Abstract
BACKGROUND AND PURPOSE
The two longest C-termini of the purinergic P2X receptors occur in the P2X2 and P2X7 receptors and are thought to interact with multiple cytoplasmic proteins, among which are members of the cytoskeleton, including microtubules. In this work we asked whether disrupting the microtubule cytoskeleton might affect the functions of these receptors.
EXPERIMENTAL APPROACH
Functions of heterologously expressed P2X2 and P2X7 receptors were evaluated with electrophysiology and dye uptake following ATP application. Permeabilization and secretion of pro-inflammatory agents were quantified from fresh or cultured peritoneal mouse macrophages, treated in vitro or in vivo with colchicine.
KEY RESULTS
Disrupting the microtubule network with colchicine did not affect currents generated by ATP in P2X2 and P2X7 receptor-expressing cells but inhibited uptake of the dye Yo-Pro-1 in Xenopus oocytes and HEK293 cells expressing these channels. Peritoneal mouse macrophages showed less ATP-induced permeabilization to ethidium bromide in the presence of colchicine, and less reactive oxygen species (ROS) formation, nitric oxide (NO) and interleukin (IL)-1β release. Colchicine treatment did not affect ATP-evoked currents in macrophages. Finally, in vivo assays with mice inoculated with lipopolysaccharide and ATP showed diminished ROS, IL-1β, interferon-γ and NO production after colchicine treatment.
CONCLUSIONS AND IMPLICATIONS
Colchicine has known anti-inflammatory actions and is used to treat several conditions involving innate immunity, including gout and familial Mediterranean fever. Here we propose a new mechanism of action – inhibition of pore formation induced by activation of P2X receptors – which could explain some of the anti-inflammatory effects of colchicine.
LINKED ARTICLE
This article is commented on by Pelegrín, pp. 908–911 of this issue. To view this commentary visit http://dx.doi.org/10.1111/j.1476-5381.2011.01325.x
Keywords: pore dilation, gout, microtubule, ATP, P2X7
Introduction
Purinergic receptors of the P2X family are ion channels gated by extracellular ATP that are present in many cell types and tissues. The family comprises seven members, namely P2X1-7 (nomenclature follows Alexander et al., 2009), and each receptor subunit is formed by two transmembrane domains, an extracellular loop and intracellular C- and N-termini (North, 2002). Some members of the family have been extensively studied in many aspects, including biophysical and functional features (Egan et al., 2006). For instance, P2X2 receptor involvement in pain pathways modulation is well known, as is the role of the P2X7 receptor in immune cells (North, 2002).
Upon activation of most P2X receptors, a current carried by Na+ and Ca2+ is generated, but the degree of current desensitization is highly variable. For instance, P2X2, 4 and 7 receptors are considered slow desensitizers, while for other receptors, the currents decrease when the agonist is still present (North, 2002). In addition, some members of the P2X family such as P2X2, 4 and 7 receptors, when activated by prolonged exposure to high concentrations of ATP, undergo the formation of a pore that allows passage of large cations, including fluorescent dyes such as ethidium bromide (EB; Egan et al., 2006). The functional consequences of this pore have been established for the P2X7 receptors in the immune system. Pore formation is necessary to trigger ATP-induced NALP3/NLRP3 inflammasome activation and consequently caspase-1 maturation, pro-interleukin (IL)-1β cleavage and IL-1β release by immune cells (Schroder and Tschopp, 2010). Therefore, the formation of P2X7 pores is necessary to the innate immune response induced by ATP, a danger-associated molecular pattern (DAMP) that signals tissue damage (Petrilli et al., 2007).
The two longest C-termini of P2X receptors are found in the P2X2 and P2X7 receptors and this part of their structure was thought to mediate interactions with other intracellular proteins. These proteins might affect the function of these channels or be effectors of P2X receptor activation. In fact, cytoskeletal proteins such as tubulin and actin were found to interact with P2X2 and P2X7 receptor C-termini respectively (Kim et al., 2001; Guimaraes, 2008). The functional consequence, if any, of the interaction between P2X2 or P2X7 receptors and the cytoskeleton on the receptors themselves, is not known.
In the present work, we sought to examine the role of the microtubule cystoskeleton in the function of P2X receptors by disrupting it. While doing so, we found out that colchicine, a drug used to treat gout and familial Mediterranean fever, is capable of inhibiting pore formation without affecting ATP-gated currents.
Methods
Oocyte electrophysiology
Defolliculated stage VI Xenopus laevis oocytes were injected with approximately 2.0 ng of rat P2X2, rat P2X3 or rat P2X7 receptor in vitro transcribed RNA obtained with Amplicap T7 (Epicentre Technologies, Madison, WI, USA). Oocytes were maintained in modified Barth's saline solution for 3–6 days before analysis. Two-electrode voltage-clamp analysis was performed at −60 mV holding potential using a Geneclamp 500 amplifier (Axon Instruments, Union City, CA, USA) and PowerLab A/D converter (ADInstruments, Colorado Springs, CO, USA). During recording oocytes were perfused at 1 mL·min−1 with ND-96 solution (composition, in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2 and 5 HEPES, pH 7.5) or nominally Ca2+ and Mg2+-free ND-96 solution (with 1 mM BaCl2), as indicated. Peak amplitudes of digitized current traces were measured with Chart software. Sigmoidal concentration-response curves were fitted with GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
Fresh and cultured macrophages
All animal care and experimental procedures followed the principles of good laboratory animal care and complied with the institutional ethical regulations. Animals were kept in microisolated cages under a constant 12 h/12 h light–dark cycle with free access to food and water. This study was carried out with adult male Swiss Webster mice weighing between 20 and 25 g. Animals were killed by exposure to CO2 and death was ensured by cervical dislocation.
Macrophages were obtained from a peritoneal cavity wash with 5 mL phosphate-buffered saline (PBS). Cell viability following peritoneal wash was above 95% in all cases, as measured by Trypan blue exclusion. Cells were transferred at 2 × 106 cells·mL−1 to Dulbecco's modified Eagle's medium (DMEM; from Gibco BRL, Paisley, Scotland) containing 5% heat-inactivated FCS, 2 g·L−1 sodium bicarbonate, 100 U·mL−1 penicillin and 100 µg·mL−1 streptomycin until use. Then cells were plated on 35 mm plastic Petri dishes (Corning, NY, USA) (cultured macrophages) or added to 1.5 mL microtubes (fresh macrophages) at a density of 5 × 105 cells per sample. In order to enrich for macrophages in the peritoneal lavage cell population, cells were allowed to adhere for 60 min at 37°C and 5% CO2, then washed three times with PBS to remove non-adherent cells. Then the macrophages were maintained until use at 37°C in a 5% CO2 humidified atmosphere. In the non-adherent cell preparation (fresh macrophages), other cell types were eliminated from the cytometric analyses, via exclusion gates based on granulosity and size.
HEK 293 transfection
HEK293T were maintained in DMEM (supplemented with 10% fetal bovine serum, penicillin, streptomycin and L-glutamine) and were transfected with rat P2X2, rat P2X7, human P2X4 and human P2X7 plasmid DNAs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in 35 mm Petri dishes. In the following day, cells were replated onto 96-well white plates and allowed to adhere for 6 h for Yo-Pro-1 uptake experiments. For ethidium bromide permeabilization, transfected cells were resuspended in a Ca2+/Mg2+-free solution and used as indicated next.
In vivo experiments
Mice were injected with 200 µL of lipopolysaccharide (LPS) (1 mg·kg−1) or PBS i.p., and all other subsequent administrations were by the same route. Then, 5 h later, mice were injected with 200 nmol colchicine (in 200 µL PBS) or with 200 µL PBS, followed 1 h by 2 µmol ATP (in 200-µL PBS). Four hours later, mice were killed and the peritoneal wash was performed as reported previously. The rectal temperature of mice was measured with an electronic thermometer immediately after injections and before death.
Dye uptake assays
Uptake of EB/sulforhodamine B
Cells were kept at 37°C for 5 min, in a solution containing (in mM): 145 NaCl, 5 KCl, 1 MgCl2 and 10 Na-HEPES, pH 7.4 (normal extracellular solution). Cells were treated with or without 50 µM colchicine and washed twice with extracellular solution. EB (10 µM final concentration) or sulforhodamine B (3 mM) and ATP were then added and cells were kept under the same conditions for an additional period of 10 min. Total plated cells were counted with phase contrast, and permeabilized cells were counted with fluorescence microscopy. Images were obtained with an Axiovert 100 microscope (Karl Zeiss, Oberkochen, Germany) equipped with an HBO lamp, a digital camera (Olympus American Inc., Center Valley, PA, USA) and Image-pro Plus v 6.2 software (Media Cybernetics, Bethesda, MD, USA). The excitation/emission wavelengths used for EB and sulforhodamine B were 535/602 and 520/640 nm respectively.
Uptake of Yo-Pro-1
P2X2 or P2X7 receptor-expressing oocytes were transferred to ND-96 solution without the addition of Ca2+ (nominally Ca2+-free) and incubated with the dye Yo-Pro-1 (5 µM for 10 min). Imaging was performed using a Nikon Diaphot fluorescence microscope equipped with 4× objective (Melville, NY, USA) and an intensified CCD camera (Hamamatsu, Japan). Images were collected using excitation at 485 nm and emission at 538 nm and pseudo-colour images were obtained every 5 s (240 s total) during the experiment (Metafluor software, Molecular Devices Corp., Sunnyvale, CA, USA). Cells were initially imaged for basal fluorescence and stimulated with 100 µM or 1 mM ATP for P2X2- and P2X7-expressing oocytes respectively. Fluorescence was quantified using NIH Image J.
Transfected HEK293 cells in 96-well plates were washed using a saline solution without the addition of calcium (in mM: 130 NaCl, 3 KCl, 0.6 MgCl2, 1.2 NaHCO3, 10 glucose, 10 HEPES, pH 7.45) and incubated in the same solution supplemented with 5 µM Yo-Pro-1 for 15 min. Using a fluorescent plate reader (SpectraMax Gemini XS, Molecular Devices) (excitation: 485 nm; emission: 538 nm), the basal fluorescence was determined (endpoint). After adding ATP with a multipipetter, a kinetic measurement was run for 30 min, with 2 min intervals.
Sulforhodamine B fluorescence microscopy
Macrophages were plated on glass coverslips, then dye uptake was measured as described previously. Subsequently, cells were exposed to 4′,6-diamidino-2-phenylindole (DAPI) to stain nuclei in blue for 10 min and then observed under the microscope. The microscopic imaging of sulforhodamine uptake by macrophages was carried out with an Axiovert 200 M microscope equipped with an Apotome slide and fluorescence optics (Zeiss, GmbH, Germany).
Reactive oxygen species (ROS) measurement
The accumulation of dichlorofluorescein (DCF) inside cells was measured by an increase in fluorescence at 530 nm when the sample was excited at 485 nm. Peritoneal macrophages in normal extracellular solution were incubated or not with 50 µM colchicine and dichlorodihydrofluorescein diacetate (DCFH2DA; 20 µg·mL−1) for 30 min at 37°C, 5% CO2 in a humidified incubator, to allow loading of the dye. Afterwards, cells were exposed to 5 mM ATP, 50 nM phorbol 12-myristate 13-acetate (PMA) or 500 µM hydrogen peroxide for 10 min at 37°C and the conversion of non-fluorescent DCFH2DA into the highly fluorescent DCF was measured using flow cytometry (FACScan, Becton Dickinson, Rutherford, NJ, USA).
Measurement of IL1-β and interferon (IFN)-γ
Murine peritoneal macrophages were plated at a density of 3 × 106 cells per well in 24-well tissue culture plates the day before each experiment. Then cells were incubated with or without 100 ng·mL−1 LPS for an additional 24 h, followed by treatment with the caspase inhibitor, Z-YVAD-FMK (1:1000) for 3 h. Cells were then treated with 50 µM colchicine for 1 h, followed by a 60 min exposure to 3 mM ATP. Finally, the supernatants were collected 6 h after the last treatment and stored at −20°C until use.
The supernatants obtained with the peritoneal wash of the treated animals were collected and stored at −20°C until use. The concentrations of IL1-β and IFN-γ in culture medium or peritoneal wash were determined with mouse IL1-β or IFN-γelisa kits according to the manufacturer's instructions.
Assay for nitric oxide (NO)
The supernatants obtained from cell culture or peritoneal wash of the treated animals were collected and stored at −20°C until use. Griess reagent (100 µL) (a 1:1 mix of 0.1% N-1-naphthylethylenediamine- HCl and 1.0% sulfanilamide in 60% acetic acid) was added to 100 µL of the sample, and incubated for 10 min in the dark, as described previously (Denlinger et al., 1996). Absorbance was measured at 550 nm with a microplate reader (Molecular Devices). Nitrite concentrations were determined using a standard curve generated with sodium nitrite.
Cell death assays
Lactate dehydrogenase assay
To measure cell death of peritoneal macrophages induced by ATP, cells were treated with or without colchicine for 1 h followed by 5 mM ATP for another hour in cell culture medium at 37°C and 5% CO2. The supernatant was collected and the activity of the enzyme lactate dehydrogenase (LDH) was measured with a colorimetric assay kit in a microplate reader in 96-well plates at 510 nm. Results were expressed as percentage of maximal lysis obtained with 0.1% Triton X-100 (100% cell death).
Trypan blue exclusion
Cells collected as described from animals after different treatments (see ‘in vivo experiments’) were centrifuged and then resuspended in 1 mL of DMEM medium. This was mixed 1:1 with a 0.4% Trypan blue solution. After 1–2 min, stained and unstained cells were counted in a haemocytometer, and the percentage of non-viable cells was determined.
Macrophage electrophysiology
Whole-cell currents activated by pulses of 1 mM Na-ATP were recorded using experimental apparatus and recording solutions as previously described (Castro et al., 1999). Petri dishes containing adherent macrophages were washed with external recording solution and mounted in a chamber that allowed linear continuous perfusion (0.5 mL·min−1) and fast, focal microperfusion of the cells through a motorized capillary tube array. Membrane potential was clamped at −70 mV and recordings were made at room temperature (23–24°C). Colchicine was added to the recording solution, alone or with Na-ATP. Currents were low-pass filtered at 1 kHz, digitized, then digitally filtered at 100 Hz for display and analysis with pCLAMP software (Axon Instruments).
Statistical analyses
Averaged data are reported as mean ± SEM and significance of the differences were determined with appropriate tests performed with Prism software, as detailed in the figure legends. Differences were considered significant when P < 0.05.
Materials
DMEM, fetal bovine serum, penicillin and streptomycin were obtained from Gibco/BRL (São Paulo, SP, Brazil). ATP, DAPI, colchicine, taxol, EB, sulforhodamine B and PMA were purchased from Sigma-Aldrich (St Louis, MO, USA). Lipofectamine 2000 was from Invitrogen. LPS, vincristine and Yo-Pro-1 were purchased from Molecular Probes (Eugene, OR, USA) and 2,7-dichlorodihydrofluorescein diacetate (DCFH2DA) was from Calbiochem (La Jolla, CA, USA). IL-1β and IFN-γ kits were from Peprotech (Rocky Hill, NJ, USA) and LDH kit was from Doles (Goiás, Brazil). Caspase-1 inhibitor Z-YVAD-FMK was purchased from BioVision, Inc. (Mountain View, CA, USA).
Rat P2X2 and P2X3 cDNAs were obtained from Dr David Julius' laboratory and those for rat P2X7, human P2X4 and human P2X7 receptors were from Dr Alan North.
Results
Microtubule disruption does not affect currents carried by heterologously expressed P2X2 and P2X7 receptors
The P2X2 receptor was found to physically interact with tubulin (Guimaraes, 2008) and there are reports that P2X7 receptors also bind to cytoskeletal elements including β-tubulin (Kim et al., 2001; Pfeiffer et al., 2004; Gu et al., 2009). As an initial attempt to investigate the physiological relevance of the P2X receptor interaction with tubulin, we performed electrophysiological experiments with Xenopus oocytes expressing P2X2 or P2X7 receptors, after treatment with or without colchicine for 1 h. Figure 1A shows representative recordings with P2X2 receptors where no difference in amplitude or kinetics of currents elicited by ATP was observed between colchicine-treated and -untreated oocytes. In addition, the ability of P2X2 receptors to form heteromultimers with P2X3 receptors was not changed as well, as oocytes expressing these two receptors presented the expected currents elicited by ATP and αβ-methylATP (not shown). P2X7 receptor-expressing oocytes also did not show differences in current amplitude following colchicine treatment (Figure 1B). In Figure 1C, the average maximum current amplitude after ATP application is shown, comparing control and colchicine-treated oocytes. No statistically significant difference was observed between treatments for P2X2 and P2X7 receptor-expressing oocytes (n = 8 and n = 6 respectively). Furthermore, colchicine did not affect the concentration-response curve of P2X7 receptor-expressing oocytes to ATP compared with control-untreated oocytes, with EC50 values of 0.30 and 0.29 mM respectively (Figure 1D, n = 3–8 oocytes per concentration/group).
Figure 1.
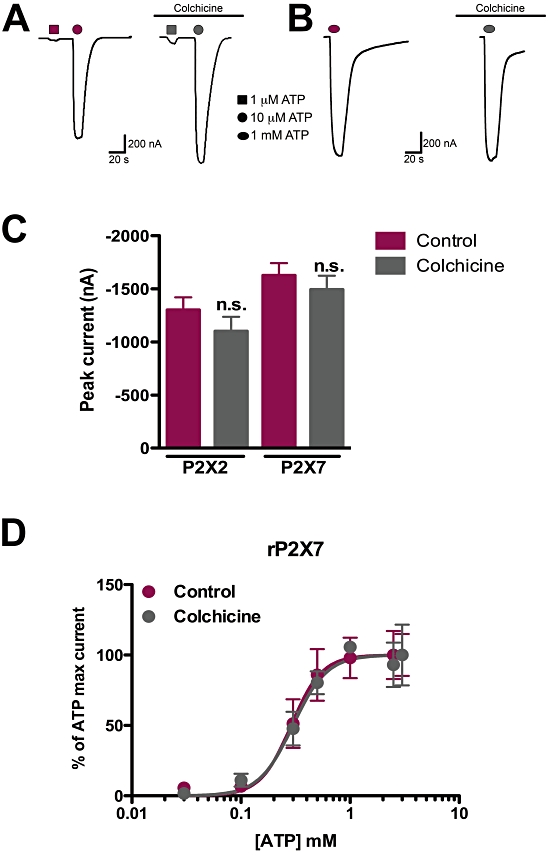
Colchicine does not affect currents evoked by ATP in P2X2 and P2X7 receptors in Xenopus oocytes. Oocytes were injected with cRNAs encoding rat P2X2 and rat P2X7 receptors and subjected to electrophysiological recordings following 1 h 50 µM colchicine or ND-96 solution (control) treatment. (A) Representative recordings of P2X2 receptor-expressing oocytes in control (left) and following colchicine treatment (right). (B) Typical traces of P2X7 receptor-expressing oocytes, following incubation with colchicine (right) or not (left). (C) Quantification of ATP-evoked current amplitudes in P2X2 and P2X7 receptor-expressing oocytes, with or without colchicine. The differences were not statistically significant (n.s.) as tested by one-way anova followed by Bonferroni's post hoc test (P = 0.29, n = 8 and P = 0.46, n = 6 for P2X2 and P2X7 receptors respectively). (D) Concentration-response curves of ATP-evoked currents in oocytes expressing P2X7 receptors, after treatment with colchicine or with control solution. Estimated EC50s were 0.30 mM (control) and 0.29 mM (colchicine); n = 3–8 oocytes per concentration/group.
Colchicine caused diminished Yo-Pro-1 uptake in P2X2 and P2X7 receptor-expressing oocytes
In addition to inward currents gated by ATP, cells expressing certain P2X receptors, including P2X2 and P2X7, also go through a phenomenon called pore dilation or permeabilization, in which sustained activation by high concentrations of ATP allows large molecules to enter the cell (Khakh et al., 1999; Virginio et al., 1999; Egan et al., 2006). Thus, oocytes expressing P2X2 receptors were treated with or without 50 µM colchicine for 1 h and evaluated for their capacity to take up the dye Yo-Pro-1 in the presence of 100 µM ATP. Untreated P2X2 receptor-expressing oocytes were able to show a substantive increase in Yo-Pro-1 uptake after treatment with ATP, whereas colchicine pre-incubation seemed to prevent this increment in ATP-induced uptake (Figure 2A). The permeabilization phenomenon is better known and characterized for the P2X7 receptor, and its functional physiological importance is known (da Cruz et al., 2006; Pelegrin and Surprenant, 2006; Schachter et al., 2008). Therefore we asked whether Yo-Pro-1 uptake in oocytes expressing rat P2X7 receptors was also inhibited by colchicine, and indeed this was the case (Figure 2B). These results are quantified in Figure 2C, which shows that colchicine treatment reduced ATP-evoked induction of fluorescence in P2X2 and P2X7 receptor-expressing oocytes by approximately 73 and 89%, respectively.
Figure 2.
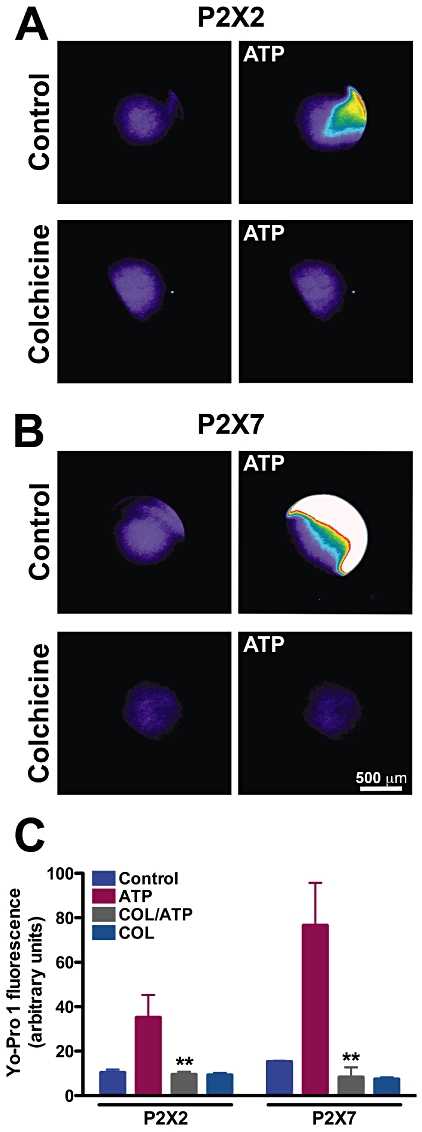
Colchicine inhibits Yo-Pro-1 uptake elicited by ATP in P2X2 and P2X7 receptor-expressing oocytes. Oocytes were treated with 50 µM colchicine for 1 h prior to Yo-Pro-1 exposure and the presence of ATP. (A) Pseudo-colour photographs of oocytes expressing P2X2 (A) or P2X7 (B) before and after 100 µM or 1 mM ATP respectively. Lower micrographs are of colchicine-treated oocytes in panels A and B. Scale bar is 500 µm. (C) Quantification of Yo-Pro-1 uptake in P2X2 and P2X7 receptor-expressing oocytes. **P < 0.01, one-way anova followed by Bonferroni's post hoc test; n = 8 (P2X2) and n = 3 (P2X7).
Kinetics of colchicine inhibition of ATP-induced Yo-Pro-1 uptake in P2X2 receptor-transfected HEK-293 cells
After determining that colchicine interfered with ATP-induced permeabilization of oocytes expressing either P2X2 or P2X7 receptors, we investigated whether mammalian cells transfected with P2X2 receptors would also show diminished dye uptake after ATP. In addition, we wanted to know if there was a delay in the uptake or simply a decrease. HEK293 cells were transfected with P2X2 receptors and plated on 96-well plates, treated with or without colchicine for 1 h and stimulated with ATP in the presence of Yo-Pro-1. Figure 3A shows that colchicine did prevent dye entry from the beginning of the stimulation and maintained the decrease in uptake, for up to 30 min following 300 µM ATP.
Figure 3.

Colchicine effects on the permeabilization of HEK293 cells transfected with rat P2X2, rat P2X7, human P2X4 and human P2X7 receptors. (A) Time course of Yo-Pro-1 uptake following ATP administration (300 µM) in P2X2 receptor-transfected HEK293 cells. Cells were previously treated with or without 50 µM colchicine for 1 h and then used in a plate-reader assay. P < 0.001 when comparing ATP plus colchicine to ATP alone, two-way anova followed by Bonferroni's post hoc test, n = 3 experiments each in triplicate. (B) Permeabilization to 10 µM ethidium bromide (EB) after 5 mM ATP in cells transfected with different P2X receptor subunits, as indicated. The percentage of cells taking up EB was measured with FACS. *P < 0.05 and ***P < 0.001 when comparing ATP plus colchicine to ATP alone, two-way anova followed by Bonferroni's post hoc test, n = 3 experiments each in triplicate.
Colchicine also inhibits ATP-induced permeabilization in HEK-293 cells transfected with other P2X receptor species and subtypes
Next we asked whether the effects of colchicine on cationic dye uptake were exclusive to rat P2X2 and rat P2X7 receptors, or if other species and/or subtypes would also have diminished pore formation in the presence of this microtubule disruptor. Due to several factors, such as the use of high concentrations of ATP for P2X7 receptors, over-expression of receptors, addition of colchicine (which rounds-up cells) and low Ca2+ and Mg2+, plated transfected cells were not staying attached after these stimuli. Therefore, we used an alternative permeabilization assay, with suspended cells analysed by flow cytometry, which is widely used to investigate pore formation in macrophages, and it was also used in the following experiments.
Therefore, rat P2X2, rat P2X7, human P2X7 and human P2X4 receptor-transfected cells were resuspended and assayed for permeabilization to EB in the presence of corresponding concentrations of ATP. All clones used showed significant increases in permeabilization after ATP. In addition, ATP-induced permeabilization was decreased by colchicine pre-treatment in all cases, except for human P2X4 receptors, possibly due to a modest ATP response in the first place, as noticed by others (Virginio et al., 1999) (Figure 3B).
Colchicine inhibits ATP-induced cationic dye uptake in macrophages
The said results suggested that colchicine was able to interfere with permeabilization of cationic dyes in cells expressing P2X2 or P2X7 receptors. We then asked whether this would happen in cells natively expressing P2X receptors and or if the inhibition observed was perhaps due to an over-expression artefact. Therefore, we performed permeabilization experiments using freshly obtained or 24 h-cultured macrophages. About 60% of freshly harvested resident macrophages took up EB after treatment with 5 mM ATP; however, in colchicine-pretreated cultures, only approximately 26% incorporated EB, a 58.5% reduction (Figure 4 B,C,G). P2X receptor expression can be down-regulated after adhesion (Sim et al., 2007 and C. Marques-da-Silva et al., unpubl. obs.), and because colchicine is known to affect adhesion and migration of immune cells (Nuki, 2008), we performed the same assay in 24 h-plated macrophages. Again colchicine caused a 39.3% reduction in the percentage of cells taking up EB after ATP (Figure 4G). Another cationic dye, sulforhodamine-B, was also used to test whether the DNA-binding property of Yo-Pro-1 and EB was important for colchicine action. In panel 4F, it is noticeable that fewer cells became fluorescent after colchicine followed by ATP, when compared with ATP alone (Figure 4E). Increasing concentrations of ATP overcame only partially the ability of 50 µM colchicine to inhibit permeabilization (Figure 4H); in other words, colchicine decreased the ATP efficacy in permeabilizing fresh macrophages. The effect of colchicine on the permeabilization induced by ATP in freshly isolated macrophages was steeply concentration dependent, with a tendency to inhibit at 25 µM (but not statistically significant) and maximal inhibition at 50 µM (Figure 4I). Higher concentrations (500 µM) began to cause toxicity, as demonstrated by an increase in permeability beyond the ATP effect. Finally, we asked whether this effect of colchicine was specific or shared by other microtubule-disrupting/stabilizing agents, such as vincristine and taxol. Vinscristine was unable to inhibit permeabilization (Figure 4J) at a concentration known to completely depolymerize macrophage microtubules (Li et al., 1996). Taxol, on the other hand, increased permeabilization by itself (without ATP), possibly due to toxicity. But more importantly, taxol did not prevent a further increase in permeabilization when co-administered with ATP, suggesting that it did not affect P2X7 receptor-induced dye uptake (Figure 4J).
Figure 4.
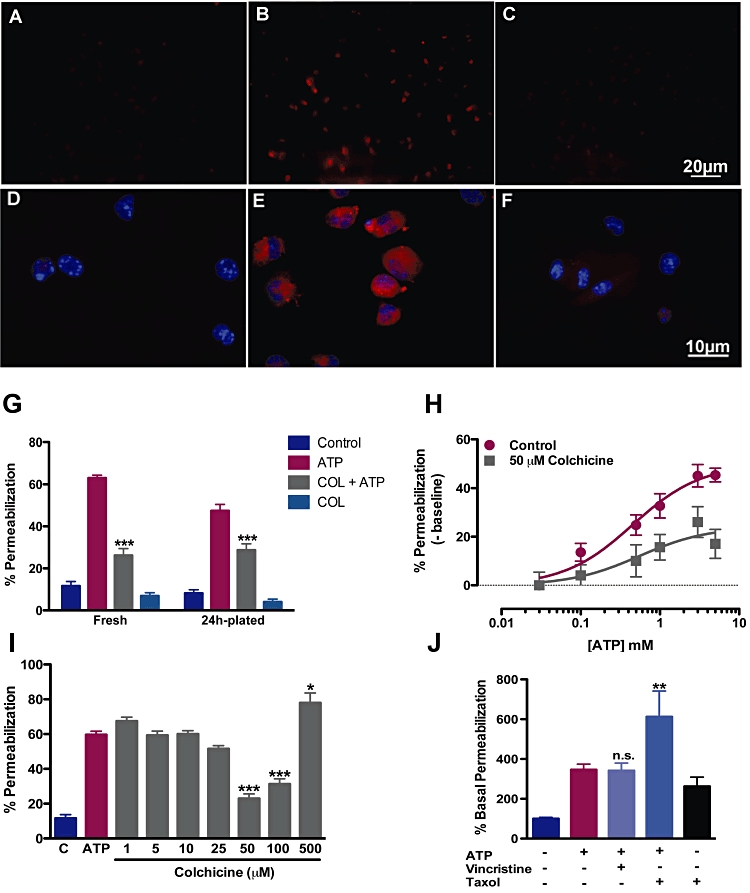
Colchicine inhibits cationic dye uptake in fresh and plated macrophages. Fresh or plated macrophages obtained by peritoneal wash were exposed or not exposed to 50 µM colchicine for 30 min. Then cells were treated with 5 mM ATP and 10 µM ethidium bromide (EB) or 3 mM sulphorhodamine for additional 15 min at same conditions. Cells were then washed and counted by optical microscopy (G and H), images acquired (A–F) or quantified by flow cytometry (I and J). (A–C) Micrographs of ATP-induced permeabilization to EB (A: control; B: ATP; C: colchicine pretreatment and then ATP). Micrographs of ATP-induced sulforhodamine-B permeabilization (D: control; E: ATP; F: colchicine pretreatment and then ATP). (G) Quantification of EB uptake induced by ATP in freshly isolated and 24 h plated macrophages (P < 0.001, two-way anova followed by Bonferroni's post hoc test, n = 5 experiments each in triplicate). (H) Concentration-response curves of ATP-induced permeabilization following colchicine or control incubation. Estimated EC50s were 0.45 and 0.54 mM for control and colchicine-treated macrophages (n = 4 experiments each in triplicate). (I) Quantification of permeabilization to EB induced by ATP in the presence of different colchicine concentrations in freshly isolated macrophages (*P < 0.05 and ***P < 0.001, relative to ATP only, one-way anova followed by Bonferroni'spost hoc test, n = 3 experiments ieach in triplicate). (J) Quantification of permeabilization to EB induced by ATP in the presence of vincristine or taxol in freshly isolated macrophages (**P < 0.01, one-way anova followed by Bonferroni's post hoc test, n = 3 experiments each in triplicate).
Colchicine inhibits formation of ROS induced by ATP
As mentioned previously, the functional consequences of the opening of the P2X7 pore are beginning to be understood, in contrast to the other pore-forming P2X receptors. One of the implications of this phenomenon in macrophages is the generation of ROS triggered by ATP (Pfeiffer et al., 2007), which precedes inflammasome activation (see Schroder and Tschopp, 2010). The importance of ROS generation to the activation of the inflammasome can be highlighted by findings that ROS scavengers prevent ATP-induced IL-1β release (Cruz et al., 2007). Therefore, to probe a cellular functional consequence of the permeabilization blockade, we examined ROS formation in fresh macrophages treated with or without colchicine. In Figure 5A, basal and ATP-induced ROS formation were quantified by FACS and compared with the induction of ROS by PMA. Cells that had been previously treated with colchicine produced approximately 55% less ROS with ATP treatment, quantified in Figure 5B. This ability to inhibit ROS generation seemed to be specific for the ATP stimulus as the reactive species formation induced by a different agent, PMA, was not inhibited by colchicine (Figure 5A). Colchicine used alone did not affect the basal levels of ROS (Figure 5B).
Figure 5.
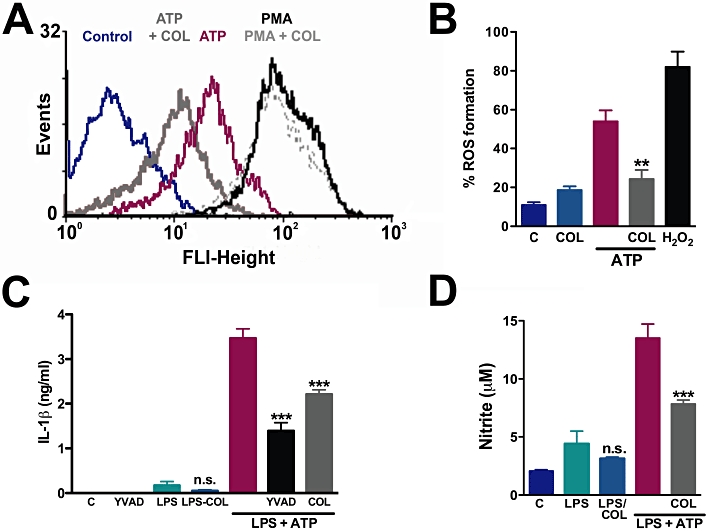
ROS, NO and IL-1β production induced by ATP are inhibited by colchicine. Macrophages obtained by peritoneal wash were pretreated with or without 50 µM colchicine for 30 min, followed by incubation with H2CFDA for additional 30 min. ATP (5 mM) was applied and 5–10 min later cells were analysed by FACScan. As a positive control, we exposed cells to 50 nM PMA (A) or 500 µM H2O2 (B) in substitution to ATP (**P < 0.01, relative to ATP only, one-way anova followed by Bonferroni's post hoc test, n = 2 experiments each in triplicate). (C and D) Peritoneal macrophages were plated for 24 h and stimulated with LPS for additional 24 h, then treated with 50 µM colchicine or Z-YVAD (1:1000) for 30 min, then exposed for 60 min to 5 mM ATP. The supernatants were collected to measure IL-1β (C) and NO (D). The concentrations of IL-1β and NO were determined in ng·mL−1 and µM, respectively, for each experimental group. ***P < 0.001, relative to LPS plus ATP, one-way anova followed by Bonferroni's post hoc test, n = 3 experiments each in triplicate. Differences between LPS only and LPS with colchicine were not significant (P = 0.26 and P = 0.36 for IL-1β and NO, respectively). NO, nitric oxide; LPS, lipopolysaccharide; PMA, phorbol 12-myristate 13-acetate; ROS, reactive oxygen species.
Colchicine inhibits release of pro-inflammatory agents in vitro
P2X7 receptors activated by ATP in macrophages, in conjunction with LPS signalling, induce the production of NO (Sperlagh et al., 1998) and other factors (Tonetti et al., 1995). It is already reported that ATP at millimolar concentrations is capable of inducing the release of mature IL-1β by macrophages (Pelegrin and Surprenant, 2006) and microglia (Takenouchi et al., 2008) pre-exposed to LPS, a phenomenon that is controlled by inflammasomes and caspase-1 activation (Martinon et al., 2002). When colchicine was co-administered with LPS plus ATP to macrophages (Figure 5C), there was an inhibition of 34% in the release of IL-1βin vitro. This IL-1β release was caspase-1 dependent, because after incubation with Z-YVAD, a caspase-1 inhibitor, IL-1β release was inhibited by 70% (Figure 5C). In addition, colchicine did not significantly reduce LPS-induced IL-1β release in vitro (Figure 5C).
P2X7 receptor activation is known to further increase NO production caused by LPS (Tonetti et al., 1995; Denlinger et al., 1996) and we asked whether colchicine affected ATP and LPS-induced NO release. We observed that when treated in vitro with colchicine, macrophages produced less NO (43%) induced by LPS and ATP, as shown in Figure 5D. However, as demonstrated for IL-1β, colchicine's effect was specific for the ATP-induction of NO release, as it did not inhibit LPS-evoked NO production (Figure 5D).
Time course of the effects of colchicine on macrophages: electrophysiology and permeabilization
Colchicine can affect other ligand-gated ion channels acutely, such as the GABAA and 5-HT3 receptors, either negatively or positively (Weiner et al., 1998; de Oliveira-Pierce et al., 2009). All the previous experiments were performed under the assumption that the microtubule depolymerizing property of colchicine was important for its effect on permeabilization, and therefore, a 1 h pre-incubation with this agent was justifiable. Indeed, a time-course analysis of colchicine treatment revealed that 15 min of preincubation before ATP was added sufficed to inhibit permeabilization by approximately 27%, whereas 5 min of pre-incubation or concomitant addition of colchicine and ATP (t = 0 pre-incubation) did not affect EB uptake (Figure 6A). Following 30 min of pre-incubation, colchicine reached its maximum effect on permeabilization (inhibition of about 50%) and this was essentially the same after 1 h pretreatment.
Figure 6.
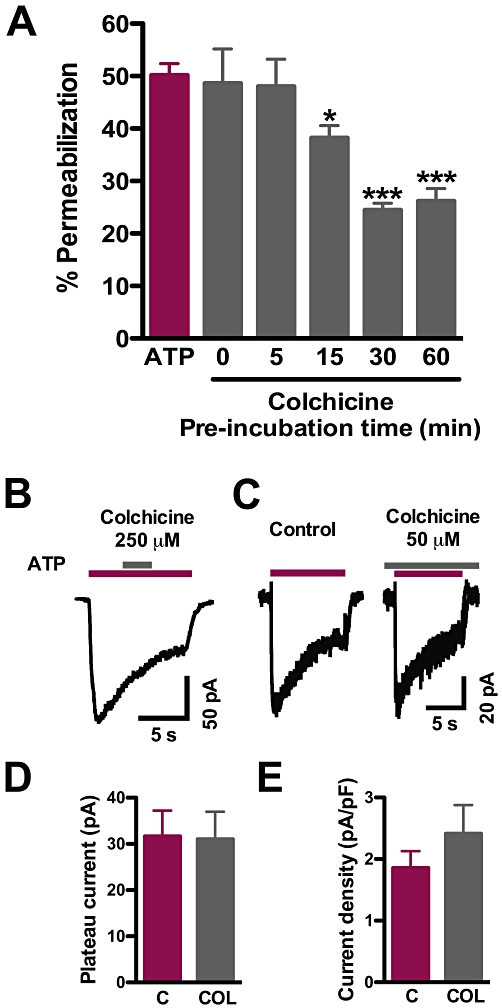
Time course of colchicine action and lack of acute and long-term modulatory effect on ATP-gated currents in macrophages. (A) Time course of colchicine inhibition of ATP-evoked permeabilization. Macrophages were incubated with 50 µM colchicine for the indicated times and then used in permeabilization assays (*P < 0.05 and ***P < 0.001, one-way anova followed by Bonferroni's post hoc test, n = 3 experiments each in triplicate for each time). (B) Whole-cell patch-clamp recording of an acute application of 250 µM colchicine during a 1 mM ATP pulse. (C) Representative recordings of ATP-evoked currents in control (left) and 50 µM colchicine-treated (right) cultured macrophages. (D) Quantification of plateau currents from cells in C (P = 0.47) and of current density (E) (P = 0.35, Student's t-test, n = 8–9 cells).
Next, although colchicine did not affect P2X receptor currents in oocytes, we wanted to verify whether its effects on permeabilization were associated with direct blockade of native P2X receptors in macrophages, as was shown for other receptors. For that purpose, we measured ATP-gated currents in resident macrophages under whole-cell patch-clamp at room temperature, to avoid activation of the high-conductance pore (Coutinho-Silva and Persechini, 1997). First, colchicine was transiently applied at 50–250 µM during the 1 mM ATP pulse, using a fast perfusion system. The brief pulses of colchicine did not affect the time course of the currents (n = 4), showing a lack of acute modulatory effect (Figure 6B). In another experiment, cells were pretreated with 50 µM colchicine for 30 min in the incubator and then transferred to the recording set-up where they were continuously perfused with a solution containing 50 µM colchicine during the recording of ATP responses. Currents activated by 1 mM ATP in macrophages exposed to colchicine for 40–160 min were similar in shape and magnitude to untreated controls from the same culture batch (Figure 6C). The peak amplitudes in treated and control cells were 73 ± 15 and 89 ± 16 pA respectively (P = 0.47, n = 8–9). After 9 s, the currents decayed to about 40% of the peak and tended to stabilize. This response pattern is probably due to a combination of slowly desensitizing P2X4 receptors and non-desensitizing P2X7 receptors (Brone et al., 2007; Sim et al., 2007). The plateau currents were not different in the treated cells (Figure 6D). Because colchicine led to considerable shrinking and rounding of the cells, we also estimated the density of the plateau current, dividing it by the whole-cell capacitance (which reflects membrane area). In fact, the current density was slightly larger in the colchicine-treated cells, albeit not significantly (P = 0.35, Figure 6E). These data show that colchicine did not inhibit the ATP-induced gating of the intrinsic channel of slow-desensitizing P2X receptors in mouse peritoneal macrophages, although it inhibited permeabilization.
Colchicine hinders pro-inflammatory pathways in vivo
In vitro, colchicine was able to block ROS formation and permeabilization induced by ATP in fresh macrophages. However, other cell types, particularly neutrophils, and signalling molecules are involved in inflammatory processes in vivo, where colchicine is known to exert anti-inflammatory actions (Chia et al., 2008; Nuki, 2008). Therefore, we primed mice for macrophage activation via inoculation with or without LPS and then treated with or without ATP (i.p.) (see scheme in Figure 7A). Macrophages obtained from the peritoneal wash of animals that received LPS and ATP presented an increased production of ROS, IL-1β, NO and IFN-γ, as described earlier (Sperlagh et al., 1998; Pfeiffer et al., 2007; Pelegrin et al., 2008; Raices et al., 2008) (Figure 7B). However, when colchicine (200 nmol) was given to the animals 1 h before ATP injection, the production of all four pro-inflammatory mediators, measured at 12 h, was clearly diminished (Figure 7B). Levels of ROS in vivo were decreased by about 45% and the same profile was found for IL-1β production, being reduced by approximately 60%. NO release was also inhibited by 60% and IFN-γ release was markedly decreased by 90% after colchicine treatment.
Figure 7.

In vivo treatment with colchicine alters cytokine and other pro-inflammatory agents in mice. (A) Diagram depicting the experimental groups and respective treatments in B and C. At time point I, mice were inoculated with LPS or PBS (i.p.), then 5 h later inoculated with colchicine or PBS (II), following inoculation with ATP or PBS after 1 h (III), and measurements were made at time IV. (B) Cells obtained by peritoneal wash were incubated with DCFH2DA for 30 min, and ROS production was analyzed by FACScan, collecting 10 000 events. The supernatants obtained by peritoneal wash were assayed with Griess reagent or cytokine kits. The concentrations of ROS, cytokines and nitrite were normalized to the production induced by LPS + ATP (100%). Representative measurements of IL-1 [in pg·mL−1 were 860 ± 15 (control), 1460 ± 24 (LPS + ATP), 721 ± 21 (LPS + COL + ATP), 933 ± 40 (LPS only) and 708 ± 22 (LPS + COL)]. Typical nitrite levels were (in µM; mean ± standard deviation): 0.49 ± 0.18 (control), 3.62 ± 0.86, (LPS + ATP), 1.45 ± 0.43 (LPS + COL + ATP), 0.94 ± 0.18 (LPS only) and 0.52 ± 0.14 (LPS + COL). Representative measurements of IFN- (in pg·mL−1) were: 81 ± 5.2 (control), 1077.1 ± 34.3 (LPS + ATP), 156.8 ± 31.2 (LPS + COL + ATP), 108.3 ± 11 (LPS only) and 95.5 ± 22.4 (LPS + COL).***P < 0.001, one-way anova followed by Bonferroni's post hoc test, at least three experiments with two animals were performed for each experimental group. The difference between LPS only and LPS + colchicine was non-significant (n.s.) in all measurements (ROS: P = 0.155; IL-1β: P = 0.160; nitrite: P = 0.33; and IFNγ: P = 0.51). (C) Time course of variation in body temperature (Δ) of mice, relative to values before treatments. Temperature was measured soon after each treatment with a rectal thermometer. Arrows indicate when each agent was administered. Some error bars are shown only in one direction for clarity. ***P < 0.001 two-way anova followed by Bonferroni's post hoc test, n = 3 animals in each group. DCFH2DA, dichlorodihydrofluorescein diacetate; LPS, lipopolysaccharide; ROS, reactive oxygen.
As noticed by others (Gourine et al., 2002; 2005), the administration of LPS and ATP to mice induced a rise of almost 6°C in their body temperature when compared with PBS-treated mice, and this raised temperature was maintained for up to 12 h (Figure 7C). Although this temperature rise still occurred after colchicine treatment, it was not maintained beyond 6 h and returned to normal levels by 12 h (Figure 7C).
Colchicine treatments in vitro and in vivo did not significantly affect cell death
Extracellular ATP is known to cause macrophage cell death via P2X7 receptors (Lammas et al., 1997), in a process recently described as pyroptosis (Brough and Rothwell, 2007). Therefore, we wanted to know if, in our experimental conditions, colchicine was influencing cell death in vitro and in vivo. LDH release was measured from cultured macrophages following the same conditions used in permeabilization assays. Under these conditions, namely 5 mM ATP treatment of resident macrophages for 15 min, no significant increment in LDH release was observed compared with controls. Extended ATP treatments for up to 1 h also did not significantly increase LDH release (Figure 8A). When colchicine was co-applied, ATP-induced LDH release was not significantly changed. However, ATP plus colchicine did increase LDH release, compared with control cultures.
Figure 8.
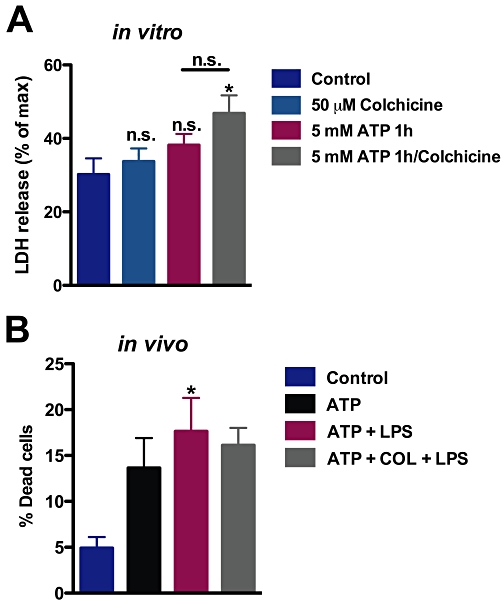
Cell death was not significantly affected by colchicine. (A) Following treatment in vitro, with 5 mM ATP for 1 h, LDH release was measured, relative to maximum release induced by Triton. In the colchicine group, cells were incubated with this agent for 1 h before addition of ATP. *P < 0.05, significantly different from control levels, one-way anova followed by Bonferroni's post hoc test, n = 3 experiments each in triplicate. (B) Animals were treated as in Figure 7; cells were obtained by peritoneal wash and used in Trypan blue exclusion assays. *P < 0.05, significantly different from control levels, one-way anova followed by Bonferroni's post hoc test, n = 2 experiments each in triplicate.
In vivo, after injecting animals with LPS and ATP and obtaining peritoneal macrophages, these treatments caused a significant increase in non-viable cells over control levels as measured by Trypan Blue exclusion (Figure 8B). However, when animals were also injected with colchicine, there was no difference between the proportion of non-viable cells in this group and that in cells treated with LPS with ATP (Figure 8B). Altogether, these data show that colchicine did not significantly affect cell death in the experimental conditions of the present study.
Discussion
P2X receptors and the cytoskeleton
Purinergic receptors have long been associated with the cytoskeleton matrix. Among the intracellular proteins known to interact with P2X receptors are several members of the cytoskeleton. P2X2 and P2X7 receptors have been shown to bind β-tubulin (Gendreau et al., 2003; Guimaraes, 2008; Gu et al., 2009) and P2X7 receptors are able to form a protein complex that includes actin filaments (Kim et al., 2001). Functionally not much is known about the outcomes of molecular interactions between cytoskeletal proteins and P2X receptors. In some cases, such as the one between P2X1 receptors and actin filaments, modifying these proteins with cytochalasin D prevents changes in channel kinetics in culture (Parker, 1998). In other cases, such as with P2X7 receptors and actin, there is extensive literature showing that channel activation causes actin filaments mobilization and substantial changes in the cytoskeleton and therefore in the shape and motility of cells. Through actin reorganization, P2X7 receptors induce bleb formation via MAPK p38 and Rho activation (Pfeiffer et al., 2004). In an attempt to find a functional significance for the binding of P2X2 receptors to β-tubulin, we disrupted the microtubule cytoskeleton with colchicine and found that one aspect of P2X2 receptor function, permeabilization to cationic dyes, was affected. Because the function of this pore is well known for the P2X7 receptor, we also investigated colchicine's effect in this phenomenon, to find that it inhibited permeabilization to dyes induced by ATP via P2X7 receptors. This effect was specific for colchicine, as vincristine or taxol did not affect permeabilization at concentrations known to interfere with the microtubule cytoskeleton. Recently, others have found that colchicine inhibits high conductance pore formation triggered by ATP in macrophages, thereby confirming our findings (Faria et al., 2010). Other authors have observed that cytochalasin D does not affect Yo-Pro-1 uptake (Pfeiffer et al., 2004). Together with our results, these data suggest that microtubules and not actin filaments are able to influence permeabilization, indicating a possible function for the physical interaction between P2X receptors and tubulin. However, the finding that vincristine did not affect permeabilization was not compatible with this idea and suggested that microtubule depolymerization might be necessary but not sufficient by itself, to inhibit permeabilization and all its cellular consequences.
Colchicine targets
Colchicine and related plant extracts have been used to treat gout flares and prevent new attacks for more than 2000 years. Its main molecular target is β-tubulin, with which it forms a complex that prevents microtubule polymerization and, at high concentrations, causes microtubule depolymerization (Terkeltaub, 2009). Another known molecular target is the drug transporter ABCB1, also known as P-glycoprotein (PGP), through which colchicine can be pumped out of cells (Terkeltaub, 2009). However, the anti-inflammatory effects of colchicine are most notable and they underlie this drug's effectiveness as an anti-gout treatment. Other structurally related compounds such as β-lumicolchicine are ineffective as microtubule depolymerizing agents and as anti-inflammatory drugs, suggesting a relationship between the two actions (Gaudry et al., 1993). On the other hand, vincristine, a known microtubule disruptor, is not currently used to treat gout and also does not inhibit dye uptake by macrophages, as shown here (Sundy, 2010). On the other hand, colchicine might target an as yet unidentified cellular element.
The anti-inflammatory actions of colchicine are well characterized in various models of inflammation, including some that involve ATP signalling. It is known that P2X7 receptor activation in microglia leads to microtubule and actin reorganization, being the first related to IL-1β cleavage and release and the latter to blebs formation (Takenouchi et al., 2009). In these cells, colchicine treatment blocks microtubule rearrangement induced by ATP via P2X7 receptors and consequently IL-1β release (Takenouchi et al., 2009). In other cell types, such as THP-1, colchicine was unable to inhibit ATP-driven IL-1β release but did diminish ureate-stimulated inflammasome recruitment (Martinon et al., 2006). However, in the latter work, besides involving a different cell type, the authors used lower micromolar colchicine concentrations, which might explain the absence of effect. The concentrations used by Martinon et al. (2006) were also ineffective in inhibiting permeabilization in our experiments.
When used therapeutically, colchicine typically achieves low nM concentrations in plasma (Wallace and Ertel, 1973), which makes our results and those of Martinon et al. (2006) and Takenouchi et al. (2008) difficult to interpret in terms of therapeutic relevance. However, colchicine can accumulate in monocytes, reaching concentrations above that in plasma (Wallace and Ertel, 1973). In addition, the amount of colchicine that enters a particular cell type is correlated with the expression level of PGP, which makes it difficult to predict, based on plasma levels, what concentration of colchicine could be achieved by macrophages (Decleves et al., 2006). This is emphasized by the findings that some patients with familial Mediterranean fever will not respond to colchicine therapy because of PGP polymorphisms (Bezalel et al., 2009).
The ability of colchicine to inhibit inflammasome activation is directly related to its efficacy as a treatment for gout and familial Mediterranean fever syndrome (Sundy, 2010). Both diseases have been correlated with excess release of pro-inflammatory cytokines such as IL-1β, although P2X7 receptor malfunction has not been directly correlated with these conditions. However, in another type of joint inflammation, a model of monoclonal anti-collagen-induced arthritis, known to be dependent on the release of IL-1β, P2X7 receptor knockout mice displayed diminished susceptibility and severity compared with wild-type animals (Labasi et al., 2002). ATP is believed to be released by macrophages when they are activated by other danger signals and to have autocrine actions (Zhang and Mosser, 2008). In fact, uric acid stimulation of human macrophages induces IL-1β release via extracellular release of endogenous ATP (Piccini et al., 2008). This finding provides strong evidence that indeed P2X7 receptors are involved in the pathogenesis of gout. Therefore, it is tempting to hypothesize that at least some of the anti-inflammatory actions of colchicine, necessary to its therapeutic efficacy, might be due to the inhibition of P2X7 pore formation.
Colchicine and the P2X pore
In the present work, we provide evidence that strongly suggest that P2X2 and P2X7 pore formation have elements in common, as both were inhibited by colchicine. Furthermore, the fact that colchicine inhibits permeabilization but not ATP-gated currents in oocytes and macrophages supports the idea that the pore is formed by another protein (or proteins) or by a complex alteration of the channel such as elongation of the pore. Pannexin-1 has been pointed out as responsible for the permeabilization in a number of cell types (Pelegrin and Surprenant, 2006; 2009). However, because pannexin is not natively expressed by Xenopus oocytes (Silverman et al., 2008), it is unlikely that permeabilization and colchicine's ability to inhibit this phenomenon are dependent on this protein. Nevertheless, at this point we cannot rule out the participation of pannexin in the effects of colchicine in other cell types, especially macrophages. Interestingly, probenecid, another treatment for gout, is able to inhibit pannexin pores (Silverman et al., 2008). In addition, it will be interesting to know if other DAMPs that promote opening of pores, such as maitotoxin, are also inhibited by colchicine (Petrilli et al., 2007).
Functional consequences of P2X7 pore formation
P2X7 pore formation has been linked to recruitment of the inflammasome culminating with IL-1β release, in addition to the classic lytic pathway (Pelegrin and Surprenant, 2006). In brief, LPS-primed cells shed microvesicles when further stimulated with ATP and these vesicles contain IL-1β. This ATP effect clearly involves the P2X7 receptor, as macrophages from knockout mice do not release IL-1β after ATP stimulation (Solle et al., 2001). In addition, there is considerable evidence suggesting that P2X7 receptor activation, through cytoskeletal rearrangement and K+ efflux, causes caspase-1 activation, which in turn cleaves pro-IL-1β into mature IL-1β, which is then released (Ferrari et al., 2006; Takenouchi et al., 2009). The microtubule rearrangement was interpreted as necessary for IL-1β release, because disrupting microtubules with colchicine inhibited the discharge of this cytokine stimulated by ATP in microglia (Takenouchi et al., 2008). Nevertheless, if colchicine had any effects upstream to microtubule depolymerization, such as inhibition of permeabilization, it was not investigated by these authors. Here we show that both in vitro and in vivo colchicine inhibit several responses related to the pro-inflammatory actions of ATP. These responses are not necessarily in a single sequence of events, suggesting that perhaps the actions of colchicine are in the core of pro-inflammatory intracellular pathways induced by ATP. For instance, ROS generation might or might not be necessary to activate the inflammasome (Petrilli et al., 2007), although there is evidence suggesting that ATP-induced ROS generation in macrophages is a mandatory step to IL-1β release (Cruz et al., 2007). Colchicine does not scavenge ROS in phagocytes stimulated with zymosan (Muller-Peddinghaus and Wurl, 1987) or preclude PMA-induced ROS formation, as shown here. This indicates that colchicine action in preventing ATP-induced ROS augmentation is not non-specific scavenging, but rather a restricted action on an upstream step in ROS induction. However, the link between P2X7 pore formation and ROS generation is currently unclear (Schroder and Tschopp, 2010). P2X7 receptor activation induces ROS production via modulation of NADPH oxidase activity and assembly (Hewinson et al., 2008). These authors found that the EC50s for permeabilization and ROS formation induced by ATP are similar, which might indicate that both signalling pathways share common effectors. Interestingly, colchicine inhibits NADPH oxidase stimulation by monosodium urate crystals (Gaudry et al., 1993). However, further studies are required to clarify the role of permeabilization in ATP-evoked ROS production. Nevertheless, our findings that colchicine inhibits both suggest that they might be in the same pathway of P2X7 receptor-triggered events.
Here we show evidence from molecular to in vivo approaches strongly suggesting that colchicine exerts at least some of its anti-inflammatory properties through inhibition of the pore elicited by activation of P2X7 receptors. Colchicine has been used for many years as a medicine to treat inflammatory conditions involving the innate immune system, such as gout and pseudo-gout. Here we propose a new mechanism of action through which colchicine inhibits pore formation following P2X7 receptor activation, which can explain its anti-inflammatory effects. In addition, colchicine might prove to be a useful tool in studies of pore formation involving P2X receptors.
Acknowledgments
The authors thank Dr David Julius and members of his laboratory for advice and assistance in the beginning of this work. The authors also thank Dr Vivian Rumjanek for providing vincristine and Pryscila Braga for technical assistance. This work was supported by a postdoctoral fellowship of the Pew Latin American Fellows Program, by a travel grant from CNPq/Brazil (MZPG), CNPq and FAPERJ.
Glossary
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- DCFH2DA
dichlorodihydrofluorescein diacetate
- DMEM
Dulbecco's modified Eagle's medium
- EB
ethidium bromide
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- LPS
lipopolysaccharide
- PGP
P-glycoprotein
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
Conflicts of interest
None.
Supporting Information
Teaching Materials; Figs 1–8 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezalel Y, Gershoni-Baruch R, Dagan E, Lidar M, Livneh A. The 3435T polymorphism in the ABCB1 gene and colchicine unresponsiveness in familial Mediterranean fever. Clin Exp Rheumatol. 2009;27:S103–S104. [PubMed] [Google Scholar]
- Brone B, Moechars D, Marrannes R, Mercken M, Meert T. P2X currents in peritoneal macrophages of wild type and P2X4 -/- mice. Immunol Lett. 2007;113:83–89. doi: 10.1016/j.imlet.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120:772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- Castro NG, de Mello MC, de Mello FG, Aracava Y. Direct inhibition of the N-methyl-D-aspartate receptor channel by dopamine and (+)-SKF38393. Br J Pharmacol. 1999;126:1847–1855. doi: 10.1038/sj.bjp.0702479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia EW, Grainger R, Harper JL. Colchicine suppresses neutrophil superoxide production in a murine model of gouty arthritis: a rationale for use of low-dose colchicine. Br J Pharmacol. 2008;153:1288–1295. doi: 10.1038/bjp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-Silva R, Persechini PM. P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am J Physiol. 1997;273:C1793–C1800. doi: 10.1152/ajpcell.1997.273.6.C1793. [DOI] [PubMed] [Google Scholar]
- Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz CM, Ventura AL, Schachter J, Costa-Junior HM, da Silva Souza HA, Gomes FR, et al. Activation of ERK1/2 by extracellular nucleotides in macrophages is mediated by multiple P2 receptors independently of P2X7-associated pore or channel formation. Br J Pharmacol. 2006;147:324–334. doi: 10.1038/sj.bjp.0706559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decleves X, Niel E, Debray M, Scherrmann JM. Is P-glycoprotein (ABCB1) a phase 0 or a phase 3 colchicine transporter depending on colchicine exposure conditions? Toxicol Appl Pharmacol. 2006;217:153–160. doi: 10.1016/j.taap.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Denlinger LC, Fisette PL, Garis KA, Kwon G, Vazquez-Torres A, Simon AD, et al. Regulation of inducible nitric oxide synthase expression by macrophage purinoreceptors and calcium. J Biol Chem. 1996;271:337–342. doi: 10.1074/jbc.271.1.337. [DOI] [PubMed] [Google Scholar]
- Egan TM, Samways DS, Li Z. Biophysics of P2X receptors. Pflugers Arch. 2006;452:501–512. doi: 10.1007/s00424-006-0078-1. [DOI] [PubMed] [Google Scholar]
- Faria RX, Cascabulho CM, Reis RA, Alves LA. Large-conductance channel formation mediated by P2X7 receptor activation is regulated through distinct intracellular signaling pathways in peritoneal macrophages and 2BH4 cells. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:73–87. doi: 10.1007/s00210-010-0523-8. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Gaudry M, Roberge CJ, de Medicis R, Lussier A, Poubelle PE, Naccache PH. Crystal-induced neutrophil activation. III. Inflammatory microcrystals induce a distinct pattern of tyrosine phosphorylation in human neutrophils. J Clin Invest. 1993;91:1649–1655. doi: 10.1172/JCI116373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau S, Schirmer J, Schmalzing G. Identification of a tubulin binding motif on the P2X2 receptor. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;786:311–318. doi: 10.1016/s1570-0232(02)00743-2. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Melenchuk EV, Poputnikov DM, Gourine VN, Spyer KM. Involvement of purinergic signalling in central mechanisms of body temperature regulation in rats. Br J Pharmacol. 2002;135:2047–2055. doi: 10.1038/sj.bjp.0704679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Poputnikov DM, Zhernosek N, Melenchuk EV, Gerstberger R, Spyer KM, et al. P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br J Pharmacol. 2005;146:139–145. doi: 10.1038/sj.bjp.0706287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu BJ, Rathsam C, Stokes L, McGeachie AB, Wiley JS. Extracellular ATP dissociates nonmuscle myosin from P2X(7) complex: this dissociation regulates P2X(7) pore formation. Am J Physiol Cell Physiol. 2009;297:C430–C439. doi: 10.1152/ajpcell.00079.2009. [DOI] [PubMed] [Google Scholar]
- Guimaraes MZ. Isoform specificity of P2X2 purinergic receptor C-terminus binding to tubulin. Neurochem Int. 2008;52:314–320. doi: 10.1016/j.neuint.2007.06.032. [DOI] [PubMed] [Google Scholar]
- Hewinson J, Moore SF, Glover C, Watts AG, MacKenzie AB. A key role for redox signaling in rapid P2X7 receptor-induced IL-1 beta processing in human monocytes. J Immunol. 2008;180:8410–8420. doi: 10.4049/jimmunol.180.12.8410. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci. 1999;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- Kim M, Jiang LH, Wilson HL, North RA, Surprenant A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 2001;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Davis GS, Mohr C, Nain M, Gemsa D. Suppression of LPS-induced tumor necrosis factor-alpha gene expression by microtubule disrupting agents. Immunobiology. 1996;195:640–654. doi: 10.1016/s0171-2985(96)80028-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Muller-Peddinghaus R, Wurl M. The amplified chemiluminescence test to characterize antirheumatic drugs as oxygen radical scavengers. Biochem Pharmacol. 1987;36:1125–1132. doi: 10.1016/0006-2952(87)90423-0. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. 2008;10:218–227. doi: 10.1007/s11926-008-0036-3. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Pierce AN, Zhang R, Machu TK. Colchicine: a novel positive allosteric modulator of the human 5-hydroxytryptamine3A receptor. J Pharmacol Exp Ther. 2009;329:838–847. doi: 10.1124/jpet.108.146522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KE. Modulation of ATP-gated non-selective cation channel (P2X1 receptor) activation and desensitization by the actin cytoskeleton. J Physiol. 1998;510:19–25. doi: 10.1111/j.1469-7793.1998.019bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. The P2X(7) receptor-pannexin connection to dye uptake and IL-1beta release. Purinergic Signal. 2009;5:129–137. doi: 10.1007/s11302-009-9141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Pfeiffer ZA, Aga M, Prabhu U, Watters JJ, Hall DJ, Bertics PJ. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol. 2004;75:1173–1182. doi: 10.1189/jlb.1203648. [DOI] [PubMed] [Google Scholar]
- Pfeiffer ZA, Guerra AN, Hill LM, Gavala ML, Prabhu U, Aga M, et al. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radic Biol Med. 2007;42:1506–1516. doi: 10.1016/j.freeradbiomed.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raices RM, Kannan Y, Sarkar A, Bellamkonda-Athmaram V, Wewers MD. A synergistic role for IL-1beta and TNFalpha in monocyte-derived IFNgamma inducing activity. Cytokine. 2008;44:234–241. doi: 10.1016/j.cyto.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J, Motta AP, de Souza Zamorano A, da Silva-Souza HA, Guimaraes MZ, Persechini PM. ATP-induced P2X7-associated uptake of large molecules involves distinct mechanisms for cations and anions in macrophages. J Cell Sci. 2008;121:3261–3270. doi: 10.1242/jcs.029991. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Park CK, Oh SB, Evans RJ, North RA. P2X1 and P2X4 receptor currents in mouse macrophages. Br J Pharmacol. 2007;152:1283–1290. doi: 10.1038/sj.bjp.0707504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- Sundy JS. Progress in the pharmacotherapy of gout. Curr Opin Rheumatol. 2010;22:188–193. doi: 10.1097/BOR.0b013e3283369014. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Iwamaru Y, Sugama S, Sato M, Hashimoto M, Kitani H. Lysophospholipids and ATP mutually suppress maturation and release of IL-1 beta in mouse microglial cells using a Rho-dependent pathway. J Immunol. 2008;180:7827–7839. doi: 10.4049/jimmunol.180.12.7827. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-induced release and processing of IL-1beta in microglial cells. Crit Rev Immunol. 2009;29:335–345. doi: 10.1615/critrevimmunol.v29.i4.40. [DOI] [PubMed] [Google Scholar]
- Terkeltaub RA. Colchicine update: 2008. Semin Arthritis Rheum. 2009;38:411–419. doi: 10.1016/j.semarthrit.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Tonetti M, Sturla L, Giovine M, Benatti U, De Flora A. Extracellular ATP enhances mRNA levels of nitric oxide synthase and TNF-alpha in lipopolysaccharide-treated RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 1995;214:125–130. doi: 10.1006/bbrc.1995.2265. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- Wallace SL, Ertel NH. Plasma levels of colchicine after oral administration of a single dose. Metabolism. 1973;22:749–753. doi: 10.1016/0026-0495(73)90247-3. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Buhler AV, Whatley VJ, Harris RA, Dunwiddie TV. Colchicine is a competitive antagonist at human recombinant gamma-aminobutyric acid A receptors. J Pharmacol Exp Ther. 1998;284:95–102. [PubMed] [Google Scholar]
- Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


