Abstract
We have recently developed an animal model of fibromyalgia syndrome in the rat. In this model, rats exposed to unpredictable sound stress develop a delayed onset enhancement and prolongation of cytokine-induced mechanical hyperalgesia in muscle and skin. In this study, we tested the hypothesis that our model also manifests symptoms of common co-morbid diagnoses: irritable bowel syndrome, temporomandibular disorder and anxiety. Both visceral sensitivity and cytokine hyperalgesia in masseter muscle were present in the stressed rats. Furthermore, in an established model of irritable bowel syndrome, water avoidance, we observed significant muscle hyperalgesia. Finally, using the elevated plus maze to assess for anxiety level, we observed a significantly higher anxiety level in sound stress exposed rats. Thus, unpredictable sound stress produces a condition in the rat with several features — delayed onset visceral and temporomandibular hyperalgesia and increased anxiety, as well as cutaneous and muscle hyperalgesia — commonly found in patients with fibromyalgia syndrome.
Keywords: Unpredictable sound stress, visceral hyperalgesia, colorectal distension, anxiety, irritable bowel syndrome, temporomandibular disorder
Introduction
While the research diagnostic criteria for fibromyalgia syndrome is based on the presence of widespread chronic musculoskeletal pain 17, 28, 47, patients commonly report other symptoms, including anxiety 1, sleep disturbance 29, fatigue 39, and co-morbid pain syndromes, especially irritable bowel syndrome 48 and temporomandibular disease 2, 4. Irritable bowel syndrome has been reported to be present in 32–80% of fibromyalgia syndrome patients 2, anxiety in 47–62% 3, 50, and temporomandibular disorder in 44–80% 4, 23, 34 of patients with fibromyalgia syndrome making these three of the most common co-morbidities in this patient population. In patients with fibromyalgia syndrome and other generalized pain syndromes, stress also plays an important role 6, 8, 15, 31, 35, and there is a close association between exacerbation of pain symptoms, increased anxiety 46 and exposure to stressors, (e.g. noise 6, 13, 45).
We recently developed a model of fibromyalgia syndrome in the rat produced by exposure to unpredictable sound stress 19. After a delay of ~2 weeks, rats develop enhancement and prolongation of nociceptive response to algesic cytokines in cutaneous and musculoskeletal domains 19. While this model shares characteristics features with fibromyalgia, it has not been shown to replicate all features. Therefore, in the current study we endeavored to provide additional support to validate the utility of this model. We show that this sound stress model also produces visceral hyperalgesia, the cardinal manifestation of irritable bowel syndrome, temporomandibular hyperalgesia and increased anxiety, and that a model of irritable bowel syndrome produces skeletal muscle hyperalgesia, clinically important co-morbidities observed in patients with fibromyalgia syndrome.
Methods
Animals
Experiments were performed on adult male Sprague Dawley rats (300–400 g, approximately 9 – 12 weeks old) from Charles River (Hollister, CA). Rats were housed two per cage (30 × 75 × 20 cm) in the Laboratory Animal Resource Center of the University of California, San Francisco, under a 12-hour light/dark cycle. Animal care and use conformed to NIH guidelines. The University of California, San Francisco Institutional Animal Care and Use Committee approved experimental protocols. Concerted effort was made to reduce the number of animals used and their suffering.
Visceral nociception
We employed the widely used colorectal distension method for evaluating visceral nociception in awake rats 9. Three days prior to nociceptive assessment, rats were anesthetized with isoflurane (3%) and implanted with electrodes for electromyographic recording (EMG) from the abdominal musculature (visceromotor response). On the day of the experiment rats were briefly anesthetized with isoflurane, and a lubricated latex balloon (~6 cm in length) attached to Tygon® tubing, was inserted per rectum into the descending colon, and the tubing secured to the base of the tail using Micropore medical tape. The balloon was distended to 20, 40, 60, or 80 mm Hg (pressure was monitored via a pressure transducer), each for 20 s separated by 4-minute inter-inflation intervals. EMG activity of the external oblique muscle was amplified, rectified, and recorded for 10 s before colon distension (baseline EMG activity), 20 s during each distension and 10 s post-inflation. The EMG signal was integrated and normalized as a change over baseline activity using Spike-2/CED 1401 data acquisition (CED 1401; Cambridge Electronic Design, Cambridge, UK) as previously described 9.
Gastrocnemius muscle nociception
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a Chatillon digital force transducer (model DFI2, Ametek Inc., Largo, FL) 10–12, 19. Rats were lightly restrained in a cylindrical acrylic holder with openings on the sides that allow for easy access to the hind limb. A 6-mm diameter probe attached to the force transducer was applied to the gastrocnemius muscle to deliver an increasing compression force; this width of probe allows for selective evaluation of muscle pain (vis-à-vis overlying skin pain) 30. The nociceptive threshold was defined as the force, in Newtons, at which the rat withdrew its hind leg. Baseline withdrawal threshold was defined as the mean of 2 readings taken at 5-min intervals. was injected into the belly of the Each hind limb is treated as an independent measure and each experiment performed on a separate group of rats.
Masseter muscle nociception
Mechanical nociceptive threshold in the orofacial region has previously been assessed using von Frey hair monofilamaents to measure nociceptive threshold 36, 51, but these small-diameter monofilaments preferentially activate cutaneous nociceptors 42. In order to measure nociceptive threshold in the masseter muscle, we employed a much larger diameter probe. Rats were lightly restrained in an acrylic holder that allowed access to the head, and a Chatillon digital force transducer force transducer with a 6-mm diameter probe was applied to the masseter muscle. Mechanical nociceptive threshold defined as the threshold force required to elicit head withdrawal, using the mean of 6 stimuli (2 trials of 3 stimuli applied at 30-s intervals between trials).
PGE2 hyperalgesia
Prostaglandin E2 (PGE2; 1 μg in 20 μl 0.9% saline) was injected into the belly of the gastrocnemius muscle. The 1 Lg dose of PGE2 was determined in earlier studies as sufficient to produce robust muscle primary mechanical hyperalgesia.
Sound stress
Exposure to sound stress occurred over 4 days as described previously 20, 41. In brief, animals were placed 3 per cage 25 cm from a speaker that emitted pure tones at four frequencies (5, 11, 15 and 19 kHz); amplitudes varied through time independently for each frequency from 20–110 dB sound pressure level at random times each minute, lasting 5 or 10 s; total time in the chamber was 30 min. Sham stressed animals were placed in the sound chamber for 30 minutes, but without exposure to the sound stimulus. Following sound or sham sound stress, rats were returned to their home cages in the animal care facility. They were exposed to the stressor on days 1, 3, and 4. Rats were used for nociceptive studies, 1 or 14 days after the last sound stress exposure.
Water avoidance stress
We used the water-avoidance rat model for visceral hypersensitivity with irritable bowel syndrome-like features 7. For 1 h/day, for 10 consecutive days, rats were placed on an acrylic platform (8 × 8 cm, 10 cm high) in the center of a clear plastic tank (45 cm length × 25 cm width × 25 cm height) filled with room temperature tap water to a depth of 9 cm. One or 14 days after the 10-day stress period, rats were tested for gastrocnemius muscle hyperalgesia.
Anxiety assessment
The elevated plus maze 33 was used to assess the effects of sound stress on anxiety in the rat. The maze is a plus-shaped platform with two opposing open and two opposing closed arms (50 × 10 cm (L × W) at an angle of 90% to each other; closed arms are surrounded by 40 cm high opaque walls on three sides). Rats were placed at the intersection of the arms, facing a closed arm and allowed to explore the maze for 5 minutes. During this time rats were videotaped so that behavioral measures could be scored at a later time. Behaviors assessed were: time spent in open and closed arms, and on the central platform (intersection between open and closed arms); number of open and closed arm entries (all four paws had to cross into the arm); total exploration (entries into any arm). “Anxiety Index” 26, an index that integrates the elevated plus behavioral measures, was calculated as follows:
A higher Anxiety Index value (range 0–1) indicates increased anxiety-like behavior26.
All behavioral testing was performed between 10 am and 4 pm.
Statistics
Group data are expressed as percentage change from baseline nociceptive threshold (mean ± SEM of n distinct observations). Statistical comparisons were made by a two-tailed Student’s t-test (for one or two independent populations) and by one-way ANOVA for comparing multiple treatments, using StatView statistical software. P<0.05 was considered statistically significant. To compare change from baseline, one-way repeated measures ANOVA with a Greenhouse-Geisser adjusted p-value was used (SPSS statistical software).
Results
Visceral nociception following sound stress
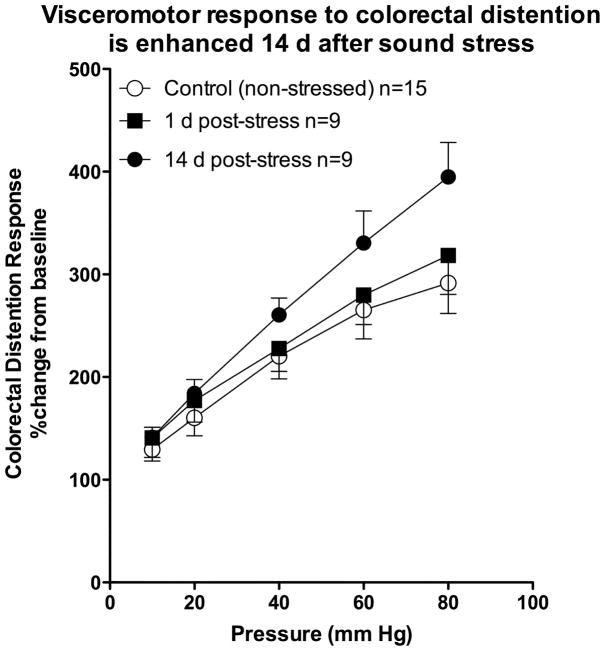
In our sound stress model of fibromyalgia syndrome, visceromotor response to graded intensities of colorectal distention was recorded. A significant increase in visceromotor response to pressure stimuli was observed 14, but not 1, day after the last exposure to sound stress, compared to a non-stressed control group or rats (Figure 1). Two-way repeated measures ANOVA, with one within-subjects factor (intra-colon pressure with four levels, i.e. 20, 40, 60, and 80 mm Hg) and one between-subjects factor (treatment with two levels, i.e. stress/no stress), demonstrated a significant treatment × pressure interaction (F4,88 = 2.85; P < 0.029), indicating a significant difference in CRD response of the two groups (stress and no stress) to increasing pressure. In contrast to the response 14 d after stress, visceromotor response to increasing pressure stimulus 1 d after the last sound stress session was not significantly different compared to the non-stressed group of rats. While not statistically significant, the visceromotor response 1 d after stress exposure did show an apparent decrease, similar to the short-latency (1 d) antinociception that precedes hyperalgesia that we have observed following stress 18.
Figure 1. Sound stress increases visceromotor response.
Rats were exposed to unpredictable sound stress and visceromotor responses were determined in the colorectal distension model, 1 or 14 d later. Compared to naïve (non-stressed) rats (open circles, n=15), the visceromotor response was significantly greater 14 (filled circles, n=9), but not 1 d after the last exposure to sound stress (filled squares, n=9).
Masseter muscle hyperalgesia in a model of fibromyalgia
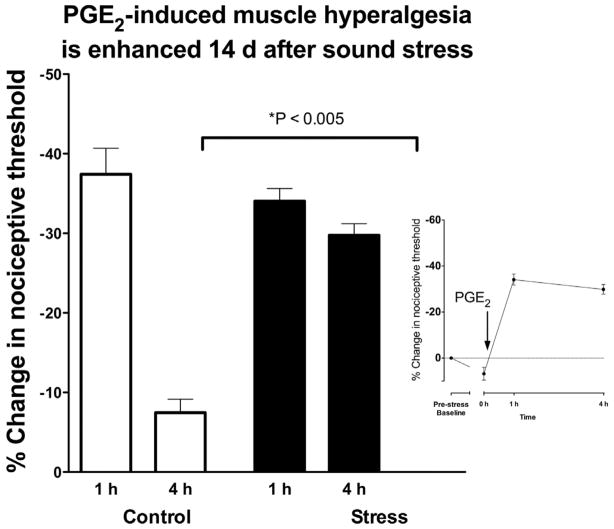
Administration of PGE2 (1 Lg in 20 μl) into the masseter muscle produced a marked decrease (~35–40%) in mechanical nociceptive threshold in the masseter muscle 1 h post-injection. In control, non-stressed rats, nociceptive threshold returned to near baseline when tested 4 h post-injection. Similar to non-stressed rats, 1 d after the last sound stress exposure, PGE2 hyperalgesia was present 1 h post injection, but thresholds returned to baseline by 4 h post injection. In contrast, 14 d after sound stress, hyperalgesia remained undiminished 4 h post-PGE2 injection (Figure 2; P<0.005).
Figure 2. Sound stress increases PGE2 hyperalgesia in masseter muscle.
Administration of PGE2 (1 μg in 20 μl) produced a marked decrease in mechanical nociceptive threshold in the masseter muscle 1 h post-injection that returns to near baseline in control (non-stressed) rats (n=8), when tested 4 h post-injection. A similar PGE2 hyperalgesia was present in rats 1 d after the last session of chronic unpredictable sound stress (n=6). In contrast, 14 d after sound stress (n=8), PGE2 hyperalgesia was still near maximal 4 h post-injection (P<0.001). Inset figure shows that exposure to sound stress had little effect on nociceptive threshold (6.8% increase).
Anxiety-like behavior following sound stress
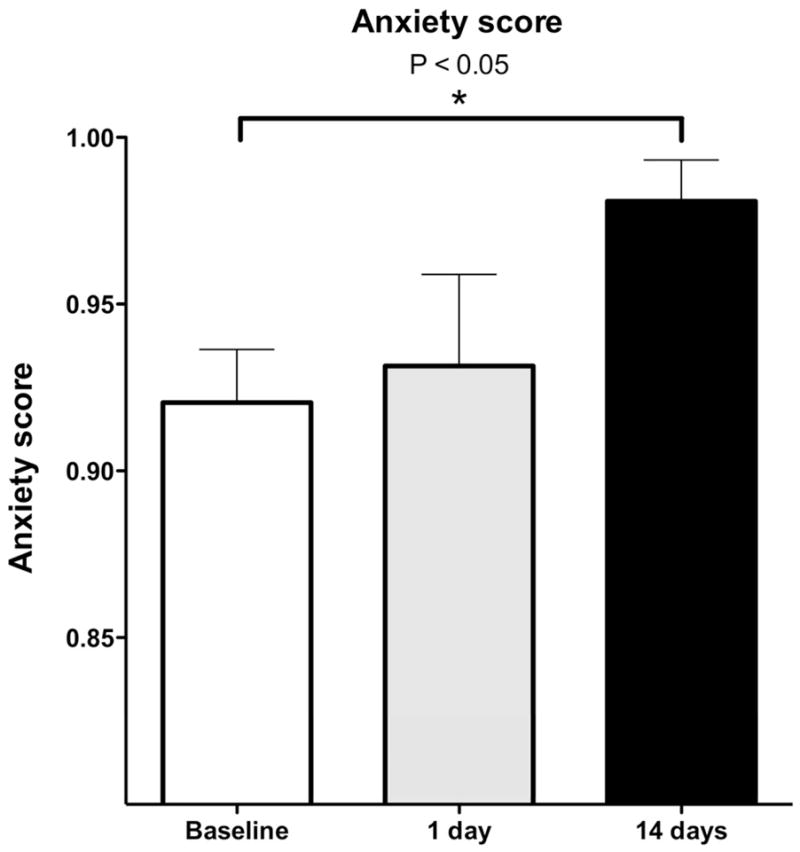
To determine if our model of fibromyalgia syndrome is associated with anxiety-like behavior, we assessed the behavior of rats in the elevated plus maze at baseline (pre-stress), and again 1 and 14 d after exposure to the stress protocol used to induce fibromyalgia syndrome. The Anxiety Index (see Methods) was significantly greater 14 d, (P<0.05) but not 1 d (P= NS), after the last exposure to sound stress, compared with pre-stress baseline (Figure 3).
Figure 3. Sound stress increases anxiety-like behavior.
Rats were exposed to unpredictable sound stress and anxiety-like behavior was evaluated using the elevated plus maze, 1 and 14 d later. Compared to pre-stress, rats had a significantly lower open arm/total entries (i.e. both arms combined) score 14 d, but not 1 d, after stress, which indicates an increased anxiety.
Gastrocnemius muscle hyperalgesia in a model of irritable bowel syndrome
The water-avoidance stress protocol, a model of irritable bowel syndrome 7, produced a statistically significant (33.6%) decrease in mechanical nociceptive threshold in the gastrocnemius muscle 1 d after the last stress exposure, compared to baseline (pre-exposure) thresholds. In contrast to sound stress exposure, lowered nociceptive threshold was observed in the absence of exogenously administered cytokines. 14 d after the last exposure to water stress, mechanical nociceptive threshold in the gastrocnemius muscle was 26.8% lower than pre-stress baseline threshold.
To determine if the water-avoidance protocol induces enhanced mechanical hyperalgesia, as observed in our sound stress model of fibromyalgia syndrome 19, we assessed hyperalgesia produced by the proinflammatory cytokine, PGE2 (1 μg) injected into the gastrocnemius muscle 12. PGE2 hyperalgesia in control, non-stressed rats, was observed at 1 h, but returned to near baseline levels 4 h after administration, while in rats 1 d after the last water-avoidance stress exposure, PGE2 hyperalgesia (compared to post-stress, pre-PGE2 administration threshold) was still present, unattenuated, 4 h after administration. 14 d after the last water-avoidance stress exposure, PGE2 hyperalgesia was also present at 1 and 4 h post PGE2 administration. Absolute values for nociceptive threshold in gastrocnemius and masseter muscle are shown in Table 1.
Table 1.
Nociceptive thresholds for masseter and gastrocnemius muscle
| Masseter muscle (threshold, N) | Gastrocnemius muscle (threshold, N) | |
|---|---|---|
| Pre-stress | 1481 ± 23 | 2646 ± 6 |
| Post-stress | 1582 ± 34 | 1757 ± 30* |
| PGE2 (1 h) | 976 ± 25† | 1244 ± 28† |
P<0.05 post-stress vs. pre-stress
P<0.05 PGE2 vs. post-stress
Discussion
We tested the hypothesis that a model of fibromyalgia syndrome, sound stress-induced delayed onset enhanced musculoskeletal and cutaneous mechanical hyperalgesia 19, also demonstrates other important features of this syndrome: co-morbidity with irritable bowel syndrome (as indicated by presence of gastrointestinal hypersensitivity), increased anxiety (as indicated by increased Anxiety Index in the elevated plus maze test) and co-morbidity with temporomandibular disorder, indicated by hyperalgesia in the masseter muscle. Of note, visceral hyperalgesia, prolonged musculoskeletal hyperalgesia and anxiety are present, 14 d, but not 1 d, after exposure to stress, similar to the post-stress delay for the enhancement of cutaneous and muscle cytokine-induced hyperalgesia 19. If anything, 1 d after stress exposure there is a decrease, albeit not statistically significant, in visceral nociception. We have previously reported a similar effect — short-latency (1 d) antinociception followed by later onset hyperalgesia — following unpredictable sound stress 18. Of clinical note, generalized pain syndromes tend to occur with delayed onset following stress exposure 32, 37. While statistical significance was not reached for the 40 and 60 mmHg pressures, which are considered to be noxious, the responses at these pressures in 14 d post-stress rats were greater than in the control group of rats. Since the variance in these groups is fairly large, in a bigger experimental group, a significant difference might have been observed at less noxious stimulus intensities.
Our observation of visceral hyperalgesia and anxiety-like behavior, both with delayed onset post-stress, indicate that key features of fibromyalgia syndrome, in addition to stress-induced delayed onset enhanced mechanical hyperalgesia, are present in this model. While sound stress markedly increased cytokine-induced muscle hyperalgesia, this stress alone did not affect mechanical nociceptive threshold 19. In contrast, visceromotor response was enhanced without the administration of cytokines. This may be explained by the presence of tonic levels of cytokines in intestinal mucosa and muscle in naïve rats 21, 38. Since many patients with an initial diagnosis of irritable bowel syndrome also meet criteria for a diagnosis of fibromyalgia syndrome, we also evaluated the impact of a well-established model of visceral hypersensitivity with irritable bowel syndrome-like features, water avoidance stress 7, on musculoskeletal nociception. We found that water-avoidance stress also induced hyperalgesia in the somatic domain. However, unlike sound stress 19, water-avoidance produced mechanical hyperalgesia in skeletal muscle without the additional requirement for cytokine administration. The basis for the differential effects of these two stress protocols, which is currently being investigated, may be related to duration of exposure stressor (for sound stress, three 30 min exposures over 4 d, versus water avoidance stress, ten 60 min exposures over 10 d), producing differential activation of stress axes. However, we have previously shown that rats exposed to this sound stress protocol have markedly increased plasma epinephrine levels at least 28 days after the last exposure to sound stress 19, indicating a state of chronic stress persists beyond exposure to the initiating stressor. Another possibility is that central mechanisms may contribute to muscle hyperalgesia, perhaps with water avoidance stress procedure producing an inhibition of descending pain inhibitory circuits or an enhancement of descending pain facilitatory controls 43 more than that produced by exposure to sound stress.
We have previously shown that chronic unpredictable sound stress produces an upregulation in catecholamine synthesizing enzymes in the adrenal medulla and prolonged increase in plasma catecholamine levels 19. It is possible that the hypothalamic pituitary adrenal (HPA) axis also plays a role, as we have previously shown that both sympathoadrenal and HPA axes are necessary to induce enhanced cytokine hyperalgesia 19. Although exposure to sound stress does not change muscle nociceptive threshold, water avoidance stress does. While the differences in mechanism(s) that explains this is unknown, it may reflect increased production of endogenous cytokines following exposure to water avoidance stress. Of note in this regard, the water avoidance stress protocol significantly increases circulating interleukin (IL)-2 levels in TNBS colitis 25 as well as increasing IL-4 49 and IL-1beta and IFN-gamma 7 expression. It is possible that an increase in cytokines following water avoidance stress contributes to the observed decrease in nociceptive threshold, while such an increase in cytokines does not occur following sound stress.
While not as well studied as pain, anxiety is another prominent feature of fibromyalgia and other chronic widespread pain syndromes 3, 24, 50. In the present study, not only was an increase in anxiety-like behavior observed, it had a delayed onset post-stress, very similar to the nociceptive effects of stress. There is an established association between stress and fibromyalgia syndrome. Acute stress can induce long-term changes in pain sensitivity with delayed onset (e.g., following a motor vehicle accident), response to acute stress is both the strongest predictor of maintenance of pain symptoms weeks later 14 and increased pain symptoms at a later date in individuals with fibromyalgia and other forms of chronic widespread pain 27.
The present findings together with those from the initial characterization of this model of fibromyalgia syndrome 19, 20, provides a relatively robust model of this clinical condition, in terms of our ability to reproduce multiple of its clinical features. Thus, in addition to demonstrating the ability of unpredictable stress to produce an enhancement and prolongation of cytokine-induced hyperalgesia in skin as well as muscle 19 and delayed onset of symptoms following exposure to stress, we now provide evidence for three other common features of fibromyalgia syndrome, namely an anxiety phenotype, which is seen in 47–62% of patients 3, 50, visceral hyperalgesia, seen in 32–80% of patients 2 and enhanced cytokine hyperalgesia in the masseter muscle, a model of temporomandibular disease, seen in 44–80% of patients 4, 23, 34. The observation that the hypernociception in muscle and skin requires exposure to proinflammatory cytokines, shown to also be present in patients with fibromyalgia 5, 16, 44, while the visceral hypernociception does not require addition of exogenous cytokines, may help explain why while there is considerable overlap between irritable bowel syndrome and fibromyalgia syndrome 22, 40 some patients have only one of these two co-morbid conditions. Finally, we also observed evidence for another common co-morbid condition, temporomandibular disorder 23, 34.
In conclusion, our results indicate that the fibromyalgia syndrome model produced by unpredictable sound stress in the rat exhibits several features in addition to musculoskeletal pain induced by chronic unpredictable stress, found in patients with fibromyalgia syndrome sufficient to justify the use of this model to test hypotheses about the underlying mechanisms and response to therapy in patients with fibromyalgia. Taken together with our previous studies of stress-induced enhancement of musculoskeletal hyperalgesia, this model provides one of the most well-validated animal models of fibromyalgia syndrome.
Perspective.
A stress model, unpredictable sound, in the rat exhibits several features (cutaneous, musculoskeletal and visceral hyperalgesia, as well as anxiety) that are found in patients with fibromyalgia syndrome. Thus, this model may be used to test hypotheses about the underlying mechanisms and response to therapy in patients with fibromyalgia.
Figure 4. Water-avoidance stress increases PGE2 hyperalgesia in gastrocnemius muscle.
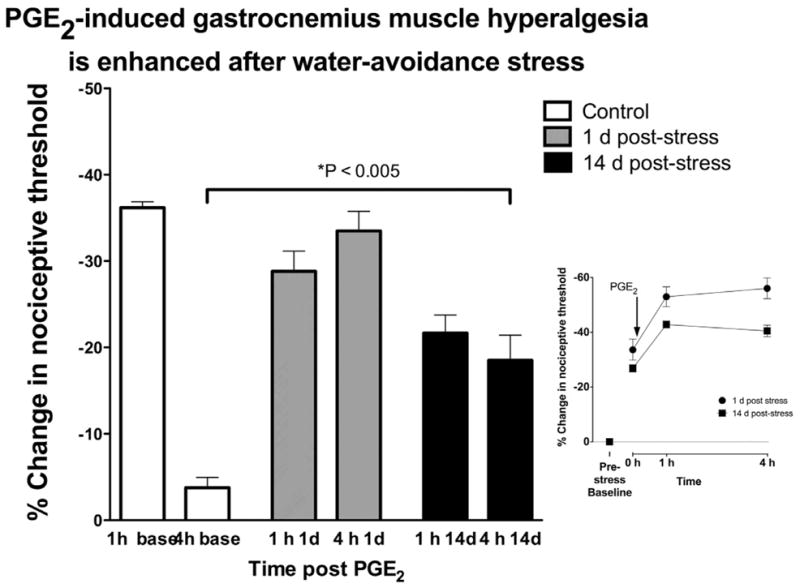
Mechanical hyperalgesia in the gastrocnemius muscle was measured 1 and 4 h after intramuscular administration of PGE2, in control (non-stressed) water-avoidance protocol exposed rats. In control, non-stressed rats, PGE2–induced decrease in mechanical nociception was present 1 h post injection, but had returned to near baseline at the 4 h time point. A similar PGE2 hyperalgesia was present in rats 1 d or 14 d after the last session of water avoidance stress (n=12). In water-avoidance stressed rats, the decrease in nociceptive threshold remained undiminished 4 h post PGE2 administration. Inset figure shows that exposure to water avoidance stress had a large effect on nociceptive threshold, decreasing it by 33.6% and 26.8% 1 and 14 day post-stress, respectively.
Acknowledgments
The authors thank Jerry Gebhart for helping us establish the colorectal distention model and Emeran Mayer for choice of water avoidance model of irritable bowel syndrome. The authors declare no conflicts of interest. This work was supported by NIH grant AR054635.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aaron LA, Bradley LA, Alarcon GS, Alexander RW, Triana-Alexander M, Martin MY, Alberts KR. Psychiatric diagnoses in patients with fibromyalgia are related to health care-seeking behavior rather than to illness. Arthritis Rheum. 1996;39:436–445. doi: 10.1002/art.1780390311. [DOI] [PubMed] [Google Scholar]
- 2.Aaron LA, Buchwald D. Chronic diffuse musculoskeletal pain, fibromyalgia and co-morbid unexplained clinical conditions. Best Pract Res Clin Rheumatol. 2003;17:563–574. doi: 10.1016/s1521-6942(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 3.Arnold LM, Hudson JI, Keck PE, Auchenbach MB, Javaras KN, Hess EV. Comorbidity of fibromyalgia and psychiatric disorders. J Clin Psychiatry. 2006;67:1219–1225. doi: 10.4088/jcp.v67n0807. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramaniam R, de Leeuw R, Zhu H, Nickerson RB, Okeson JP, Carlson CR. Prevalence of temporomandibular disorders in fibromyalgia and failed back syndrome patients: a blinded prospective comparison study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:204–216. doi: 10.1016/j.tripleo.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Bazzichi L, Rossi A, Massimetti G, Giannaccini G, Giuliano T, De Feo F, Ciapparelli A, Dell’Osso L, Bombardieri S. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol. 2007;25:225–230. [PubMed] [Google Scholar]
- 6.Bennett RM. Fibrositis: misnomer for a common rheumatic disorder. West J Med. 1981;134:405–413. [PMC free article] [PubMed] [Google Scholar]
- 7.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 8.Buskila D, Ablin JN, Ben-Zion I, Muntanu D, Shalev A, Sarzi-Puttini P, Cohen H. A painful train of events: increased prevalence of fibromyalgia in survivors of a major train crash. Clin Exp Rheumatol. 2009;27:S79–85. [PubMed] [Google Scholar]
- 9.Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc. 2007;2:2624–2631. doi: 10.1038/nprot.2007.392. [DOI] [PubMed] [Google Scholar]
- 10.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms Mediating Vibration-induced Chronic Musculoskeletal Pain Analyzed in the Rat. J Pain. 2010;11:369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase cepsilon-dependent chronic-latent muscle pain. J Pain. 2008;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohrenbusch R, Sodhi H, Lamprecht J, Genth E. Fibromyalgia as a disorder of perceptual organization? An analysis of acoustic stimulus processing in patients with widespread pain. Z Rheumatol. 1997;56:334–341. doi: 10.1007/s003930050047. [DOI] [PubMed] [Google Scholar]
- 14.Drottning M, Staff PH, Levin L, Malt UF. Acute emotional response to common whiplash predicts subsequent pain complaints: A prospective study of 107 subjects sustaining whiplash injury. Nordic Journal of Psychiatry. 2009;49:293–300. [Google Scholar]
- 15.Geenen R, Bijlsma JW. Deviations in the endocrine system and brain of patients with fibromyalgia: cause or consequence of pain and associated features? Ann N Y Acad Sci. 2010;1193:98–110. doi: 10.1111/j.1749-6632.2009.05290.x. [DOI] [PubMed] [Google Scholar]
- 16.Gur A, Karakoc M, Nas K, Remzi Cevik, Denli A, Sarac J. Cytokines and depression in cases with fibromyalgia. J Rheumatol. 2002;29:358–361. [PubMed] [Google Scholar]
- 17.Henriksson KG, Bengtsson A. Fibromyalgia--a clinical entity? Can J Physiol Pharmacol. 1991;69:672–677. doi: 10.1139/y91-100. [DOI] [PubMed] [Google Scholar]
- 18.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain. 2009;10:1073–1077. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Knapp HR, Oelz O, Sweetman BJ, Oates JA. Synthesis and metabolism of prostaglandins E2, F2alpha and D2 by the rat gastrointestinal tract. Stimulation by a hypertonic environment in vitro. Prostaglandins. 1978;15:751–757. doi: 10.1016/0090-6980(78)90141-7. [DOI] [PubMed] [Google Scholar]
- 22.Kurland JE, Coyle WJ, Winkler A, Zable E. Prevalence of irritable bowel syndrome and depression in fibromyalgia. Dig Dis Sci. 2006;51:454–460. doi: 10.1007/s10620-006-3154-7. [DOI] [PubMed] [Google Scholar]
- 23.Leblebici B, Pektas ZO, Ortancil O, Hurcan EC, Bagis S, Akman MN. Coexistence of fibromyalgia, temporomandibular disorder, and masticatory myofascial pain syndromes. Rheumatol Int. 2007;27:541–544. doi: 10.1007/s00296-006-0251-z. [DOI] [PubMed] [Google Scholar]
- 24.Lepine JP, Briley M. The epidemiology of pain in depression. Hum Psychopharmacol. 2004;19 (Suppl 1):S3–7. doi: 10.1002/hup.618. [DOI] [PubMed] [Google Scholar]
- 25.Liebregts T, Adam B, Bertel A, Lackner C, Neumann J, Talley NJ, Gerken G, Holtmann G. Psychological stress and the severity of post-inflammatory visceral hyperalgesia. Eur J Pain. 2007;11:216–222. doi: 10.1016/j.ejpain.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Mazor A, Matar MA, Kaplan Z, Kozlovsky N, Zohar J, Cohen H. Gender-related qualitative differences in baseline and post-stress anxiety responses are not reflected in the incidence of criterion-based PTSD-like behaviour patterns. World J Biol Psychiatry. 2009;10:856–869. doi: 10.1080/15622970701561383. [DOI] [PubMed] [Google Scholar]
- 27.McLean SA, Clauw DJ, Abelson JL, Liberzon I. The development of persistent pain and psychological morbidity after motor vehicle collision: integrating the potential role of stress response systems into a biopsychosocial model. Psychosom Med. 2005;67:783–790. doi: 10.1097/01.psy.0000181276.49204.bb. [DOI] [PubMed] [Google Scholar]
- 28.Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol Suppl. 2005;75:6–21. [PubMed] [Google Scholar]
- 29.Moldofsky H. The significance, assessment, and management of nonrestorative sleep in fibromyalgia syndrome. CNS Spectr. 2008;13:22–26. doi: 10.1017/s1092852900026808. [DOI] [PubMed] [Google Scholar]
- 30.Murase S, Terazawa E, Queme F, Ota H, Matsuda T, Hirate K, Kozaki Y, Katanosaka K, Taguchi T, Urai H, Mizumura K. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness) J Neurosci. 2010;30:3752–3761. doi: 10.1523/JNEUROSCI.3803-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsen KB, Westgaard RH, Stovner LJ, Helde G, Ro M, Sand TH. Pain induced by low-grade stress in patients with fibromyalgia and chronic shoulder/neck pain, relation to surface electromyography. Eur J Pain. 2006;10:615–627. doi: 10.1016/j.ejpain.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and post-traumatic stress disorder. J Rehabil Res Dev. 2003;40:397–405. doi: 10.1682/jrrd.2003.09.0397. [DOI] [PubMed] [Google Scholar]
- 33.Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 34.Plesh O, Wolfe F, Lane N. The relationship between fibromyalgia and temporomandibular disorders: prevalence and symptom severity. J Rheumatol. 1996;23:1948–1952. [PubMed] [Google Scholar]
- 35.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 37.Roy-Byrne P, Smith WR, Goldberg J, Afari N, Buchwald D. Post-traumatic stress disorder among patients with chronic pain and chronic fatigue. Psychol Med. 2004;34:363–368. doi: 10.1017/s0033291703008894. [DOI] [PubMed] [Google Scholar]
- 38.Schaeffer C, Habold C, Martin E, Lignot JH, Kedinger M, Foltzer-Jourdainne C. Cytokine expression in rat colon during postnatal development: regulation by glucocorticoids. J Pediatr Gastroenterol Nutr. 2006;43:439–450. doi: 10.1097/01.mpg.0000239989.27893.f1. [DOI] [PubMed] [Google Scholar]
- 39.Schur EA, Afari N, Furberg H, Olarte M, Goldberg J, Sullivan PF, Buchwald D. Feeling bad in more ways than one: comorbidity patterns of medically unexplained and psychiatric conditions. J Gen Intern Med. 2007;22:818–821. doi: 10.1007/s11606-007-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sperber AD, Atzmon Y, Neumann L, Weisberg I, Shalit Y, Abu-Shakrah M, Fich A, Buskila D. Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. Am J Gastroenterol. 1999;94:3541–3546. doi: 10.1111/j.1572-0241.1999.01643.x. [DOI] [PubMed] [Google Scholar]
- 41.Strausbaugh HJ, Green PG, Dallman MF, Levine JD. Repeated, non-habituating stress suppresses inflammatory plasma extravasation by a novel, sympathoadrenal dependent mechanism. Eur J Neurosci. 2003;17:805–812. doi: 10.1046/j.1460-9568.2003.02493.x. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi K, Taguchi T, Itoh K, Okada K, Kawakita K, Mizumura K. Influence of surface anesthesia on the pressure pain threshold measured with different-sized probes. Somatosens Mot Res. 2005;22:299–305. doi: 10.1080/08990220500420475. [DOI] [PubMed] [Google Scholar]
- 43.Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006;140:1311–1320. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Moser M, Schiltenwolf M, Buchner M. Circulating cytokine levels compared to pain in patients with fibromyalgia -- a prospective longitudinal study over 6 months. J Rheumatol. 2008;35:1366–1370. [PubMed] [Google Scholar]
- 45.Waylonis GW, Heck W. Fibromyalgia syndrome. New associations. Am J Phys Med Rehabil. 1992;71:343–348. doi: 10.1097/00002060-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Wilson HD, Starz TW, Robinson JP, Turk DC. Heterogeneity within the fibromyalgia population: theoretical implications of variable tender point severity ratings. J Rheumatol. 2009;36:2795–2801. doi: 10.3899/jrheum.090432. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe F. Fibromyalgia: the clinical syndrome. Rheum Dis Clin North Am. 1989;15:1–18. [PubMed] [Google Scholar]
- 48.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 49.Yang PC, Jury J, Soderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104–14. doi: 10.2353/ajpath.2006.050575. quiz 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yunus MB. Fibromyalgia Syndrome: -- Clinical Features and Spectrum. J Musculoskeletal Pain. 1994;2:5–21. [Google Scholar]
- 51.Zhou Q, Imbe H, Dubner R, Ren K. Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: implications for persistent orofacial pain. J Comp Neurol. 1999;412:276–291. doi: 10.1002/(sici)1096-9861(19990920)412:2<276::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]