Abstract
Background
The prevalence of diagnosed celiac disease is less than 1 in 2,000 in the United States, but screening studies undertaken in European and other populations have revealed a much higher prevalence.
Objectives
To determine the prevalence of celiac disease and the utility of screening in the general adult population of a geographically isolated area.
Methods
Serum tissue transglutaminase antibodies (tTG-IgA) were measured in volunteer health care participants aged 18 years and over at Annual Casper, Wyoming Blue Envelope Health Fair Blood Draw. Subjects with positive tTG-IgA tests had their endomysial IgA antibodies checked. Double positives were offered endoscopy with small bowel biopsy. All subjects completed a short GI symptom questionnaire.
Results
3850 residents of the Natrona County had serologic evaluation for celiac disease, 34 of whom tested positive for both tTG and EMA IgA. Excluding three individuals with previous diagnosis of celiac disease, the overall prevalence of celiac serology positive in this community sample was 0.8%. All 31 subjects were offered a small bowel biopsy. Seventeen of the 18 biopsied subjects (94%) had at least partial villous atrophy. Symptoms that were reported by the fair attendees did not predict positivity.
Conclusions
Screening for celiac disease was widely accepted in this preventative healthcare setting. Undiagnosed celiac disease affects 1 in 126 individuals in this Wyoming community. Most were asymptomatic or had atypical presentations. Serologic testing can readily detect this disease in a general population.
INTRODUCTION
The prevalence of “suspected” celiac disease in Western nations varies from 1 in 87 to 1 in 500 individuals.1–5 The prevalence of diagnosed celiac disease is much lower, however, being somewhere between 1 in 500 and 1 in 9,000 individuals.6–8 In the past, diagnosis has been more frequent in patients with classic symptoms, such as diarrhea, steatorrhea, weight loss or failure to thrive, and multiple vitamin deficiencies.9 Increasing awareness of the condition and availability of serologic tests have led to augmented case finding,10–12 with a corresponding change in the types of presentations that lead to the diagnosis. Consequently, an increasing proportion of patients without classic features of malabsorption or diarrheal illness have been identified.8,13
For many years, celiac disease has been considered rare in the United States even though an increased frequency of celiac disease was being reported in European countries. 14 Recently a large multi-center study in the United States, that included a substantial cohort of subjects regarded as “healthy,” suggested a celiac disease prevalence of 1 in 133.15 However, there has been much debate about the most efficient strategy for detecting these additional celiac disease cases.16,17 If physicians only test those with severe, classic, malabsorptive symptoms, patients with atypical symptoms, who make up the majority of cases, would be missed; even testing those in whom celiac disease is more likely to occur (e.g., those with an affected family member, iron deficient anemia, type one diabetes, or premature osteoporosis) will miss most cases.18 Other than some well defined clinical associations,9 little is known about the symptoms that may provide clues to early diagnosis. In this regard, it has been suggested that the presence of symptoms suggestive of IBS may predict celiac disease,20–23 but this has remained controversial. However based on cost-benefit considerations and lack of an ideal screening strategy for celiac disease, to date a case-finding approach rather than population screening has been advocated by others.12, 24
The aims of this study were to determine the prevalence of undiagnosed celiac disease in a large population of adults in a geographically predefined area, and to identify any symptoms that predict celiac disease. Our secondary aim was to determine the efficiency and acceptability of screening for celiac disease in a health fair setting.
SUBJECTS AND METHODS
Study Design
Annual health fairs have been a longstanding practice and a traditional mode of providing preventative health care and information in Wyoming. This practice grew out of the seminal studies on community-based testing and treatment of streptococcal infection in the 1950’s.25 Traditionally, there has been wide participation of the adult population in these fairs, which provide information regarding disease-specific prevention and low cost tests for health screening purposes. This study took advantage of the well-established health fair in Natrona County, Wyoming. This county is a mixed urban and rural community of approximately 66,500 individuals (~49,000 adults) that is 91% Caucasian with the rest predominantly Hispanic or American Indian (US census 2000). This site was chosen for several reasons: First, it is geographically isolated, with virtually all hospital-based care being delivered by the Wyoming Medical Center. Secondly, there is a single GI practice that provides most of the gastrointestinal consultation and endoscopy services for the area, allowing prevalent cases of celiac disease to be readily enumerated. Lastly, there is a very active support group available for the patients with celiac disease in the area.
The laboratory component of the Health Fairs consisted of offering routine screening blood tests to individuals on an appointment basis. Individual participants select from a menu of tests. Typically, these tests include prostate specific antigen, cholesterol, homocysteine, hemoglobin and a full blood count, liver chemistries, and measures of iron stores.
Subject Recruitment
Individuals participating in the laboratory component of the health fair 2003 were invited to participate in this study when they presented for a blood test. This strategy avoided recruiting individuals with a predetermined interest in testing for celiac disease. Each subject was given a short GI symptom questionnaire and a consent form on arrival at the blood draw site. Consenting subjects completed the questionnaire and had their serum saved for serologic examination. All participating individuals were informed about their test results. Subjects with previous history of celiac disease and those with serious medical problems high risk for invasive procedures were excluded from further evaluation and statistical analysis. Study procedures were approved by the Institutional Review Boards of the Wyoming Medical Center and the Mayo Foundation.
Questionnaire
The questionnaire used in the study was adapted from the internationally validated Nepean Dyspepsia Index (NDI), which is designed to be a short, rapidly completed questionnaire suitable for general population surveys.26 The questionnaire asked subjects to report if they experienced upper GI (a full feeling after meals, food stays in stomach, nausea, vomiting, heartburn, dysphagia) or lower GI (diarrhea, greater than three bowel movements/day, watery, urgent or incontinent stools, constipation, less than three bowel movements/week, hard stools, feeling of anal blockage, bloating) symptoms, and to rate the frequency and severity of any symptoms from 1 to 5 (rare and mild to frequent and severe). Each symptom was treated as separate parameter in order to determine if there was a clinically relevant severity.
Serological Screening
Serum samples were shipped overnight to the testing laboratory in Rochester, Minnesota. A two-step sequential serologic testing strategy was used. First, the samples were tested for tissue transglutaminase IgA antibodies (tTG-IgA)using a commercially available ELISA kit that used human recombinant tissue transglutaminase as the substrate (INOVA, San Diego, California) as described in detail previously.21 Any test results in the positive or indeterminate range underwent subsequent testing with endomysial indirect immunofluorescence assay using monkey esophagus as the substrate at two screening dilutions (1:5 and 1:20) as described elsewhere.27
Biopsy Confirmation
All individuals who were positive, both on primary tissue transglutaminase and subsequently on secondary endomysial IgA testing, were informed of their results and if there were no contraindications were offered endoscopic biopsies. Those consenting to undergo endoscopy had six distal duodenal biopsies obtained for histology. Pathologists in Wyoming who were not aware of the serologic results interpreted the biopsies. Pathological results were expressed in terms of architectural changes and inflammation as well as Marsh scoring. 28
Statistical Analysis
Prevalence rates for positive serology are expressed as percentages, accompanied by 95% exact (binomial) confidence intervals (CIs). The distribution of categorical variables, such as the patient-reported symptoms, is summarized as counts and percentages. For laboratory parameters and other continuous measures, means and standard deviations (SD) are provided for variables that are approximately normally-distributed, or medians and interquartile ranges (IQRs) otherwise. The descriptive statistics reported for all variables included the number of subjects with missing data. Patient-reported symptoms, originally captured on a 5-point scale, were characterized in the analysis as present for responses of “sometimes”, “often”, or “very often”, and absent for responses of “never” or “rarely” to reflect the likely clinical relevance of the response for each symptom. Logistic regression was used to test for association between serology status and individual symptoms or laboratory parameters, controlling for age and gender. From these models, odds ratios (OR) and 95% CIs are presented to convey the strength of association between each factor and having positive serology, independent of age and gender. A P value of less than 0.05 was considered statistically significant. Analyses were carried out using the SAS statistical software package (Version 8.2, SAS Institute Inc., Cary, NC).
RESULTS
82% (4,131 of 5,037) of health fair participants volunteered for this study. Of these, 3850 lived in Natrona County and were included in the analysis. The ethnic composition of the cohort was predominantly Caucasian (94% Caucasian, 1% African American, 1% Native American, <1% Asian, <1% Pacific Islander, 2% from other races, and 2% from two or more races).
Prevalence Estimate
The 3850 Natrona County residents who underwent serologic testing for tTG IgA in their serum formed the denominator used for analysis. Seventy five subjects were positive for tTG assay, of which 34 subjects had positive sequential serologic testing. [Figure 1] However, three seropositive subjects had a previous diagnosis of celiac disease and were consequently excluded from the estimate of undiagnosed celiac disease. Two seronegative subjects had a prior diagnosis of celiac disease.
Figure 1.
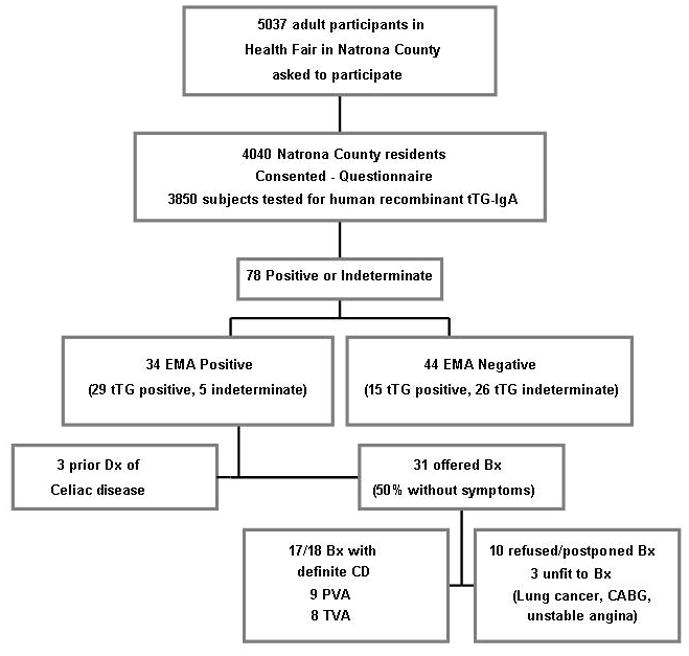
Study flow chart, (PVA: Partial Villous Atrophy, TVA: Total Villous Atrophy)
Overall, the prevalence of undiagnosed celiac disease based on positive tTG and EMA tests in this population-based sample in Natrona County was 0.8% ( 95% CIs: 0.6–1.1%). The mean age (±SD) of 54.5 (±14.7) years in the seropositive subjects was not statistically different from that in the seronegative group (57.5 ±14.7 years, p = 0.23), though the numbers in the extreme age groups were small [Table 1]. Sixty-five percent (n=20) of the 31 seropositive subjects were female, compared to 59% among the seronegative population (p=0.55).
Table 1.
Prevalence of undiagnosed celiac disease by age-group
| Age-group | #Positive/Group | Proportion % ( 95% CI) |
|---|---|---|
| 18–30 | 2/130 | 1.5 (0.2–5.5) |
| 30–40 | 2/260 | 0.8 (0.1–2.8) |
| 40–50 | 7/730 | 1.0 (0.4–2.0) |
| 50–60 | 8/998 | 0.8 (0.4–1.6) |
| 60–70 | 6/844 | 0.7 (0.3–1.5) |
| 70–80 | 5/698 | 0.7 (0.2–1.7) |
| 80+ | 1/187 | 0.5 (0.0–3.0) |
| Overall | 31/3847 | 0.8 (0.6–1.1) |
All individuals who were double positive and had no prior diagnosis of celiac disease were offered biopsies except for three subjects not felt to be medically fit for intervention (ischemic heart disease, heart failure and metastatic lung cancer). Seventeen of the eighteen subjects who underwent intestinal biopsy demonstrated at least partial villous atrophy, crypt hyperplasia, and intraepithelial lymphocytosis. Eight subjects had Marsh 3c, 6 had Marsh 3b and 3 had Marsh 3a. One subject who had a negative duodenal biopsy was Asian, and his tTG-IgA and EMA tests were both weakly positive. This latter subject’s included one biopsy with Brunner glands suggesting that a sample from the bulb had been included. Based on these findings the positive predictive value for biopsy confirmed celiac disease of this sequential serologic testing strategy was 94% for celiac disease.
Disease Prediction by Symptoms
From logistic regression modeling, adjusting for age and gender, no symptom was found to be positively associated with seropositivity [Table 2]. The most common symptoms reported by subjects with positive serology included feeling full (46%) and heartburn (40%) [Table 2]. Of the symptomatic subjects who were offered intestinal biopsy, eleven subjects complained of constipation, five reported diarrhea and two had a family history of celiac disease. Conversely, eight (44%) of the 18 subjects who underwent intestinal biopsy were asymptomatic.
Table 2.
Patient-Reported Symptoms by Serology Status. (BM : Bowel Movement)
| Symptom† | Negative (n=3816) | Positive (n=31) | Logistic Result‡ OR (95% CI) [p- value] |
|---|---|---|---|
| Feeling full | 1253 (34%) [180] | 14 (47%) [1] | 1.58 (0.76, 3.29) [0.22] |
| Bloating | 1305 (37%) [249] | 9 (30%) [1] | 0.69 (0.31, 1.52) [0.35] |
| Heartburn | 1463 (41%) [226] | 12 (40%) [1] | 0.94 (0.45, 1.95) [0.86] |
| Nausea | 501 (14%) [208] | 4 (14%) [2] | 0.87 (0.30, 2.56) [0.80] |
| Vomiting | 132 (4%) [274] | 1 (4%) [3] | 0.91 (0.12, 6.77) [0.92] |
| Diarrhea | 964 (27%) [223] | 5 (17%) [1] | 0.52 (0.20, 1.38) [0.19] |
| Constipation | 1114 (31%) [239] | 11 (38%) [2] | 1.33 (0.61, 2.89) [0.47] |
| >3 BMs a day | 810 (23%) [253] | 2 (7%) [1] | 0.24 (0.06, 1.01) [0.05] |
| <3 BMs a week | 431 (12%) [296] | 4 (13%) [1] | 1.01 (0.35, 2.96) [0.98] |
| Lumpy or hard BMs | 1137 (32%) [245] | 10 (34%) [2] | 1.09 (0.50, 2.39) [0.82] |
| Watery BMs | 870 (24%) [256] | 5 (17%) [2] | 0.63 (0.24, 1.66) [0.35] |
| Urgent need to have BM | 1259 (35%) [242] | 9 (31%) [2] | 0.84 (0.38, 1.85) [0.66] |
| Any symptoms | 2924 (81%) [216] | 24 (80%) [1] | 0.87 (0.35, 2.17) [0.77] |
| Sum of symptomsρ | 3.0 ± 2.6 [120] | 2.9 ± 2.8 [1] | 0.96 (0.83, 1.11) [0.56] |
| NDI symptom scoreρ | 28.6 ± 8.7 [117] | 27.8 ± 9.7 [1] | 0.98 (0.94, 1.03) [0.46] |
Unless indicated otherwise, count (%) [# subjects with missing data] values provided
Adjusted for age and gender
Mean ± SD [# subjects with missing data]
Associated Laboratory Abnormalities
Among the 2438 (63%) subjects who had hemoglobin tested, the mean (± SD) hemoglobin level in 23 seropositive subjects was 15.0 (± 1.4) g/dL compared with 15.2 (± 1.3) g/dL in 2415 sero-negative subjects (p = 0.38 adjusted for age and gender). Three (13%) seropositive subjects had a hemoglobin value less than 13 g/dl compared with 104 (4%) seronegative subjects (p = 0.06). All but twelve subjects were tested for total cholesterol, with a mean level of 198.9 (± 31.3) in the 31 seropositive subjects and 206.3 (± 38.9) in 3804 seronegative subjects (p=0.32). Only 882 subjects (23%) underwent ferritin testing. Within this subgroup, seven seropositive subjects had a median (IQR) ferritin level of 10.0 (8.0, 117.0) compared with a median of 53.0 (19.0, 145.0) in 875 seronegative subjects (p = 0.23). Five out of the seven (71%) seropositive subjects had ferritin values less than 20 g/dL compared with 230 of 875 (26%) seronegative subjects (p=0.037). In addition, seropositive status was associated with lower levels of HDL and calcium, though the latter was not statistically significant (p = 0.02 and 0.08, respectively) [Supplemental Table,]
Discussion
This study confirms that celiac disease is relatively common in the general population. This conclusion is further reinforced by histological confirmation in the majority of individuals with positive celiac serology. However, individual GI symptoms did not identify the seropositive cases. Interestingly, despite a relative lack of awareness of celiac disease in the general population, there was a wide acceptance of screening for this condition among the health fair participants in our study.
One of the major concerns regarding screening protocols in the general population is the likelihood of false positive results that may lead to unnecessary invasive evaluations; however, this potential problem was minimized here as the serologic strategy applied in our study took advantage of relatively sensitive anti-tTG-IgA testing (94%) followed by highly specific EMA measurement (100%).29 Our findings are consistent with previous studies on prevalence of celiac disease which have demonstrated that screening for celiac disease with this sequential method is an effective method to detect undiagnosed cases.30,31 In our study, the positive predictive value of this sequential testing was acceptable, suggesting this method as an efficient way to carry out population-based screening, while minimizing the false positives. However due to the invasive nature of endoscopy, biopsy was exclusively offered to double positive individuals. We acknowledge that, due to the rigorous serologic standards required by this approach, we may have missed some individuals with otherwise undiagnosed celiac disease. In other words, even if a few cases of celiac disease were missed because of false negative serologic test, those numbers are not likely to be great.31 Of course, the overall prevalence of previously undiagnosed celiac disease would be even higher if such patients were included. While we have been able to achieve this level of sensitivity with this technique, a recent meta-analysis suggests a lower sensitivity of 89% for celiac disease serology.32 In addition as tTG-IgG testing were not undertaken, the few subjects with IgA deficiency that could be completely seronegative might have been missed by this strategy. As such, this study may underestimate the true prevalence of celiac disease, although such a miss rate is minimal. Moreover, for the purpose of population-based screening, serology provides the only practical means of detecting the disease.
Is screening the general population justified? If we are to detect most patients with celiac disease, a community -based screening strategy such as this study could be applied. Celiac disease may meet the five WHO criteria used for determining the need for a screening program. First of all it is relatively common. It can be detected by a non-invasive strategy with high sensitivity and specificity based on sequential serological testing. Celiac disease can be also detected at an early, often asymptomatic state, and is readily treated. Certain morbidities such as fractures are more frequent in celiac disease both before and following the diagnosis, 33–36 but this increased risk is not seen in patients diagnosed as children.37 A recent study by Kurppa et al. has showed that even subjects with normal villous structure, but a positive endomysial antibody, may suffer from complications of the disease and may benefit from a gluten-free diet.38
Retrospective studies have been conducted using archival sera to diagnose celiac disease. In one study of 9133 subjects using this same robust serological strategy, subjects with serologic evidence of undiagnosed celiac disease had a 4-fold increased mortality compared to seronegative subjects39 This is consistent with a large European study suggesting that subjects with positive anti-TTG antibodies are significantly at increased risk of cancer related death.40 However a study from Finland, where the highest proportion of celiac disease is detected clinically, did not find increased mortality. Malignant complications of celiac disease - such as lymphoma or adenocarcinoma – may be the first presentation of celiac disease,42, 43 but the increased risk usually rises later in life and is low in absolute terms.
This study addressed 2 main issues pertinent to screening for celiac disease. The first, it demonstrated that despite the relative rarity of the disease most people approached were happy to undergo testing for celiac disease when participating in community-wide preventative health event. Secondly, it did not identify any simple GI symptoms that would predict those most likely to have celiac disease. The results of this study tell us that testing for celiac disease in the general population is a complex issue. On the one hand symptoms do not predict well who will have celiac disease. Suggesting that general population screening may be needed to find celiac disease but also tell us that the disease may have had little effect on the subjects. However a recent study suggest that 30% of patients discovered by screening without apparent symptoms at the time of detection felt better after starting the gluten free diet though some reduction in their self perception of health.44
This study has several limitations. It was conducted in a geographically isolated area with a predominately Caucasian population which may not be representative of the entire North American population and would probably have lower yields across non-Caucasian population. Nonetheless, as a mixed Caucasian population makes up the majority of subjects, it probably reflects the Caucasian U.S. population that is most prone to celiac disease. Additionally, not all subjects who were serum positive were fit enough or agreed to have endoscopy. It was entirely the patient’s decision not to undergo endoscopy and not, as was reported in a large prior study, due to refusal of insurance company coverage or unwillingness of the primary physician to order endoscopy.15 Furthermore considering the costs associated with the serologic testing in routine diagnostic use and the number of subjects identified to have celiac disease, the cost effectiveness of this screening strategy is unclear. Conversely, the strengths of this study depend on the geographic isolation, the high acceptance rate among participants of the health fair, the collection of symptom data prior to testing using a standardized, validated questionnaire, and the lack of any prior advertising that might have biased participation by encouraging those particularly concerned about the possibility of celiac disease.
A complete long term follow up of the undiagnosed patients identified by celiac serologic screening is needed to further clarify the usefulness and benefit of population screening for celiac disease, as well as the associated impact on quality of life of the otherwise asymptomatic individuals. Considering the restrictive aspect of adherence to gluten free diet, evaluation of the compliance with the treatment in the absence of typical symptoms for celiac disease would be the crucial component of such a study. We are now following these subjects regarding compliance with a gluten free diet, the response to treatment and the potential changes in laboratory parameters and mucosal histologic features. In fact we have observed that many of those who initially did not consent to biopsies did commence on a gluten-free diet. (data not shown)
In conclusion, celiac disease is common in the U.S. population. In general, symptoms do not appear to predict the occurrence of celiac disease in the population. Members of the general population are agreeable to serology screening and subsequent management but not all will agree to endoscopy. Screening of the general population can be accomplished with a high degree of specificity using sequential serological testing however, the who, when, and if, screening of the general population are justified are still unanswered and require further study.
Supplementary Material
Laboratory parameters of undiagnosed celiac disease cases compared to serology-negative subjects
Acknowledgments
We thank the Wyoming Celiac Sprue Association, for their help and support in this study. We thank the Blue envelope health fairs, and the many volunteers from the Wyoming celiac sprue community in Casper, WY.
Financial support: This study was supported in part by research grants DK 57982 from the National Institutes of Health, US Public Health Service. ELISA kits were provided by the Binding Site (Now Inova Diagnostics, San Diego, CA)
Footnotes
Potential competing interests: None
Specific author contributions:
Kent D. Katz: conception, planning, study design, execution, collection and interpretation of data, overseeing the study and drafting the manuscript.
Shahrooz Rashtak: conception, statistical analysis, interpretation of data, and drafting the manuscript.
Brian D. Lahr: statistical analysis.
L Joseph Melton III: study design, statistical analysis and interpretation of data Patricia K. Krause: laboratory testing
Kristine Maggi: data collection and overseeing the study
Nicholas J. Talley: study design
Joseph A. Murray: conception, planning, study design, analysis and interpretation of data, laboratory testing, securing funding and drafting manuscript.
All authors have reviewed and approved the final draft submitted.
References
- 1.Carlsson AK, Axelsson IE, Borulf SK, Bredberg AC, Ivarsson SA. Serological screening for celiac disease in healthy 2.5-year-old children in Sweden. Pediatrics. 2001;107:42–5. doi: 10.1542/peds.107.1.42. [DOI] [PubMed] [Google Scholar]
- 2.Dube C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, Macneil J, Mack D, Patel D, Moher D. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology. 2005;128:S57–67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Catassi C, Ratsch IM, Fabiani E, Rossini M, Bordicchia F, Candela F, Coppa GV, Giorgi PL. Coeliac disease in the year 2000: exploring the iceberg. Lancet. 1994;343:200–3. doi: 10.1016/s0140-6736(94)90989-x. [DOI] [PubMed] [Google Scholar]
- 4.Cook HB, Burt MJ, Collett JA, Whitehead MR, Frampton CM, Chapman BA. Adult coeliac disease: prevalence and clinical significance. J Gastroenterol Hepatol. 2000;15:1032–6. doi: 10.1046/j.1440-1746.2000.02290.x. [DOI] [PubMed] [Google Scholar]
- 5.Corazza GR, Andreani ML, Biagi F, Corrao G, Pretolani S, Giulianelli G, Ghironzi G, Gasbarrini G. The smaller size of the ‘coeliac iceberg’ in adults. Scand J Gastroenterol. 1997;32:917–9. doi: 10.3109/00365529709011202. [DOI] [PubMed] [Google Scholar]
- 6.Bode SH, Gudmand-Hoyer E. Incidence and prevalence of symptomatic celiac disease among adults in Denmark. Ugeskr Laeger. 1998;160:2100–4. [PubMed] [Google Scholar]
- 7.Carrington JM, Hewitt CJ, Dowsett LR, Barbezat GO. The prevalence of coeliac disease in Otago. N Z Med J. 1987;100:460–2. [PubMed] [Google Scholar]
- 8.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 9.Corazza GR, Di Sario A, Sacco G, Zoli G, Treggiari EA, Brusco G, Gasbarrini G. Subclinical coeliac disease: an anthropometric assessment. J Intern Med. 1994;236:183–7. doi: 10.1111/j.1365-2796.1994.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 10.Collin P, Reunala T, Rasmussen M, Kyronpalo S, Pehkonen E, Laippala P, Maki M. High incidence and prevalence of adult coeliac disease. Augmented diagnostic approach Scand. J Gastroenterol. 1997;32:1129–33. doi: 10.3109/00365529709002992. [DOI] [PubMed] [Google Scholar]
- 11.Collin P, Rasmussen M, Kyronpalo S, Laippala P, Kaukinen K. The hunt for coeliac disease in primary care. Qjm. 2002;95:75–7. doi: 10.1093/qjmed/95.2.75. [DOI] [PubMed] [Google Scholar]
- 12.Catassi C, Kryszak D, Louis-Jacques O, Duerksen DR, Hill I, Crowe SE, Brown AR, Procaccini NJ, Wonderly BA, Hartley P, Moreci J, Bennett N, Horvath K, Burk M, Fasano A. Detection of Celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol. 2007;102:1454–60. doi: 10.1111/j.1572-0241.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 13.Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128:S74–8. doi: 10.1053/j.gastro.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Fasano A. Where have all the American celiacs gone? Acta Paediatr Suppl. 1996;412:20–4. doi: 10.1111/j.1651-2227.1996.tb14242.x. [DOI] [PubMed] [Google Scholar]
- 15.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 16.Fasano A. European and North American populations should be screened for coeliac disease. Gut. 2003;52:168–9. doi: 10.1136/gut.52.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar PJ. European and North American populations should be screened for coeliac disease. Gut. 2003;52:170–1. doi: 10.1136/gut.52.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray JA. Celiac disease in patients with an affected member, type 1 diabetes, iron-deficiency, or osteoporosis? Gastroenterology. 2005;128:S52–6. doi: 10.1053/j.gastro.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Farrell RJ, Kelly CP. Celiac Sprue 10.1056/NEJMra010852. N Engl J Med. 2002;346:180–188. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 20.Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–94. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locke GR, 3rd, Murray JA, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Celiac disease serology in irritable bowel syndrome and dyspepsia: a population-based case-control study. Mayo Clin Proc. 2004;79:476–82. doi: 10.4065/79.4.476. [DOI] [PubMed] [Google Scholar]
- 22.Sanders DS, Carter MJ, Hurlstone DP, Pearce A, Ward AM, McAlindon ME, Lobo AJ. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504–8. doi: 10.1016/S0140-6736(01)06581-3. [DOI] [PubMed] [Google Scholar]
- 23.Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BM, Moayyedi P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009;169:651–8. doi: 10.1001/archinternmed.2009.22. [DOI] [PubMed] [Google Scholar]
- 24.Spiegel BM, DeRosa VP, Gralnek IM, Wang V, Dulai GS. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: a cost-effectiveness analysis. Gastroenterology. 2004;126:1721–32. doi: 10.1053/j.gastro.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Denny FW, Wannamaker LW, Brink WR, Rammelkamp CH, Jr, Custer EA. Prevention of rheumatic fever; treatment of the preceding streptococcic infection. J Am Med Assoc. 1950;143:151–3. doi: 10.1001/jama.1950.02910370001001. [DOI] [PubMed] [Google Scholar]
- 26.Talley NJ, Haque M, Wyeth JW, Stace NH, Tytgat GN, Stanghellini V, Holtmann G, Verlinden M, Jones M. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Aliment Pharmacol Ther. 1999;13:225–35. doi: 10.1046/j.1365-2036.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar V, Lerner A, Valeski JE, Beutner EH, Chorzelski TP, Rossi T. Endomysial antibodies in the diagnosis of celiac disease and the effect of gluten on antibody titers. Immunol Invest. 1989;18:533–44. doi: 10.3109/08820138909112261. [DOI] [PubMed] [Google Scholar]
- 28.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 29.Volta U, Granito A, Parisi C, Fabbri A, Fiorini E, Piscaglia M, Tovoli F, Grasso V, Muratori P, Pappas G, De Giorgio R. Deamidated gliadin Peptide antibodies as a routine test for celiac disease: a prospective analysis. J Clin Gastroenterol. 44:186–90. doi: 10.1097/MCG.0b013e3181c378f6. [DOI] [PubMed] [Google Scholar]
- 30.Gomez JC, Selvaggio G, Pizarro B, Viola MJ, La Motta G, Smecuol E, Castelletto R, Echeverria R, Vazquez H, Mazure R, Crivelli A, Sugai E, Maurino E, Bai JC. Value of a screening algorithm for celiac disease using tissue transglutaminase antibodies as first level in a population-based study. Am J Gastroenterol. 2002;97:2785–90. doi: 10.1111/j.1572-0241.2002.07023.x. [DOI] [PubMed] [Google Scholar]
- 31.Walker MM, Murray JA, Ronkainen J, Aro P, Storskrubb T, D’Amato M, Lahr B, Talley NJ, Agreus L. Detection of Celiac Disease and Lymphocytic Enteropathy by Parallel Serology and Histopathology in a Population-Based Study. Gastroenterology. 2010;139(1):112–9. doi: 10.1053/j.gastro.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Windt DAWM, Jellema P, Mulder CJ, Kneepkens CMF, van der Horst HE. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. J Am Med Assoc. 2010;303(17):1738–1746. doi: 10.1001/jama.2010.549. [DOI] [PubMed] [Google Scholar]
- 33.Jafri MR, Nordstrom CW, Murray JA, Van Dyke CT, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Long-term fracture risk in patients with celiac disease: a population-based study in Olmsted County, Minnesota. Dig Dis Sci. 2008;53:964–71. doi: 10.1007/s10620-007-9976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davie MW, Gaywood I, George E, Jones PW, Masud T, Price T, Summers GD. Excess non-spine fractures in women over 50 years with celiac disease: a cross-sectional, questionnaire-based study. Osteoporos Int. 2005;16:1150–5. doi: 10.1007/s00198-004-1822-z. [DOI] [PubMed] [Google Scholar]
- 35.Heneghan MA, Feeley KM, Stevens FM, Little MP, McCarthy CF. Precipitation of iron overload and hereditary hemochromatosis after successful treatment of celiac disease. Am J Gastroenterol. 2000;95:298–300. doi: 10.1111/j.1572-0241.2000.01556.x. [DOI] [PubMed] [Google Scholar]
- 36.Fickling WE, McFarlane XA, Bhalla AK, Robertson DA. The clinical impact of metabolic bone disease in coeliac disease. Postgrad Med J. 2001;77:33–6. doi: 10.1136/pmj.77.903.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomason K, West J, Logan RF, Coupland C, Holmes GK. Fracture experience of patients with coeliac disease: a population based survey. Gut. 2003;52:518–22. doi: 10.1136/gut.52.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurppa K, Collin P, Sievanen H, Huhtala H, Maki M, Kaukinen K. Gastrointestinal symptoms, quality of life and bone mineral density in mild enteropathic coeliac disease: a prospective clinical trial. Scand J Gastroenterol. 45:305–14. doi: 10.3109/00365520903555879. [DOI] [PubMed] [Google Scholar]
- 39.Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, Brantner TL, Kim WR, Phelps TK, Lahr BD, Zinsmeister AR, Melton LJ, 3rd, Murray JA. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metzger MH, Heier M, Maki M, Bravi E, Schneider A, Lowel H, Illig T, Schuppan D, Wichmann HE. Mortality excess in individuals with elevated IgA anti-transglutaminase antibodies: the KORA/MONICA Augsburg cohort study 1989–1998. Eur J Epidemiol. 2006;21:359–65. doi: 10.1007/s10654-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 41.Lohi S, Maki M, Montonen J, Knekt P, Pukkala E, Reunanen A, Kaukinen K. Malignancies in cases with screening-identified evidence of coeliac disease: a long-term population-based cohort study. Gut. 2009;58:643–7. doi: 10.1136/gut.2007.140970. [DOI] [PubMed] [Google Scholar]
- 42.Egan LJ, Walsh SV, Stevens FM, Connolly CE, Egan EL, McCarthy CF. Celiac-associated lymphoma. A single institution experience of 30 cases in the combination chemotherapy era. J Clin Gastroenterol. 1995;21:123–9. [PubMed] [Google Scholar]
- 43.Potter DD, Murray JA, Donohue JH, Burgart LJ, Nagorney DM, van Heerden JA, Plevak MF, Zinsmeister AR, Thibodeau SN. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004;64:7073–7. doi: 10.1158/0008-5472.CAN-04-1096. [DOI] [PubMed] [Google Scholar]
- 44.Ukkola A, Mäki M, Kurppa K, Collin P, Huhtala H, Kekkonen L, Kaukinen K. Diet improves perception of health and well-being in symptomatic, but not asymptomatic, patients with celiac disease. Clin Gastroenterol Hepatol. 2010 doi: 10.1016/j.cgh.2010.10.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Laboratory parameters of undiagnosed celiac disease cases compared to serology-negative subjects


