fits with published reports and our own observations that even in the longest nerves, there appear to be just a few nuclei of wrapping and subperineurial glia, which can be identified by their large and oblong shape (Auld 1996).
Unlike their internal counterparts, the perineurial glia divide during larval development. In the first instar larvae, nerve perineurial glia are relatively few in number and do not completely envelop the nerves (Stork et al. 2008). Their number subsequently increases through cell division, and they are readily labeled during the 3rd instar with a pulse of BrdU (Leiserson et al. 2000) or identified in MARCM clones (Stork et al. 2008). The origin of the perineurial cells is controversial. Although Edwards et al. (1993) concluded that the CNS perineurial glia were of mesodermal origin, they based their conclusion on the observation that twist mutant embryos, which lack mesoderm, also lack perineurial cells. However, the loss of mesoderm causes secondary and non-autonomous effects (e.g., induction of trachea morphogenesis; Sutherland et al. 1996), so other approaches, such as lineage analysis, are needed to establish the origin of perineurial glia.
Blood barriers of larval nerves
The paracellular barriers to diffusion in larval nerves are provided by pleated septate junctions (pSJs). These are visible as ladder-like structures by transmission electron microscopy. First described in hydra over 50 years ago (Wood 1959), pSJs are found in many epithelial and secretory tissues of invertebrates (Noirot-Timothee and Noirot 1980). There is good evidence that they provide a barrier to diffusion, analogous to tight junctions found in mammals and other vertebrates. Significantly, pSJs are also found in mammals at the nodes of Ranvier of myelinated axons (reviewed in Banerjee et al. 2006b).
The evidence that pSJs provide a barrier to diffusion is abundant. Early investigators proposed a barrier function based on their location near the apical membrane of the cells that form barriers. Lanthanum impregnation studies showed that the ion could not penetrate across the pSJs (Carlson et al. 2000; Juang and Carlson 1994; Lane 1991; Noirot-Timothee and Noirot 1980). The response to treatment with non-isosmotic solutions was also similar to that of tight junctions (Lord and DiBona 1976; Noirot-Timothee and Noirot 1980). More recently, the molecular components of Drosophila pSJs have been identified, along with mutations in the corresponding genes (Banerjee et al. 2006b; Tepass et al. 2001). Mutations in many of these genes disrupt pSJs and/or compromise the paracellular barriers (Banerjee et al. 2006a; Baumgartner et al. 1996; Behr et al. 2003; Faivre-Sarrailh et al. 2004; Genova and Fehon 2003; Lamb et al. 1998; Llimargas et al. 2004; Paul et al. 2003; Schulte et al. 2003; Strigini et al. 2006; Wu et al. 2004; Wu et al. 2007).
In Drosophila, pSJs are found in a wide variety of tissues of ectodermal origin, including epidermis, salivary glands, foregut, hindgut, trachea, and subperineurial glia of the nervous system (Tepass and Hartenstein 1994; Tepass et al. 2001). Consequently, mutations that affect pSJ formation or structure often affect many tissues. Although it is tempting to extrapolate findings about pSJs from one tissue to another, we should do so with caution because pSJs from different tissues may have different proteins and functions. One such difference involves the beta subunit of the Na/K ATPase, Nrv2, a component of ventral ganglion, tracheal, and epidermal pSJs, which is absent in the nerve SPG. Interestingly, Nrv2 is strongly expressed in the wrapping glia which are not thought to form pSJs (Stork et al. 2008).
Regulation of the ion environment and extracellular volume
The presence of a blood barrier requires a physiological system to maintain ionic balance and volume homeostasis (Treherne and Schofield 1981). Such a mechanism is particularly important in the nervous system because electrical activity leads to a rise in extracellular K, which if left unchecked, would block action potential firing. The idea that neural activity would lead to increased extracellular K dates back to the first descriptions of the ionic basis of the action potential. In squid, tonic excitation of the nerve results in a reduction in the hyperpolarizing after-potential and reduced amplitudes of the action potential, both of which result from increased extracellular K and a more positive equilibrium potential for K (Frankenhaeuser and Hodgkin 1956). Recordings from glia (Schwann cells) in the mud puppy optic nerve indicated that there was a corresponding depolarization of the glial membrane (Orkand et al. 1966). Recordings from Muller glia of the Salamander optic nerve indicated the presence of a “K siphon,” where K is ejected from the glial endfeet in response to increased K concentrations at distal processes (Newman et al. 1984). These observations provide strong evidence that one role of glia is to remove excess K from the extracellular environment of axons, maintaining K homeostasis.
The mechanism often invoked to explain how glia accomplish this task can be broken down into two steps: K uptake and spatial buffering. It has been shown that glia take up K via mechanisms that involve K channels, Na/K ATPase, and NKCC cotransporters (Coles and Dietmer 2005; Kofuji and Newman 2004; Walz 2000). Because glia are often large cells, they could provide a reservoir for excess K, which the glial cell would slowly release into the extracellular space. By contrast, the concept of spatial buffering or “K siphoning,” pioneered by Newman (1984), is a dynamic process, in which K flows out of the glia away from the site of absorption (Kamasawa et al. 2005; Kofuji and Newman 2004; Menichella et al. 2006; Orkand 1986).
In systems as diverse as cats, rats, and bees, it has been found that increases in extracellular K (e.g., as the result of neural activity) not only causes a flux of K into the glia, but also glial cell swelling, and a decrease in extracellular volume (Coles et al. 1986; Dietzel et al. 1980; Dietzel et al. 1982; Ransom et al. 1985; Svoboda and Sykova 1991; Walz and Hinks 1985). The volume changes are thought to result from osmotic forces arising from the influx of ions across the cell membrane. Inhibition of the NKCC1 cotransporter in optic nerves and primary cultures of astrocytes reduces the glial swelling that arises in response to increased extracellular K, suggesting that NKCC1 mediates the change in volume (MacVicar et al. 2002; Su et al. 2002a; Su et al. 2002b; Walz and Hinks 1986). Analysis of the kinetics of extracellular volume change in response to neural activity suggests that during high frequency firing (100 Hz), the extracellular volume increases by about 20%, and only decreases once the activity has stopped (Svoboda and Sykova 1991).
The evidence to date suggests that some of the same mechanisms of extracellular ion and volume regulation that have been found in vertebrates also apply to Drosophila. We have shown that cotransport by an NKCC1 ortholog regulates the extracellular space of larval nerves (Leiserson et al. 2010). Recently, we obtained evidence that mutants that have chronically active nerves throughout larval life show a corresponding increase in the extracellular volume of their nerves.
The ether-a-gogo (eag) and Shaker (Sh) mutations each reduce the expression of specific K channels, and double mutants have enhanced neuronal activity throughout development (Budnik et al. 1990; Ganetzky and Wu 1983; Haugland and Wu 1990). The larval nerves of these mutants are swollen, owing to the accumulation of extracellular fluid between axons and glia (Figure 2B and E). This effect is likely the result of neural activity, as mlenapts, a mutation that reduces the hyperactivity of eag Sh, also reduces nerve widths back to wild type levels (Figure 2E).
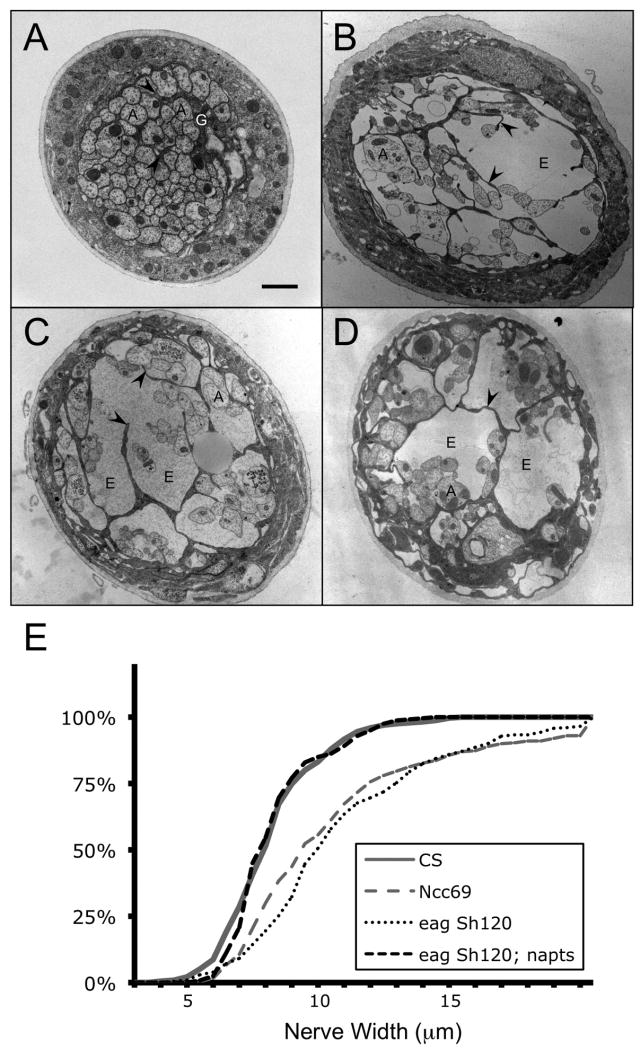
Figure 2.
Drosophila bulging nerve mutants. A-D: Transmission electron micrographs of nerves from 3rd instar larvae. In wild type A, axons (“A”) and glial cell processes (“G” and arrowheads) are closely associated, with little extracellular space. By contrast, in eag Sh B, Ncc69 C, and fray D mutant nerve bulges, axons and glia may be separated by a large amount of extracellular space (“E”). E. Cumulative frequency graphs of nerve widths. Curves from animals with a higher proportion of large nerve widths are shifted to the right; those with a lower proportion are shifted to the left. Both Ncc69 and eag Sh120 mutant larvae have larger nerve widths than wild type (CS). Introducing the mlenapts mutation into eag Sh120 suppresses neuronal hyperactivity and restores nerve widths to wild type levels. Bar in A: 1 micron; A-D are to the same scale. Genotypes: A, CS. B, eag Sh120. C, w; Ncc69r2. D, ry frayr1. E, eag Sh120; mlenapts.
The ultrastructure of eag Sh larval nerves resembles that of fray and Ncc69, mutations that reduce Na-K-Cl cotransport through Ncc69, the ortholog of human NKCC1 (Figure 2). Based on a large body of work on its mammalian homologs as well as genetic data in flies, Fray is a Ser/Thr kinase that activates Ncc69 cotransport (Leiserson et al. 2010; Leiserson et al. 2000). Nerves of 3rd instar larvae mutant for fray or Ncc69 have fluid between axons and glia. Mosaic knockdown (RNAi) and rescue experiments show that the fray and Ncc69 genes are genetically required in SPG, the cells that form the blood barrier. Mammalian orthologs of Fray and Ncc69, rat PASK and human NKCC1 respectively, can rescue the corresponding fly mutants, demonstrating a strong conservation of the molecular function of these molecules.
In mammalian systems, PASK (also known as SPAK) has been shown to bind, phosphorylate, and activate NKCC1 transport (Dowd and Forbush 2003; Gagnon et al. 2006; Gagnon et al. 2007; Piechotta et al. 2003; Vitari et al. 2006). Given that the fly orthologs are expressed and required in the same nerve glia, and cause a near identical phenotype in the nerves, it is likely the mammalian results also apply to Drosophila. In support of this view, Fray and Ncc69 have been shown to interact in a yeast-two-hybrid assay (Leiserson et al. 2010).
Although nerve bulges in Ncc69 and fray mutants can exceed 70 μm in diameter, 10 times the normal nerve width, the impact on neural transmission is minimal, as judged by behavioral, genetic, and electrophysiological criteria (Leiserson et al. 2010). Mutant larvae crawl normally, are not temperature-sensitive paralytic, and respond normally to nociceptive stimuli. Even when combined with mutations that affect neural activity, Ncc69 loss of function causes no detectable change in activity. This suggests that the K concentration within the bulges is low, and that the primary abnormality is the accumulation of extracellular fluid with otherwise normal ionic composition.
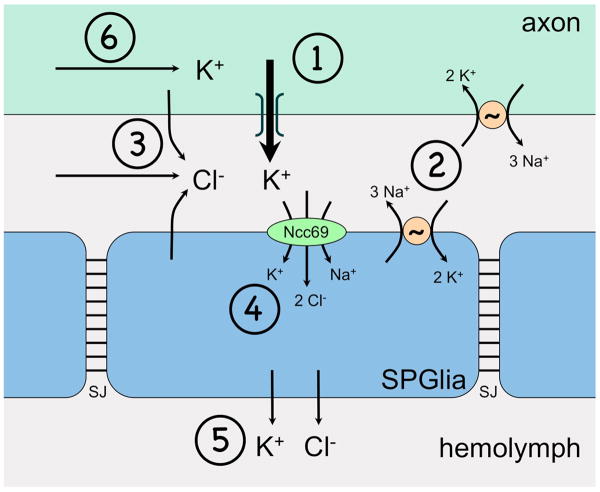
A model that incorporates these results is shown in Figure 3. Neural activity causes K to flow out of the axon through voltage-gated K channels, resulting in increased concentrations of K. The Na/K ATPase acts to restore the Na and K ion gradients, moving 3 Na ions out of the cell for every 2 K ions into the cell. The net effect is to accumulate solutes in the extracellular space. We propose that Ncc69 functions to remove excess solutes from the extracellular space, importing them into the SPG. Ncc69 would also reduce extracellular K, because it moves 1 K ion along with 1 Na and 2 Cl. In this model, for every two cycles of the Na/K ATPase, Ncc69 cycles once to maintain osmotic balance, exchanging 5 Na for 5 K.
Figure 3.
A model of Ncc69 function in larval nerves, showing how the Na/K ATPase could create the need for solute removal from the extracellular space in larval nerves. The diagram shows a simplified view of the nerve, consisting of an axon and the subperineurial glia, whose septate junctions (SJ) restrict paracellular flow. The model leaves out two classes of glia whose roles are unknown: the wrapping glia, which are located inside the nerve, and the perineurial cells which are located on the outside. They are not essential in this model because they do not form septate junctions, and would not pose much of a barrier to paracellular ion flow. The ion flows in the diagram are represented by arrows. 1: The flow of ions is initiated by the action potential which leaves increased extracellular K in its wake through voltage-gated K channels. 2: In each cycle, the Na/K ATPase removes 2 K ions from the extracellular space, and replaces them with 3 Na ions. 3: Cl ions move to balance the gain in positive charge. These Cl ions could flow from other parts of the extracellular space and/or from intracellular sources, e.g., through Cl channels or transporters. The net effect of the Na/K ATPase is to accumulate NaCl in the extracellular space. If left unchecked (as in Ncc69 mutants), the accumulation of NaCl draws water into the extracellular space through osmosis, causing swelling. 4: Ncc69 relieves the pressure by transporting solutes into the subperineurial glia, causing it to swell. 5: The subperineurial cell exports solutes, presumably into the hemolymph, to maintain volume homeostasis. 6: K flows down the axoplasm to replace the K that is lost (From Leiserson et al. 2010 with permission.).
Relationship of Drosophila nerves to mammalian systems
The genetic rescue of fray and Ncc69 mutants by expression of the mammalian orthologs demonstrates the conservation of molecular function. The homologies extend beyond the molecular, however, and can be classified at the level of physiology, morphology, molecular mechanisms, and mutant phenotypes.
Physiology
The nervous systems of flies and mammals are isolated by blood barriers that pose similar physiological problems of ion and volume homeostasis. All organisms use Na and K gradients and fluxes to generate action potentials. There is thus a universal need to regulate extracellular K and maintain ion and volume homeostasis.
Morphology
Mammalian and larval nerves share the common feature of glia that wrap axons. At the ultrastructural level, larval nerves closely resemble immature unmyelinated mammalian fibers, in which a single Schwann cell wraps many axons. While unmyelinated fibers predominate during early development, they remain common in adult mammals, involving nearly 50% of motor axons and 75% of cutaneous axons (Berthold and Rydmark 1995).
Molecular mechanisms
The molecules known to mediate extracellular volume regulation, including Fray, Ncc69, and the pSJ proteins, have human orthologs. Human NKCC1 can substitute for Ncc69 in flies, and rat PASK for Fray, demonstrating the functional conservation of these molecules. Such experiments demonstrate not only that the effector function of each molecule is conserved, but that the regulatory elements are also conserved. Otherwise the alien molecule would not be able to respond appropriately to the regulatory signals in the glial cell. In most cases when a human protein can substitute for the fly homolog, entire pathways have been found to be conserved. Thus, we are optimistic that as further glial molecules are identified that function in the fly Fray-Ncc69 pathway, many will have human orthologs.
The other molecules demonstrated to have a role in extracellular ion and volume regulation in larval nerves are the components of the pSJs. In the absence of pSJs, the paracellular blood barrier fails allowing ions and solutes to exchange with the hemolymph. Although pSJs have not been observed at the blood barriers in mammals, they are found in the paranodal regions of the nodes of Ranvier, where they form “axo-glial” junctions between the axons and the terminal loops of myelin. Components of the axo-glial pSJs have fly homologs that are found in fly pSJs (Table 2). Knocking down the mammalian pSJ components causes a corresponding reduction in axo-glial pSJs (Bhat et al. 2001; Boyle et al. 2001).
Table 2. Mammalian homologs of Drosophila pleated septate junction molecules.
| Drosophila | Mammal |
|---|---|
| NeurexinIV | NCP1 (Nrx/Caspr1/Paranodin) |
| Coracle | Band4.1 |
| Neuroglian | Neurofascin (NF155) |
| D-Contactin | Contactin |
| Sinuous, Megatrachea | Claudins (tight junction proteins) |
| Gliotactin | Neuroligin3 |
| Discs Large | Dlg1-4 |
| varicose | VAM-1/PALS2 |
| Na/K ATPase subunits | Na/K ATPase subunits |
| Lachesin | IgLONs |
The first four Drosophila pSJ molecules have been found in mammalian pSJs; the others have not. Sinous and Megatrachea are related to Claudins, molecules that have been found at tight junctions, the predominant paracellular barrier in mammals.
The other presumed components of the apparatus that regulates ion and volume homeostasis in larval nerves have not been tested in vivo. These include the Na/K ATPase, K and Cl channels, transporters, and exchangers, all of which have potential orthologs in Drosophila (Table 3). The Na/K ATPase is of central importance, because it not only generates the Na and K ion gradients, but is also a structural component of pSJs in fly epithelia, trachea, and salivary glands.
Table 3. Drosophila homologs of mammalian ion/volume homeostasis molecules.
| Function | Drosophila | Mammal |
|---|---|---|
| Na-K-Cl cotransport | Ncc69, Ncc83 | NKCC1 |
| K-Cl cotransport | Kcc | KCC3, KCC4 |
| Ser/Thr kinase | Fray | OSR1 and SPAK/PASK |
| K channel (inward rectifier) | Ir, Irk2, Irk3 | Kir4.1 |
| K channel (voltage-gated) | KCNQ | Kv7.3, Kv7.4 |
| Cl channel | ClC-a | ClC-Ka, ClC-Kb |
| Gap junction | (Innexin family) | Connexin family |
| Aquaporin | Drip, CG4019, CG17662, CG17664, CG7777, Bib* | AQP4 |
Table shows mammalian molecules, described and referenced in the text, that have been shown to function in ion or volume homeostasis in the nervous system or inner ear with corresponding functions. The only Drosophila orthologs with genetic rescue data are Ncc69 and Fray (Leiserson et al. 2010; Leiserson et al. 2000). The candidate orthologous Drosophila molecules were obtained from OrthoDB (Kriventseva et al. 2008); where no putative orthologs were found, related molecules of similar function are shown in parentheses.
Evidence suggests that while Bib is homologous to Aquaporins, it may not function as a water channel (Tatsumi et al. 2009).
Ion/volume misregulation phenotypes
In mice and humans, there is a growing number of ion regulation phenotypes that have been observed in the nervous system and inner ear. Knockdowns of Connexins Cx32 and Cx47 (Anzini et al. 1997; Menichella et al. 2003; Menichella et al. 2006; Odermatt et al. 2003; Oh et al. 1997; Scherer et al. 1998), Kir4.1 (Djukic et al. 2007; Kofuji et al. 2000; Neusch et al. 2001), and KCC3 (Byun and Delpire 2007) result in “extracellular vacuoles” in and around myelinated fibers of the nervous system. Genetic analysis and expression studies have provided a model in which these molecules function together to remove excess K from paranodal regions (Kofuji and Newman 2004; Somjen 2002; Walz 2000).
The gene encoding Cx32 is of particular interest because it is associated with a human inherited neuropathy, Charcot-Marie-Tooth disorder (CMT1X). In mice mutant for this gene, the primary nerve phenotype is a progressive demyelinating neuropathy, characterized by proliferating Schwann cells (“onion bulbs”) and abnormal myelinated fibers (Anzini et al. 1997; Oh et al. 1997; Scherer et al. 1998). Mutants of Cx47 or Kir4.1 develop extracellular vacuoles in white matter of the CNS (Neusch et al. 2001; Odermatt et al. 2003), and interact genetically with Cx32 (Menichella et al. 2006). In the optic nerves of Cx32 Cx47 double mutants, the size of the vacuoles varies with neural activity (Menichella et al. 2006). Mutation of KCC3, one isoform of the K-Cl cotransporter, causes a peripheral neuropathy in mice, with periaxonal swelling (Byun and Delpire 2007; Sun et al. 2010). These results suggest that the vacuoles and swelling in these various mutant combinations are the result of the accumulation of extracellular solutes, similar to the phenotype seen in fray and Ncc69 larval nerves.
Mouse SPAK and NKCC1, orthologs of Fray and Ncc69, are expressed in peripheral nerves, but their function there has not been demonstrated (Alvarez-Leefmans et al. 2001; Piechotta et al. 2003). By contrast, NKCC1 plays a demonstrably essential role in the inner ear, where it is required for a process known as K recycling (Kikuchi et al. 2000; Wangemann 2002). Indeed, the most prominent phenotype of NKCC1 mouse mutants is deafness and vestibular malfunction (Delpire et al. 1999; Dixon et al. 1999; Flagella et al. 1999), similar to a deafness syndrome in humans caused by loop diuretics that target NKCC1 (Arnold et al. 1981). NKCC1 is expressed in the basolateral membrane of marginal cells of the stria vascularis of the inner ear (Crouch et al. 1997). These cells secrete K into the endolymph through an apical channel, Kv7.1 (isK; Vetter et al. 1996). In response to mechanical stimuli, K from the endolymph passes through apical channels into hair cells, after which it is expelled from the basolateral regions of the hair cells into the extracellular space. From there, it takes a route through and between cells back to the stria vascularis.
Pharmacological or genetic knockdowns of the molecules that mediate K recycling have ultrastructural defects that look remarkably similar to fly fray and Ncc69 mutants. Mice defective for NKCC1 or Kv7.1 accumulate extracellular fluid in the stria vasculari (Santi and Lakhani 1983; Vetter et al. 1996; Wangemann 2002). Similarly, the Kv7.3 and Kv7.4 K channels; ClC-K Cl channels; a variety of Connexins; and KCC3 and KCC4; all participate in this recycling, and give mutant phenotypes that include the accumulation of extracellular fluid (Boettger et al. 2002; Boettger et al. 2003; Wangemann 2002; Zdebik et al. 2009; Zhao et al. 2006).
Prospects
The homologies between ion and volume regulation in flies and mammals suggest findings in one will provide insights into the other. Drosophila has a rich tool kit for discovering and analyzing molecular mechanisms of biological processes. For most genes, mutations and/or RNAi lines are available that can be used to knock down gene function. Transgenic gain and loss of function experiments are particularly powerful, as it is possible to drive expression of a dominant-acting construct in specific cells or tissues at any time during development. This is done using bipartite expression systems such as GeneSwitch or TARGET (Han et al. 2000; McGuire et al. 2003; Osterwalder et al. 2001; Roman et al. 2001). Other tools, such as the FLP and MARCM allow sophisticated lineage and genetic mosaic analysis (Lee and Luo 2001; Struhl and Basler 1993; Xu and Rubin 1993). Perhaps the most exciting prospect for Drosophila is to use it to screen for mutations that by themselves, or in combination with other mutations, affect ion/volume homeostasis. Given the dramatic phenotypes associated with homeostatic dysfunction in the larval nerves, it should be possible to rapidly extend the relevant signaling cassettes to new molecular players. The genes identified in the fly would then point to the relevant candidate genes to study in mammals.
One major advance from a screen for glial genes would be to identify the molecules upstream of Ncc69 that trigger its activation. In mice, members of the WNK kinase family of Ser/Thr kinases have been shown to work with SPAK and OSR1 to regulate NKCC1 activity in response to changes in osmolarity and intracellular Cl, but the sensing mechanism that activates these kinases remain elusive (Kahle et al. 2008; Richardson and Alessi 2008). What exactly are the molecules that sense the need to activate cotransport, and what exactly are they sensing? Is it low Cl, stress on the cytoskeleton from osmotic forces, a combination of those two, or something else entirely? Answering these questions would be very helpful in understanding how extracellular ion/volume is regulated in larval nerves. In mammals, NKCC1 is expressed in a wide variety of cells and locations, including parts of the nervous system, including neurons (Dzhala et al. 2005; Plotkin et al. 1997b; Wang et al. 2002), glia (Alvarez-Leefmans et al. 2001; Su et al. 2002b; Wang et al. 2003), endothelial cells of brain capillaries (O'Donnell et al. 2004), and the epithelium of the choroid plexus (Plotkin et al. 1997a). In these different cell types, a variety of cellular functions have been ascribed to NKCC1, including setting the internal Cl concentration in neurons and glia (Hubner and Rust 2006; Wang et al. 2003), K homeostasis and volume regulation in glia (Chen and Sun 2005), and the secretion of cerebrospinal fluid and brain interstitial fluid (Davson et al. 1987; O'Donnell et al. 2004).
NKCC1 has been implicated in the swelling that is associated with stroke and traumatic brain injury (Chen and Sun 2005; Yan et al. 2001). Endothelial cells of the brain capillaries respond to ischemia by an elevated secretion of NaCl and water into the brain parenchyma, resulting in swelling (O'Donnell et al. 2004). This response precedes the breakdown of the blood-brain barrier, and is responsible for the initial swelling following ischemic attacks. NKCC1 also mediates swelling in astrocytes in response to ischemia, which likely restricts blood flow (Chen and Sun 2005).
Yet despite the widespread expression of NKCC1, the knockout phenotype is relatively modest. This is probably due to functional redundancy among the proteins involved in maintaining the blood-brain barrier, complicating the genetic analysis. By contrast, the homologous Ncc69 cotransporter of Drosophila is principally expressed by the glia of the blood-nerve barrier (the SPG), and has a pronounced loss of function phenotype. The genetic analysis of Drosophila orthologs, such as fray or Ncc69, is a good way to understand the mechanisms that regulate homeostasis in the mammalian nervous system. A better understanding of the molecular basis of ion/volume homeostasis would in turn speed the development of effective clinical treatments for the swelling associated with stroke and brain injury.
Acknowledgments
We thank Judy Cole for technical assistance; Barry Piekos for help with the EM; Louise Nicholson and Brett Berke for helpful discussions. We are grateful for the support of Flybase, the Berkeley Drosophila Genome Project, the Bloomington and Vienna Stock Centers, and the many members of the Fly community who shared protocols, stocks, reagents and ideas. The research was supported by grants to HK from the NIH (5R01NS031651 and 1R21NS053807), and the NSF (IBN 0641915).
References
- Alvarez-Leefmans FJ, Leon-Olea M, Mendoza-Sotelo J, Alvarez FJ, Anton B, Garduno R. Immunolocalization of the Na(+)-K(+)-2Cl(-) cotransporter in peripheral nervous tissue of vertebrates. Neuroscience. 2001;104(2):569–82. doi: 10.1016/s0306-4522(01)00091-4. [DOI] [PubMed] [Google Scholar]
- Anzini P, Neuberg DH, Schachner M, Nelles E, Willecke K, Zielasek J, Toyka KV, Suter U, Martini R. Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein connexin 32. J Neurosci. 1997;17(12):4545–51. doi: 10.1523/JNEUROSCI.17-12-04545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W, Nadol JB, Jr, Weidauer H. Ultrastructural histopathology in a case of human ototoxicity due to loop diuretics. Acta Otolaryngol. 1981;91(5-6):399–414. doi: 10.3109/00016488109138521. [DOI] [PubMed] [Google Scholar]
- Auld V. The role of glia in the development of the insect nervous system. In: Jessen KR, Richardson WD, editors. Glial Cell Development: basic principles and clinical relevance. Oxford: Bios Scientific Publishers Ltd.; 1996. pp. 229–250. [Google Scholar]
- Auld VJ, Fetter RD, Broadie K, Goodman CS. Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood-nerve barrier in Drosophila. Cell. 1995;81(5):757–67. doi: 10.1016/0092-8674(95)90537-5. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. 2005;123(1):145–56. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Pillai AM, Paik R, Li J, Bhat MA. Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J Neurosci. 2006a;26(12):3319–29. doi: 10.1523/JNEUROSCI.5383-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006b;46(1):65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87(6):1059–68. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Begg M, Cruickshank WJ. A partial analysis of Drosophila larval haemolymph. Proceedings of the Royal Society of Edinbergh Series B-Containing Papers of a Biological Character. 1963;68:215–236. [Google Scholar]
- Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell. 2003;5(4):611–20. doi: 10.1016/s1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Berthold CH, Rydmark M. Morphology of normal peripheral axons. In: Waxman SG, Kocsis JD, Stys PK, editors. The axon: structure, function, and pathophysiology. New York: Oxford University Press; 1995. pp. 13–48. [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30(2):369–83. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Bishop GH, Briggs AP, Ronzoni E. Body fluids of the honey bee larva. II. Chemical constituents of the blood, and their osmotic effects. Journal of Biological Chemistry. 1925;66(1):77–88. [Google Scholar]
- Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature. 2002;416(6883):874–8. doi: 10.1038/416874a. [DOI] [PubMed] [Google Scholar]
- Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, Faulhaber J, Ehmke H, Pfeffer C, Scheel O, et al. Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. Embo J. 2003;22(20):5422–34. doi: 10.1093/emboj/cdg519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone GJ. Le rapport de sodium/potassium dans le liquide coelomique des insectes. Annales de la Société Royale Zoologique de Belgique. 1944;75:123. [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30(2):385–97. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Brecher L. The inorganic properties of chrysalis blood (Sphynx pinastri, pieris brassicae) Changes in the content of inorganic properties in pupation (Pieris brassicae) Biochemische Zeitschrift. 1929;211:40–64. [Google Scholar]
- Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10(11):3754–68. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun N, Delpire E. Axonal and periaxonal swelling precede peripheral neurodegeneration in KCC3 knockout mice. Neurobiol Dis. 2007;28(1):39–51. doi: 10.1016/j.nbd.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. Berlin; New York: Springer; 1997. p. xvii.p. 405. [Google Scholar]
- Carlson SD, Juang JL, Hilgers SL, Garment MB. Blood barriers of the insect. Annu Rev Entomol. 2000;45:151–74. doi: 10.1146/annurev.ento.45.1.151. [DOI] [PubMed] [Google Scholar]
- Chen H, Sun D. The role of Na-K-Cl co-transporter in cerebral ischemia. Neurol Res. 2005;27(3):280–6. doi: 10.1179/016164105X25243. [DOI] [PubMed] [Google Scholar]
- Coles JA, Dietmer JW. Glial cell regulation of extracellular potassium. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford; New York: Oxford University Press; 2005. pp. 334–345. [Google Scholar]
- Coles JA, Orkand RK, Yamate CL, Tsacopoulos M. Free concentrations of Na, K, and Cl in the retina of the honeybee drone: stimulus-induced redistribution and homeostasis. Ann N Y Acad Sci. 1986;481:303–17. doi: 10.1111/j.1749-6632.1986.tb27160.x. [DOI] [PubMed] [Google Scholar]
- Croghan PC, Lockwood APM. The Composition of the Haemolymph of the Larva of Drosophila-Melanogaster. Journal of Experimental Biology. 1960;37(2):339–343. [Google Scholar]
- Crouch JJ, Sakaguchi N, Lytle C, Schulte BA. Immunohistochemical localization of the Na-K-Cl co-transporter (NKCC1) in the gerbil inner ear. J Histochem Cytochem. 1997;45(6):773–8. doi: 10.1177/002215549704500601. [DOI] [PubMed] [Google Scholar]
- Davson H, Welch K, Segal MB. Physiology and Pathophysiology of the Cerebrospinal Fluid. New York: Churchill Livingstone; 1987. p. 1013. [Google Scholar]
- Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet. 1999;22(2):192–5. doi: 10.1038/9713. [DOI] [PubMed] [Google Scholar]
- Dietzel I, Heinemann U, Hofmeier G, Lux HD. Transient changes in the size of the extracellular space in the sensorimotor cortex of cats in relation to stimulus-induced changes in potassium concentration. Exp Brain Res. 1980;40(4):432–9. doi: 10.1007/BF00236151. [DOI] [PubMed] [Google Scholar]
- Dietzel I, Heinemann U, Hofmeier G, Lux HD. Stimulus-induced changes in extracellular Na+ and Cl- concentration in relation to changes in the size of the extracellular space. Exp Brain Res. 1982;46(1):73–84. doi: 10.1007/BF00238100. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Gazzard J, Chaudhry SS, Sampson N, Schulte BA, Steel KP. Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum Mol Genet. 1999;8(8):1579–84. doi: 10.1093/hmg/8.8.1579. [DOI] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27(42):11354–65. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1) J Biol Chem. 2003;278(30):27347–53. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- Drilhon A. The organic fluids of moths. Comptes Rendus Des Seances De La Societe De Biologie Et De Ses Filiales. 1934;115:1194–1195. [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11(11):1205–13. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Edenfeld G, Volohonsky G, Krukkert K, Naffin E, Lammel U, Grimm A, Engelen D, Reuveny A, Volk T, Klambt C. The splicing factor crooked neck associates with the RNA-binding protein HOW to control glial cell maturation in Drosophila. Neuron. 2006;52(6):969–80. doi: 10.1016/j.neuron.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Edwards JS, Swales LS, Bate M. The differentiation between neuroglia and connective tissue sheath in insect ganglia revisited: the neural lamella and perineurial sheath cells are absent in a mesodermless mutant of Drosophila. J Comp Neurol. 1993;333(2):301–8. doi: 10.1002/cne.903330214. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131(20):4931–42. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, et al. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274(38):26946–55. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B, Hodgkin AL. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956;131(2):341–76. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredieu JR, Mahowald AP. Glial interactions with neurons during Drosophila embryogenesis. Development. 1989;106(4):739–48. doi: 10.1242/dev.106.4.739. [DOI] [PubMed] [Google Scholar]
- Gagnon KB, England R, Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol. 2006;26(2):689–98. doi: 10.1128/MCB.26.2.689-698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KB, England R, Delpire E. A single binding motif is required for SPAK activation of the Na-K-2Cl cotransporter. Cell Physiol Biochem. 2007;20(1-4):131–42. doi: 10.1159/000104161. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983;1(1):17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol. 2003;161(5):979–89. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DD, Stein D, Stevens LM. Investigating the function of follicular subpopulations during Drosophila oogenesis through hormone-dependent enhancer-targeted cell ablation. Development. 2000;127(3):573–83. doi: 10.1242/dev.127.3.573. [DOI] [PubMed] [Google Scholar]
- Haugland FN, Wu CF. A voltage-clamp analysis of gene-dosage effects of the Shaker locus on larval muscle potassium currents in Drosophila. J Neurosci. 1990;10(4):1357–71. doi: 10.1523/JNEUROSCI.10-04-01357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle G. High blood potassium in insects in relation to nerve conduction. Nature. 1952;169(4294):281–2. doi: 10.1038/169281a0. [DOI] [PubMed] [Google Scholar]
- Hubner CA, Rust MB. Physiology of Cation-Chloride Cotransporters. Advances in Molecular and Cell Biology. 2006;38:241–277. [Google Scholar]
- Hummel T, Attix S, Gunning D, Zipursky SL. Temporal control of glial cell migration in the Drosophila eye requires gilgamesh, hedgehog, and eye specification genes. Neuron. 2002;33(2):193–203. doi: 10.1016/s0896-6273(01)00581-5. [DOI] [PubMed] [Google Scholar]
- Juang JL, Carlson SD. Analog of vertebrate anionic sites in blood-brain interface of larval Drosophila. Cell Tissue Res. 1994;277(1):87–95. doi: 10.1007/BF00303084. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol. 2008;70:329–55. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- Kamasawa N, Sik A, Morita M, Yasumura T, Davidson KG, Nagy JI, Rash JE. Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience. 2005;136(1):65–86. doi: 10.1016/j.neuroscience.2005.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Adams JC, Miyabe Y, So E, Kobayashi T. Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med Electron Microsc. 2000;33(2):51–6. doi: 10.1007/s007950070001. [DOI] [PubMed] [Google Scholar]
- Klambt C. Modes and regulation of glial migration in vertebrates and invertebrates. Nat Rev Neurosci. 2009;10(11):769–79. doi: 10.1038/nrn2720. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20(15):5733–40. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience. 2004;129(4):1045–56. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriventseva EV, Rahman N, Espinosa O, Zdobnov EM. OrthoDB: the hierarchical catalog of eukaryotic orthologs. Nucleic Acids Res. 2008;36(Database issue):D271–5. doi: 10.1093/nar/gkm845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RS, Ward RE, Schweizer L, Fehon RG. Drosophila coracle, a member of the protein 4.1 superfamily, has essential structural functions in the septate junctions and developmental functions in embryonic and adult epithelial cells. Mol Biol Cell. 1998;9(12):3505–19. doi: 10.1091/mbc.9.12.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane NJ. Morphology of glial blood-brain barriers. Ann N Y Acad Sci. 1991;633:348–62. doi: 10.1111/j.1749-6632.1991.tb15626.x. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24(5):251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Leiserson WM, Forbush B, Keshishian H. Drosophila glia use a conserved cotransporter mechanism to regulate extracellular volume. Glia. 2011;59(2):320–32. doi: 10.1002/glia.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiserson WM, Harkins EW, Keshishian H. Fray a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron. 2000;28:793–806. doi: 10.1016/s0896-6273(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Llimargas M, Strigini M, Katidou M, Karagogeos D, Casanova J. Lachesin is a component of a septate junction-based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system. Development. 2004;131(1):181–90. doi: 10.1242/dev.00917. [DOI] [PubMed] [Google Scholar]
- Lord BA, DiBona DR. Role of the septate junction in the regulation of paracellular transepithelial flow. J Cell Biol. 1976;71(3):967–72. doi: 10.1083/jcb.71.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Feighan D, Brown A, Ransom B. Intrinsic optical signals in the rat optic nerve: role for K(+) uptake via NKCC1 and swelling of astrocytes. Glia. 2002;37(2):114–23. doi: 10.1002/glia.10023. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302(5651):1765–8. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J Neurosci. 2003;23(13):5963–73. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Majdan M, Awatramani R, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J Neurosci. 2006;26(43):10984–91. doi: 10.1523/JNEUROSCI.0304-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci. 2001;21(15):5429–38. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Frambach DA, Odette LL. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science. 1984;225(4667):1174–5. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noirot-Timothee C, Noirot C. Septate and Scalariform Junctions in Arthropods. International Review of Cytology. 1980;63:97–140. doi: 10.1016/s0074-7696(08)61758-1. [DOI] [PubMed] [Google Scholar]
- O'Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab. 2004;24(9):1046–56. doi: 10.1097/01.WCB.0000130867.32663.90. [DOI] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, et al. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci. 2003;23(11):4549–59. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Ri Y, Bennett MV, Trexler EB, Verselis VK, Bargiello TA. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron. 1997;19(4):927–38. doi: 10.1016/s0896-6273(00)80973-3. [DOI] [PubMed] [Google Scholar]
- Orkand RK. Glial-interstitial fluid exchange. Ann N Y Acad Sci. 1986;481:269–72. doi: 10.1111/j.1749-6632.1986.tb27157.x. [DOI] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98(22):12596–601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RJ, Auld VJ. Roles of glia in the Drosophila nervous system. Semin Cell Dev Biol. 2006;17(1):66–77. doi: 10.1016/j.semcdb.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Paul SM, Ternet M, Salvaterra PM, Beitel GJ. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development. 2003;130(20):4963–74. doi: 10.1242/dev.00691. [DOI] [PubMed] [Google Scholar]
- Piechotta K, Garbarini N, England R, Delpire E. Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl- cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J Biol Chem. 2003;278(52):52848–56. doi: 10.1074/jbc.M309436200. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na(+)-K(+)-2Cl- cotransporter BSC2 in the nervous system. Am J Physiol. 1997a;272(1 Pt 1):C173–83. doi: 10.1152/ajpcell.1997.272.1.C173. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997b;33(6):781–95. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Yamate CL, Connors BW. Activity-dependent shrinkage of extracellular space in rat optic nerve: a developmental study. J Neurosci. 1985;5(2):532–5. doi: 10.1523/JNEUROSCI.05-02-00532.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci. 2008;121(Pt 20):3293–304. doi: 10.1242/jcs.029223. [DOI] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98(22):12602–7. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Canada C, Budnik V. Introduction on the use of the Drosophila embryonic/larval neuromuscular junction as a model system to study synapse development and function, and a brief summary of pathfinding and target recognition. Int Rev Neurobiol. 2006;75:1–31. doi: 10.1016/S0074-7742(06)75001-2. [DOI] [PubMed] [Google Scholar]
- Santi PA, Lakhani BN. The effect of bumetanide on the stria vascularis: a stereological analysis of cell volume density. Hear Res. 1983;12(2):151–65. doi: 10.1016/0378-5955(83)90103-x. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Xu YT, Nelles E, Fischbeck K, Willecke K, Bone LJ. Connexin32-null mice develop demyelinating peripheral neuropathy. Glia. 1998;24(1):8–20. doi: 10.1002/(sici)1098-1136(199809)24:1<8::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Schmid A, Chiba A, Doe CQ. Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development. 1999;126(21):4653–89. doi: 10.1242/dev.126.21.4653. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol. 1997;189(2):186–204. doi: 10.1006/dbio.1997.8660. [DOI] [PubMed] [Google Scholar]
- Schulte J, Tepass U, Auld VJ. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J Cell Biol. 2003;161(5):991–1000. doi: 10.1083/jcb.200303192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood-brain barrier formation in drosophila. Cell. 2005;123(1):133–44. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Schulte J, Auld VJ. The Developmental Dynamics of Peripheral Glia in Drosophila melanogaster. Glia. 2000 doi: 10.1002/(sici)1098-1136(200004)30:2<122::aid-glia2>3.0.co;2-b. in press. [DOI] [PubMed] [Google Scholar]
- Silies M, Edenfeld G, Engelen D, Stork T, Klambt C. Development of the peripheral glial cells in Drosophila. Neuron Glia Biology. 2007;3:35–43. doi: 10.1017/S1740925X07000622. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Ion regulation in the brain: implications for pathophysiology. Neuroscientist. 2002;8(3):254–67. doi: 10.1177/1073858402008003011. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A. 1994;175(2):179–91. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28(3):587–97. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M, Cantera R, Morin X, Bastiani MJ, Bate M, Karagogeos D. The IgLON protein Lachesin is required for the blood-brain barrier in Drosophila. Mol Cell Neurosci. 2006;32(1-2):91–101. doi: 10.1016/j.mcn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72(4):527–40. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Su G, Kintner DB, Flagella M, Shull GE, Sun D. Astrocytes from Na+-K+-Cl- cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am J Physiol Cell Physiol. 2002a;282(5):C1147–1160. doi: 10.1152/ajpcell.00538.2001. [DOI] [PubMed] [Google Scholar]
- Su G, Kintner DB, Sun D. Contribution of Na+-K+-Cl- cotransporter to high-[K+]o- induced swelling and EAA release in astrocytes. Am J Physiol Cell Physiol. 2002b;282(5):C1136–1146. doi: 10.1152/ajpcell.00478.2001. [DOI] [PubMed] [Google Scholar]
- Sun YT, Lin TS, Tzeng SF, Delpire E, Shen MR. Deficiency of electroneutral K+-Cl- cotransporter 3 causes a disruption in impulse propagation along peripheral nerves. Glia. 2010;58(13):1544–52. doi: 10.1002/glia.21028. [DOI] [PubMed] [Google Scholar]
- Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87(6):1091–101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- Svoboda J, Sykova E. Extracellular space volume changes in the rat spinal cord produced by nerve stimulation and peripheral injury. Brain Res. 1991;560(1-2):216–24. doi: 10.1016/0006-8993(91)91235-s. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Tsuji S, Miwa H, Morisaku T, Nuriya M, Orihara M, Kaneko K, Okano H, Yasui M. Drosophila big brain does not act as a water channel, but mediates cell adhesion. FEBS Lett. 2009;583(12):2077–82. doi: 10.1016/j.febslet.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161(2):563–96. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–84. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- Tobias JM. The high potassium and low sodium in the body fluid and tissues of a phytophagous insect, the silkworm Bombyx mori and the change before pupation. J Cell Physiol. 1948;31(2):143–8. doi: 10.1002/jcp.1030310204. [DOI] [PubMed] [Google Scholar]
- Treherne JE, Schofield PK. Mechanisms of ionic homeostasis in the central nervous system of an insect. J Exp Biol. 1981;95:61–73. doi: 10.1242/jeb.95.1.61. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Mann JR, Wangemann P, Liu J, McLaughlin KJ, Lesage F, Marcus DC, Lazdunski M, Heinemann SF, Barhanin J. Inner ear defects induced by null mutation of the isk gene. Neuron. 1996;17(6):1251–64. doi: 10.1016/s0896-6273(00)80255-x. [DOI] [PubMed] [Google Scholar]
- Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397(1):223–31. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hilchen CM, Beckeruordersandforth RM, Rickert C, Technau GM, Altenhein B. Identity, origin, and migration of peripheral glial cells in the Drosophila embryo. Mechanisms of Development. 2008;125(3-4):337–352. doi: 10.1016/j.mod.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int. 2000;36(4-5):291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Walz W, Hinks EC. Carrier-mediated KCl accumulation accompanied by water movements is involved in the control of physiological K+ levels by astrocytes. Brain Res. 1985;343(1):44–51. doi: 10.1016/0006-8993(85)91156-4. [DOI] [PubMed] [Google Scholar]
- Walz W, Hinks EC. A transmembrane sodium cycle in astrocytes. Brain Res. 1986;368(2):226–32. doi: 10.1016/0006-8993(86)90565-2. [DOI] [PubMed] [Google Scholar]
- Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res Dev Brain Res. 2002;139(1):59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Yan Y, Kintner DB, Lytle C, Sun D. GABA-mediated trophic effect on oligodendrocytes requires Na-K-2Cl cotransport activity. J Neurophysiol. 2003;90(2):1257–65. doi: 10.1152/jn.01174.2002. [DOI] [PubMed] [Google Scholar]
- Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165(1-2):1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- Wood RL. Intercellular attachment in the epithelium of Hydra as revealed by electron microscopy. J Biophys Biochem Cytol. 1959;6:343–52. doi: 10.1083/jcb.6.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164(2):313–23. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu VM, Yu MH, Paik R, Banerjee S, Liang Z, Paul SM, Bhat MA, Beitel GJ. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development. 2007 doi: 10.1242/dev.02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117(4):1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yan Y, Dempsey RJ, Sun D. Na+-K+-Cl- cotransporter in rat focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21(6):711–21. doi: 10.1097/00004647-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Zdebik AA, Wangemann P, Jentsch TJ. Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology (Bethesda) 2009;24:307–16. doi: 10.1152/physiol.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HB, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J Membr Biol. 2006;209(2-3):177–86. doi: 10.1007/s00232-005-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]