Abstract
BACKGROUND AND PURPOSE
Systemic glucocorticoid therapy may effectively attenuate lung inflammation but also induce severe side-effects. Delivery of glucocorticoids by liposomes could therefore be beneficial. We investigated if liposome-encapsulated dexamethasone inhibited ventilator-induced lung inflammation. Furthermore, we evaluated whether targeting of cellular Fcγ-receptors (FcγRs) by conjugating immunoglobulin G (IgG) to liposomes, would improve the efficacy of dexamethasone-liposomes in attenuating granulocyte infiltration, one of the hallmarks of lung inflammation.
EXPERIMENTAL APPROACH
Mice were anaesthetized, tracheotomized and mechanically ventilated for 5 h with either ‘low’ tidal volumes ∼7.5 mL·kg−1 (LVT) or ‘high’ tidal volumes ∼15 mL·kg−1 (HVT). At initiation of ventilation, we intravenously administered dexamethasone encapsulated in liposomes (Dex-liposomes), dexamethasone encapsulated in IgG-modified liposomes (IgG-Dex-liposomes) or free dexamethasone. Non-ventilated mice served as controls.
KEY RESULTS
Dex-liposomes attenuated granulocyte infiltration and IL-6 mRNA expression after LVT-ventilation, but not after HVT-ventilation. Dex-liposomes also down-regulated mRNA expression of IL-1β and KC, but not of CCL2 (MCP-1) in lungs of LVT and HVT-ventilated mice. Importantly, IgG-Dex-liposomes inhibited granulocyte influx caused by either LVT or HVT-ventilation. IgG-Dex-liposomes diminished IL-1β and KC mRNA expression in both ventilation groups, and IL-6 and CCL2 mRNA expression in the LVT-ventilated group. Free dexamethasone prevented granulocyte influx and inflammatory mediator expression induced by LVT or HVT-ventilation.
CONCLUSIONS AND IMPLICATIONS
FcγR-targeted IgG-Dex-liposomes are pharmacologically more effective than Dex-liposomes particularly in inhibiting pulmonary granulocyte infiltration. IgG-Dex-liposomes inhibited most parameters of ventilator-induced lung inflammation as effectively as free dexamethasone, with the advantage that liposome-encapsulated dexamethasone will be released locally in the lung thereby preventing systemic side-effects.
Keywords: mechanical ventilation, ventilator-induced lung injury, lung inflammation, Fcγ-receptor targeting, dexamethasone-liposomes, mouse
Introduction
Mechanical ventilation has the potential to induce or worsen lung injury, a phenomenon referred to as ventilator-induced lung injury (VILI) (Dreyfuss and Saumon, 1998; Slutsky, 1999). VILI is characterized by enhanced inflammation, vascular leakage and impaired gas exchange (Parker et al., 1993). It has been hypothesized that a ventilator-induced increase in granulocyte infiltration and inflammatory mediator expression in the lung is crucial in the development of pulmonary injury (Haitsma et al., 2003; Wilson et al., 2003; 2005). One of the most potent group of drugs to treat lung inflammation are the glucocorticoids, which exert their effects by binding to intracellular glucocorticoid receptors (GRs) (Brower et al., 2001; Luce, 2002). After binding, the GR complex migrates from the cytosol to the nucleus where it regulates a wide range of gene activity, including inhibition of nuclear factor kappa B (NF-κB) and activator protein (AP)-1 driven expression of inflammatory genes (Barnes, 2006).
Previous research in experimental models of VILI showed that synthetic glucocorticoids have the potential to attenuate ventilator-induced lung inflammation (Held et al., 2001; Ohta et al., 2001). However, systemic administration of glucocorticoids is associated with severe side-effects like increased blood glucose levels (Weinstein et al., 1995; Feldman-Billard et al., 2006). Local delivery of glucocorticoids by liposomal formulations could therefore be of therapeutic importance. In this respect, we previously demonstrated that delivery of liposome-encapsulated dexamethasone (Dex-liposomes) inhibits pro-inflammatory gene expression in a murine model of glomerulonephritis without affecting blood glucose levels (Asgeirsdottir et al., 2007). Liposomes are valuable drug delivery systems for treatment of VILI as they can act as a depot from which the encapsulated drug will be slowly released to enable prolonged drug exposure at low concentrations (Storm and Crommelin, 1998). Furthermore, liposomes extravasate into tissues that experience increased capillary permeability (Storm and Crommelin, 1998), which facilitates delivery at sites of inflammation or mechanical stretch.
The present study was designed to examine whether Dex-liposomes are capable of down-regulating ventilator-induced lung inflammation. Moreover, we hypothesized that conjugation of immunoglobulin G (IgG) to the Dex-liposomes would promote binding of the IgG Fc-fragment on Dex-liposomes to Fcγ-receptors (FcγRs) on macrophages and granulocytes, thereby improving the efficacy of Dex-liposomes to inhibit granulocyte infiltration, one of the hallmarks of VILI. We investigated the effects of dexamethasone encapsulated in liposomes (Dex-liposomes), dexamethasone encapsulated in IgG-modified liposomes (IgG-Dex-liposomes) and free dexamethasone in an established murine model of VILI (Wolthuis et al., 2009).
Methods
Animals
All animal care and experimental procedures were approved by the animal use and care committees of the University Medical Center Utrecht and Academic Medical Center Amsterdam. Adult male C57Bl6 mice (n = 126; Charles River, Maastricht, the Netherlands), weighing 20–24 g, were randomly assigned to different experimental groups.
Healthy mice (n = 108) were exposed to mechanical ventilation as described previously (Wolthuis et al., 2009). General anaesthesia was achieved with an intraperitoneal injection of 126 mg·kg−1 ketamine (Eurovet Animal Health BV, Bladel, the Netherlands), 0.2 mg·kg−1 medetomidine (Pfizer Animal Health BV, Capelle a/d IJssel, the Netherlands) and 0.5 mg·kg−1 atropine (Pharmachemie, Haarlem, the Netherlands). Maintenance anaesthesia consisted of 36 mg·kg−1 ketamine, 0.04 mg·kg−1 medetomidine and 0.075 mg·kg−1 atropine and was administered every hour via an intraperitoneal catheter (PE 10 tubing; BD, Breda, the Netherlands).
Groups of six mice were ventilated simultaneously for 5 h in a pressure-controlled mode, at a fractional inspired oxygen concentration (FiO2) of 50 %, inspiration-to-expiration ratio of 1:1 and positive end-expiratory pressure (PEEP) of 2 cmH2O. Mechanical ventilation was initiated with either an inspiratory pressure of 10 cmH2O (resulting in ‘low’ tidal volumes (VT) ∼7.5 mL·kg−1; LVT) or 18 cmH2O (resulting in ‘high’ VT∼15 mL·kg−1; HVT). Respiratory rate was set at 100 and 50 breaths·min−1 respectively. Body temperature was kept constant between 36.5–37.5°C. Non-ventilated mice (n = 18) served as controls [non-ventilated controls (NVC)]. At the end of the 5 h experimental period, animals were killed by exsanguination.
Synthesis of liposome-encapsulated dexamethasone
The glucocorticoid dexamethasone was encapsulated in liposomes (Dex-liposomes) or IgG-modified liposomes (IgG-Dex-liposomes) as described previously (Asgeirsdottir et al., 2007). Lipids from stock solutions of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, cholesterol, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000]-maleimide in chloroform/methanol (9:1), were mixed in a molar ratio of 55:40:4:1, dried under reduced nitrogen pressure, dissolved in cyclohexane, and lyophilized. The lipids were then hydrated in 10 mM HEPES and 135 mM NaCl, pH 6.7, or, when appropriate, in an aqueous solution of 75 to 100 mg·mL−1 dexamethasone disodium phosphate. The liposomes formed were sized by repeated extrusion (13 times) through polycarbonate filters (Costar; Corning Life Sciences, Acton, MA, USA), pore size 50 nm, using a high-pressure extruder (Lipex, Vancouver, BC, Canada). The rat IgG was thiolated by N-succinimidyl-S-acetylthioacetate and coupled to a maleimide group at the distal end of the polyethylene glycol chain. The liposomes were characterized by determining protein content, using mouse IgG as a standard (Peterson, 1977) and phospholipid phosphorus content (Bottcher et al., 1961). Total liposomal lipid concentrations were adjusted for the amount of cholesterol present in liposome preparations. The amount of coupled rat IgG was 34.2 g per mol of lipid. Particle size was analysed by dynamic light scattering using a Nicomp model 370 submicron particle analyser (Santa Barbara, CA) in the volume weighing model. The diameter of Dex-liposomes was 88.6 nm and that of IgG-Dex-liposomes 103.0 nm. The content of encapsulated dexamethasone disodium phosphate was determined after Bligh and Dyer extraction in the resulting methanol/H2O phase by high-performance liquid chromatography (Melgert et al., 2000). The amount of encapsulated dexamethasone phosphate was 28.0 g per mol of lipid (Dex-liposomes) or 34.8 g per mol of lipid (IgG-Dex-liposomes).
Dexamethasone treatment
At initiation of ventilation, we intravenously administered either Dex-liposomes, IgG-Dex-liposomes (0.4 µmol of lipid per animal; 11.2 and 13.9 µg dexamethasone, respectively) or free dexamethasone (20 µg per animal). In addition, LVT or HVT-ventilated mice were intravenously treated with liposomal formulations lacking entrapped dexamethasone. Control LVT and HVT-ventilated mice received the same volume of sterile saline (vehicle) intravenously.
Haemodynamics and blood gas analysis
After 0, 2.5 and 5 h, systolic blood pressure and heart rate were non-invasively monitored using a murine tail-cuff system (ADInstruments, Spenbach, Germany). After 5 h, arterial blood was taken from the carotid artery for blood gas analysis (Rapidlab 865; Bayer, Mijdrecht, the Netherlands).
Histopathology and immunohistochemistry
The left lung was filled with Tissue-Tek (Sakura Finetek, Zoeterwoude, the Netherlands), snap frozen and cut to 5 µm cryosections using a cryostat. To assess lung histopathology, longitudinal sections were stained with haematoxylin and eosin (H&E; Klinipath, Duiven, the Netherlands). To assess granulocyte infiltration in lung tissue, longitudinal sections were incubated with rabbit antibody to polymorphonuclear cells (PMNs) (Accurate Antibodies, Westbury, NY, USA) followed by horseradish peroxidase (HRP) goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA) and visualization with diaminobenzamidine.
Homogenates
Lung tissue was pulverized using a liquid nitrogen-cooled mortar and pestle, and divided in several fractions allowing us to perform multiple analyses (as described in the following).
Myeloperoxidase (MPO) activity
MPO activity was determined as described previously (Hegeman et al., 2009). In short, pulmonary tissue was homogenized in 50 mM HEPES buffer (pH 8.0), centrifuged and pellets were homogenized again in water and 0.5% cetyltrimethylammonium chloride (CTAC; Merck, Darmstadt, Germany). After centrifugation, supernatants were diluted in 10 mM citrate buffer (pH 5.0) and 0.22% CTAC. Substrate solution containing 3 mM 3′,5,5′-tetramethylbenzidine dihydrochloride (TMB; Sigma-Aldrich, Steinheim, Germany), 120 µM resorcinol (Merck) and 2.2 mM H2O2 in distilled water was added. Reaction mixtures were incubated for 20 min at room temperature and stopped by addition of 4 M H2SO4 followed by determination of optical density at 450 nm. MPO activity of a known amount of MPO units per mL (Sigma-Aldrich) was used as reference. MPO activity was corrected for total protein (BCA protein assay; Pierce Biotechnology, Rockford, IL, USA) using BSA as standard.
Real-time RT-PCR
Total RNA was isolated with TRIzol® reagent (Invitrogen, Paisley, UK). cDNA was synthesized with SuperScript Reverse Transcriptase (Invitrogen). PCR reaction was performed with iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA) using primers for interleukin (IL)-1β, IL-6, keratinocyte-derived chemokine (KC) and CC chemokine ligand (CCL) 2 [monocyte chemotatic protein (MCP-1); nomenclature follows Alexander et al., 2009]. PCR product size was verified on gel to confirm appropriate amplification. Data were normalized for expression of internal controls, that is, the average value of β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primers: IL-6, FW ACCgCTATgAAgTTCCTCTC, RV CTCTgTgAAgTCTCCTCTCC; CCL2 FW ggTCCCTgTCATgCTTCTg, RV CATCTTgCTggTgAATgAgTAg; for other sequences see Hegeman et al. (2009).
Statistical analysis
Data are expressed as mean ± standard error of the mean. Blood gas variables (LVT vs. HVT) were analysed by independent T-test. All other parameters were analysed by one-way analysis of variance (anova) with least significant difference (LSD) post hoc test. P-values less than 0.05 were considered as statistically significant.
Materials
Ketamine was obtained from Eurovet Animal Health BV, Bladel, the Netherlands; medetomidine from Pfizer Animal Health BV, Capelle a/d IJssel, the Netherlands and atropine from Pharmachemie, Haarlem, the Netherlands. Dexamethasone disodium phosphate was from Bufa, Hilversum, the Netherlands; rat IgG and cholesterol from Sigma-Aldrich Chemie, Zwijndrecht, the Netherlands and the other lipids from Avanti Polar Lipids, Alabaster, AL, USA.
Results
Stability of the murine model of VILI
All animals survived the ventilation procedures and were killed after 5 h of mechanical ventilation. Systolic blood pressures and heart rates were stable throughout the experiment (Table 1).
Table 1.
Haemodynamic characteristics over 5 h of mechanical ventilation
| LVT | HVT | |||||||
|---|---|---|---|---|---|---|---|---|
| Veh | Dex lip | IgG-Dex lip | Dex | Veh | Dex lip | IgG-Dex lip | Dex | |
| HRt=0h | 370 ± 11 | 391 ± 19 | 399 ± 30 | 381 ± 17 | 370 ± 21 | 387 ± 16 | 395 ± 19 | 388 ± 6 |
| HRt=2,5h | 385 ± 19 | 408 ± 22 | 360 ± 17 | 371 ± 15 | 350 ± 15 | 358 ± 12 | 376 ± 14 | 347 ± 20 |
| HRt=5h | 409 ± 10 | 386 ± 28 | 407 ± 18 | 402 ± 17 | 367 ± 7 | 354 ± 12 | 378 ± 11 | 357 ± 26 |
| BPt=0h | 103 ± 5 | 119 ± 7 | 103 ± 4 | 112 ± 11 | 103 ± 6 | 110 ± 13 | 108 ± 8 | 113 ± 7 |
| BPt=2,5h | 64 ± 4 | 66 ± 4 | 62 ± 3 | 76 ± 8 | 69 ± 4 | 68 ± 5 | 61 ± 5 | 69 ± 7 |
| BPt=5h | 68 ± 7 | 66 ± 7 | 70 ± 5 | 64 ± 4 | 67 ± 5 | 70 ± 7 | 67 ± 6 | 78 ± 8 |
Data are presented as mean ± standard error of the mean (LVTn = 6–8, HVTn = 6–8).
BP, systolic blood pressure in mmHg; Dex, LVT or HVT-ventilated mice intravenously treated with free dexamethasone; Dex lip, LVT or HVT-ventilated mice intravenously treated with liposomes containing dexamethasone; HR, heart rate in beats min−1; HVT, mice ventilated with high tidal volumes; IgG-Dex lip, LVT or HVT-ventilated mice intravenously treated with IgG-liposomes containing dexamethasone; LVT, mice ventilated with low tidal volumes; Veh, LVT or HVT-ventilated mice intravenously treated with vehicle (sterile saline).
Arterial oxygen tension (PaO2) was reduced in HVT-ventilated mice in comparison with LVT-ventilated mice (Table 2). In both ventilation groups, carbon dioxide tension (PaCO2), pH and base excess (BE) remained within the physiological range.
Table 2.
Arterial blood gas analysis after 5 h of mechanical ventilation
| LVT | HVT | |||||||
|---|---|---|---|---|---|---|---|---|
| Veh | Dex lip | IgG-Dex lip | Dex | Veh | Dex lip | IgG-Dex lip | Dex | |
| PaO2 | 229.0 ± 20.8 | 230.0 ± 14.0 | 228.9 ± 17.9 | 224.6 ± 23.3 | 171.2 ± 15.2* | 175.6 ± 14.7 | 177.7 ± 19.5 | 170.5 ± 13.5 |
| PaCO2 | 31.8 ± 3.7 | 32.2 ± 2.7 | 33.6 ± 6.5 | 31.8 ± 4.0 | 33.2 ± 2.3 | 35.9 ± 1.3 | 34.1 ± 2.6 | 36.5 ± 1.7 |
| pH | 7.52 ± 0.03 | 7.50 ± 0.04 | 7.49 ± 0.06 | 7.50 ± 0.04 | 7.52 ± 0.03 | 7.48 ± 0.02 | 7.48 ± 0.03 | 7.49 ± 0.02 |
| BE | 2.52 ± 1.25 | 1.60 ± 1.44 | 0.19 ± 0.98 | 0.61 ± 1.38 | 3.70 ± 1.37 | 2.78 ± 1.00 | 1.35 ± 0.79 | 3.76 ± 0.86 |
Data are presented as mean ± standard error of the mean (LVTn = 8–10, HVTn = 8–10).
P < 0.05 versus LVT Veh.
BE, base excess in mmol·L−1; Dex, LVT or HVT-ventilated mice intravenously treated with free dexamethasone; Dex lip, LVT or HVT-ventilated mice intravenously treated with liposomes containing dexamethasone; HVT, mechanically ventilated with high tidal volumes; IgG-Dex lip, LVT or HVT-ventilated mice intravenously treated with IgG-liposomes containing dexamethasone; LVT, mechanically ventilated with low tidal volumes; PaO2, partial pressure of arterial oxygen in mmHg; PaCO2, partial pressure of arterial carbon dioxide in mmHg; Veh, LVT or HVT-ventilated mice intravenously treated with vehicle (sterile saline).
Effect of Dex-liposomes and IgG-Dex-liposomes on pulmonary architecture
Mice were intravenously treated with either saline (vehicle), Dex-liposomes, IgG-Dex-liposomes or free dexamethasone at initiation of mechanical ventilation and subsequently ventilated for 5 h. No changes in haemodynamic and blood gas variables were observed after administration of (liposome-encapsulated) dexamethasone (Tables 1 and 2). Lung sections were stained for H&E to analyse histopathological changes after mechanical ventilation (Figure 1). We observed that both LVT and HVT-ventilation (vehicle treatment) induced damage to pulmonary architecture compared with NVC, that is, thickening of the alveolar wall and cellular infiltrate. Immunohistochemical staining revealed that the infiltrated immune cells were granulocytes. Administration of Dex-liposomes, IgG-Dex-liposomes or free dexamethasone at the initiation of ventilation preserved pulmonary architecture during 5 h of mechanical ventilation.
Figure 1.
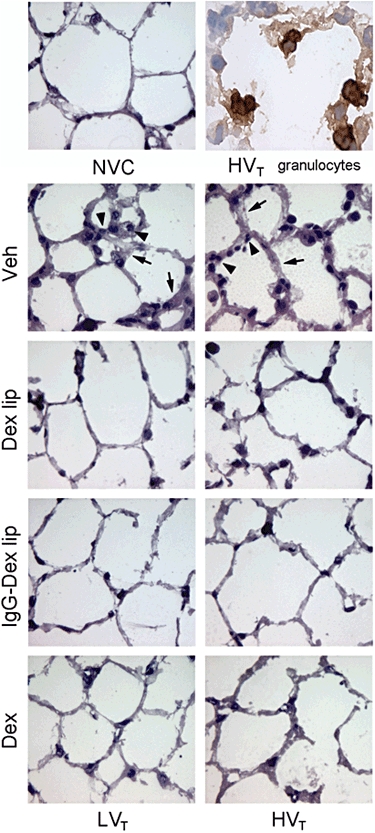
Effect of Dex-liposomes, IgG-Dex-liposomes and free dexamethasone on changes in lung histology induced by mechanical ventilation. Lung sections were stained with haematoxylin and eosin (H&E) to analyse lung histopathology and presence of cellular infiltrate in pulmonary tissue. Magnification ×500. Black arrow indicates the typical thickening of the alveolar wall and black arrowhead indicates cellular infiltrate. Lung sections were immunohistochemically stained for polymorphonuclear cells (PMNs) to assess if the cellular infiltrate consisted of granulocytes. Magnification ×1000. Dex, LVT or HVT-ventilated mice intravenously treated with free dexamethasone; Dex lip, LVT or HVT-ventilated mice intravenously treated with liposomes containing dexamethasone; HVT, mice ventilated with high tidal volumes; IgG-Dex lip, LVT or HVT-ventilated mice intravenously treated with IgG-liposomes containing dexamethasone; LVT, mice ventilated with low tidal volumes; NVC, non-ventilated controls; Veh, LVT or HVT-ventilated mice intravenously treated with vehicle (sterile saline).
Effect of Dex-liposomes and IgG-Dex-liposomes on ventilator-induced granulocyte infiltration
To assess ventilator-induced lung inflammation, we determined influx of granulocytes into pulmonary tissue. Granulocyte infiltration was quantified by measuring MPO activity in total lung homogenates. MPO activity was significantly increased after LVT and HVT-ventilation in comparison with NVC (Figure 2), which correlated with the margination of granulocytes to the blood vessel wall observed in lung sections of LVT and HVT-ventilated mice (Figure 1).
Figure 2.
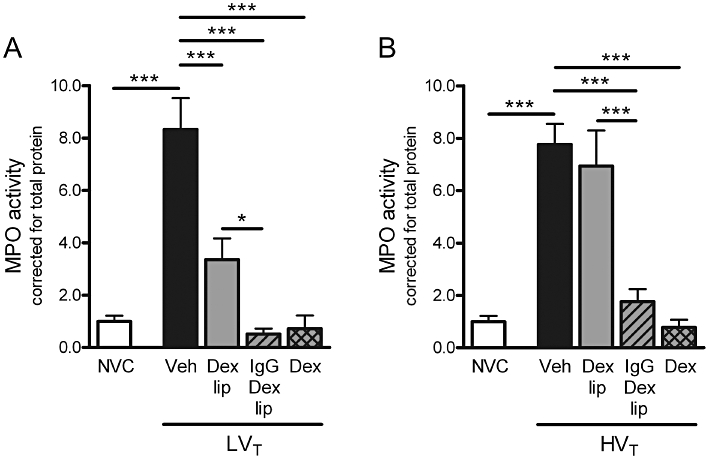
Effect of Dex-liposomes, IgG-Dex-liposomes and free dexamethasone on granulocyte influx induced by mechanical ventilation. (A–B) In total lung homogenates, myeloperoxidase (MPO) activity was determined as a measure of granulocyte infiltration. Levels were normalized for total protein levels. Data are expressed as mean ± SEM, and shown relative to NVC. (A/B) NVC n = 18/18, Veh n = 20/14, Dex lip n = 15/14, IgG-Dex lip n = 10/9; Dex n = 7/7. *P < 0.05, ***P < 0.001 versus NVC, LVT/HVT Veh or LVT/HVT Dex lip. Dex, LVT or HVT-ventilated mice intravenously treated with free dexamethasone; Dex lip, LVT or HVT-ventilated mice intravenously treated with liposomes containing dexamethasone; HVT, mice ventilated with high tidal volumes; IgG-Dex lip, LVT or HVT-ventilated mice intravenously treated with IgG-liposomes containing dexamethasone; LVT, mice ventilated with low tidal volumes; NVC, non-ventilated controls; Veh, LVT or HVT-ventilated mice intravenously treated with vehicle (sterile saline).
Next, we investigated if administration of Dex-liposomes at the initiation of ventilation was capable of inhibiting lung inflammation induced by 5 h of LVT or HVT-ventilation. Treatment with Dex-liposomes attenuated MPO activity in mice exposed to LVT-ventilation (Figure 2A) although MPO activity was still above baseline level in these mice (P < 0.05 vs. NVC). In mice exposed to HVT-ventilation, however, no inhibitory effect of Dex-liposomes on MPO activity was observed (Figure 2B). In contrast, treatment with IgG-Dex-liposomes effectively inhibited granulocyte infiltration in both LVT and HVT-ventilated mice. Furthermore, IgG-Dex-liposomes were as effective as free dexamethasone in down-regulating MPO activity in lungs of LVT and HVT-ventilated mice. H&E staining of lung sections confirmed the quantitative measures for granulocyte influx after treatment with Dex-liposomes, IgG-Dex-liposomes and free dexamethasone (Figure 1).
Effect of Dex-liposomes and IgG-Dex-liposomes on ventilator-induced cytokine expression
To examine the effect of LVT and HVT-ventilation on cytokine expression, we determined de novo synthesis of the prototypic pro-inflammatory cytokines IL-1β and IL-6 in total lung homogenates. Compared with NVC, both ventilation strategies induced mRNA expression of IL-1β (Figure 3A and B) and IL-6 (Figure 3C and D).
Figure 3.
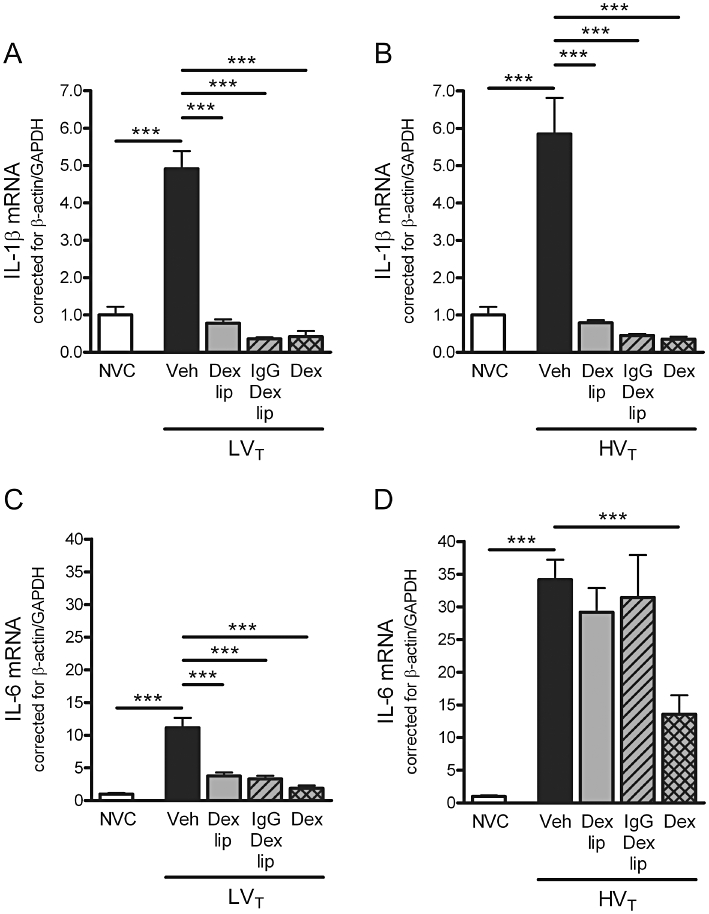
Effect of Dex-liposomes, IgG-Dex-liposomes and free dexamethasone on cytokine mRNA expression induced by mechanical ventilation. (A–B) In total lung homogenates, mRNA expression of interleukin (IL)-1β was determined by real-time RT-PCR. (C–D) In addition, mRNA expression of IL-6 was determined. Levels were normalized for expression of internal controls, that is, the average value of β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are expressed as mean ± SEM, and shown relative to NVC. (A/B) NVC n = 15/15, Veh n = 22/17, Dex lip n = 15/12, IgG-Dex lip n = 10/8; Dex n = 7/7; (C/D) NVC n = 15/15, Veh n = 21/18, Dex lip n = 15/13, IgG-Dex lip n = 10/10; Dex n = 7/7. ***P < 0.001 versus NVC or LVT/HVT Veh. Dex, LVT or HVT-ventilated mice intravenously treated with free dexamethasone; Dex lip, LVT or HVT-ventilated mice intravenously treated with liposomes containing dexamethasone; HVT, mice ventilated with high tidal volumes; IgG-Dex lip, LVT or HVT-ventilated mice intravenously treated with IgG-liposomes containing dexamethasone; LVT, mice ventilated with low tidal volumes; NVC, non-ventilated controls; Veh, LVT or HVT-ventilated mice intravenously treated with vehicle (sterile saline).
Dex-liposomes and IgG-Dex-liposomes attenuated IL-1β mRNA expression after LVT and HVT-ventilation. IL-6 mRNA levels were down-regulated in LVT-ventilated mice, while both liposomal formulations were fully devoid of IL-6 mRNA inhibitory activity in HVT-ventilated mice. Administration of free dexamethasone prevented the increase in IL-1β and IL-6 mRNA expression in lungs of LVT and HVT-ventilated mice, although IL-6 mRNA expression was still above baseline level in HVT-ventilated mice (P < 0.05 vs. NVC; Figure 3D).
Effect of Dex-liposomes and IgG-Dex-liposomes on ventilator-induced chemokine expression
To study the effect of LVT and HVT-ventilation on de novo synthesis of chemokines, we determined KC and CCL2 mRNA expression in total lung homogenates. mRNA expression of KC (Figure 4A and B) and CCL2 (Figure 4C and D) was increased after LVT and HVT ventilation. Treatment with Dex-liposomes diminished KC mRNA expression induced by LVT or HVT-ventilation. However, Dex-liposomes did not affect CCL2 mRNA levels in LVT-ventilated mice while they enhanced CCL2 mRNA levels in HVT-ventilated mice (204.5%; P < 0.001 vs. HVT vehicle; Figure 4D). IgG-Dex-liposomes diminished KC mRNA expression after both LVT and HVT-ventilation and CCL2 mRNA expression only after LVT-ventilation. Administration of free dexamethasone inhibited KC and CCL2 mRNA levels in lungs of LVT and HVT-ventilated mice.
Figure 4.
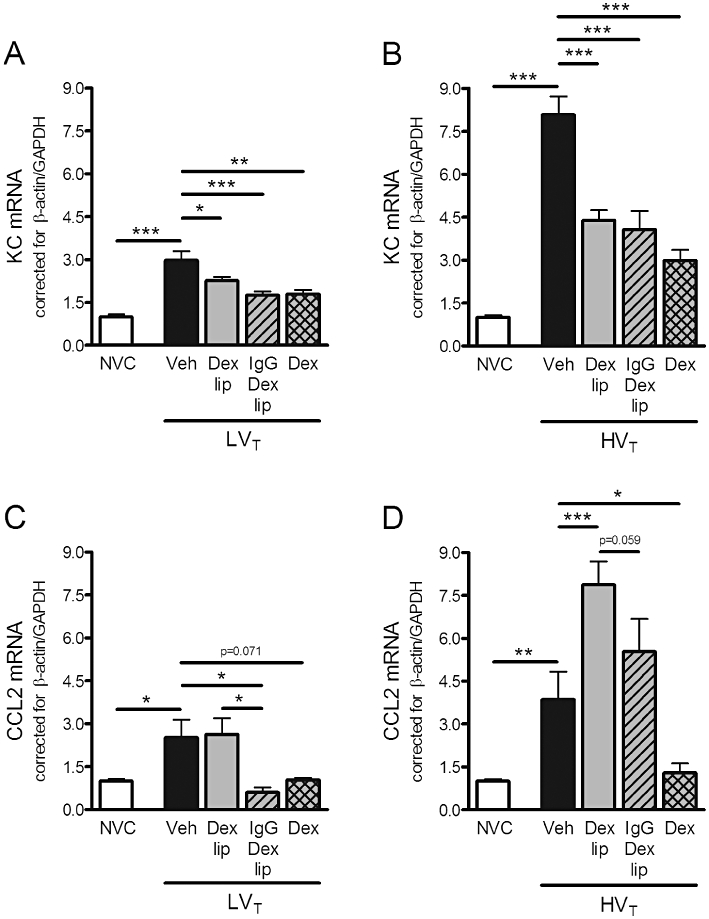
Effect of Dex liposomes, IgG-Dex-liposomes and free dexamethasone on chemokine mRNA expression induced by mechanical ventilation. (A–B) In total lung homogenates, mRNA expression of keratinocyte-derived chemokine (KC) was determined by real time RT-PCR. (C–D) In addition, mRNA expression of CC chemokine ligand (CCL) 2 was determined. Levels were normalized for expression of internal controls, that is, average value of β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are expressed as mean ± SEM, and shown relative to NVC. (A/B) NVC n = 15/15, Veh n = 20/17, Dex lip n = 15/13, IgG-Dex lip n = 10/9; Dex n = 8/7; (C/D) NVC n = 15/15, Veh n = 21/16, Dex lip n = 15/13, IgG-Dex lip n = 10/9; Dex n = 8/7. *P < 0.05, **P < 0.01, ***P < 0.001 versus NVC, LVT/HVT Veh or LVT/HVT Dex lip. Dex, LVT or HVT-ventilated mice intravenously treated with free dexamethasone; Dex lip, LVT or HVT-ventilated mice intravenously treated with liposomes containing dexamethasone; HVT, mice ventilated with high tidal volumes; IgG-Dex lip, LVT or HVT-ventilated mice intravenously treated with IgG-liposomes containing dexamethasone; LVT, mice ventilated with low tidal volumes; NVC, non-ventilated controls; Veh, LVT or HVT-ventilated mice intravenously treated with vehicle (sterile saline).
Effect of empty liposomes on lung inflammation
Liposomal formulations without entrapped dexamethasone were administered at initiation of ventilation to evaluate whether lipid carriers as such would influence the inflammatory response caused by LVT or HVT-ventilation. We used empty IgG-modified liposomes as these liposomal formulations were most efficient in down-regulating ventilator-induced lung inflammation. Empty IgG-liposomes were ineffective in alleviating the increase in MPO activity, IL-1β, IL-6, KC and CCL2 mRNA expression in lungs of LVT and HVT-ventilated mice (Figure 5A to J).
Figure 5.
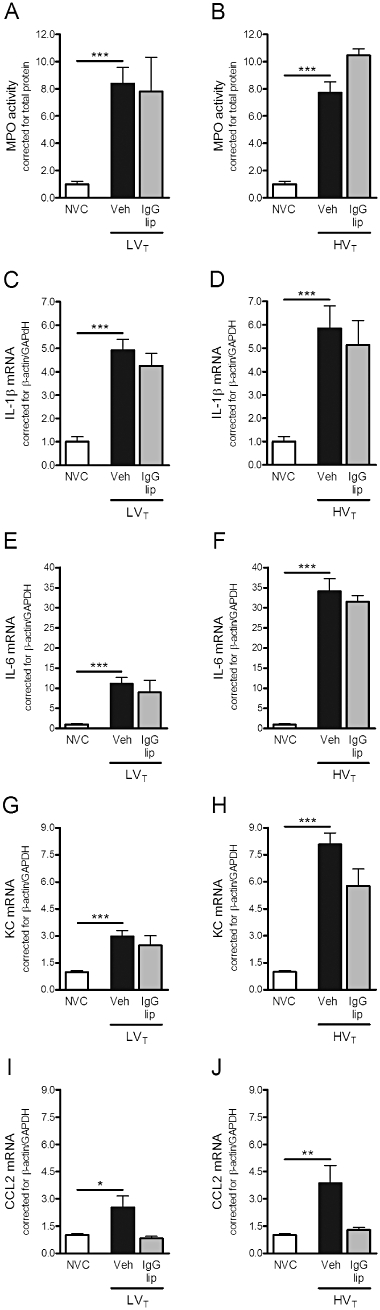
Effect of empty IgG-liposomes on granulocyte infiltration, cytokine and chemokine mRNA expression induced by mechanical ventilation. (A–B) In total lung homogenates, myeloperoxidase (MPO) activity was determined as a measure of granulocyte infiltration. Levels were normalized for total protein levels. (C–F) In total lung homogenates, mRNA expression of the cytokines interleukin (IL)-1β and IL-6 was determined by real-time RT-PCR. G–J: In addition, mRNA expression of the chemokines keratinocyte-derived chemokine (KC) and CC chemokine ligand (CCL) 2 was determined. Levels were normalized for expression of internal controls, that is, the average value of β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are expressed as mean ± SEM, and depicted relative to NVC. (A/B) NVC n = 18/18, Veh n = 20/14, IgG-lip n = 4/2; (C/D) NVC n = 15/15, Veh n = 22/17, IgG-lip n = 4/2; (E/F) NVC n = 15/15, Veh n = 21/18, IgG-lip n = 4/2; (G/H) NVC n = 15/15, Veh n = 20/17, IgG-lip n = 4/2; (I/J) NVC n = 15/15, Veh n = 21/16, IgG-lip n = 4/2. *P < 0.05, **P < 0.01, ***P < 0.001 versus NVC. HVT, mice ventilated with high tidal volumes; IgG-lip, LVT or HVT-ventilated mice intravenously treated with empty IgG-liposomes; LVT, mice ventilated with low tidal volumes; NVC, non-ventilated controls; Veh, LVT or HVT-ventilated mice intravenously treated with vehicle (sterile saline).
Discussion and conclusions
The present study was designed to evaluate whether liposome-encapsulated dexamethasone attenuated lung inflammation induced by mechanical ventilation. Our major finding is that FcγR-targeted IgG-Dex-liposomes were pharmacologically more effective than Dex-liposomes particularly in preventing influx of granulocytes, a major hallmark of VILI. We demonstrated that treatment with IgG-Dex-liposomes successfully protected against granulocyte influx induced by LVT or HVT-ventilation, which was accompanied by inhibition of IL-1β and KC mRNA expression in both ventilation groups, and IL-6 and CCL2 mRNA expression in the LVT-ventilated group. Moreover, IgG-Dex-liposomes inhibited most parameters of ventilator-induced lung inflammation as effectively as free dexamethasone. In contrast, Dex-liposomes only attenuated IL-1β and KC mRNA during both ventilation strategies and IL-6 mRNA and granulocyte influx during LVT-ventilation. All administration formulae of dexamethasone preserved pulmonary architecture during mechanical ventilation without inducing changes in haemodynamic and blood gas variables.
Both LVT and HVT-ventilation induced inflammation in pulmonary tissue as measured by increased granulocyte infiltration and cytokine/chemokine mRNA expression. Since we used non-perfused lung homogenates, we cannot exclude that part of this enhanced cytokine/chemokine response was derived from blood cells. Previous studies described that the combined effects of high PaO2 levels and mechanical ventilation worsen VILI (Sinclair et al., 2004; Li et al., 2007). Since we used moderate hyperoxia (FiO2 of 50 %) in our experimental model of VILI, it cannot be excluded that the higher oxygen levels may aggravate the stretch-induced inflammatory response in the lung. However, we have already shown that hyperoxia (FiO2 of 100 %) by itself did not lead to pulmonary inflammation (Hegeman et al., 2009). Therefore, it is tempting to speculate that the moderate hyperoxia in our present study will not be the primary cause of lung inflammation during mechanical ventilation.
In agreement with prior reports (Held et al., 2001; Ohta et al., 2001), we showed that free dexamethasone successfully protects against lung inflammation induced by 5 h of mechanical ventilation. It has been recognized that systemic administration of glucocorticoids may lead to unwanted side-effects like hyperglycaemia, deposition of body fat, suppressed systemic immunity and increased susceptibility to infections (Schacke et al., 2002). The advantage of using liposome-encapsulated dexamethasone is that the drug will be released more locally in the lung thereby inhibiting ventilator-induced inflammatory responses without inducing hyperglycaemia, one of the first clinically relevant side-effects of free dexamethasone treatment (Weinstein et al., 1995; Feldman-Billard et al., 2006). Unfortunately, we were not able to investigate the effects of dexamethasone on blood glucose levels in this experimental model of VILI as the anaesthesia procedure itself was affecting blood glucose levels (Zuurbier et al., 2008). However, our previous study in a murine model of glomerulonephritis clearly showed that liposome-encapsulated dexamethasone prevents the occurrence of hyperglycaemia associated with administration of the free drug (Asgeirsdottir et al., 2007).
An important feature of liposomes is their preferential extravasation at sites with increased capillary permeability (Storm and Crommelin, 1998). As ventilator-induced stretch of lung tissue enhances vascular permeability (Egan, 1982; Parker et al., 1984; Dreyfuss et al., 1985), liposomes may accumulate in cells of the lung and slowly release their encapsulated dexamethasone locally. Indeed, we observed that administration of Dex-liposomes significantly inhibited IL-1β and KC mRNA expression in lungs of LVT and HVT-ventilated mice and IL-6 mRNA expression in lungs of LVT-ventilated mice. More importantly, Dex-liposomes diminished granulocyte infiltration induced by LVT-ventilation.
Even though Dex-liposomes were capable of attenuating important parameters of VILI, especially in LVT-ventilated mice, they were not as effective as free dexamethasone in preventing granulocyte infiltration. Since granulocytes are known to be important in the pathogenesis of VILI (Kawano et al., 1987), active delivery of the drug into these circulating granulocytes might be advantageous. Therefore, we hypothesized that IgG-Dex-liposomes may be more efficient in inhibiting ventilator-induced lung inflammation than Dex-liposomes due to interaction with the FcγRs on activated granulocytes and macrophages (McKenzie and Schreiber, 1998). We found that IgG-Dex-liposomes significantly inhibited granulocyte influx in lungs of both LVT and HVT-ventilated mice whereas Dex-liposomes only attenuated granulocyte infiltration in lungs of LVT-ventilated mice. Based on this result, we propose that IgG-Dex-liposomes interact more efficiently with granulocytes in the systemic circulation or in the marginal zone of vessels, thereby preventing granulocyte activation and infiltration into lung tissue. The observation that glucocorticoids suppress granulocyte activation and recruitment into the alveolar space (Ohta et al., 2001) supports our current findings. Moreover, (IgG)-Dex-liposomes may also be internalized more efficiently by macrophages in the lung, where they primarily prevent de novo synthesis of IL-1β and KC. It should be noted that the lipid carriers themselves were ineffective in attenuating lung inflammation, as IgG-liposomes without entrapped dexamethasone did not affect the ventilator-induced increase in inflammatory mediator expression.
Previously, Suntres and Shek described that liposome-encapsulated dexamethasone diminished neutrophil activation and infiltration in a rat model of lipopolysaccharide (LPS)-induced acute lung injury (ALI) (Suntres and Shek, 2000). Moreover, these authors demonstrated that liposome-encapsulated dexamethasone was even more effective in down-regulating LPS-induced lung inflammation than free dexamethasone. Here we show that IgG-Dex-liposomes inhibited granulocyte influx in lungs of LVT and HVT-ventilated mice as efficiently as free dexamethasone. An explanation for this discrepancy in efficacy might be that Suntres and Shek investigated the effects of liposome-encapsulated and free dexamethasone at 24 h after LPS-challenge (Suntres and Shek, 2000). Because liposomes are considered as slow-release systems (Suntres and Shek, 1998), it may well be that liposomes become more effective than free dexamethasone after longer periods of time. Moreover, it is important to note that the underlying mechanisms of LPS-induced and ventilator-induced lung inflammation are likely to be different as well, especially with respect to the consequences of mechanical stretch on lung tissue. Taken together, our present data show that liposome-encapsulated dexamethasone may effectively prevent infiltration of granulocytes and de novo synthesis of pro-inflammatory cytokines and chemokines, in particular during LVT-ventilation.
Interestingly, Dex-liposomes and IgG-Dex-liposomes did not influence IL-6 and CCL2 mRNA expression induced by HVT-ventilation. These data may indicate that the target cells of our liposomal formulations may not be the only source of IL-6 and CCL2 production. In vitro studies have demonstrated that alveolar epithelial and capillary endothelial cells are also activated by mechanical stretch, in a stretch-amplitude dependent matter, thereby releasing inflammatory mediators into the surrounding pulmonary tissue (Vlahakis et al., 1999; Iwaki et al., 2009). Iwaki et al. demonstrated that high cyclic stretch of microvascular endothelial cells led to increased production of IL-6 and CCL2 (Iwaki et al., 2009). In agreement with these previous findings, our study shows that IL-6 and CCL2 levels were elevated in HVT-ventilated mice which were exposed to high levels of mechanical stretch. It is therefore tempting to speculate that alveolar epithelial and capillary endothelial cells will not internalize the liposomal formulations and therefore be responsible for the lack of IL-6 and CCL2 down-regulation after liposome treatment. The fact that liposome-encapsulated dexamethasone was effective in inhibiting IL-1β and KC, which are primarily produced by alveolar macrophages (Bhatia and Moochhala, 2004), supports this explanation. Future studies are needed to obtain further insights into the working mechanism of Dex-liposomes and IgG-Dex-liposomes with respect to the enhancement of CCL2 mRNA expression in HVT-ventilated mice after liposome treatment. Our present data, however, suggest that the increase in CCL2 is not caused by the liposomes themselves, as treatment with empty IgG-liposomes did not enhance CCL2 mRNA expression.
In conclusion, our study shows for the first time that liposomal formulation of the glucocorticoid dexamethasone prevents important parameters of ventilator-induced lung inflammation. Importantly, we observed that the selective targeting of Dex-liposomes to cells expressing FcγRs, by conjugating IgG to the liposomes, markedly improved their efficacy. In this respect, IgG-Dex-liposomes inhibited most parameters of ventilator-induced lung inflammation as effectively as free dexamethasone with the advantage that liposome-encapsulated dexamethasone will be released locally in the lung thereby preventing systemic side-effects. In view of our data, we suggest that IgG-Dex-liposomes may be an attractive therapeutic strategy to treat critically ill patients diagnosed with serious inflammatory lung diseases such as VILI.
Acknowledgments
This study was financially supported by the Catharijne Foundation of the University Medical Center Utrecht, the Netherlands. The authors thank Henriëtte Morselt for her expert technical assistance.
Glossary
Abbreviations
- ALI
acute lung injury
- AP
activator protein
- BE
base excess
- CCL
CC chemokine ligand
- Dex-liposomes
dexamethasone encapsulated in liposomes
- FcγR
Fcγ-receptor
- FiO2
fractional inspired oxygen concentration
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GR
glucocorticoid receptor
- H&E
haematoxylin and eosin
- HVT
high tidal volume
- IgG-Dex-liposomes
dexamethasone encapsulated in IgG-modified liposomes
- IL
interleukin
- KC
keratinocyte-derived chemokine
- LPS
lipopolysaccharide
- LVT
low tidal volume
- MCP
monocyte chemotactic protein
- MPO
myeloperoxidase
- NF-κB
nuclear factor kappa B
- NVC
non-ventilated controls
- PaCO2
partial pressure of arterial carbon dioxide
- PaO2
partial pressure of arterial oxygen
- PEEP
positive end-expiratory pressure
- PMN
polymorphonuclear cell
- RT-PCR
reverse transcriptase polymerase chain reaction
- VILI
ventilator-induced lung injury
Conflicts of interest
None.
Supporting Information
Teaching Materials; Figs 1–5 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgeirsdottir SA, Kamps JA, Bakker HI, Zwiers PJ, Heeringa P, van der Weide K, et al. Site-specific inhibition of glomerulonephritis progression by targeted delivery of dexamethasone to glomerular endothelium. Mol Pharmacol. 2007;72:121–131. doi: 10.1124/mol.107.034140. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol. 2006;148:245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- Bottcher CDF, van Gent CM, Pries C. Rapid and sensitive submicro phosphorus determination. Anal Chim Acta. 1961;24:203–204. [Google Scholar]
- Brower RG, Ware LB, Berthiaume Y, Matthay MA. Treatment of ARDS. Chest. 2001;120:1347–1367. doi: 10.1378/chest.120.4.1347. [DOI] [PubMed] [Google Scholar]
- Dreyfuss D, Saumon G. From ventilator-induced lung injury to multiple organ dysfunction? Intensive Care Med. 1998;24:102–104. doi: 10.1007/s001340050529. [DOI] [PubMed] [Google Scholar]
- Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis. 1985;132:880–884. doi: 10.1164/arrd.1985.132.4.880. [DOI] [PubMed] [Google Scholar]
- Egan EA. Lung inflation, lung solute permeability, and alveolar edema. J Appl Physiol. 1982;53:121–125. doi: 10.1152/jappl.1982.53.1.121. [DOI] [PubMed] [Google Scholar]
- Feldman-Billard S, Du Pasquier-Fediaevsky L, Heron E. Hyperglycemia after repeated periocular dexamethasone injections in patients with diabetes. Ophthalmology. 2006;113:1720–1723. doi: 10.1016/j.ophtha.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Haitsma JJ, Uhlig S, Verbrugge SJ, Goggel R, Poelma DL, Lachmann B. Injurious ventilation strategies cause systemic release of IL-6 and MIP-2 in rats in vivo. Clin Physiol Funct Imaging. 2003;23:349–353. doi: 10.1046/j.1475-0961.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- Hegeman MA, Hennus MP, Heijnen CJ, Specht PA, Lachmann B, Jansen NJ, et al. Ventilator-induced endothelial activation and inflammation in the lung and distal organs. Crit Care. 2009;13:R182. doi: 10.1186/cc8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held HD, Boettcher S, Hamann L, Uhlig S. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-kappaB and is blocked by steroids. Am J Respir Crit Care Med. 2001;163:711–716. doi: 10.1164/ajrccm.163.3.2003001. [DOI] [PubMed] [Google Scholar]
- Iwaki M, Ito S, Morioka M, Iwata S, Numaguchi Y, Ishii M, et al. Mechanical stretch enhances IL-8 production in pulmonary microvascular endothelial cells. Biochem Biophys Res Commun. 2009;389:531–536. doi: 10.1016/j.bbrc.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Mori S, Cybulsky M, Burger R, Ballin A, Cutz E, et al. Effect of granulocyte depletion in a ventilated surfactant-depleted lung. J Appl Physiol. 1987;62:27–33. doi: 10.1152/jappl.1987.62.1.27. [DOI] [PubMed] [Google Scholar]
- Li LF, Liao SK, Ko YS, Lee CH, Quinn DA. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: a prospective, controlled animal experiment. Crit Care. 2007;11:R25. doi: 10.1186/cc5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce JM. Corticosteroids in ARDS. An evidence-based review. Crit Care Clin. 2002;18:79–89. doi: 10.1016/s0749-0704(03)00066-6. [DOI] [PubMed] [Google Scholar]
- McKenzie SE, Schreiber AD. Fc gamma receptors in phagocytes. Curr Opin Hematol. 1998;5:16–21. doi: 10.1097/00062752-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Melgert BN, Olinga P, Jack VK, Molema G, Meijer DK, Poelstra K. Dexamethasone coupled to albumin is selectively taken up by rat nonparenchymal liver cells and attenuates LPS-induced activation of hepatic cells. J Hepatol. 2000;32:603–611. doi: 10.1016/s0168-8278(00)80222-6. [DOI] [PubMed] [Google Scholar]
- Ohta N, Shimaoka M, Imanaka H, Nishimura M, Taenaka N, Kiyono H, et al. Glucocorticoid suppresses neutrophil activation in ventilator-induced lung injury. Crit Care Med. 2001;29:1012–1016. doi: 10.1097/00003246-200105000-00027. [DOI] [PubMed] [Google Scholar]
- Parker JC, Townsley MI, Rippe B, Taylor AE, Thigpen J. Increased microvascular permeability in dog lungs due to high peak airway pressures. J Appl Physiol. 1984;57:1809–1816. doi: 10.1152/jappl.1984.57.6.1809. [DOI] [PubMed] [Google Scholar]
- Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med. 1993;21:131–143. doi: 10.1097/00003246-199301000-00024. [DOI] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med. 2004;32:2496–2501. doi: 10.1097/01.ccm.0000148231.04642.8d. [DOI] [PubMed] [Google Scholar]
- Slutsky AS. Lung injury caused by mechanical ventilation. Chest. 1999;116:9S–15S. doi: 10.1378/chest.116.suppl_1.9s-a. [DOI] [PubMed] [Google Scholar]
- Storm G, Crommelin DJA. Liposomes: quo vadis? Pharm Sci Technol Today. 1998;1:19–31. [Google Scholar]
- Suntres ZE, Shek PN. Liposomes promote pulmonary glucocorticoid delivery. J Drug Target. 1998;6:175–182. doi: 10.3109/10611869808997891. [DOI] [PubMed] [Google Scholar]
- Suntres ZE, Shek PN. Prophylaxis against lipopolysaccharide-induced lung injuries by liposome-entrapped dexamethasone in rats. Biochem Pharmacol. 2000;59:1155–1161. doi: 10.1016/s0006-2952(99)00411-6. [DOI] [PubMed] [Google Scholar]
- Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;277:L167–L173. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- Weinstein SP, Paquin T, Pritsker A, Haber RS. Glucocorticoid-induced insulin resistance: dexamethasone inhibits the activation of glucose transport in rat skeletal muscle by both insulin- and non-insulin-related stimuli. Diabetes. 1995;44:441–445. doi: 10.2337/diab.44.4.441. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Choudhury S, Goddard ME, O'Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol. 2003;95:1385–1393. doi: 10.1152/japplphysiol.00213.2003. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Choudhury S, Takata M. Pulmonary inflammation induced by high-stretch ventilation is mediated by tumor necrosis factor signaling in mice. Am J Physiol Lung Cell Mol Physiol. 2005;288:L599–L607. doi: 10.1152/ajplung.00304.2004. [DOI] [PubMed] [Google Scholar]
- Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ, Juffermans NP, Schultz MJ. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care. 2009;13:R1. doi: 10.1186/cc7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier CJ, Keijzers PJ, Koeman A, Van Wezel HB, Hollmann MW. Anesthesia's effects on plasma glucose and insulin and cardiac hexokinase at similar hemodynamics and without major surgical stress in fed rats. Anesth Analg. 2008;106:135–142. doi: 10.1213/01.ane.0000297299.91527.74. table. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


