Abstract
Atrial fibrillation (AF) is associated with substantial morbidity, mortality, and economic burden and confers a lifetime risk of up to 25%. Current medical management involves thromboembolism prevention, rate, and rhythm control. An increased understanding of AF pathophysiology has led to enhanced pharmacological and medical therapies; however this is often limited by toxicity, variable symptom control, and inability to modulate the atrial substrate. Surgical AF ablation has been available since the original description of the Cox Maze procedure, either as a standalone or concomitant intervention. Advances in novel energy delivery systems have allowed the development of less technically demanding procedures potentially eliminating the need for median sternotomy and cardiopulmonary bypass. Variations in the definition, duration, and reporting of AF have produced methodological limitations impacting on the validity of interstudy comparisons. Standardization of these parameters may, in future, allow us to further evaluate clinical endpoints and establish the efficacy of these techniques.
1. Introduction
Atrial fibrillation (AF) is associated with significant morbidity and mortality in both medical and surgical patients. Results from the Framingham Heart study found AF to be associated with an overall lifetime risk of 1 in 4 adults aged 40–95 years or 1 in 6 of those without previous myocardial infarction or congestive cardiac failure [1]. Coronary artery bypass grafting alone has been associated with an incidence of AF reaching 30% in multicentre observational studies [2, 3] and may be significantly higher following valvular surgery [4]. More importantly, postoperative AF has been found to triple the risk of death from cardiac causes and quadruple the risk of stroke and other disabling embolic events [2].
The original “corridor” procedure for the treatment of AF was described by Guiraudon in 1985, but for a number of reasons was soon superseded by the “Maze” procedure as described by Cox et al. in 1991 [5, 6]. His work outlined a series of “cut and sew” lesions which aimed to direct electrical impulses in one direction through the atrium, disrupting the macro reentrant circuits which allow the development and propagation of AF. This procedure, whilst effective, was not without its complications, and the resultant inability to mount a tachycardic response to exercise and left atrial dysfunction led to two further adaptations of this procedure culminating in the Cox Maze III lesion set [5]. These modifications resulted in an improvement in the rates of postoperative sinus rhythm and long-term sinus node function, leading to fewer pacemaker implantations after surgery. Furthermore, improved long-term atrial transport function, low rates of arrhythmia recurrence, and a low incidence of thromboembolic complications could support the application of the maze procedure in patients where nonsurgical therapy has failed.
However, the maze procedure has been limited to a few specialist centres due to its technical difficulty and a lack of widespread experience. Its requirement for median sternotomy and cardiopulmonary bypass (CPB) also fuelled the search for an alternative, less invasive technique. Surgical ablative devices and modifications to the lesion set now allow, in some cases, for a minimally invasive, off-pump, beating heart approach. However, it is important to note that at the present time these techniques may not attain a similar level of long-term freedom from AF as the classical Maze III procedure [7].
This review describes the evolution of AF surgery, from “cut and sew” to ablative techniques, using novel energy delivery systems. We discuss its role both as a standalone and concomitant procedure and highlight the current indications and outcomes for the most common techniques described in the literature.
2. Corridor Procedure
Originally proposed by Guiraudon in 1985, the corridor procedure isolated the atria, allowing only a single conduction pathway between the sinoatrial (SA) and atrioventricular (AV) node, thereby re-establishing a regular ventricular rhythm [8]. The procedure achieved good rates of freedom from AF, however isolation of the atria from the SA node allowed the remaining atria to continue to fibrillate and did not restore atrial transport function. The thromboembolic risk of AF therefore remained and, as such, the procedure has since been superseded by the “cut and sew” Maze.
3. The “Cut and Sew” Maze
Cox et al. described the original maze procedure in 1991 [6, 9]. Between September 1987 and 1994, a total of 123 patients were included in his trial. The first 32 patients underwent the Cox-Maze I procedure, however, at late followup several limitations were revealed. The first of these was the inability to generate an appropriate sinus tachycardia in response to exercise. This was felt to be a consequence of placing the surgical incision at the junction of the superior vena cava (SVC) with the right atrium (RA) too anteriorly. This incision was removed in Maze II procedure; however, several other modifications necessary to maintain the efficacy of Maze I resulted in significantly more technical difficulty with this procedure. The left-sided exposure with Maze II was very limited and required division of the SVC. Furthermore, it became necessary to patch the SVC with autologous pericardium to prevent stenosis. Additionally, Maze II resulted in a similar degree of left atrial (LA) dysfunction as Maze I. Division of Bachmann's bundle during both procedures markedly slowed inter-atrial conduction, in turn causing synchronous contraction of the left atrium and ventricle, eliminating the LA “kick”. Further modification to the lesion set therefore culminated in Maze III procedure, which both eliminated the need for an SVC patch and improved left-sided exposure [5].
The “cut and sew” Maze III procedure has been associated with good long-term results, in Cox's original study 93% of patients remained free from AF or flutter recurrence at 8.5-year followup and all recurrences were successfully cardioverted with one antiarrhythmic drug [10]. Similarly encouraging results have been published by other groups (Table 1).
Table 1.
Freedom from AF at final followup after “cut and sew” maze procedure.
| Study | Type of procedure | Number of patients | Duration of followup (years) (Mean ± SD) | Freedom from AF at final followup (%) |
|---|---|---|---|---|
| Cox et al., 1996 [10] | Lone maze | 178 | 8.5 | 93 |
| Lönnerholm et al., 2008 [11] | Lone maze | 52 | 4.7 ± 1.0 | 86.5 |
| Prasad et al., 2003 [75] | Lone maze | 98 | 5.4 ± 3.0 | 79.6 (no AAD*) |
| 95.9 (with AAD) | ||||
| Concomitant | 86 | 5.4 ± 2.7 | 73.4 (no AAD) | |
| 97.5 (with AAD) | ||||
| Ad et al., 2009 [59] | Lone Maze | 33 | 9.8 ± 7.7 | 91.0 |
| Lone and Concomitant maze | 76 | 9.8 ± 7.7 | 84.0 | |
| Stulak et al., 2007 [7] | Concomitant | 56 | 0.7 (2.75–7)** | 92.0 |
| Gaynor et al., 2005 [13] | Concomitant | 253*** | 6.1 (0.5–15.5)** | 92.2 |
*AAD: Anti Arrhythmic Drugs, **median (range), ***includes 33 Maze I, 16 Maze II, 197 Maze III, and 30 Maze IV.
Despite its efficacy at terminating AF, several aspects of the Maze III procedure have limited its uptake to a few specialist centres. Primarily this reflects the technical difficulty of the procedure and the requirement for median sternotomy and cardiopulmonary bypass. Many surgeons feel that such an approach is too invasive for the sole treatment of cardiac arrhythmia, limiting it to patients undergoing concomitant coronary or valve surgery. In addition, even with the Maze III procedure, the early and late effects on atrial mechanical function have raised concern [11]. However, as yet the long-term effects of reduced atrial contraction on potential thromboembolic complications have not been studied in depth and remain a focus for further research.
4. Surgical Ablation and the Cox Maze IV
The introduction of novel energy delivery systems (Table 4) allowed for the development of Maze IV procedure as described by Damiano and Gaynor in 2004 [12]. This provided the potential advantages of a reduction in postoperative morbidity, without reducing efficacy or completeness of the lesion set. During Maze IV procedure, whilst both left and right arteriotomies are performed surgically, radiofrequency ablation reproduces many of the surgical incisions of Maze III procedure with additional cryoablation added to complete the lesion set to the mitral annulus (Figures 1(a) and 1(b)). In their original study, Gaynor and colleagues reported a 93.1% freedom from AF at 6 months but admitted that followup at the time of publication was insufficient to allow comparison to Maze III lesion set [13]. Since this time various permutations on Maze IV lesion set have been implemented, these are summarised in Table 3.
Table 4.
Comparison of ablative modalities.
| Transmurality | Endocardial | Epicardial | Advantages | Potential complications | Use outside research and clinical trials | Accuracy (width/depth ratio) | |
|---|---|---|---|---|---|---|---|
| Radiofrequency | Variable improved with bipolar devices | Yes | Yes | Able to produce fast and effective lesion set | Risk of inter-cavity thrombus formation, char formation, collateral damage to circumflex artery and oesophagus and PV stricture | Yes | Moderate |
| Cryoablation | Good | Yes | Yes | Preserves cellular architecture and capable of producing mitral and tricuspid isthmus lesions. Minimal collateral damage, able to produce well-demarcated lesion, adheres to myocardium to produce good contact with tissue, low risk of bleeding or perforation | Potential risk of coronary artery damage | Yes | Moderate |
| Microwave | Variable | Yes | Yes | Lower risk of thromboembolism, minimal char formation, and minimal collateral damage | Potential for circumflex artery damage | Yes | Good |
| High Frequency Ultrasound | Excellent | No | Yes | Advantage of fast, transmural epicardial lesions with theoretical potential to visualize wall thickness and perform tailor made lesion | Risk of collateral damage and perforation | No | Poor |
| Laser | Excellent | Yes | Yes | Able to produce fast, deep, and uniform lesions | Risk of crater formation and perforation | No | Poor |
Figure 1.
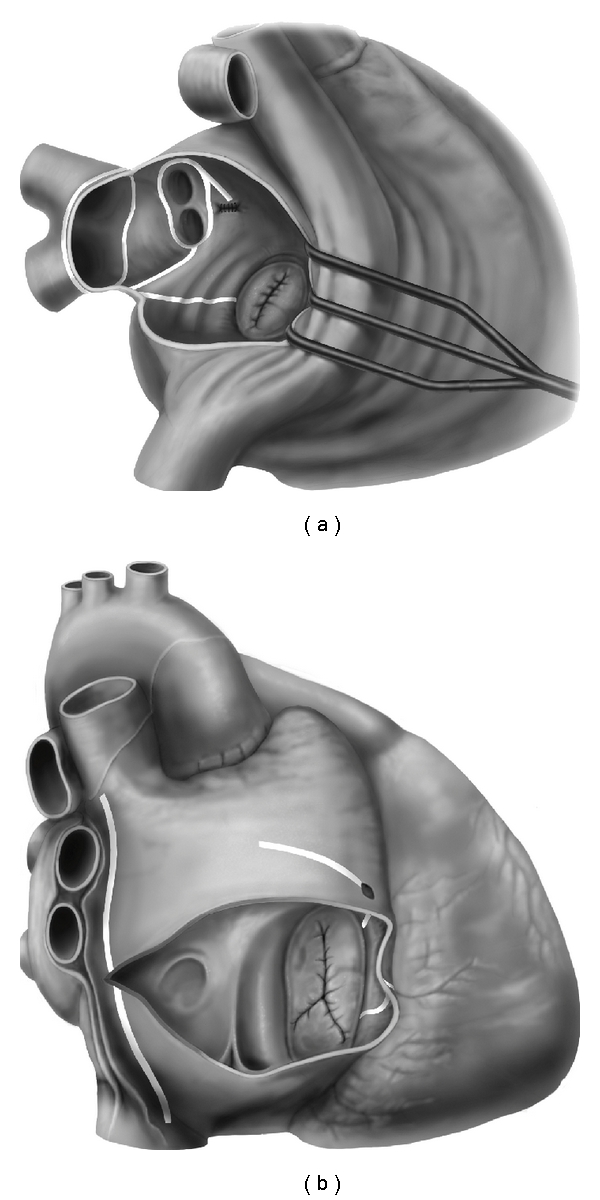
(a) Left atrial Maze III lesion set. (b) Right atrial Maze III lesion set.
Table 3.
Summary of results from alternative ablative technique.
| Study | Modality | n | Lesion set | Approach | Lone or concomitant | Type of AF and aetiology | Mean f/u ± SD (months) | Freedom from AF | Mortality at last f/u |
|---|---|---|---|---|---|---|---|---|---|
| Vicol et al., 2008 [21] | Microwave | 41 | Left atrial (endocardial) | Median sternotomy | Concomitant | Permanent | 5.37 ± 0.91 | 39.3% at 5 years |
17% |
| Pruitt et al., 2007 [22] | Microwave | 100 | PVI + LAA line | Thoracoscopic | Lone | Paroxysmal (64%) Persistent (11%) Permanent (25%) |
23.1 | 42% at last followup |
3% |
| Topkara et al., 2006 [20] | Microwave | 85 | PV lesion 98.8% Mitral annulus lesion 68.2% LA ablation 32.9% Flutter lesion 15.3% |
Median sternotomy 78.8% Minimally invasive 21.2% |
Concomitant 99% Lone 1% |
Persistent | 16.8 ± 12 | 66.7% at 12 months |
3.5% 30 day mortality |
| Radiofrequency | 120 | PV lesion 94.2% Mitral annulus lesion 64.2% LA ablation 65.8% Flutter lesion 30.0% |
Median sternotomy 95.0% Minimally invasive 5.0% |
9.6 ± 7.2 | 71.4% at 12 months |
3.4% 30 day mortality |
|||
| Knaut et al., 2006 [23] | Microwave | 59 | PVI + mitral annulus (Allessie's lesion set, endocardial) |
Median sternotomy | Concomitant | Permanent | — | 52% at 1 year |
11.5% at 1 year |
| Microwave | 43 | PV box lesion + LAA line (endocardial) | 74% at 1 year |
||||||
| Molloy 2005 [24] | Microwave | 29 | Left Atrial + Ligament of Marshall (epicardial) | Median sternotomy | Concomitant | Permanent 86% Paroxysmal 14% |
315 days | 82% at mean followup | 3.6% |
| Hurlé et al., 2004 [25] | Microwave | 9 | Biatrial (endocardial) | Median sternotomy | Concomitant | Permanent | 5.2 ± 3.3 | 62.5% at mean followup | 11% |
| Wisser et al., 2004 [26] | Microwave | 23 | Biatrial (endocardial) | Median sternotomy | Concomitant | Permanent | 24.2 ± 1.3 | *81% at 12 months |
8.7% |
| Radiofrequency | 29 | 12.1 ± 1.2 | *80% at 12 months |
0% | |||||
| Mitnovetski et al., 2009 [27] | High frequency ultrasound | 10 | Epicardial | Median sternotomy | Concomitant | Permanent 71% | 9 (3–13) | 75% at mean followup | 7.1% |
| 4 | Paroxysmal 29% | 78% at mean followup | |||||||
| Topkara et al., 2006 [20] | Microwave | 143 | PV lesion 96.8% Mitral Annulus 48.7% LAA 33.0% |
Median sternotomy |
Concomitant 96.8% Lone |
Persistent 75.8% Paroxysmal |
Recorded data at 3, 6, 12, and 24 months followup | 75.3% | 4.9% postoperative |
| Radiofrequency | 169 | Flutter lesion 17.7% PV box lesion only 41.9% |
73.8% | ||||||
| Laser | 27 | (82.9% endocardial; 17.9% epicardial) | 71.4% | ||||||
| Baek et al., 2006 [28] | Cryoablation | 93 | Cox Maze III | Median sternotomy | Concomitant | Chronic | 26.6 ± 15.2 | 84% | 3.2% |
| 77 | Kosakai-Maze | 86% at last followup | 1.3% | ||||||
| Gillinov et al., 2006 [29] | Cryoablation | 31 | PVI alone | Median sternotomy | Concomitant | Paroxysmal | Median 13.5 | Prevalence of AF of flutter 9% at 1 year f/u | 8.5% at 6 months |
| 80 | PVI + connecting lesions | ||||||||
| 41 | Cox Maze III | ||||||||
| Chen et al., 2001 [30] | Cryoablation and Radiofrequency | 13 | Maze II, III | Median Sternotomy | Concomitant | Chronic | 3 Months | 73% at 3 months | 15.4% |
| 48 | Maze IV | 81% at 3 months | 2.1% | ||||||
| 58 | No Maze (Control) | 11% at 3 months | 6.9% | ||||||
*Sinus Rhythm: Microwave—59.0% at 12 months; 60.0% at 24 months; Radiofrequency—57.1% at 12 months.
5. Radiofrequency Ablation
In the attempt to achieve a less invasive technique capable of producing results comparable to the cut and sew Maze III, procedure the use of radiofrequency (RF) ablation has grown rapidly in popularity. RF techniques are capable of producing a continuous and transmural lesion set in conjunction with a reduction in both operating time and technical difficulty [14]. Whilst RF ablation is safe, it should be carefully applied, avoiding direct contact with the pulmonary veins (PV), to prevent PV stenosis. The risk of intercavity thrombus formation and the potential for collateral oesophageal or circumflex injury should also be considered and bipolar devices used where possible [14].
Along with the advent of this innovation came multiple changes to the lesion set creating difficulties in evaluating its results against those of the “cut and sew” Maze III (Table 2). In their multicentre study closely replicating Maze III lesion set, Raman et al. achieved excellent results with concomitant RF ablation reporting 84% freedom from AF at 3 months, 90% at 6 months, and 100% at 12 and 18 months [15]. Notably however, only 15 of the 110 patients had reached 12-month followup at the time of publication and following the procedure all patients were kept on a regimen 200 mg amiodarone for 6 months unless contraindicated.
Table 2.
Summary of results obtained by radiofrequency ablation.
| Study | N | Lesion set | Type of AF and aetiology | Concomitant or lone surgery | Mean followup ± SD (months) | Freedom from AF at mean followup (%) | Overall mortality at last f/u (%) |
|---|---|---|---|---|---|---|---|
| Srivastava et al., 2008 [16] | 160 | Overall | Persistent >3 months Rheu matic | Concomitant | 40 | ||
| 40 | Biatrial | 62.5 | 10 | ||||
| 40 | LA only | 57.5 | 7.5 | ||||
| 40 | PVI | 67.5 | 10 | ||||
| 40 | None | 20.0 | 5 | ||||
| Wang et al., 2009 [17] | 299 | Overall | Permanent | Concomitant | 28 ± 5 | 85.0 | 2.3 |
| 149 | LA+ cavotricuspid | 85.2 | 1.3 | ||||
| 150 | Biatrial | 84.1 | 4.7 | ||||
| Chiappini et al., 2004 [18] | 40 | CM III | Chronic | Concomitant | 16.5 ± 2.5 | 88.5 at last f/u | 7.5 |
| Beukema et al., 2008 [19] | 285 | Modified Maze (biatrial) | Permanent | Concomitant | 43.6 ± 25.4 | 57.1 at last f/u | 27.4% |
| Topkara et al., 2006 [20] |
Endocardial 82.9% epicardial 17.1%. Variable lesion set |
Paroxysmal | Concomitant and Lone | Last f/u 24 months | 75.3 | Not specified for RF only | |
| 168 | Persistent | ||||||
| Lone | |||||||
Pulmonary Vein Isolation (PVI) alone has been performed by a number of groups and has produced good results in paroxysmal AF; however the results in longstanding AF have been suboptimal with significantly higher recurrence rates reported [31–34]. Several small studies report freedom from AF after RF PVI ranging from 87% at 6 months [31] and 71% at 3.3-year mean followup [35]. When combined with left atrial appendage (LAA) excision, ligament of Marshall (LoM) or ganglionic plexus ablation, PVI has yielded rates of freedom from AF ranging from 87–87.5% at 6 months [31, 32] and 65% at 1-year followup [36].
As with all ablative techniques, these interstudy variations in success rate must be considered cautiously whilst taking into account the criteria for diagnosis and reporting of AF. The move from telephone questionnaire and one-off ECGs to long-term monitoring after the publication of the heart rhythm society (HRS) consensus guidelines may have influenced reporting of AF recurrence rates. Incompletely transmural lesions may also explain the higher recurrence rates seen with RF over the “cut and sew” technique. Innovations such as cooled tip and bipolar electrodes have allowed for greater efficiency with RF ablation; however, confirmation of transmurality remains a problem. Factors related to the electrode (duration of application, contact with tissue), the myocardial tissue (tissue convection and conductance), and the surrounding environment (convective cooling due to blood flow) all play an important role [14]. As such, it is difficult to produce uniform settings for each instrument and the need for feedback to confirm transmurality remains important. Whilst some of these groups report the use of impedance measurements or entry and exit stimulation as confirmatory mechanisms [36, 37] others lack any form of feedback [31, 35, 38]. It is also possible that even in the presence of complete transmurality early postoperative reinnervation of the myocardium may precipitate AF recurrence. In 2006, Kangavari and colleagues highlighted an upregulation of nerve growth factor (NGF) following RF ablation [39], potentially resulting in nerve sprouting and AF recurrence. Further work is now required to identify whether this phenomenon translates into a clinically significant effect.
6. Cryoablation
Cryoablative devices use argon and helium delivered under high pressure to producing cooling of −55 to −60°C. This initially results in cellular disruption which is followed by inflammation and fibrosis to produce a homogeneous, full thickness disruption to cellularity without causing stromal damage [14].
The results with cryoablation have been variable from 60% at 3.6-year followup [40] to 82.3% at 3.8-year followup [41]. With their more extensive lesion set, Funatsu et al. report a freedom from AF of 84.1% and 80.2% at 3- and 5 years, respectively [42]. These results can be seen to be comparable to RF ablation, however outcomes of large directly comparative studies are still awaited [43].
Several advantages have been described with the use of cryoablation over other ablative techniques. The first of these is a visual confirmation of transmurality provided by frosting along the ablation line. Secondly, by maintaining the integrity of vascularity and preserving collagen, less damage to the surrounding tissues has been reported [14]. Cryoablation also causes significantly less endocardial thrombus volume potentially reflecting the preservation of the endothelial cell layer. Additionally, cryoablation has practical benefits, technically enabling the surgeon to create an isthmus lesion from the pulmonary veins to the mitral annulus and allows an electrical isolation of the atrium that cannot easily be achieved with RF or microwave ablation.
7. Microwave Ablation
Microwave ablation produces a well-demarcated area of thermal injury and is not only capable of producing transmural lesions when applied to the epicardial surface but may be more easily applicable to minimally invasive techniques. However, the overall results for this new technology have been less encouraging (Table 3). Whether this is a reflection of the technology or the quality of lesion set is uncertain. Current data reflects a combination of LA and biatrial procedures, each with slightly different patterns of ablation and in different types of AF. In a trial of 41 patients, Vicol and colleagues performed a series of LA endocardial ablations in patients with chronic secondary AF (>1 year) undergoing concomitant cardiac surgery via median sternotomy [21]. Whilst the 1-year freedom from AF was 80%, by a mean followup of 5.37 years only 39% had long-term freedom from AF. Similarly, thoracoscopic studies have reported relatively high incidences of AF recurrence. Both Puritt [22] and Koistenen [44] combined a population of lone paroxysmal, persistent and permanent AF in their studies. Pruitt reported freedom from AF to be 42% at long-term followup (mean 23.1 months) whereas Koistinen reported 59% at 1 year. A lack of homogeneity is clearly exemplified here in both patient selection and treatment strategies. Pruitt's group performed PVI alone, ligating the left atrial appendage (LAA) only when it was found to be enlarged, whereas Koistinen performed PVI with an LAA extension, ligating the LAA in 85% and including a right atrial and intercaval line in 25%. However, their published results reflect the group as a whole, and it is therefore impossible to identify the precise cause of such high rates of AF recurrence.
It is indeed possible that part of this explanation may arise from inadequacies in the technology itself. “Heat sink” secondary to poor contact with atrial tissue and the absence of a feedback mechanism may have resulted in inadequate transmurality and incomplete disruption of reentrant circuits. Alternatively, these results may reflect a drawback in their study design. It is recognised that structural remodelling seen in longstanding AF makes PVI alone suboptimal and necessitates a more extensive lesion set of the kind described in the Maze III procedure. Furthermore, by limiting ablation lines to the LA, the potential for development of RA flutter or macro-reentrant circuits is not excluded, potentially explaining a degree of this recurrence. This data should therefore be interpreted with caution, calling for a more homogeneous approach to study design before definitive conclusions can be drawn.
8. High-Frequency Ultrasound Ablation
High-Frequency Ultrasound (HIFU) creates localised hyperthermic lesions and is capable of producing transmurality when applied epicardially. It is a relatively new ablative modality and as such current guidelines from the National Institute for Health and Clinical Excellence (NICE) only approve it for use in specially organised audit or research [45]. Current results are however encouraging, reporting a freedom from AF of 85% at 6 months [46, 47] and 86.2% at 18 months [48]. However, evidence of oesophageal and mediastinal injury has been documented following HIFU catheter ablation, with one group reporting a case of fatal atriooesophageal fistula reported at 31 days following this technique [49]. Whilst no such problems have been identified with concomitant HIFU ablation during other cardiac surgery a potential for collateral damage is recognised in the literature [45].
Despite these concerns, the theoretical possibility of combining the imaging benefits of ultrasound with ablative techniques could potentially produce an effective device which not only allows the surgeon to quantify atrial wall thickness and deliver a tailor made ablation, but also confirms transmurality [14].
9. Laser Ablation
Laser ablation uses high-energy optical beams to create a narrow, well-demarcated, and nonarrhythmogenic thermal lesion [14]. Animal studies have demonstrated that laser ablation is able to produce rapid and histologically transmural lesions capable of electrophysiologically isolating the atrium [20]. Whilst there is a lack of large multicentre human trials in this modality, smaller studies have reported positive results. Hamman and coworkers examined 28 patients with variable types of AF. They performed a limited left-sided lesion set in patients with paroxysmal AF but extended this to include right-sided lesions in those with persistent or permanent AF. At a mean followup of 18 months, 76% were free from all tachyarrhythmias. At present, no device-related complications have been reported in the above studies however, some potential concerns have been raised with this technology. Poor visibility of the scar necessitates careful monitoring of the path and extent of the lesion, and it should be remembered the excess heat produces the potential for crater formation, perforation, and tissue loss [14].
10. Thoracoscopic Maze Procedures
The invasive nature of median sternotomy combined with endocardial ablation, atriotomy, and cardiopulmonary bypass has historically limited the standalone surgical treatment of AF. Over the past decade, the development of flexible, epicardial ablative devices has allowed for the evolution of minimally invasive procedures, thereby offering a surgical strategy in symptomatic patients failing catheter ablation. By reducing surgical trauma, totally thoracoscopic approaches offer the advantage of improved postoperative recovery and a reduction in hospital stay. More recently, the application of robotic techniques may offer both improved surgical dexterity and increased visualization [50, 51].
However, early thoracoscopic techniques have been subject to a number of limitations. The use of a range of ablative modalities, as well as the variability and limited nature of the lesion set, has led to difficulties in interpretation of the overall results. Whilst freedom from AF reaching 91% has been obtained at 3 and 6 months [52, 53], long-term followup has been less encouraging [22, 36, 54]. Presently, thoracoscopic modalities are also largely limited to PVI in patients with paroxysmal AF. Furthermore, whilst less invasive than median sternotomy, the majority of these procedures still require minithoracotomy, carrying with it its own postoperative morbidity [32]. Finally, thoracoscopic and robotic techniques are associated with higher operative cost and a steep learning curve for the surgeon [55]; consequentially prolonging operative time and limiting their application to centres with sufficient resource.
The role of thoracoscopic and robotic procedures in the surgical management of AF therefore remains an exciting area of development. At present whilst it should be considered in the treatment of pAF, further larger studies are required to quantify its long-term outcomes.
11. Lesion Sets
Consideration of the pathophysiology of the underlying arrhythmia is vital when deciding upon an appropriate treatment plan [56]. The onset and type of AF (primary/lone AF versus secondary AF), right and left atrial dimensions are of paramount importance in determining a surgical strategy. For example, it is well recognised that patients with persistent AF have poorer results with PVI in comparison to those with paroxysmal AF. In 1998, Haïssaguerre et al. mapped the majority of triggering foci in paroxysmal AF to the pulmonary vein ostia [57] and demonstrated the potential for RF ablation of these foci to treat AF. In permanent or persistent AF however, the self-perpetuating nature of the arrhythmia and presence of macro-reentrant circuits eliminates the requirement for these triggers to repeatedly initiate the arrhythmia [56]. As such, the physical size of these circuits in comparison to the triggering foci seen in paroxysmal AF often necessitates the need for intervention beyond PVI alone.
Atrial size has also recurrently been implicated as an independent risk factor for the recurrence of AF following surgical ablation [58–60]. In patients with a normal RA, the prolonged refractory period only allows for the development of a single macro-reentrant circuit. However, patients with RA enlargement have the potential for the development of multiple macro-reentrant circuits and as such biatrial intervention should be considered [56]. Conversely, in secondary AF without concomitant RA enlargement, macro reentrant drivers are mainly confined to the LA and consequently isolated LA procedures may be sufficient. However, it should be noted that standalone LA procedures do not eliminate the potential to generate RA flutter. An additional cavotricuspid “flutter lesion” may therefore be required in LA only lesion sets [56].
12. Permanent AF
Permanent AF is associated with changes in the atrial substrate including a reduction in the ERP, shortened action potential and wavelength. Furthermore, myocardial fibrotic changes slow conduction velocity and perpetuate the arrhythmia. As such, permanent AF presents further challenges in maintaining long-term freedom from the arrhythmia following surgical intervention. Isolated PVI is not recommended in the treatment of primary persistent AF due to high rates of AF recurrence [61]. Higher failure rates have also been seen in the treatment of primary chronic AF with standalone left atrial procedures. Following their standalone LA radiofrequency lesion set, Speziale et al. describe a recurrence rate of 18.5% in persistent lone AF compared to 5.3% in paroxysmal AF at 6-month followup (P < .001) [31]. Similarly, Cui et al. report a 67.7% freedom from AF at 12 months in long-standing persistent “lone” AF compared to 80% in paroxysmal “lone” AF following minimally invasive RF ablation [62]. Haïssaguerre and Cox et al. provide a potential explanation to this recurring problem based on a change in the right atrial substrate. Mapping atrial electrograms in the majority of patients reveals prolonged right atrial AF cycle lengths, reflecting a driving mechanism from the LA. However, approximately 20% of patients with persistent AF exhibit shorter cycle lengths indicating a right atrial driver [63]. It follows that shorter AF cycle lengths may allow for more than one macro reentrant circuit to be set up and as such neither standalone LA procedures nor the addition of a right atrial cavotricuspid lesion will prevent AF recurrence [56, 63]. In such patients, a complete biatrial Maze III procedure may be the only way to ensure long-term freedom from AF.
13. Atrial Size Reduction
The concept of an atrial critical mass above which the propagation and maintenance of AF is favoured was originally proposed by Garrey in 1914 [64]. More recent work has quantifiably demonstrated this hypothesis, with both increased left atrial area [58, 65] and reduced effective refractory period (ERP) favouring sustained AF [65]. By reducing the area in which the macro reentrant circuits that propagate AF can be set up, atrial reduction surgery may therefore potentially sustain freedom from AF. In their study of 80 patients with enlarged left atrium (ELA) undergoing concomitant atrial reduction at the time of the CryoMaze III procedure, Marui and colleagues demonstrate a significant improvement in long-term freedom from AF at both 12 and 24 months [66]. Scherer et al. also show improved freedom from AF in patients undergoing concomitant LA reduction (61.1 versus 70% at 36 months), although this did not reach statistical significance [67]. The overall results of atrial reduction surgery found within the literature are outlined in Table 5. A wide range in freedom from AF is seen here (58–100% at last followup), reflecting the variability in the duration of preoperative AF, atrial reduction techniques used and the concomitant procedures performed. Whilst a role for atrial reduction procedures is therefore apparent in patients with ELA, further evidence is required to define clear clinical guidelines.
Table 5.
Freedom from AF following atrial size reduction.
| Reference | Group | Procedure** | Duration of Pre op AF (months) | n | Atrial diameter (mm) | Followup (months) | Freedom from AF | |
|---|---|---|---|---|---|---|---|---|
| Pre op | Post op | |||||||
| Wang et al., [68] | ELA | Modified full RF Cox Maze III + biatrial reduction procedure with reef imbricate technique | 45 ± 87 | 83 | 64 ± 12 | 49 ± 8 | 19 ± 16 (Last f/u) | 90% at last f/u |
| GLA | 56 ± 67 | 39 | 86 ± 17 | 51 ± 11 | 58% at last f/u | |||
| Scherer et al., [67] | Study | LA reduction procedure + RF Maze III | >12 | 20 | 60 ± 15 | 57 ± 5 | 36 (Last f/u) | 70.0% |
| Control | RF Maze III only | >12 | 20 | 69 ± 19 | 55 ± 6 | 61.1% | ||
| Badhwar et al., [69] | RF Maze III + LA reduction | 49.3 ± 58 | 70 | 67 ± 12 | 43 ± 6 | 10.7 ± 8.4 (Mean f/u) | 92.6% at 0–6/12 93.8% at 7–12/12 92% at 1 year |
|
| Marui et al., [66] | Study | Cryoablation modified LA maze III + volume reduction | 169.2 ± 64.8 | 44 | 67.1 ± 7.8 | 47.6 ± 6.3 | 36 (Last f/u) | 90% at 12/12 87% at 24/12 100% at 36/12 |
| Control | Cryoablation modified LA maze III only | 114 ± 61.2 | 36 | 64.5 ± 6.7 | 62.1 ± 7.9 | 69% at 12/12 67% at 24/12 64% at 36/12 |
||
| Scherer et al., [70] | LA reduction | >12 | 27 | 60.2 ± 9.8 | 44.5 ± 7.0 | 12 (Last f/u) | 63% at 1 year* | |
| García-Villarreal et al., [71] | LA reduction | 46.8 ± 34.8 | 23 | 81 ± 14.7 | 48 ± 7.7 | 13.9 ± 11 (Mean f/u) | 100% at last f/u | |
| Sankar and Farnsworth, [72] | MV replacement + CABG + LA reduction | 228 | 1 | 69 | 41 | 7 | Pt remained in SR at last f/u | |
*19% of patients described as “free from AF at 1 year” suffered from intermittent, symptomatic pAF during the 1-year followup period.
**All patients underwent concomitant MV ± TV ± AV procedures ± CABG at the time of procedure outlined.
14. Success Rate Monitoring
With the advent of a multitude of new technologies it has become more important than ever to produce a drive towards a homogeneous definition of the criteria constituting AF recurrence or surgical failure. A number of studies report results based on telephone questionnaires and single ECG strips with a lack of long-term monitoring, others report surgical success based upon postoperative thromboembolic events without defining recurrence at all. This has been the focus for a number of criticisms surrounding a potential over-representation of success rates with the Cox Maze III “cut and sew” procedure [36]. Since the publication of the Heart Rhythm Society expert consensus guidelines [73] there is now a move to report any period of AF or flutter recorded for greater than 30 seconds on Holter monitoring as a recurrence of AF. However, the use of long-term monitoring devices is not without its associated shortcomings. Patient compliance may be poor, and in those systems requiring self-triggering, asymptomatic paroxysms may not be adequately captured. Some long-term systems solely determining AF on the basis of R-R variability may not register paroxysms that do not produce such a variation. Conversely, some long-term systems may register irregular premature atrial beats as AF, resulting in premature atrial beats caused by nonisolated triggers outside the PV cuff being recorded as AF recurrence [59]. When assessing the current data and planning further work, one should therefore keep this under consideration. The question has also been raised that if episodes of un-sustained AF are asymptomatic and last between 30 seconds and 5 mins, are these of clinical significance? The answer to this certainly involves a number of factors and in part will depend on the frequency of these episodes and the risk of thromboembolic complications although relevant information appears to be sparse and inconclusive. Indeed, quality of life (QoL) scores have been shown to improve significantly following surgery for AF, with health-related QoL scores equivalent to an age-matched general population at long-term followup (mean 4.6 years) [74]. We therefore believe that whilst clinical significance should be considered when discussing AF recurrence rates, we must be cautious to comply with uniform reporting criteria to allow meaningful interstudy comparisons to be made.
15. Anticoagulation
The duration of anticoagulation following AF surgery either by ablative or “cut and sew” techniques remains variable within the literature. Surgical ligation of the left atrial appendage and lower AF recurrence rates have resulted in low rates of thromboembolic complications following surgical intervention [75, 76] and whilst concomitant valve surgery may necessitate the need for permanent anticoagulation, varying strategies have been implemented in standalone procedures. In their 1999 study of the “cut and sew” maze procedure reporting a 0.4% stroke rate at 11-year followup, Cox and colleagues did not advocate anticoagulation without a prior history of thromboembolism. Whilst these results are encouraging, a consensus for short-term anticoagulation can be found throughout the literature. As such, in 2008 Henry and Ad produced guidelines on the management of anticoagulation following the Maze procedure. These recommendations advise all patients to be commenced on warfarin for 3 months postoperatively unless otherwise contraindicated. Before the discontinuation of anticoagulation, confirmation of sinus rhythm should take place by means of long term holter monitoring. At this point, any patients found to be in AF should continue anticoagulation until this has been resolved [24].
16. Summary
Surgical treatment for AF has been available for two decades since the original description of the Cox-Maze procedure. Technical advances, including novel energy delivery systems for the creation of atrial lesion sets, and a better understanding of the pathogenesis of AF have also validated surgical ablation as an efficacious concomitant procedure and, occasionally, as a standalone treatment. These advances have paved the way for the development of less invasive approaches, some of which eliminate the need for median sternotomy and CPB.
However, comparative studies of patients undergoing surgical ablation versus either established antiarrhythmic therapy or between different lesion sets and varying energy sources have endured certain methodological limitations. AF definition (and duration) has remained variable in numerous studies, when it is well established that AF burden can significantly influence outcomes. Secondly, the most common endpoint, freedom from AF after surgery, must be evaluated in view of the use (or not) of antiarrhythmic medications and the duration of followup. Finally, what is often considered as procedural success or “cure” for symptomatic persistent AF may occasionally represent its transformation to silent paroxysmal AF that has not been adequately captured. Many of these issues are highlighted in the systematic review by Khargi and colleagues, who demonstrate that whilst sinus rhythm conversion rates in patients undergoing the conventional Cox-Maze III versus alternative energy sources are equivalent, there is significant heterogeneity between studies [77].
Despite this, there is a consensus towards the usefulness of surgical AF ablation especially in patients with structural heart disease. The report of the Heart Rhythm Society (HRS) Task Force indicates that AF ablation must be offered to all patients undergoing other cardiac surgery, as long as the risk of this concomitant procedure remains low, there is a reasonable chance of success, and the surgeon has appropriate experience in antiarrhythmia surgery. With respect to standalone surgical ablation, the HRS Task Force suggests that it may be considered for symptomatic patients willing to undergo surgery, who are either not candidates for catheter-ablation or in whom catheter ablation has failed [20]. However, since these recommendations were published in 2007 there have been no robust multicentre randomised clinical trials to overcome some of the reported limitations or to evaluate tangible endpoints such as functional capacity and long-term mortality.
Despite encouraging long-term success rates with open interventions, the invasiveness of median sternotomy in the standalone treatment of AF continues to remain an important consideration. Conversely, whilst percutaneous catheter based techniques offer a minimally invasive approach, the long-term freedom from AF may be variable. As such, there has been growing interest in establishing a minimally invasive approach, either thoracoscopically or by minithoracotomy which may potentially offer a “middle ground”, combining the success rates of conventional open surgery with reduced procedural trauma. However, at the present time the application of these techniques is limited to a few specialist centres, and long-term outcome data is awaited before recommendations can be made.
In conclusion, this review highlights the widespread acceptance of both “cut and sew” and ablative techniques in the restoration of sinus rhythm, via both open and minimally invasive approaches. Equally we raise the need for well-conducted studies to establish a comparative efficacy in the different types of AF and more accurately evaluate clinical endpoints. The advances in minimally invasive technologies and robotics render the future of surgical AF management an encouraging prospect.
References
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the framingham heart study. Circulation. 2004;110(9):1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Mariscalco G, Klersy C, Zanobini M, et al. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008;118(16):1612–1618. doi: 10.1161/CIRCULATIONAHA.108.777789. [DOI] [PubMed] [Google Scholar]
- 3.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk Index for atrial fibrillation after cardiac surgery. Journal of the American Medical Association. 2004;291(14):1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 4.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Annals of Thoracic Surgery. 1993;56(3):539–549. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 5.Cox JL, Boineau JP, Schuessler RB, Jaquiss RDB, Lappas DG. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. Journal of Thoracic and Cardiovascular Surgery. 1995;110(2):473–484. doi: 10.1016/S0022-5223(95)70244-X. [DOI] [PubMed] [Google Scholar]
- 6.Cox JL, Schuessler RB, D’Agostino HJ, Jr., et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. Journal of Thoracic and Cardiovascular Surgery. 1991;101(4):569–583. [PubMed] [Google Scholar]
- 7.Stulak JM, Dearani JA, Sundt TM, III, et al. Superiority of cut-and-sew technique for the Cox maze procedure: comparison with radiofrequency ablation. Journal of Thoracic and Cardiovascular Surgery. 2007;133(4):1022–1027. doi: 10.1016/j.jtcvs.2006.09.115. [DOI] [PubMed] [Google Scholar]
- 8.Guiraudon GM, Campbell CS, Jones DL, Mclellan DG, Macdonald JL. Combined sinoatrial node atrioventricular isolation: a surgical alternative to His-bundle ablation in patients with atrial-fibrillation. Circulation. 1985;72:p. 220. [Google Scholar]
- 9.Cox JL, Boineau JP, Schuessler RB, et al. Electrophysiologic basis, surgical development, and clinical results of the maze procedure for atrial flutter and atrial fibrillation. Advances in Cardiac Surgery. 1995;6:1–67. [PubMed] [Google Scholar]
- 10.Cox JL, Schuessler RB, Lappas DG, Boineau JP. An 8 1/2-year clinical experience with surgery for atrial fibrillation. Annals of Surgery. 1996;224(3):267–273. doi: 10.1097/00000658-199609000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lönnerholm S, Blomström P, Nilsson L, Blomström-Lundqvist C. Long-term effects of the Maze procedure on atrial size and mechanical function. Annals of Thoracic Surgery. 2008;85(3):916–920. doi: 10.1016/j.athoracsur.2007.10.090. [DOI] [PubMed] [Google Scholar]
- 12.Damiano RJ, Gaynor SL. Atrial fibrillation ablation during mitral valve surgery using the AtricureTM device. Operative Techniques in Thoracic and Cardiovascular Surgery. 2004;9:24–33. [Google Scholar]
- 13.Gaynor SL, Schuessler RB, Bailey MS, et al. Surgical treatment of atrial fibrillation: predictors of late recurrence. Journal of Thoracic and Cardiovascular Surgery. 2005;129(1):104–111. doi: 10.1016/j.jtcvs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 14.Comas GM, Imren Y, Williams MR. An overview of energy sources in clinical use for the ablation of Aatrial fibrillation. Seminars in Thoracic and Cardiovascular Surgery. 2007;19(1):16–24. doi: 10.1053/j.semtcvs.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Raman J, Ishikawa S, Storer MM, Power JM. Surgical radiofrequency ablation of both atria for atrial fibrillation: results of a multicenter trial. Journal of Thoracic and Cardiovascular Surgery. 2003;126(5):1357–1366. doi: 10.1016/s0022-5223(03)01185-1. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava V, Kumar S, Javali S, et al. Efficacy of three different ablative procedures to treat atrial fibrillation in patients with valvular heart disease: a randomised trial. Heart Lung and Circulation. 2008;17(3):232–240. doi: 10.1016/j.hlc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Meng X, Li H, Cui Y, Han J, Xu C. Prospective randomized comparison of left atrial and biatrial radiofrequency ablation in the treatment of atrial fibrillation. European Journal of Cardio-Thoracic Surgery. 2009;35(1):116–122. doi: 10.1016/j.ejcts.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Chiappini B, Martìn-Suàrez S, LoForte A, Arpesella G, Di Bartolomeo R, Marinelli G. Cox/Maze III operation versus radiofrequency ablation for the surgical treatment of atrial fibrillation: a comparative study. Annals of Thoracic Surgery. 2004;77(1):87–92. doi: 10.1016/s0003-4975(03)01463-2. [DOI] [PubMed] [Google Scholar]
- 19.Beukema WP, Sie HT, Misier AR, Delnoy PP, Wellens HJJ, Elvan A. Predictive factors of sustained sinus rhythm and recurrent atrial fibrillation after a radiofrequency modified Maze procedure. European Journal of Cardio-Thoracic Surgery. 2008;34(4):771–775. doi: 10.1016/j.ejcts.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Topkara VK, Williams MR, Cheema FH, et al. Surgical ablation of atrial fibrillation: the Columbia Presbyterian experience. Journal of Cardiac Surgery. 2006;21(5):441–448. doi: 10.1111/j.1540-8191.2006.00273.x. [DOI] [PubMed] [Google Scholar]
- 21.Vicol C, Kellerer D, Petrakopoulou P, Kaczmarek I, Lamm P, Reichart B. Long-term results after ablation for long-standing atrial fibrillation concomitant to surgery for organic heart disease: is microwave energy reliable? Journal of Thoracic and Cardiovascular Surgery. 2008;136(5):1156–1159. doi: 10.1016/j.jtcvs.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 22.Pruitt JC, Lazzara RR, Ebra G. Minimally invasive surgical ablation of atrial fibrillation: the thoracoscopic box lesion approach. Journal of Interventional Cardiac Electrophysiology. 2007;20(3):83–87. doi: 10.1007/s10840-007-9172-3. [DOI] [PubMed] [Google Scholar]
- 23.Knaut M, Tugtekin SM, Spitzer SG, Jung F, Matschke K. Intraoperative endocardial microwave ablation for treatment of permanent atrial fibrillation during coronary artery bypass surgery: 1-year follow-up. Europace. 2006;8(1):16–20. doi: 10.1093/europace/euj011. [DOI] [PubMed] [Google Scholar]
- 24.Molloy TA. Midterm clinical experience with microwave surgical ablation of atrial fibrillation. Annals of Thoracic Surgery. 2005;79(6):2115–2118. doi: 10.1016/j.athoracsur.2004.06.104. [DOI] [PubMed] [Google Scholar]
- 25.Hurlé A, Ibáñez A, Parra JM, Martínez JG. Preliminary results with the microwave-modified Maze III procedure for the treatment of chronic atrial fibrillation. Pacing and Clinical Electrophysiology. 2004;27(12):1644–1646. doi: 10.1111/j.1540-8159.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- 26.Wisser W, Khazen C, Deviatko E, et al. Microwave and radiofrequency ablation yield similar success rates for treatment of chronic atrial fibrillation. European Journal of Cardio-Thoracic Surgery. 2004;25(6):1011–1017. doi: 10.1016/j.ejcts.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 27.Mitnovetski S, Almeida AA, Goldstein J, Pick AW, Smith JA. Epicardial high-intensity focused ultrasound cardiac ablation for surgical treatment of atrial fibrillation. Heart Lung and Circulation. 2009;18(1):28–31. doi: 10.1016/j.hlc.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Baek MJ, Na CY, Oh SS, et al. Surgical treatment of chronic atrial fibrillation combined with rheumatic mitral valve disease: effects of the cryo-maze procedure and predictors for late recurrence. European Journal of Cardio-Thoracic Surgery. 2006;30(5):728–736. doi: 10.1016/j.ejcts.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Gillinov AM, Bakaeen F, McCarthy PM, et al. Surgery for paroxysmal atrial fibrillation in the setting of mitral valve disease: a role for pulmonary vein isolation? Annals of Thoracic Surgery. 2006;81:19–26. doi: 10.1016/j.athoracsur.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 30.Chen MC, Chang JP, Guo GBF, Chang HW. Atrial size reduction as a predictor of the success of radiofrequency maze procedure for chronic atrial fibrillation in patients undergoing concomitant valvular surgery. Journal of Cardiovascular Electrophysiology. 2001;12(8):867–874. doi: 10.1046/j.1540-8167.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 31.Speziale G, Bonifazi R, Nasso G, et al. Minimally invasive radiofrequency ablation of lone atrial fibrillation by monolateral right minithoracotomy: operative and early follow-up results. Annals of Thoracic Surgery. 2010;90(1):161–167. doi: 10.1016/j.athoracsur.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 32.Sirak J, Jones D, Sun B, Sai-Sudhakar C, Crestanello J, Firstenberg M. Toward a definitive, totally thoracoscopic procedure for atrial fibrillation. Annals of Thoracic Surgery. 2008;86(6):1960–1964. doi: 10.1016/j.athoracsur.2008.07.066. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Li Y, Sun L, et al. Minimally invasive surgical pulmonary vein isolation alone for persistent atrial fibrillation: preliminary results of epicardial atrial electrogram analysis. Annals of Thoracic Surgery. 2008;86(4):1219–1225. doi: 10.1016/j.athoracsur.2008.04.081. [DOI] [PubMed] [Google Scholar]
- 34.Nitta T. Invited commentary. Annals of Thoracic Surgery. 2008;86:1225–1226. doi: 10.1016/j.athoracsur.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Maltais S, Forcillo J, Bouchard D, et al. Long-term results following concomitant radiofrequency modified maze ablation for atrial fibrillation. Journal of Cardiac Surgery. 2010;25(5):608–613. doi: 10.1111/j.1540-8191.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 36.Han FT, Kasirajan V, Kowalski M, et al. Results of a minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation: single-center experience with 12-month follow-up. Circulation: Arrhythmia and Electrophysiology. 2009;2(4):370–377. doi: 10.1161/CIRCEP.109.854828. [DOI] [PubMed] [Google Scholar]
- 37.Sirak J, Jones D, Schwartzman D. The five-box thoracoscopic maze procedure. Annals of Thoracic Surgery. 2010;90(3):986–989. doi: 10.1016/j.athoracsur.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Benussi S, Cini R, Gaynor SL, Alfieri O, Calafiore AM. Bipolar radiofrequency maze procedure through a transseptal approach. Annals of Thoracic Surgery. 2010;90(3):1025–1027. doi: 10.1016/j.athoracsur.2009.10.063. [DOI] [PubMed] [Google Scholar]
- 39.Kangavari S, Oh YS, Zhou S, et al. Radiofrequency catheter ablation and nerve growth factor concentration in humans. Heart Rhythm. 2006;3(10):1150–1155. doi: 10.1016/j.hrthm.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 40.Gammie JS, Didolkar P, Krowsoski LS, et al. Intermediate-term outcomes of surgical atrial fibrillation correction with the CryoMaze procedure. Annals of Thoracic Surgery. 2009;87(5):1452–1458. doi: 10.1016/j.athoracsur.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Kim JB, Cho WC, Jung SH, Chung CH, Choo SJ, Lee JW. Alternative energy sources for surgical treatment of atrial fibrillation in patients undergoing mitral valve surgery: microwave ablation vs cryoablation. Journal of Korean Medical Science. 2010;25(10):1467–1472. doi: 10.3346/jkms.2010.25.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funatsu T, Kobayashi J, Nakajima H, Iba Y, Shimahara Y, Yagihara T. Long-term results and reliability of cryothermic ablation based maze procedure for atrial fibrillation concomitant with mitral valve surgery. European Journal of Cardio-Thoracic Surgery. 2009;36(2):267–271. doi: 10.1016/j.ejcts.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 43.Luik A, Merkel M, Hoeren D, Riexinger T, Kieser M, Schmitt C. Rationale and design of the FreezeAF trial: a randomized controlled noninferiority trial comparing isolation of the pulmonary veins with the cryoballoon catheter versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation. American Heart Journal. 2010;159(4):555–560. doi: 10.1016/j.ahj.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Koistinen J, Valtonen M, Savola J, Airaksinen J. Thoracoscopic microwave ablation of atrial fibrillation. Interactive Cardiovascular and Thoracic Surgery. 2007;6(6):695–698. doi: 10.1510/icvts.2006.147942. [DOI] [PubMed] [Google Scholar]
- 45.NICE. High-Intensity Focused Ultrasound for Atrial Fibrillation in Association with Other Cardiac Surgery. London, UK: National Institute for Health and Clinical Excellence; 2006. [Google Scholar]
- 46.Groh MA, Binns OA, Burton HG, III, Champsaur GL, Ely SW, Johnson AM. Epicardial ultrasonic ablation of atrial fibrillation during concomitant cardiac surgery is a valid option in patients with ischemic heart disease. Circulation. 2008;118(14):S78–S82. doi: 10.1161/CIRCULATIONAHA.107.750927. [DOI] [PubMed] [Google Scholar]
- 47.Ninet J, Roques X, Seitelberger R, et al. Surgical ablation of atrial fibrillation with off-pump, epicardial, high-intensity focused ultrasound: results of a multicenter trial. Journal of Thoracic and Cardiovascular Surgery. 2005;130(3):803–809. doi: 10.1016/j.jtcvs.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Groh MA, Binns OA, Burton HG, III, Ely SW, Johnson AM. Ultrasonic cardiac ablation for atrial fibrillation during concomitant cardiac surgery: long-term clinical outcomes. Annals of Thoracic Surgery. 2007;84(6):1978–1983. doi: 10.1016/j.athoracsur.2007.06.081. [DOI] [PubMed] [Google Scholar]
- 49.Neven K, Schmidt B, Metzner A, et al. Fatal end of a safety algorithm for pulmonary vein isolation with use of high-intensity focused ultrasound. Circulation: Arrhythmia and Electrophysiology. 2010;3:260–265. doi: 10.1161/CIRCEP.109.922930. [DOI] [PubMed] [Google Scholar]
- 50.Argenziano M, Williams MR. Robotic atrial septal defect repair and endoscopic treatment of atrial fibrillation. Seminars in Thoracic and Cardiovascular Surgery. 2003;15(2):130–140. [PubMed] [Google Scholar]
- 51.Bolotin G, Kypson AP, Nifong LW, Chitwood WR., Jr. Robotically-assisted left atrial fibrillation ablation and mitral valve repair through a right mini-thoracotomy. Annals of Thoracic Surgery. 2004;78(4):e63–e64. doi: 10.1016/j.athoracsur.2003.12.093. [DOI] [PubMed] [Google Scholar]
- 52.Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. Journal of Thoracic and Cardiovascular Surgery. 2005;130(3):797–802. doi: 10.1016/j.jtcvs.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 53.Wudel JH, Chaudhuri P, Hiller JJ. Video-assisted epicardial ablation and left atrial appendage exclusion for atrial fibrillation: extended follow-up. Annals of Thoracic Surgery. 2008;85(1):34–38. doi: 10.1016/j.athoracsur.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Edgerton JR, Brinkman WT, Weaver T, et al. Pulmonary vein isolation and autonomic denervation for the management of paroxysmal atrial fibrillation by a minimally invasive surgical approach. Journal of Thoracic and Cardiovascular Surgery. 2010;140:823–828. doi: 10.1016/j.jtcvs.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 55.Charokopos N, Rouska E, Styliadis I, Antonitsis P, Papakonstantinou C, Spanos P. Totally endoscopic microwave ablation for lone atrial fibrillation: an alternative method of treatment. Hellenic Journal of Cardiology. 2006;47(6):377–380. [PubMed] [Google Scholar]
- 56.Cox JL. The longstanding, persistent confusion surrounding surgery for atrial fibrillation. Journal of Thoracic and Cardiovascular Surgery. 2010;139(6):1374–1386. doi: 10.1016/j.jtcvs.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 57.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. New England Journal of Medicine. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 58.Chen MC, Chang JP, Chang HW. Preoperative atrial size predicts the success of radiofrequency maze procedure for permanent atrial fibrillation in patients undergoing concomitant valvular surgery. Chest. 2004;125(6):2129–2134. doi: 10.1378/chest.125.6.2129. [DOI] [PubMed] [Google Scholar]
- 59.Ad N, Henry L, Hunt S, Barnett S, Stone L. The Cox-Maze III procedure success rate: comparison by electrocardiogram, 24-hour holter monitoring and long-term monitoring. Annals of Thoracic Surgery. 2009;88(1):101–105. doi: 10.1016/j.athoracsur.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Je HG, Lee JW, Jung SH, et al. Risk factors analysis on failure of maze procedure: mid-term results. European Journal of Cardio-Thoracic Surgery. 2009;36(2):272–278. doi: 10.1016/j.ejcts.2009.02.058. [DOI] [PubMed] [Google Scholar]
- 61.Pagé P. Canadian cardiovascular society atrial fibrillation guidelines 2010: surgical therapy. The Canadian Journal of Cardiology. 2011;27:67–73. doi: 10.1016/j.cjca.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Cui YQ, Li Y, Gao F, et al. Video-assisted minimally invasive surgery for lone atrial fibrillation: a clinical report of 81 cases. Journal of Thoracic and Cardiovascular Surgery. 2010;139(2):326–332. doi: 10.1016/j.jtcvs.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 63.Haïssaguerre M, Wright M, Hocini M, Jaïs P. The substrate maintaining persistent atrial fibrillation. Circulation: Arrhythmia and Electrophysiology. 2008;1(1):2–5. doi: 10.1161/CIRCEP.108.764233. [DOI] [PubMed] [Google Scholar]
- 64.Garrey WE. The nature of fibrillatory contraction of the heart: its relation to tissue mass and form. American Journal of Physiology. 1914;33:397–414. [Google Scholar]
- 65.Byrd GD, Prasad SM, Ripplinger CM, et al. Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. Circulation. 2005;112(9):I7–I13. doi: 10.1161/CIRCULATIONAHA.104.526210. [DOI] [PubMed] [Google Scholar]
- 66.Marui A, Nishina T, Tambara K, et al. A novel atrial volume reduction technique to enhance the Cox maze procedure: initial results. Journal of Thoracic and Cardiovascular Surgery. 2006;132(5):1047–1053. doi: 10.1016/j.jtcvs.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 67.Scherer M, Therapidis P, Wittlinger T, Miskovic A, Moritz A. Impact of left atrial size reduction and endocardial radiofrequency ablation on continuous atrial fibrillation in patients undergoing concomitant cardiac surgery: three-year results. The Journal of Heart Valve Disease. 2007;16(2):126–131. [PubMed] [Google Scholar]
- 68.Wang W, Guo LR, Martland AM, Feng XD, Ma J, Feng XQ. Biatrial reduction plasty with reef imbricate technique as an adjunct to maze procedure for permanent atrial fibrillation associated with giant left atria. Interactive Cardiovascular and Thoracic Surgery. 2010;10(4):577–581. doi: 10.1510/icvts.2009.220012. [DOI] [PubMed] [Google Scholar]
- 69.Badhwar V, Rovin JD, Davenport G, et al. Left atrial reduction enhances outcomes of modified maze procedure for permanent atrial fibrillation during concomitant mitral surgery. Annals of Thoracic Surgery. 2006;82(5):1758–1763. doi: 10.1016/j.athoracsur.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 70.Scherer M, Dzemali O, Aybek T, Wimmer-Greinecker G, Moritz A. Impact of left atrial size reduction on chronic atrial fibrillation in mitral valve surgery. Journal of Heart Valve Disease. 2003;12(4):469–474. [PubMed] [Google Scholar]
- 71.García-Villarreal OA, Gouveia AB, González R, Argüero R. Left atrial reduction. A new concept in surgery for chronic atrial fibrillation. Revista Espanola de Cardiologia. 2002;55(5):499–504. doi: 10.1016/s0300-8932(02)76642-6. [DOI] [PubMed] [Google Scholar]
- 72.Sankar NM, Farnsworth AE. Left atrial reduction for chronic atrial fibrillation associated with mitral valve disease. Annals of Thoracic Surgery. 1998;66(1):254–256. doi: 10.1016/s0003-4975(98)00281-1. [DOI] [PubMed] [Google Scholar]
- 73.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2009;11(1):p. 132. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 74.Lönnerholm S, Blomström P, Nilsson L, Blomström-Lundqvist C. A high quality of life is maintained late after Maze III surgery for atrial fibrillation. European Journal of Cardio-Thoracic Surgery. 2009;36(3):558–562. doi: 10.1016/j.ejcts.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 75.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. Journal of Thoracic and Cardiovascular Surgery. 2003;126(6):1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 76.Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. Journal of Thoracic and Cardiovascular Surgery. 1999;118(5):833–840. doi: 10.1016/s0022-5223(99)70052-8. [DOI] [PubMed] [Google Scholar]
- 77.Khargi K, Hutten BA, Lemke B, Deneke T. Surgical treatment of atrial fibrillation; a systematic review. European Journal of Cardio-Thoracic Surgery. 2005;27(2):258–265. doi: 10.1016/j.ejcts.2004.11.003. [DOI] [PubMed] [Google Scholar]


