Abstract
A functional balance between excitatory and inhibitory control over dopamine (DA)-dependent behavioral and neurochemical effects of cocaine is afforded by the serotonin2C receptor (5-HT2CR) located within the ventral tegmental area and the nucleus accumbens (NAc). The 5-HT2CR located in the medial prefrontal cortex (mPFC) has also been shown to inhibit cocaine-induced behaviors perhaps through inhibition of DA function in the NAc. Using in vivo microdialysis in halothane-anesthetized rats, we tested this hypothesis by assessing the influence of mPFC 5-HT2CRs on cocaine-induced DA outflow in the NAc shell. Intra-mPFC injection of the 5-HT2CR agonist Ro 60-0175 at 5 µg/0.2 µl, but not 1 µg/0.2 µl, potentiated the increase in accumbal DA outflow induced by the intraperitoneal administration of 10 mg/kg of cocaine. Conversely, cocaine-induced accumbal DA outflow was significantly reduced by the intra-mPFC injection of the selective 5-HT2CR antagonist SB 242084 (0.5 µg/0.2 µl) or SB 243213 (0.5 and 1 µg/0.2 µl).
These results show that mPFC 5-HT2CRs exert a positive control over cocaine-induced accumbal DA outflow. Observations further support the idea that the overall action of central 5-HT2CRs on accumbal DA output is dependent on the functional balance among different 5-HT2CR populations located within the mesocorticoaccumbens system, and that 5-HT2CRs can modulate DA-dependent behaviors independently of changes of accumbal DA release itself.
Keywords: 5-HT2C receptor, cocaine, medial prefrontal cortex, nucleus accumbens, accumbal dopamine release, rat
Introduction
The central serotonin2C receptor (5-HT2CR) is now well established as a modulator of dopamine (DA) neuron function in the mammalian brain (for review see Alex and Pehek, 2007; Berg et al., 2008), and the knowledge of this interaction is currently considered as a promising avenue for improved treatments of neuropsychiatric disorders related to DA neuron dysfunction (Bubar and Cunningham, 2006; Di Giovanni et al., 2006; Millan, 2005). Studies focusing on the mesoaccumbens DA pathway, in keeping with its importance in mediating the behavioral effects of drug of abuse (Kalivas and Volkow, 2005), have highlighted the potential of the 5-HT2CR to control cocaine abuse and dependence (Bubar and Cunningham, 2006; Higgins and Fletcher, 2003). In fact, 5-HT2CR ligands consistently modulate DA-dependent behavioral and neurochemical effects induced by cocaine (Bubar and Cunningham, 2006; Fletcher et al., 2006, 2008; Higgins and Fletcher, 2003; Liu and Cunningham, 2006: Navailles et al., 2004).
Intracranial microinjection studies have recently shown that cocaine-induced DA release and behaviors are under the control of distinct 5-HT2CR populations expressed in the ventral tegmental area (VTA) and the NAc, which provide inhibitory (VTA, NAc) and excitatory (NAc) controls (Filip and Cunningham, 2002; Fletcher et al., 2004; McMahon et al., 2001; Navailles et al., 2008). These findings led to the proposal that the overall inhibitory effect of 5-HT2CRs on the mesoaccumbens DA pathway results from a composite response involving a functional balance between different populations of 5-HT2CRs within multiple brain DA areas (Filip and Cunningham, 2002; Navailles et al., 2006, 2008). In line with this conclusion, 5-HT2CRs localized within the medial prefrontal cortex (mPFC) (Bubar and Cunningham, 2007; Clemett et al., 2000), a brain region functionally linked to the mesoaccumbens DA pathway and known to play a key role in reward-related mechanisms of drug abuse (Tzschentke, 2001), have been also shown to inhibit the behavioral effects of cocaine (Filip and Cunningham, 2003). Considering that cocaine-induced behaviors are thought to result from increased DA efflux in the NAc (Di Chiara, 2002; Dunnett and Robbins, 1992), they raise the possibility that mPFC 5-HT2CRs exert an inhibitory control over cocaine-induced accumbal DA outflow (Filip and Cunningham, 2003). However, direct neurochemical evidence for this hypothesis is still lacking.
The present study was therefore aimed at determining the contribution of mPFC 5-HT2CRs in the control of cocaine-induced accumbal DA outflow, to specifically identify the nature (inhibition/excitation) of this control. Experiments were performed using in vivo microdialysis in halothane-anesthetized rats, an experimental procedure allowing simultaneous implantation of a dialysis cannula in the medio-ventral subdivision (shell) of the NAc and an injection cannula in the ipsilateral mPFC (Navailles et al., 2006, 2008). According to previous behavioral studies (Filip and Cunningham, 2003), selective 5-HT2CR agonist (Ro 60-0175) and antagonists (SB 242084, SB 243213) were applied locally into the mPFC prior to the intraperitoneal administration of cocaine.
Materials and Methods
Animals
Male Sprague Dawley rats (IFFA CREDO, Lyon, France) weighing 320–350 g were used. Animals were kept at constant room temperature (21 ± 2°C) and relative humidity (60%) with a 12 hour light/dark cycle (dark from 20:00 h) and had free access to water and food. All animal use procedures conformed to International European Ethical Standards (86/609-EEC) and the French National Committee (décret 87/848) for the care and use of laboratory animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Drugs
The following compounds were used: Ro 60–0175.HCl (S-2-(6-chloro-5-fluoroindol-1-yl)-1-methylethylamine.hydrochloride) kindly donated by Dr P. Weber (F Hoffmann-La Roche, Basel, Switzerland); SB 243213-A (5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]-6-trifluoromethylindoline.hydrochloride) generously provided by Dr M. Wood (Psychiatry, Centre of Excellence for Drug Discovery, GlaxoSmithKline, Harlow, U.K); SB 242084.2HCl (6-chloro-5-methyl-1-[6-(2-methylpiridin-3-yloxy)pyridin-3-yl carbamoyl]indoline.dihydrochloride) was purchased from Sigma-RBI (Saint Quentin Fallavier, France). Cocaine hydrochloride was purchased from Cooper (Melun, France). All other chemicals and reagents were the purest commercially available (VWR, Strasbourg, France; Sigma, Illkirch, France).
Microdialysis
Surgery and perfusion procedures were performed as previously described (Navailles et al., 2008). Briefly, rats were anesthetized with a mixture of halothane and nitrous oxide-oxygen (2%; 2:1 v/v). After tracheotomy for artificial ventilation, the animals were placed in a stereotaxic frame, and their rectal temperature was monitored and maintained at 37.3°C ± 0.1 with a heating pad. A microdialysis probe (2 mm long, CMA/11, 240 µm outer diameter, Cuprophan; Carnegie Medicin, Phymep, Paris, France) was implanted in the medio-ventral part of the right NAc, corresponding to the shell subdivision (coordinates from interaural point: anteroposterior [AP] = 10.7, lateral [L] =1, ventral [V] = 2) according to the atlas of Paxinos and Watson (1986). The probe was perfused at a constant flow rate of 2 µl/min by means of a microperfusion pump (CMA 111, Carnegie Medicin, Phymep) with artificial cerebrospinal fluid (aCSF) containing (in mM): 154.1 Cl−, 147 Na+, 2.7 K+, 1 Mg2+, and 1.2 Ca2+, adjusted to pH 7.4 with 2 mM sodium phosphate buffer. Dialysates (30 µl) were collected on ice every 15 min. The in vitro recovery of the probe was about 10% for DA.
Surgical implantation of cannulae and microinjection protocol
Drug applications into the PFC were performed after the stabilization of DA levels in the perfusate (see “Pharmacological Treatments” section). A stainless steel cannula (30 G) was stereotaxically lowered (using an oblique approach, 10° from vertical) into the right PFC through a previously drilled hole. Stereotaxic coordinates were chosen according to the atlas of Paxinos and Watson (1986) to target the ventral division of the mPFC corresponding to the infralimbic (IL) subregion (coordinates from interaural point: AP = 12.7, L = 0.5, V = −4.8). Drug or corresponding vehicle was delivered into the mPFC in a final volume of 0.2 µl at a constant flow rate of 0.1 µl/min by a 10 µl Exmire syringe and a syringe pump (CMA 400, Carnegie Medicin, Phymep). After completion of the microinjection, the injection cannula was left in place for an additional 5 min before withdrawal to allow diffusion from the tip and prevent reflux of the solution injected.
Histology
At the end of each experiment, the brain was removed and fixed in NaCl (0.9%)/paraformaldehyde solution (10%). The location of the microdialysis probe into the NAc shell and the stainless steel cannula into the IL subregion of the mPFC were determined histologically on serial coronal sections (60 µm) stained with cresyl violet, and only data obtained from rats with correctly implanted probes were included in the results. No significant tissue damage was evident upon histological examination of sections.
Chromatographic Analysis
Dialysate samples were immediately analyzed by reverse-phase high-performance liquid chromatography coupled with electrochemical detection, as described previously (Navailles et al., 2008) with minor modifications. The mobile phase (containing [in mM] 70 NaH2PO4, 0.1 Na2EDTA, and 0.1 octylsulfonic acid plus 10% methanol, adjusted to pH 4.8 with orthophosphoric acid) was delivered at 0.250 ml/min flow rate (system LC-10AD-VP, Shimadzu, Champs s/ Marne, France) through an Equisil BDS column (C18; 2 × 250 mm, particle size 5 µm; Cluzeau Info Labo, France). Detection of DA was carried out with an amperometric detector (Antec Leyden DECADE II, Alpha-mos, Toulouse, France) with a glassy carbon electrode set at +450 mV versus Ag/AgCl. Output signals were recorded on a computer (system class VP-4, Shimadzu, France). Under these conditions, the sensitivity for DA was 0.3 ρg/30 µl with a signal/noise ratio of 3:1.
Pharmacological Treatments
Pharmacological treatments were performed after the stabilization of DA levels in the perfusate. A stable baseline, defined as three consecutive samples in which DA contents varied by less than 10%, was generally obtained 135 min after the beginning of the perfusion (stabilization period).
Cocaine was diluted in NaCl 0.9%, and administered intraperitoneally at 10 mg/kg in a volume of 2 ml/kg (Filip and Cunningham, 2003; Fletcher et al., 2002; Navailles et al., 2004, 2008). The 5-HT2CR agonist Ro 60-0175 and the selective 5-HT2CR antagonist SB 242084 were dissolved in NaCl 0.9%, and administered into the mPFC (Ro 60-0175: 1 and 5 µg/0.2 µl; SB 242084: 0.1 and 0.5 µg/0.2 µl) 15 min before the systemic administration of cocaine. The selective 5-HT2CR antagonist SB 243213 was dissolved in a mixture of NaCl 0.9% containing hydroxypropyl-β-cyclodextrin (HBC, 8% by weight) plus citric acid (25 mM), and administered into the PFC (0.5 and 1 µg/0.2 µl) 30 min before the systemic administration of cocaine. The final solution of each 5-HT2CR compound was adjusted to pH 6–7 prior to injection into the mPFC. Corresponding vehicle solution at pH 6–7 did not alter basal DA extracellular levels in the NAc (see control groups in figures). Doses, concentrations and pretreatment administration time of the different 5-HT2CR compounds used were chosen on the basis of dose and concentration range used in previous studies to keep both selectivity and efficiency toward the targeted sites (Filip and Cunningham, 2002; Fletcher et al., 2004; Kennett et al., 1997; Martin et al., 1998; Navailles et al., 2006, 2008). All drug doses and concentrations were calculated as the free base. In each experimental group, animals received either drugs or their appropriate vehicle.
Statistical Analysis
The DA content in each sample was expressed as the percentage of the average baseline level calculated from the three fractions preceding any treatment. Data correspond to the mean ± SEM values of the percentage obtained in each experimental group. The overall drug effect was calculated as the average of DA content from dialysates collected after their administration. The interaction between cocaine and 5-HT2CR compounds was studied by a two-way ANOVA (pre-treatment × treatment) with time as repeated measures, performed for the eight samples that followed cocaine administration. Thereafter, a one-way ANOVA (using group as the main factor) followed by the Fisher's protected least significant difference test (PLSD) was performed to allow adequate multiple comparisons between groups or, when the two-way ANOVA was not significant (p>0.05), to determine the effect of 5-HT2CR compounds in our experimental conditions.
For each experiment, statistical differences in basal DA values among groups were assessed by a one-way ANOVA (using group as a main factor).
Results
Histology
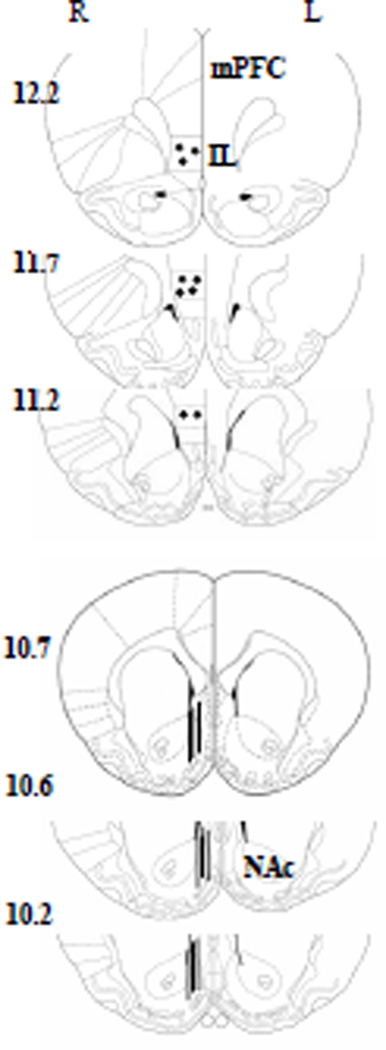
Figure 1 shows the location of microdialysis probe membranes in the NAc and injection cannula tips in the mPFC, redrawn in a schematic representation of frontal section rat brain from Paxinos and Watson atlas (1986). In the NAc, all the probe tips were located into the shell. In the mPFC injection cannula tips were located in the IL subregion. For all experiments, only animals with the probe membrane within the NAc shell and the cannula tip within the IL were included for analysis: ~ 12% of rats that underwent surgery were excluded.
Figure 1.
Histological verification of injection and perfusion sites. Filled circles in the top coronal sections indicate the location of injection cannula tips in the infralimbic (IL) subregion of the medial PFC (mPFC). Straight lines in the bottom coronal sections indicate the location of microdialysis probe membranes (2 mm) in the nucleus accumbens (NAc) shell. Plates are taken from Paxinos and Watson atlas (1986), and the number beside each plate corresponds to millimeters from interaural point. For clarity, the schematic diagram shows the representative sites of cannulae and probes placements for only a subset of the animals used. R = right hemisphere; L = left hemisphere.
Basal extracellular DA concentrations in dialysates from the NAc
All measurements were performed 150 min after the beginning of perfusion, by which time a steady state was achieved. Absolute basal levels of DA in dialysate collected from the NAc did not differ across experimental groups throughout the course of the study and were 5.3 ± 0.3 pg/30 µl (mean ± SEM, without adjusting for probe recovery; n = 168 animals).
Effect of intra-mPFC administration of 5-HT2CR agonist and antagonists on cocaine-induced increase in accumbal DA outflow
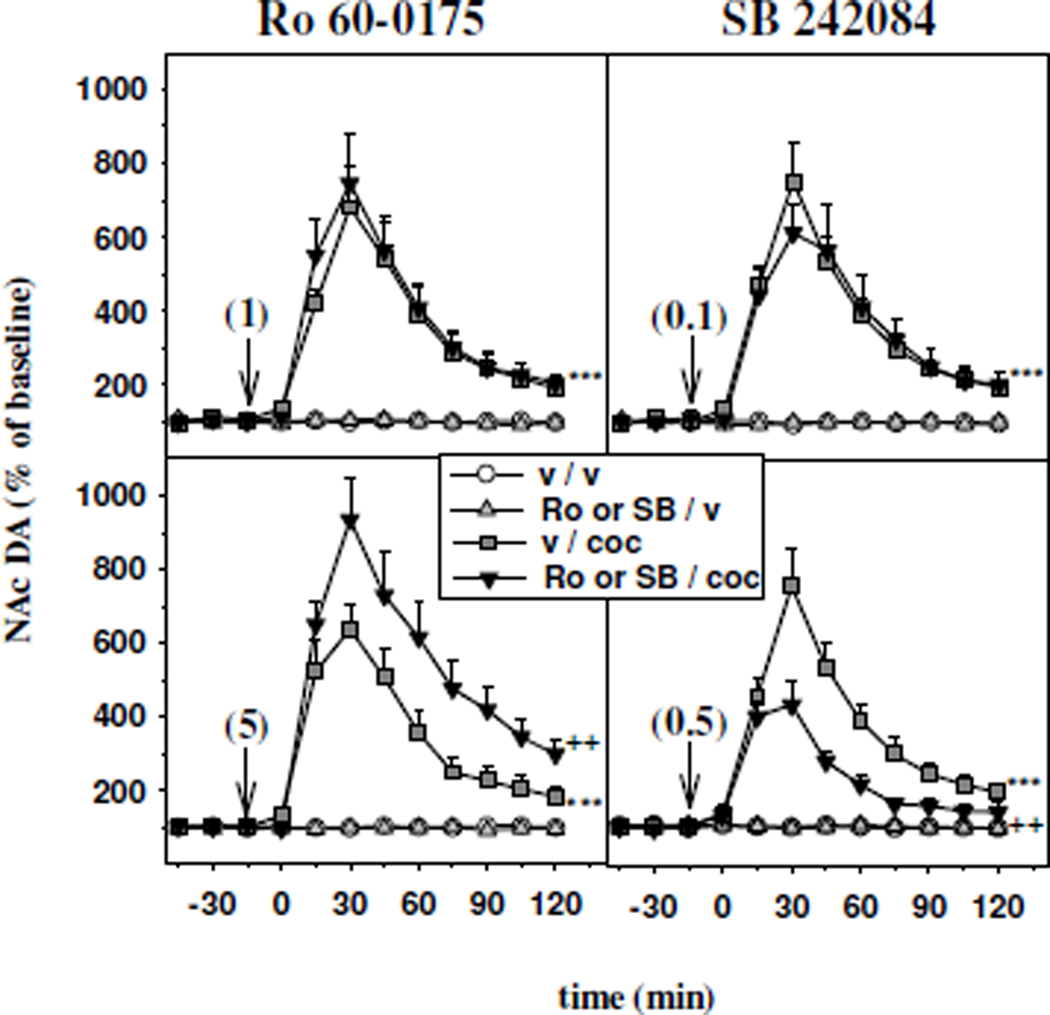
Figure 2 illustrates the effects of intra-mPFC injection of the 5-HT2CR agonist Ro 60-0175 at 1 µg/0.2 µl (upper left panel) and 5 µg/0.2 µl (lower left panel) and the 5-HT2CR antagonist SB 242084 at 0.1 µg/0.2 µl (upper right panel) and 0.5 µg/0.2 µl (lower right panel) on the increase in accumbal DA outflow induced by the intraperitoneal (ip.) administration of cocaine (10 mg/kg).
Figure 2.
Time course effect of the intra-mPFC administration of the 5-HT2CR agonist Ro 60-0175 and the 5-HT2CR antagonist SB 242084 on the increase in accumbal DA outflow induced by cocaine. Ro 60-0175 (Ro; left panels) and SB 242084 (SB; right panels) were injected into the mPFC (vertical arrows) 15 min before cocaine. The doses injected are indicated in parentheses in µg/ 0.2 µl. Cocaine (coc) was administered intraperitoneally at 10 mg/kg at time zero. Data are presented as the mean ± SEM percentages of the baseline calculated from the three samples preceding the first drug administration (n= 6–10 animals/group). ***p<0.001 versus the vehicle/vehicle (v / v) group and ++p<0.01 versus the vehicle/cocaine (v / coc) group (Fisher's PLSD test).
The systemic administration of 10 mg/kg of cocaine elicited an overall significant increase in accumbal DA efflux, reaching approximately 413% of baseline (p<0.001, Fisher's PLSD test). Indeed, the effect of cocaine peaked at 744% of baseline 30 min after its injection, and thereafter decreased progressively to about 200% of baseline at the end of the experiment. When administered at 1 µg/0.2 µl into the mPFC, Ro 60-0175 did not alter the increase in DA extracellular levels induced by cocaine in the NAc (two-way ANOVA, F1,28 = 0.11, not significant NS; upper left panel). However, when administered at 5 µg/0.2 µl into the mPFC, Ro 60-0175 significantly increased cocaine-stimulated DA efflux in the NAc (two-way ANOVA, F1,26 = 4.33, p<0.05; lower left panel). Indeed, DA extracellular levels in the NAc after intra-mPFC administration of 5 µg/0.2 µl Ro 60-0175 (Ro 60-0175/cocaine group) were significantly higher than those found after vehicle plus cocaine administration (p<0.01, Fisher’s PLSD test).
The facilitatory effect of cocaine on accumbal DA efflux was not altered by the intra-mPFC injection of SB 242084 at 0.1 µg/0.2 µl (two-way ANOVA, F1,23 = 0.01, NS; upper right panel), but significantly decreased after the intra-mPFC injection of SB 242084 at 0.5 µg/0.2 µl (two-way ANOVA, F1,22 = 5.33, p<0.05; lower right panel). Indeed, DA extracellular levels in the NAc after intra-mPFC administration of 0.5 µg/0.2 µl SB 242084 (SB 242084/cocaine group) were significantly lower than those found after vehicle plus cocaine administration (p<0.01, Fisher’s PLSD test).
Intra-PFC injections of Ro 60-0175 or SB 242084, at either dose, did not alter basal extracellular levels of DA in the NAc (NS, Fisher's PLSD test).
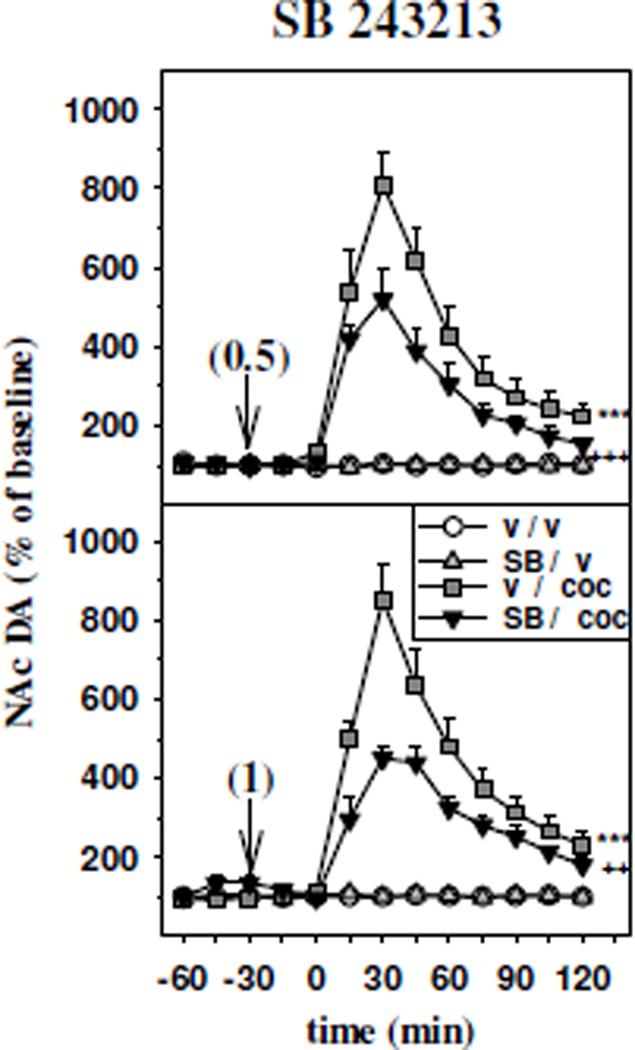
Figure 3 reports the effects of intra-mPFC injection of the 5-HT2CR antagonist SB 243213 at 0.5 µg/0.2 µl (upper panel) and 1 µg/0.2 µl (lower panel) on the increase in accumbal DA outflow induced by the administration of cocaine (10 mg/kg, ip.).
Figure 3.
Time course effect of the intra-mPFC administration of the 5-HT2CR antagonist SB 243213 on the increase in accumbal DA outflow induced by cocaine. SB 243213 was injected into the mPFC (vertical arrows) 30 min before cocaine. The doses injected are indicated in parentheses in µg/ 0.2 µl. Cocaine (coc) was administered intraperitoneally at 10 mg/kg at time zero. Data are presented as the mean ± SEM percentages of the baseline calculated from the three samples preceding the first drug administration (n= 5–8 animals/group). ***p<0.001 versus the vehicle/vehicle (v / v) group and ++p<0.01, +++p<0.001 versus the vehicle/cocaine (v / coc) group (Fisher's PLSD test).
The facilitatory effect of cocaine on accumbal DA efflux was significantly reduced by the intra-PFC injection of SB 243213 at either 0.5 µg/0.2 µl (two-way ANOVA, F1,22 = 6.20, p<0.05; upper panel), or 1 µg/0.2 µl (two-way ANOVA, F1,23 = 5.65, p<0.05; lower panel). Indeed, DA extracellular levels in the NAc after intra-mPFC administration of 0.5 µg/0.2 µl or 1 µg/0.2 µl SB 243213 (SB 243213/cocaine groups) were significantly lower than those found in their respective vehicle/cocaine groups (SB 243213 0.5 µg/0.2 µl: p<0.001; SB 243213 1 µg/0.2 µl: p<0.01, Fisher’s PLSD test).
Finally, basal DA outflow in the NAc was unaltered by either concentration of SB 243213 (NS, Fisher’s PLSD test).
Discussion
The present study provides the first neurochemical evidence that cocaine-induced DA efflux in the NAc shell undergoes a positive modulatory control by 5-HT2CR localized in the mPFC. Indeed, intra-mPFC administration of selective 5-HT2CR agonist and antagonist respectively increases and reduces accumbal DA efflux elicited by cocaine. These findings confirm and extend our previous investigations proposing that the overall inhibitory control exerted by central 5-HT2CRs over the mesoaccumbens DA pathway results from a balance between inhibitory and excitatory effects involving different 5-HT2CR populations localized to diverse brain regions (Filip and Cunningham, 2002, 2003; Navailles et al., 2006, 2008).
The systemic administration of cocaine elicited a significant increase in DA extracellular levels in the shell subregion of the NAc, as reported previously (Navailles et al., 2004, 2008). We found that cocaine-induced accumbal DA outflow was potentiated by the intra-mPFC injection of the 5-HT2CR agonist Ro 60-0175 (5 µg/0.2 µl). Despite different affinities of Ro 60-0175 for members of the 5-HT2R family (Martin et al., 1998; Porter et al., 1999), the observed effect of Ro 60-0175 is likely to result from the selective stimulation of the 5-HT2CR, over the 5-HT2AR and 5-HT2BR, in the mPFC. Indeed, Ro 60-0175 displays a 30-fold higher affinity for the 5-HT2CR over the 5-HT2AR (Martin et al., 1998; Porter et al., 1999), and 5-HT2BRs are not expressed within the PFC (Duxon et al., 1997). Furthermore, in the present study, Ro 60-0175 was locally administered at a dose regimen known to provide selective stimulation of 5-HT2CRs in the rat brain (Fletcher et al., 2004; Navailles et al., 2008; Pozzi et al., 2002). In line with previous observations (Navailles et al., 2008; Pozzi et al., 2002), the ability of the intra-mPFC injection of Ro 60-0175 to facilitate cocaine-induced DA outflow reveals the existence of a phasic control exerted by PFC 5-HT2CRs on stimulated DA release.
The influence of 5-HT2CR blockade on cocaine-induced DA outflow was assessed using two potent and selective 5-HT2CR antagonists, the SB 242084 and the SB 243213 (Berg et al., 2008). Both 5-HT2CR antagonists possess similar high affinity for the 5-HT2CR (pKi = 9 for SB 242084 and pKi = 9.4 for SB 243213) and 150-fold selectivity over the 5-HT2A and 5-HT2BRs (Kennett et al., 1997; Wood et al., 2001). Cocaine-induced overall DA efflux in the NAc was reduced to a similar extent (approximately −160%) by the intra-mPFC administration of either SB 242084 or SB 243213 at the concentration of 0.5 µg/0.2 µl. As no greater inhibitory effect was observed after the injection of a higher concentration of SB 243213 (1 µg/0.2 µl), the maximal blockade of 5-HT2CR may have been reached at the lower dose of the 5-HT2CR antagonists. Thus, the cocaine-induced accumbal DA outflow may be sustained only in part by 5-HT2CR stimulation elicited by increased endogenous 5-HT extracellular levels consequent to the blockade of 5-HT reuptake by cocaine (Müller et al., 2007). Indeed, as previously observed in the VTA and the NAc (Navailles et al., 2006, 2008), blockade of mPFC 5-HT2CRs per se has no influence on basal DA outflow in the NAc, a finding which likely reflect the existence of a low endogenous 5-HT tone at mPFC 5-HT2CR. Also, basal locomotor activity, a response typically related to increased accumbal DA release (Dunnett and Robbins, 1992), is unaltered by 5-HT2CR blockade in either brain regions (Filip and Cunningham, 2002, 2003; Fletcher et al., 2004; McMahon et al., 2001).
These findings together emphasize that the mPFC 5-HT2CR has no influence on accumbal DA outflow in resting conditions, but does contribute to the tonic and phasic excitatory controls on DA efflux elicited by cocaine. A similar positive control over cocaine-induced accumbal DA release was also observed after intra-NAc administration of low concentrations of a 5-HT2CR agonist or antagonist (Navailles et al., 2008). Nevertheless, this excitatory 5-HT2CR influence exerted at the level of the mPFC and NAc is overshadowed as the systemic administration of 5-HT2CR ligands affords essentially a net inhibitory effect (Navailles et al., 2004). In fact, cocaine-induced DA outflow in the NAc is potentiated or unaltered by the intraperitoneal administration of a 5-HT2CR antagonist or agonist, respectively (Navailles et al., 2004). Hence, the profile of effects of 5-HT2CR compounds on cocaine-induced accumbal DA efflux after systemic administration suggests that excitatory controls occurring at the level of the mPFC or the NAc are masked by a net inhibitory effect involving other 5-HT2CR populations likely located in the NAc itself or in other brain regions such as the VTA (Navailles et al., 2008). Indeed, stimulation of the VTA 5-HT2CR exerts a unidirectional inhibitory effect on accumbal DA outflow induced by cocaine, and, in the NAc, an excitatory effect is replaced by an inhibitory influence after intra-NAc infusion of higher concentrations of 5-HT2CR ligands (Navailles et al., 2008). In line with these considerations, systemic administration of 5-HT2CR agonists or antagonist has been shown to consistently result in an inhibition or excitation of DA-dependent behaviors induced by cocaine, respectively (Bubar and Cunningham, 2006; Fletcher et al., 2006; 2008; Higgins and Fletcher, 2003; Liu and Cunningham, 2006).
Our results confirm and extend the proposal that 5-HT2CR inhibitory control of the mesoaccumbens DA pathway may be considered as a composite response involving a functional balance between excitatory and inhibitory inputs to DA neurons (Filip and Cunningham, 2002, 2003; Navailles et al., 2006, 2008), related to different 5-HT2CR populations located within multiple brain areas (Clemett et al., 2000). This conclusion is further supported by the fact that, in contrast to their effects observed after systemic administration (Berg et al., 2008), local administration of 5-HT2CR ligands into the mPFC (present study), the VTA or the NAc (Navailles et al., 2006), had no influence on basal DA release in the NAc (Navailles et al., 2006, 2008, present results). Thus, inhibition of 5-HT reuptake by cocaine and consequent increase of 5-HT extracellular levels (Muller et al., 2007) appears as a permissive factor for the expression of the modulatory control of cocaine-induced accumbal DA release observed after the local administration of 5-HT2CR antagonists into either the mPFC (present study) or the NAc (Navailles et al., 2008). Furthermore, as discussed elsewhere (Navailles et al., 2008), the heterogeneous distribution of 5-HT transporter within the NAc (Brown and Molliver, 2000), by generating distinct functional 5-HT2CR populations, could account for the biphasic excitatory and inhibitory changes of accumbal DA release observed after intra-NAc administration of increasing concentrations of 5-HT2CR ligands.
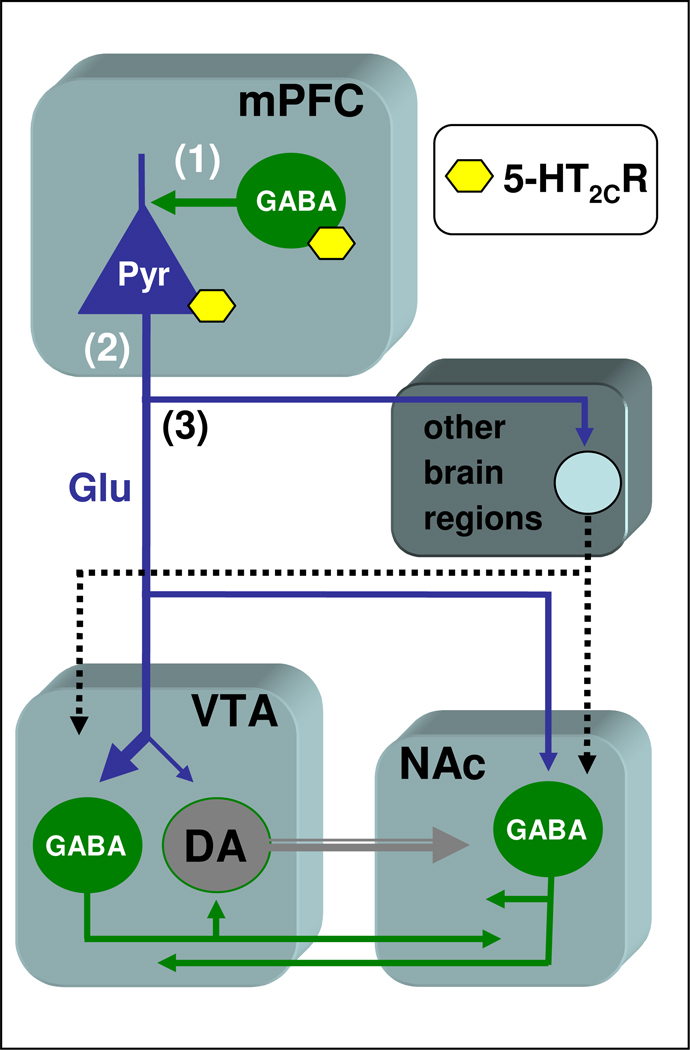
Although the mechanisms and circuits whereby mPFC 5-HT2CRs regulate DA release in the NAc cannot be specifically addressed in this study, it is conceivable that this interaction involves glutamate-containing pyramidal projection neurons connecting the mPFC to the mesoaccumbens DA pathway, at the level of both the VTA and the NAc (Sesack et al., 2003; Tzschentke, 2001), (see figure 4). In the mPFC, 5-HT2CRs are primarily localized to GABA interneurons which project to cell bodies and initial axon segments of pyramidal neurons (Liu et al., 2007; Vysokanov et al., 1998), and are known to inhibit mPFC pyramidal neurons (Bergqvist et al., 1999). Interestingly, anatomical studies demonstrate that the majority of glutamate-containing pyramidal neurons do not make direct synaptic contact with mesoaccumbens DA neurons in either the VTA or the NAc (Omelchenko and Sesack, 2007; Sesack et al., 2003), and suggest that the mesoaccumbens DA pathway is indirectly regulated through local collaterals or efferent projections originating from VTA and/or NAc GABA neurons which receive direct monosynaptic input from mPFC pyramidal cells (Sesack et al., 2003). In line with this conclusion, several studies, but not all (see Sesack et al., 2003), have reported that mPFC glutamate neurons provide an inhibitory control on NAc DA release (Jackson et al., 2001; Taber et al., 1996; Takahata and Moghaddam, 2000). Thus, it is tempting to speculate that stimulation of mPFC 5-HT2CRs would function to reduce excitatory glutamate output within the mesoaccumbens system leading to an indirect increase of accumbal DA outflow consequent to decreased GABA transmission in the VTA and/or the NAc. Nevertheless, the possible involvement of additional circuits underlying the effect of mPFC 5-HT2CRs on NAc DA release has to be considered. First, the 5-HT2CR also localizes to glutamate pyramidal neurons (Carr et al., 2002; Liu et al., 2007; Visokanov et al., 1998). Second, recent anatomical studies have shown that a small portion of mesoaccumbens DA neurons receives direct synaptic input from axons labelled for the vesicular glutamate transporter that is expressed by mPFC pyramidal neurons (Omelchenko and Sesack, 2007), Third, control of the mesoaccumbens DA pathway activity by mPFC may cover multisynaptic pathways involving brain regions, such as the amygdala, the habenula, the mediodorsal nucleus of the thalamus, the lateral hypothalamus, the brainstem laterodorsal/peduncolopontine tegmentum, the dorsal raphe nucleus, which receive projections from the mPFC pyramidal neurons (Gabbott et al., 2005; Sesack et al., 2003), and project in turn to the VTA and/or the NAc (Azmitia and Segal, 1978; Geisler et al., 2007; Omelchenko and Sesack, 2007; Pinto et al., 2003; Sesack et al., 2003). Hence, the control of mesoaccumbens DA pathway may involve a functional balance between mPFC 5-HT2CR populations providing opposite indirect (GABA-mediated) and direct effects on glutamate pyramidal cells, which in turn positively modulate accumbal DA release through polysynaptic cortico-subcortical pathways afferent to the mesoaccumbens DA system. Further research is needed to unravel the mechanisms and circuitry underlying this interaction.
Figure 4.
Schematic representation of the possible circuits involved in the control of mesoaccumbens DA neurons by mPFC 5-HT2CR. In the mPFC, the 5-HT2CR is expressed on GABA interneurons as well as on pyramidal glutamate neurons (Liu et al., 2007; Visokanov et al., 1998). The majority of pyramidal glutamate neurons do not provide direct innervation to mesoaccumbens DA neurons in either the VTA or the NAc (Sesack et al., 2003; Omelchenko and Sesack, 2007). Within the mesoaccumbens system, GABAergic neurons provide an inhibitory interface between glutamate input and mesoaccumbens DA neurons. (1) Stimulation of 5-HT2CR on GABA interneurons would function to reduce excitatory glutamate output to the mesoaccumbens DA pathway, thereby leading to an increase in DA neuron activity consequent to reduced GABA transmission within the VTA and/or NAc. (2) Stimulation of 5-HT2CR on pyramidal cells would increase excitatory glutamate output, thereby increasing GABA transmission within the VTA and/or NAc and, consequently, decreasing DA neuron activity. (3) Activation of pyramidal neurons could also provide indirect control of the mesoaccumbens DA pathway via other brain regions (amygdala, mediodorsal nucleus of the thalamus, lateral hypothalamus, brainstem laterodorsal/peduncolopontine tegmentum, dorsal raphe nucleus) receiving input from the mPFC pyramidal neurons (Gabbott et al., 2005; Sesack et al., 2003), and innervating the VTA and/or the NAc (Azmitia and Segal, 1978; Geisler et al., 2007; Omelchenko and Sesack, 2007; Pinto et al., 2003; Sesack et al., 2003). DA= dopamine; mPFC= medial prefrontal cortex; VTA= ventral tegmental area; NAc= nucleus accumbens; Pyr= pyramidal neuron; Glu= glutamate.
Finally, the obtained results suggest that NAc DA outflow cannot account for all of the effects of 5-HT2CR selective compounds on DA-dependent behaviors induced by cocaine. On the one hand, peripheral administration of 5-HT2CR antagonists increases both behavioral (Bubar and Cunningham, 2006; Higgins and Fletcher, 2003; Fletcher et al., 2006) and neurochemical (Navailles et al., 2004) effects of cocaine. Intra-VTA injection of 5-HT2CR agonist or antagonist also elicits parallel changes of accumbal DA efflux (Navailles et al., 2008) and DA-dependent behaviors evoked by cocaine (Fletcher et al., 2004; McMahon et al., 2001). Intra-NAc shell administration of 5-HT2CR agonist or antagonist facilitates both cocaine-induced behavior (Filip and Cunningham, 2002; McMahon et al., 2001) and accumbal DA release (Navailles et al., 2008), although this latter effect is observed only after the infusion of low concentration of 5-HT2CR ligands (Navailles et al., 2008). On the other hand, the intraperitoneal administration of the 5-HT2CR agonist Ro 60-0175 (1 mg/kg) has no influence on cocaine-stimulated accumbal DA outflow (Navailles et al., 2004), but potently reduces the hyperlocomotive and reinforcing properties of cocaine (Higgins and Fletcher, 2003; Fletcher et al., 2008). In addition, local injection of 5-HT2CR compounds into the mPFC facilitated accumbal DA outflow (present study), but inhibited behavioral responses induced by cocaine (Filip and Cunningham, 2003). As discussed elsewhere (Navailles et al., 2004, 2008), it is unlikely that the different experimental procedures used in behavioral and neurochemical studies, including anesthesia, may account for the different effect of 5-HT2CR agents on the neurochemical and behavioral responses induced by cocaine. More likely, our findings, together with the data mentioned above, suggest that, as already shown for the 5-HT1AR (Müller et al., 2007), 5-HT2CRs may facilitate cocaine-induced DA behaviors independently from a net action on NAc DA outflow, by controlling DA transmission downstream from DA neurons (Navailles et al., 2004, 2008; Zachariou et al., 2002).
In conclusion, the present study provides the first neurochemical evidence that mPFC 5-HT2CRs are able to modulate cocaine-evoked DA efflux in the NAc shell in an excitatory manner. This finding confirms and extends the idea that the overall inhibitory effect exerted by the 5-HT2CR on cocaine-induced DA outflow may result from a functional balance between excitatory and inhibitory effects involving different populations of 5-HT2CRs localized within the mPFC, VTA and NAc (Filip and Cunningham, 2002, 2003; Navailles et al., 2004, 2008). Furthermore, in keeping with the differential effects of 5-HT2CR agents on DA outflow and DA-dependent behaviors induced by cocaine (Filip and Cunningham, 2003; Navailles et al., 2004, 2008), our findings indicate that 5-HT2CRs can modulate mesoaccumbens DA activity by controlling NAc DA transmission independently of changes of accumbal DA release itself. Finally, the obtained results afford additional knowledge into the prominent role of the 5-HT2CR in the regulatory neurochemistry of mesoaccumbens DA functions, and its potential for improved treatments of cocaine abuse and dependence (Bubar and Cunningham, 2006; Di Giovanni et al., 2006; Higgins and Fletcher, 2003).
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse DA 00260, DA 020087, and DA13595 (KAC) and Institut National de la Recherche et de la Santé (INSERM) and Bordeaux 2 University. We are grateful to Dr. P. Weber (F Hoffmann-La Roche, Basel, Switzerland) for the gift of Ro 60-0175, and to Dr. M. Wood (Psychiatry, Centre of Excellence for Drug Discovery, GlaxoSmithKline, Harlow, U.K) for the gift of SB 243213.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol. Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Cunningham KA, Spampinato U. Fine-tuning serotonin2C receptor function in the brain: Molecular and functional implications. Neuropharmacology. 2008;2008 doi: 10.1016/j.neuropharm.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist PB, Dong J, Blier P. Effect of atypical antipsychotic drugs on 5-HT2 receptors in the rat orbito-frontal cortex: an in vivo electrophysiological study. Psychopharmacology (Berl) 1999;143:89–96. doi: 10.1007/s002130050923. [DOI] [PubMed] [Google Scholar]
- Brown P, Molliver ME. Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. J. Neurosci. 2000;20:1952–1963. doi: 10.1523/JNEUROSCI.20-05-01952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr. Top. Med. Chem. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience. 2007;146:286–297. doi: 10.1016/j.neuroscience.2006.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J. Neurosci. 2002;22:6846–6855. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav. Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Pierucci M, Benigno A, Esposito E. Central serotonin2C receptor: from physiology to pathology. Curr. Top. Med. Chem. 2006;6:1909–1925. doi: 10.2174/156802606778522113. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Robbins TW. The functional role of mesotelencephalic dopamine systems. Biol. Rev. Camb. Philos. Soc. 1992;67:491–518. doi: 10.1111/j.1469-185x.1992.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Duxon MS, Flanigan TP, Reavley AC, Baxter GS, Blackburn TP, Fone KC. Evidence for expression of the 5-hydroxytryptamine-2B receptor protein in the rat central nervous system. Neuroscience. 1997;76:323–329. doi: 10.1016/s0306-4522(96)00480-0. [DOI] [PubMed] [Google Scholar]
- Filip M, Cunningham KA. Serotonin 5-HT(2C) receptors in nucleus accumbens regulate expression of the hyperlocomotive and discriminative stimulus effects of cocaine. Pharmacol. Biochem. Behav. 2002;71:745–756. doi: 10.1016/s0091-3057(01)00741-9. [DOI] [PubMed] [Google Scholar]
- Filip M, Cunninghan KA. Hyperlocomotive and discriminative stimulus effects of cocaine are under the control of serotonin(2C) (5-HT(2C)) receptors in rat prefrontal cortex. J. Pharmacol. Exp. Ther. 2003;306:734–743. doi: 10.1124/jpet.102.045716. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004;29:308–318. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Sinyard J, Higgins GA. The effects of the 5-HT2C receptor antagonist SB242084 on locomotor activity induced by selective, or mixed, indirect serotonergic and dopaminergic agonists. Psychopharmacology. 2006;187:515–525. doi: 10.1007/s00213-006-0453-9. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J. Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PL. Serotonin and drug reward: focus on 5-HT2C receptors. Eur. J. Pharmacol. 2003;480:151–162. doi: 10.1016/j.ejphar.2003.08.102. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Frost AS, Moghaddam B. Stimulation of prefrontal cortex at physiologically relevant frequencies inhibits dopamine release in the nucleus accumbens. J. Neurochem. 2001;78:920–923. doi: 10.1046/j.1471-4159.2001.00499.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackbum TP. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Cunningham KA. Serotonin2C receptors (5-HT2CR) control expression of cocaine-induced conditioned hyperactivity. Drug Alcohol Depend. 2006;81:275–282. doi: 10.1016/j.drugalcdep.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Martin JR, Bös M, Jenck F, Moreau JL, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HH, Broekkamp CL, Ruigt GS, Köhler C, Delft AM. 5-HT2C receptor agonists: Pharmacological characteristics and therapeutic potential. J. Pharmacol. Exp. Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- McMahon LR, Filip M, Cunningham KA. Differential regulation of the mesoaccumbens circuit by serotonin 5-hydroxytryptamine 5-HT2A and 5-HT2C receptors. J. Neurosci. 2001;21:7781–7787. doi: 10.1523/JNEUROSCI.21-19-07781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Müller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog. Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Navailles S, De Deurwaerdère P, Porras G, Spampinato U. In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2004;29:319–326. doi: 10.1038/sj.npp.1300329. [DOI] [PubMed] [Google Scholar]
- Navailles S, Moison D, Cunningham KA, Spampinato U. Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology. 2008;33:237–246. doi: 10.1038/sj.npp.1301414. [DOI] [PubMed] [Google Scholar]
- Navailles S, Moison D, Ryczko D, Spampinato U. Region-dependent regulation of mesoaccumbens dopamine neurons in vivo by the constitutive activity of central serotonin2C receptors. J. Neurochem. 2006;99:1311–1319. doi: 10.1111/j.1471-4159.2006.04188.x. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic press; 1986. [DOI] [PubMed] [Google Scholar]
- Pinto A, Jankowski M, Sesack SR. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J. Comp. Neurol. 2003;459:142–155. doi: 10.1002/cne.10596. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br. J. Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L, Acconcia S, Ceglia I, Invernizzi RW, Samanin R. Stimulation of 5-hydroxytryptamine (5-HT(2C)) receptors in the ventrotegmental area inhibits stress-induced but not basal dopamine release in the rat prefrontal cortex. J. Neurochem. 2002;82:93–100. doi: 10.1046/j.1471-4159.2002.00947.x. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann. N. Y. Acad. Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Taber MT, Baker GB, Fibiger HC. Glutamate receptor agonists decrease extracellular dopamine in the rat nucleus accumbens in vivo. Synapse. 1996;24:165–172. doi: 10.1002/(SICI)1098-2396(199610)24:2<165::AID-SYN8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Target-specific glutamatergic regulation of dopamine neurons in the ventral tegmental area. J. Neurochem. 2000;75:1775–1778. doi: 10.1046/j.1471-4159.2000.0751775.x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog. Neurobiol. 2001;63:241–320. doi: 10.1016/s0301-0082(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Vysokanov A, Flores-Hernandez J, Surmeier DJ. mRNAs for clozapine-sensitive receptors co-localize in rat prefrontal cortex neurons. Neurosci. Lett. 1998;258:179–182. doi: 10.1016/s0304-3940(98)00882-9. [DOI] [PubMed] [Google Scholar]
- Wood MD, Reavill C, Trail B, Wilson A, Stean T, Kennett GA, Lightowler S, Blackburn TP, Thomas D, Gager TL, Riley G, Holland V, Bromidge SM, Forbes IT, Middlemiss DN. SB-243213, a selective 5-HT2C receptor inverse agonist with improved anxiolytic profile: lack of tolerance and withdrawal anxiety. Neuropharmacology. 2001;41:186–199. doi: 10.1016/s0028-3908(01)00054-5. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Benoit-Marand M, Allen PB, Ingrassia P, Fienberg AA, Gonon F, Greengard P, Picciotto MR. Reduction of cocaine place preference in mice lacking the protein phosphatase 1 inhibitors DARPP-32 or Inhibitor 1. Biol. Psychiatry. 2002;51:612–620. doi: 10.1016/s0006-3223(01)01318-x. [DOI] [PubMed] [Google Scholar]