Abstract
There are over 750 species of bacteria that inhabit the human oral cavity, but only a small fraction of those are attributed to causing plaque-related diseases such as caries. Streptococcus mutans is accepted as the main cariogenic agent and there is substantial knowledge regarding the specific virulence factors that render the organism a pathogen. There has been rising interest in alternative, target-specific treatment options as opposed to nonspecific mechanical plaque removal or application of broad-spectrum antibacterials that are currently in use. The impact of diet on oral health is undeniable, and this is directly observable in populations that consume high quantities of polyphenol-rich foods or beverages. Such populations have low caries incidence and better overall oral health. Camellia sinensis, the plant from which various forms of tea are derived, and Vaccinium macrocarpon (American cranberry fruit) have received notable attention both for their prevalence in the human diet as well as for their unique composition of polyphenols. The biologically active constituents of these plants have demonstrated potent enzyme-inhibitory properties without being bactericidal, a key quality that is important in developing therapies that will not cause microorganisms to develop resistance. The aim of this review is to consider studies that have investigated the feasibility of tea, cranberry, and other select plant derivatives as a potential basis for alternative therapeutic agents against Streptococcus mutans and to evaluate their current and future clinical relevance.
Key Words: Cranberry, Polyphenols, Tea
Introduction
The human oral cavity, much like the rest of the human body, is home to its own microbiota consisting of over 750 species of commensal bacteria, viruses, and fungi, including mutans streptococci (mainly Streptococcus mutans and Streptococcus sobrinus), which are associated with caries initiation and progression [Marsh, 2003; Avila et al., 2009]. The oral microbiota is a dynamic entity and is greatly impacted by oral hygiene practices and diet [Bullon et al., 2009a, b]. For example, mutans streptococci upregulate virulence genes when a high level of carbohydrate, especially sucrose, becomes available in the environment. Major virulence genes of Streptococcus mutans include gtfB, gtfC, and gtfD, which encode glucosyltransferases (GTFs) that bind sucrose as a substrate and form soluble and insoluble glucans [Yamashita et al., 1993; Vacca-Smith et al., 1996].
Current methods of combating caries-associated bacteria are mostly broad-spectrum antimicrobials [Allaker and Douglas, 2009]. Chlorhexidine, for example, is a nonspecific antimicrobial specifically used for dental applications. It acts by reducing viability of all bacteria in the oral cavity, resulting in a decreased incidence of caries. There are many undesirable aspects to such a bactericidal approach. Antibiotics that alter oral microbiota can put selective pressure on microorganisms to become resistant to drugs, kill symbiotic bacteria, and allow proliferation of other disease-causing pathogens [Banas et al., 2007; Cegelski et al., 2008].
Over the past few years, great advances have been made in identifying specific virulence properties unique to S. mutans. It is known that it produces insoluble glucans using GTFs and is both aciduric and acidogenic due to F-ATPase proton-translocating pumps [Gibbons and Qureshi, 1980; Marsh, 2005]. Targeting these unique virulence factors of S. mutans without threatening its existence, or the existence of other species in the oral cavity, is a more attractive approach compared to bactericidal methods because it inhibits pathogenesis and formation of biofilms without stimulating evolution of resistant bacteria [Cegelski et al., 2008].
There has been a rising interest in naturally derived biologically active compounds that may have potential therapeutic uses in medicine and dentistry [Groppo et al., 2008; Newman, 2008]. Plants have been used in folk medicine for thousands of years, and even with the advent of modern medicine, products derived from medicinal plants have been the basis for development of many new lead chemicals for pharmaceuticals [Cowan, 1999; Newman, 2008; Palombo, 2009]. Many currently used antibiotics were discovered by screening natural products and compound libraries against whole organisms, which identified bacteriostatic and/or bacteriocidal properties. Dental medicine has become especially amenable to plant-derived products, driven by evidence that shows populations that regularly incorporate foods or beverages containing certain phytochemicals into their diet have better oral health [Palombo, 2009]. For example, Japanese tea drinkers have been known to have decreased incidence of caries compared to other populations [Hamilton-Miller, 2001; Taylor et al., 2005]. Italian researchers examined the oral health of groups of people with specific, long-standing drinking habits (regular consumption of either coffee, barley coffee, tea, or red wine). They found that those with the drinking habits had significantly better plaque scores and had lower bacterial counts of mutans streptococci and lactobacilli compared to those who did not have drinking habits, implying that these plant-based beverages must have some active compound(s) that maintain oral health with regular consumption [Signoretto et al., 2006, 2010].
Some major classes of phytochemicals that are known to have biological activity are simple phenols and phenolic acids, quinones, flavonoids (including flavonols and flavones), and terpenoids [Cowan, 1999]. All of the aforementioned categories, with the exception of terpenoids, can be classified under the broader class of ‘polyphenols’. Polyphenols are one of the most common, widespread groups of substances produced in plants and it has long been known that they have a broad range of health-promoting activities, including antimicrobial activity [Tapiero et al., 2002]. Various in vivoand in vitro studies have shown that polyphenols are able to selectively interfere with specific virulence traits of S. mutans, preventing adherence to tooth surfaces and inhibiting GTF enzymes, without having a marked effect on bacterial viability [Koo et al., 2005; Duarte et al., 2006; Gregoire et al., 2007].
Since diet is a key factor that defines oral health, much research has focused on widely consumed food products. Tea (Camellia sinensis) and American cranberry fruit (Vaccinium macrocarpon) have received particular attention because of their unique polyphenol compositions and their prevalence in the human diet [Hamilton-Miller, 2001; Taylor et al., 2005]. An abundance of studies have reported bacteriostatic or bactericidal activities for compounds derived from hundreds of higher plants, but this review will mainly focus on studies conducted using cranberry fruit and the various forms of tea due to (i) their high rate of consumption, (ii) relative lack of toxicity, and (iii) tendency to be bacteriostatic rather than bactericidal, and discuss their applicability in the context of clinical oral health and caries management.
Table 1 summarizes the biological activities of polyphenol compounds isolated from natural sources discussed in this review.
Table 1.
Biological activities of polyphenol compounds isolated from natural sources
| Compounds | Source | Type of study | Results | Reference |
|---|---|---|---|---|
| Crude tea polyphenolic compounds |
Camellia sinensis |
in vitro and in vivo |
inhibit attachment and GTF activity; inhibit caries in rats |
Otake et al. [1991] |
| Polyphenols/oolong tea |
Camellia sinensis |
in vitro |
inhibit GTF activity |
Nakahara et al. [1993] |
| Monomeric-catechin-free fraction/oolong tea |
Camellia sinensis |
in vitro |
inhibit GTF activity |
Ooshima et al. [1993] |
| Monomeric-catechin-free fraction/oolong tea |
Camellia sinensis |
in vivo |
inhibits caries in rats |
Ooshima et al. [1998] |
| Polymeric polyphenol fraction/oolong tea |
Camellia sinensis |
in vitro |
inhibit GTF activity |
Matsumoto et al. [2003] |
| Monomeric compounds |
Camellia sinensis |
in vitro |
antibacterial activity against mutans streptococci |
Sasaki et al. [2004] |
| Cranberry juice fractions |
Vaccinum macrocarpon |
in vitro |
inhibit biofilm adhesion |
Johnson-White et al. [2006] |
| Cranberry juice |
Vaccinum macrocarpon |
in vitro |
interfere with adhesion and the initial stages of biofilm formation |
Yamanaka et al. [2004] |
| High-molecular-weight constituent/cranberry juice |
Vaccinum macrocarpon |
in vitro |
inhibit GTF and FTF activity |
Steinberg et al. [2004] |
| Flavonols, anthocyanins and proanthocyanidins |
Vaccinum macrocarpon |
in vitro |
inhibit GTF and F-ATPase activities and acid production by S. mutans |
Duarte et al. [2006] |
| Low-molecular-weight polyphenols |
Vaccinum macrocarpon |
in vitro |
inhibit GTF and F-ATPase activities and acid production by S. mutans |
Gregoire et al. [2007] |
| Proanthocyanidins of various degrees of polymerization |
Vaccinum macrocarpon |
in vitro and in vivo |
inhibit attachment and GTF activity; reduce the incidence of smooth surface caries in rats |
Koo et al. [2010] |
| Flavonoids |
propolis from Apis mellifera |
in vivo |
inhibit biofilm formation and caries induction in rats |
Koo et al. [1999] |
| Apigenin and tt-farnesol | propolis from Apis mellifera | in vitro | inhibit GTF and change the permeability or fluidity of mutans streptococci cell membrane | Koo et al. [2002, 2003] |
Polyphenols: An Overview
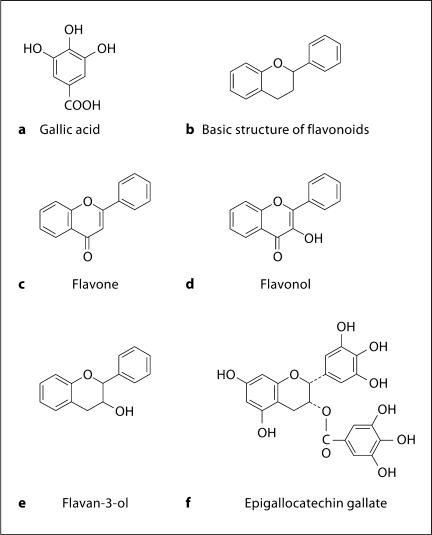
A polyphenol, in its simplest definition, is any substance that contains at least one aromatic ring with one or more hydroxyl groups (other substituents can be present). Gallic acid (fig. 1a) is an example of a simple phenol. Polyphenols are sorted into approximately 15 major classes [Harborne, 1964], and of those classes, the flavonoids family contains the largest variety of compounds that show pharmacological activities [Cushnie and Lamb, 2005; Tavares et al., 2010; Vauzour et al., 2010]. The basic structure of a flavonoid consists of two aromatic rings linked by three carbons, forming a heterocyclic ring (fig. 1b). Exact classification of flavonoid compounds varies depending on the literature; according to Bennick [2002], there are two major subclasses within the flavonoid family: 3-desoxyflavonoids, also referred to as flavones due to the presence of a ketone group at the C4 carbon (fig. 1c), and 3-hydroxyflavonoids, or flavonols (fig. 1d), which have a hydroxyl group at the C3 carbon. A subclass of the flavonols are the flavan-3-ols (fig. 1e), which resemble flavonols in structure, but do not have the ketone group at the C4 carbon. Variations in position, number, and type of substituents give rise to a great diversity of flavonoids. Flavan-3-ol units (such as catechin) can polymerize to form proanthocyanidins (sometimes referred to as ‘condensed tannins’) of varying molecular weights. It is important to note here that proanthocyanidins and polymers of flavan-3-ols are sometimes referred to as flavanols, not to be confused with flavonols.
Fig. 1.
Structures of representative polyphenols [from Bennick, 2002].
Proanthocyanidins can exist as oligomers (containing up to 6 catechin units), which are soluble, or polymers (more than 6 catechin units), which are insoluble. Plants contain varying degrees and ratios of low-molecular-weight polyphenol monomers, dimers, and trimers to high-molecular-weight polymers. Molecular weight and/or solubility of these polyphenols, in addition to number and location of substituents, seem to be correlated with biological activity [Hemingway et al., 1992; Haslam and Cai, 1994; Gross et al., 1999]. Through in vitro testing, many biological and pharmacological activities have been demonstrated for this family of compounds, ranging from direct bacteriocidal action to selective inhibition of specific enzymes. It is generally believed that one of the major modes of action of polyphenols is the ability to complex with other molecules [Haslam and Cai, 1994].
Inhibitory Effects of Tea Polyphenols
Tea is one of the most widely consumed beverages in Asian countries and it was recently reported that tea is also the major source of flavan-3-ols and flavonols in the US diet [Song and Chun, 2008]. Tea is known to be a rich source of polyphenols, but polyphenol type and levels vary depending on how the tea has been processed [Astill et al., 2001]. There are various ways a tea can be processed (green, black, oolong), but all tea comes from the plant Camellia sinensis. Green tea is unfermented and contains mostly monomeric polyphenols such as epicatechin, epicatechin gallate, and epigallocatechin (fig. 1f); black tea has been fermented and many of the simple catechins have been oxidized to larger molecules of higher molecular weight. Oolong tea is semifermented and contains a mixture of monomeric and oligomeric polyphenols [Yam et al., 1997].
Hamilton-Miller [2001] claims that tea, in any of its varieties, does not contain ‘tannins’, or what has been defined earlier in this paper as high-molecular-weight polymeric polyphenols. However, certain studies (discussed below) claim that oolong tea does contain unique, unspecified polymeric polyphenols not present in green tea, and that these polyphenols show significant inhibitory activity. A possible explanation for this inconsistency may be due to the fact that authors referring to the polymeric polyphenols in oolong tea technically meant oligomeric polyphenols (the technical difference being that oligomers are smaller and soluble and polymers are larger and insoluble). More rigorous definitions of what constitutes a ‘tannin’ must be established. Further complicating the matter is the existence of evidence suggesting that certain polyphenolic components of tea, particularly monomeric catechins, may directly be bactericidal.
Otake et al. [1991] showed that ‘Sunphenon’, a crude green tea extract, was able to cause a significant inhibition in adherence of S. mutans to saliva-coated hydroxyapatite, as well as to inhibit GTF activity in vitro. The same study showed that when rats were fed a diet enriched with Sunphenon (in both food and water), caries formation was greatly inhibited. Otake et al. [1991] attributed the results of the study to Sunphenon's ability to prevent adherence and inhibit GTF enzyme, but also mentioned that Sunphenon (or any of its constituents) may be bactericidal. The authors did not offer possible mechanisms as to how it is bactericidal nor did they corroborate such claims with bacterial viability assays.
Nakahara et al. [1993] explored the inhibitory effects of polyphenols found in the semifermented oolong tea. Preliminary chemical analyses of the polyphenolic substances in oolong tea extract showed that oolong tea extract contained significant quantities of unknown polyphenols that are not detected in green tea. A fraction of oolong tea extract, designated OTF10, did not in fact contain any low-molecular-weight compounds and showed the strongest inhibition of S. sobrinus GTF activity. The authors did not identify the exact structure of the polymeric polyphenols in oolong tea but suggested that polymerization of monomeric catechins during the fermentation process may be critical to the inhibitory effects on GTF.
Ooshima et al. [1993] examined the effects of a monomeric-catechin-free fraction of oolong tea (referred to as OT-6) on bacterial growth as well as the effects in vivo. OT-6 did not affect growth of S. mutans or S. sobrinus; however it did significantly inhibit insoluble glucan synthesis by GTFs. When rats infected with S. mutans were given food and water treated with OT-6, plaque index and caries score were significantly reduced [Ooshima et al., 1998]. Matsumoto et al. [2003] attempted to elucidate exactly how polymeric polyphenols found in oolong tea may exert inhibitory activities on bacterial GTFs by cloning functional domains of a recombinant GTF enzyme – the N-terminal catalytic domain and the C-terminal glucan-binding domain. Matsumoto et al. [2003] found that the polymeric polyphenol fraction specific for oolong tea (devoid of low-molecular-weight catechins) hindered glucan synthesis by noncompetitively inhibiting the glucan-binding domain region of the enzyme, not the N-terminal catalytic domain.
Sasaki et al. [2004] also studied the effects of oolong tea, but looked at the fractions of oolong tea that did not contain the high-molecular-weight compounds. In other words, the fraction that was studied included entirely monomeric compounds (GCG, EGCg, CG). This monomer-rich fraction, referred to as OT-2, exhibited significant antibacterial activity against mutans streptococci; however, when OT-2 was pretreated with bovine serum albumin, all bactericidal activity was abolished [Sasaki et al., 2004]. The authors postulated that the monomeric polyphenols have a high tendency to complex with proteins, and such reactions neutralize any bactericidal potential. In the human oral cavity, the monomeric polyphenols’ affinity for proline-rich salivary proteins may diminish their bactericidal potential. As mentioned earlier, oolong tea contains a mixture of both monomeric and oligo-/polymeric polyphenols so it is possible that these exert distinctive cariostatic effects.
However, there are still unanswered questions regarding the activities and structure-function relationships of tea polyphenols. Studies by Ooshima et al. [1993] and Matsumoto et al. [2003] both generated strong evidence that polymeric polyphenols inhibit GTF activity, but they did not provide any insight into whether they may or may not have any bactericidal potential by conducting bacterial viability assays. The results from Sasaki et al. [2004] also did not address the possibility of protein interaction of the polymeric polyphenol-rich fraction of oolong tea. The fact that OT-6 exhibited inhibitory effects against GTF in vivo [Ooshima et al., 1993] suggests that polymeric polyphenol activity may be preserved even in the presence of protein-rich saliva, but more evidence is necessary to corroborate this. Furthermore, monomeric catechins such as EC, EGC, and EGCg were shown to inhibit GTF in some studies [Otake et al., 1991], comparable to the unidentified polymeric compounds. It is worth exploring whether or not they share similar modes of action, as well as to what extent monomeric catechins affect cell viability.
Teas offer a great variety of bioactive compounds but characterization of specific compounds and structure-activity relationships remain elusive. Green teas, with their high monomeric, potentially bactericidal polyphenol content, may hold less use than oolong teas, which contain known polymeric enzyme inhibitors. More information is needed on how monomeric catechins of tea will interact with salivary proteins. Few studies have been conducted on black teas, mainly due to the inability of chromatographic methods to characterize its chemical components.
Inhibitory Effects of Cranberry Polyphenols
Cranberry fruit (Vaccinium macrocarpon) has well-known antiadhesive properties and holds great potential as an antiadhesion agent against cariogenic mutans streptococci. The therapeutic potential of cranberry was first appreciated with Escherichia coli, when it was observed that cranberry juice prevented urinary tract infections by inhibiting of adherence of bacteria [Avorn et al., 1994]; it was also found to be capable of inhibiting the adherence of Helicobacter pylori to host gastric cells [Burger et al., 2000]. Many fruits that are a part of the human diet contain polyphenols, but cranberry is the only fruit that has consistently and effectively been shown to inhibit biofilm formation [Johnson-White et al., 2006]. Johnson-White et al. [2006] attempted to simulate the antiadhesive properties of cranberry juice and could not find such effects in apple, orange, or grape juices. Incidentally, white cranberry juice was also devoid of antiadhesive activity.
Yamanaka et al. [2004] treated S. mutans and S. sobrinus cells with cranberry juice and found that it reduced cell surface hydrophobicity of the cells, interfering with adhesion and the initial stages of biofilm formation. The authors hypothesized that cranberry juice components may interact with hydrophobic proteins on the surface of the bacterial cell. Since hydrophobicity is an important factor in the initial attachment of the bacteria to a surface, reducing hydrophobicity decreases likelihood of adhesion. Hydrophobicity is not the only factor that is affected by treatment with cranberry. Steinberg et al. [2004] used a high-molecular-weight, nondialyzable constituent (NDM) of cranberry juice to study its effects on S. mutans GTF activity and found that NDM significantly reduced the activities of GTF as well as fructosyltransferase, both in solution and immobilized on saliva-coated hydroxyapatite. Interestingly, the antiadhesion effect of NDM was less pronounced, though still present, when sucrose was not present in the reaction mixture. The precise structure of any active components within the NDM could not be resolved by chromatographic methods due to the high molecular weight [Steinberg et al., 2004].
In addition to cell surface hydrophobicity and ability to synthesize glucans using GTFs, mutans streptococci are also acidogenic and aciduric. High metabolic activity creates an acidic environment, but the bacterium is able to maintain an alkaline intracellular pH by removing protons through the use of an F-ATPase proton pump. Duarte et al. [2006] conducted a study comparing the efficacies of the three main polyphenolic components of cranberry: flavonols (such as quercetin, kaempferol, and myricetin), anthocyanins (flavan compounds containing a glycoside moiety), and proanthocyanidins (flavan-3-ol oligomers). Results showed that, out of all the compounds tested, proanthocyanidins most efficiently inhibited bacterial GTFs [Duarte et al., 2006]. More importantly, flavonols and proanthocyanidins were shown to interfere with the bacteria's F-ATPase activity, preventing pH drop and subsequent acidification of extracellular environment. Flavonols such a quercetin are known noncompetitive inhibitors of F-ATPases, but this was the first study that demonstrated similar capabilities in proanthocyanidins. It is unknown whether or not proanthocyanidins share the same inhibitory mechanism as non-proanthocyanidin flavonols. Ultimately, cranberry polyphenols were able to suppress multiple virulence traits without affecting bacterial viability.
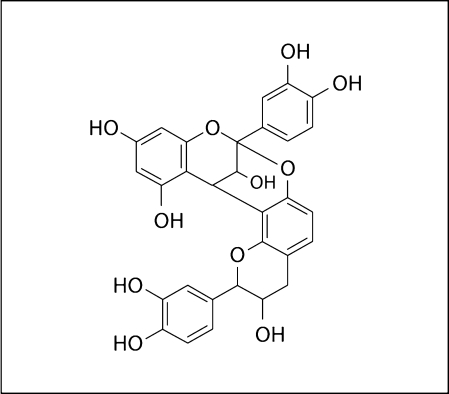
Gregoire et al. [2007] specifically examined the effects of low-molecular-weight polyphenols found in cranberry. Flavonols and their glycosides such as myricetin and myricetin 3-rhamnoside exhibited significant but moderate effects. Monomer flavan-3-ols and phenolic acids such as epicatechin, caffeic acid, and chlorogenic acid showed negligible inhibitory activity; however, procyanidin A2, a dimer of epicatechin, did show inhibitory activity against GTFs [Gregoire et al., 2007]. The epicatechin units of the dimer are linked by an A-type interflavan linkage which is believed to make the molecule rigid. This type of linkage is unique to polyphenols found in cranberry (fig. 2), which may possibly explain the lack of inhibitory activity of similar polyphenols in non-cranberry fruits such as apple, which contains procyanidin B2 lacking the A-type interflavan linkage [Johnson-White et al., 2006; Gregoire et al., 2007]. The authors also noted that the degree of polymerization may be a critical factor that determines biological activity. None of the polyphenols tested had bactericidal effects on S. mutans, which is noteworthy since low-molecular-weight components in tea were reported to have an effect on bacterial viability [Otake et al., 1991].
Fig. 2.
Procyanidin A2 contains an A-type interflavan linkage unique to cranberry [from Gregoire et al., 2007].
In the aforementioned studies, cranberry flavonols demonstrated moderate inhibitory activity but proanthocyanidins seemed to demonstrate the most significant inhibitory effects against bacterial GTF and F-ATPase. Koo et al. [2010] exclusively examined proanthocyanidins of various degrees of polymerization and their effects of GTF activity and pH drop. The lowest-molecular-weight compound tested was the proanthocyanidin monomer catechin. Catechin was the only test agent that was not an effective inhibitor of GTF. All other proanthocyanidins were able to inhibit GTF; proanthocyanidins that had a degree of polymerization between 4 and 12 were the most potent inhibitors [Koo et al., 2010].
Experiments testing for inhibitory activity against acid production yielded slightly different results. Proanthocyanidin oligomers that had degrees of polymerization greater than 3 only had modest inhibitory effects of acid production; however, the proanthocyanidin dimer procyanidin A2 was more inhibitory [Duarte et al., 2006; Gregoire et al., 2007]. It is important to note that none of the proanthocyanidins tested showed bactericidal activity [Duarte et al., 2006; Gregoire et al., 2007]. In animal studies, proanthocyanidins was able to significantly reduce the incidence of smooth surface caries only and fluoride (or fluoride in conjunction with proanthocyanidin) was more effective than the treatment with proanthocyanidins alone [Koo et al., 2010]. Nonetheless, this study elucidated many important properties of proanthocyanidins. It defined a precise range of degree of polymerization for which these compounds would have activity, and it also demonstrated that degree of polymerization is an important factor in determining what aspect of virulence would be targeted (GTF or F-ATPase). In other words, it is the combination of polyphenols of varying sizes, and not any single compound, that is able to exert multitarget-inhibitory activities. Authors [Gregoire et al., 2007; Koo et al., 2010] proposed that proanthocyanidins with degrees of polymerization between 4 and 12 were optimal for interaction and inhibition of GTF; any greater polymerization actually diminished inhibitory activity.
As with tea polyphenols, there are still questions that need to be addressed. What still remains unclear is the exact mechanism in which proanthocyanidins disrupt acid production and acid tolerance in S. mutans, a major virulence trait. Low-molecular-weight polyphenols (such as flavonols and procyanidin A2) are thought to partially inhibit the F-ATPase membrane-associated enzyme. The role of oligomeric proanthocyanidins in pH drop is more elusive, and the degree of polymerization does not seem to be related to any effects in glycolytic pH drop. Authors did not believe oligomers would interfere with any intracellular glycolytic enzymes. Nevertheless, it is evident that proanthocyanidin oligomers directly interact with bacterial membrane and though precise mechanisms are unknown, they can cause membrane disturbances which affect the glycolytic pathway. Proanthocyanidins and other compounds derived from cranberries, an already popular and sustainable fruit, have promising cariostatic properties and may potentially be developed as a therapeutic supplement to fluoride, the only proven anticaries agent currently in use.
Additional Plant Derivatives Possessing Potent Cariostatic Properties
Traditional medicinal plants help researchers narrow their focus, so as to avoid a haphazard, random search for useful compounds [Kingston and Newman, 2005; Newman, 2008]. In that sense, cranberry and tea polyphenols are the most sensible sources for polyphenols in that they are already widely consumed as part of the human diet. Toxicity to humans is unlikely and both are sustainable crops. Such factors explain why much of the research conducted is disproportionately devoted to these two plants.
Nevertheless, there are some natural bioactive compounds that are not derived from common foods per se, but have demonstrated remarkable potential to inhibit virulence traits of cariogenic bacteria. One unusual source for such compounds is propolis, produced by the Apis mellifera species of bees. Propolis is a resinous substance consisting of various plant products mixed with beeswax [Ghisalberti, 1979]. Propolis exhibits various potent bioactive properties, including antimicrobial and anti-inflammatory properties. Flavonoids (particularly aglycones) have been detected in propolis, but non-polyphenol compounds, such as terpenoids, have also demonstrated biological activity. Preliminary studies found that propolis was a potent inhibitor of plaque formation and caries induction in rats [Koo et al., 1999], possibly due to a complex mixture of bioactive compounds.
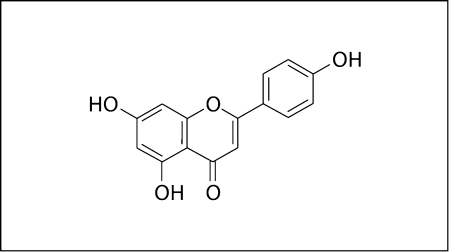
Koo et al. [2002] selected and tested 30 distinct compounds occurring in propolis on the activity of GTF enzymes of S. mutans and Streptococcus sanguinis. Flavones and flavonols were potent inhibitors of GTFs; of these, a compound called apigenin (4’,5,7-trihydroxyflavone) was the most effective inhibitor of GTFs (fig. 3). It had no bactericidal activity. Some compounds exhibited bactericidal activity; of these, tt-farnesol (a sesquiterpene) was the most effective antibacterial agent.
Fig. 3.
The structure of apigenin, a bioactive compound found in propolis [from Koo et al., 2002].
When apigenin and tt-farnesol were isolated and tested on S. mutans biofilms in vitro either separately or in combination [Koo et al., 2003], all experimental groups showed some reduction in plaque accumulation, but only the combination of test agents resulted in a significant effect. The authors noted that test agents did not appear to be bactericidal. Further investigation showed that tt-farnesol has bactericidal activity in planktonic cells of mutans streptococci, but has limited bactericidal effects on biofilms at highest therapeutic doses tested; tt-farnesol seemed to also disrupt glucan synthesis and acid product, but in a mechanism different from its chemically distinct partner apigenin. It seems most like that tt-farnesol affects the membrane integrity of bacteria by changing the permeability or fluidity of the cell membrane [Koo et al., 2003]. In a follow-up study, Koo et al. [2005] tested the possible supplementary effects that apigenin and/or tt-farnesol might have on fluoride's anticaries abilities in vitro as well as in vivo. According to results, a combination of all three agents (apigenin, tt-farnesol, and fluoride) was most effective in the reduction of insoluble glucans. No appreciable bactericidal effects were observed [Koo et al., 2005].
Novel, multifaceted combination therapies represent a more efficient method of fighting caries-causing plaque compared to what is widely used in practice now (use of only one agent, fluoride) [Koo, 2008]. The critical factor in discovering novel therapeutic compounds is that they should not have significant effects on cell viability. Because apigenin and tt-farnesol are both inhibitory without threatening cell viability, they are potential therapeutic compounds that can be evaluated for human safety and clinical efficacy.
Conclusion
The two main polyphenol sources discussed, Camellia sinensis and Vaccinium macrocarpon, both contain high levels of bioactive compounds; however, studies conducted on cranberry polyphenols have been more conclusive. Identification of specific structure-activity relationships, such as the unique A-type interflavan linkage in some cranberry polyphenols, has not been achieved for tea. Studies involving tea polyphenols are also limited in that there is little information regarding the extent to which the low-molecular-weight polyphenols are bactericidal. Low-molecular-weight components of cranberry, such as catechin (which is also found in green and oolong teas), did not seem to affect bacterial viability, as was the case in studies using tea catechins. This could be due to confounding factors such as experimental design, or there could be some inherent, uncharacterized difference between the two sources that affects biological activity. Nevertheless, obvious general patterns can be extrapolated for both tea and cranberry polyphenols. For example, size (molecular weight and/or degree of polymerization) seems to be a significant determinant of biological activity. Data for both tea and cranberry extracts indicate that inhibitory activity increases with increase in molecular weight and degree of polymerization. Studies conducted with apigenin and tt-farnesol derived from propolis support the idea that it is not one specific compound, but more likely a combination of compounds, that will have optimal, multilevel inhibitory activity against S. mutans. Finally, even if some phytochemicals, such as tea monomeric catechins and tt-farnesol, do possess some degree of bactericidal activity, if there is a possible dose that maximizes inhibitory efficiency of such compounds without significantly affecting bacterial cell viability, they should not be ruled out.
Traditional antibacterial approaches are falling out of favor due to increased incidence of resistant organisms and virulence-targeted therapies are gaining increased interest. Since a great deal is already known about the critical virulence traits of S. mutans, researchers can focus on how to manage caries by finding ways to target virulence enzymes such as GTFs and F-ATPases. Natural products discovered from medicinal plants have provided numerous clinically used medicines and there is an abundance of evidence that naturally occurring compounds, especially from foods that we already consume, may have therapeutic potential in managing caries. Once a potential plant-based therapy has been proposed, certain factors need to be taken into consideration, such as cost, sustainability of the source, effectiveness in comparison to (as well as in conjunction with) fluoride, and possible toxicity to humans. There should also be rigorous testing to ensure that the compound or derivative does not have significant bactericidal effects. A major goal of studying plant-derived polyphenols is possibility for chemical synthesis of an analog(s); but even if this turns out to be unfeasible, we know from studies in human populations that simple incorporation of these foods is correlated with better oral health and based on that knowledge, certain dietary habits can be advocated.
Disclosure Statement
No author had any conflict of interest in the conduct of this review.
Acknowledgment
The authors' research in this area was supported by grants from the National Center for Complementary and Alternative Medicine (NCCAM/NIH 1K99AT006507-01).
References
- Allaker RP, Douglas CW. Novel anti-microbial therapies for dental plaque-related diseases. Int J Antimicrob Agents. 2009;33:8–13. doi: 10.1016/j.ijantimicag.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Astill C, Birch MR, Dacombe C, Humphrey PG, Martin PT. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J Agric Food Chem. 2001;49:5340–5347. doi: 10.1021/jf010759+. [DOI] [PubMed] [Google Scholar]
- Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009;28:405–411. doi: 10.1089/dna.2009.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271:751–754. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- Banas JA, Miller JD, Fuschino ME, Hazlett KR, Toyofuku W, Porter KA, Reutzel SB, Florczyk MA, McDonough KA, Michalek SM. Evidence that accumulation of mutants in a biofilm reflects natural selection rather than stress-induced adaptive mutation. Appl Environ Microbiol. 2007;73:357–361. doi: 10.1128/AEM.02014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A. Interaction of plant polyphenols with salivary proteins. Crit Rev Oral Biol Med. 2002;13:184–196. doi: 10.1177/154411130201300208. [DOI] [PubMed] [Google Scholar]
- Bullon P, Morillo JM, Ramirez-Tortosa MC, Quiles JL, Newman HN, Battino M. Metabolic syndrome and periodontitis: is oxidative stress a common link? J Dent Res. 2009a;88:503–518. doi: 10.1177/0022034509337479. [DOI] [PubMed] [Google Scholar]
- Bullon P, Quiles JL, Morillo JM, Rubini C, Goteri G, Granados-Principal S, Battino M, Ramirez-Tortosa M. Gingival vascular damage in atherosclerotic rabbits: hydroxytyrosol and squalene benefits. Food Chem Toxicol. 2009b;47:2327–2331. doi: 10.1016/j.fct.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Burger O, Ofek I, Tabak M, Weiss EI, Sharon N, Neeman I. A high molecular mass constituent of cranberry juice inhibits Helicobacter pylori adhesion to human gastric mucus. FEMS Immunol Med Microbiol. 2000;29:295–301. doi: 10.1111/j.1574-695X.2000.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte S, Gregoire S, Singh A, Vorsa N, Schaich K, Bowen W, Koo H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol Lett. 2006;257:50–56. doi: 10.1111/j.1574-6968.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- Ghisalberti EL. Propolis – review. Bee World. 1979;60:59–84. [Google Scholar]
- Gibbons RJ, Qureshi JV. Virulence-related physiological changes and antigenic variation in populations of Streptococcus mutans colonizing gnotobiotic rats. Infect Immun. 1980;29:1082–1091. doi: 10.1128/iai.29.3.1082-1091.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire S, Singh AP, Vorsa N, Koo H. Influence of cranberry phenolics on glucan synthesis by glucosyltransferases and Streptococcus mutans acidogenicity. J Appl Microbiol. 2007;103:1960–1968. doi: 10.1111/j.1365-2672.2007.03441.x. [DOI] [PubMed] [Google Scholar]
- Groppo FC, Bergamaschi Cde C, Cogo K, Franz-Montan M, Motta RH, de Andrade ED. Use of phytotherapy in dentistry. Phytother Res. 2008;22:993–998. doi: 10.1002/ptr.2471. [DOI] [PubMed] [Google Scholar]
- Gross GG, Hemingway RW, Yoshida T. Plant Polyphenols. 2: Chemistry, Biology, Pharmacology, Ecology. New York: Kluwer Academic/Plenum Publishers; 1999. [Google Scholar]
- Hamilton-Miller JM. Anti-cariogenic properties of tea (Camellia sinensis) J Med Microbiol. 2001;50:299–302. doi: 10.1099/0022-1317-50-4-299. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Biochemistry of Phenolic Compounds. London: Academic Press; 1964. [Google Scholar]
- Haslam E, Cai Y. Plant polyphenols (vegetable tannins): gallic acid metabolism. Nat Prod Rep. 1994;11:41–66. doi: 10.1039/np9941100041. [DOI] [PubMed] [Google Scholar]
- Hemingway RW, Laks PE, Branham SJ. Plant Polyphenols: Synthesis, Properties, Significance. New York: Plenum Press; 1992. [Google Scholar]
- Johnson-White B, Buquo L, Zeinali M, Ligler FS. Prevention of nonspecific bacterial cell adhesion in immunoassays by use of cranberry juice. Anal Chem. 2006;78:853–857. doi: 10.1021/ac051700v. [DOI] [PubMed] [Google Scholar]
- Kingston DG, Newman DJ. Natural products as drug leads: an old process or the new hope for drug discovery? IDrugs. 2005;8:990–992. [PubMed] [Google Scholar]
- Koo H. Strategies to enhance the biological effects of fluoride on dental biofilms. Adv Dent Res. 2008;20:17–21. doi: 10.1177/154407370802000105. [DOI] [PubMed] [Google Scholar]
- Koo H, Duarte S, Murata RM, Scott-Anne K, Gregoire S, Watson GE, Singh AP, Vorsa N. Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Res. 2010;44:116–126. doi: 10.1159/000296306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003;52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother. 2002;46:1302–1309. doi: 10.1128/AAC.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Rosalen PL, Cury JA, Park YK, Ikegaki M, Sattler A. Effect of Apis mellifera propolis from two brazilian regions on caries development in desalivated rats. Caries Res. 1999;33:393–400. doi: 10.1159/000016539. [DOI] [PubMed] [Google Scholar]
- Koo H, Schobel B, Scott-Anne K, Watson G, Bowen WH, Cury JA, Rosalen PL, Park YK. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J Dent Res. 2005;84:1016–1020. doi: 10.1177/154405910508401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- Marsh PD. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol. 2005;32(suppl 6):7–15. doi: 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hamada S, Ooshima T. Molecular analysis of the inhibitory effects of oolong tea polyphenols on glucan-binding domain of recombinant glucosyltransferases from Streptococcus mutans mt8148. FEMS Microbiol Lett. 2003;228:73–80. doi: 10.1016/S0378-1097(03)00723-7. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Kawabata S, Ono H, Ogura K, Tanaka T, Ooshima T, Hamada S. Inhibitory effect of oolong tea polyphenols on glycosyltransferases of mutans streptococci. Appl Environ Microbiol. 1993;59:968–973. doi: 10.1128/aem.59.4.968-973.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ. Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem. 2008;51:2589–2599. doi: 10.1021/jm0704090. [DOI] [PubMed] [Google Scholar]
- Ooshima T, Minami T, Aono W, Izumitani A, Sobue S, Fujiwara T, Kawabata S, Hamada S. Oolong tea polyphenols inhibit experimental dental caries in SPF rats infected with mutans streptococci. Caries Res. 1993;27:124–129. doi: 10.1159/000261529. [DOI] [PubMed] [Google Scholar]
- Ooshima T, Minami T, Matsumoto M, Fujiwara T, Sobue S, Hamada S. Comparison of the cariostatic effects between regimens to administer oolong tea polyphenols in SPF rats. Caries Res. 1998;32:75–80. doi: 10.1159/000016433. [DOI] [PubMed] [Google Scholar]
- Otake S, Makimura M, Kuroki T, Nishihara Y, Hirasawa M. Anticaries effects of polyphenolic compounds from Japanese green tea. Caries Res. 1991;25:438–443. doi: 10.1159/000261407. [DOI] [PubMed] [Google Scholar]
- Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med. 2009 doi: 10.1093/ecam/nep067. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Matsumoto M, Tanaka T, Maeda M, Nakai M, Hamada S, Ooshima T. Antibacterial activity of polyphenol components in oolong tea extract against Streptococcus mutans. Caries Res. 2004;38:2–8. doi: 10.1159/000073913. [DOI] [PubMed] [Google Scholar]
- Signoretto C, Bianchi F, Burlacchini G, Sivieri F, Spratt D, Canepari P. Drinking habits are associated with changes in the dental plaque microbial community. J Clin Microbiol. 2010;48:347–356. doi: 10.1128/JCM.00932-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretto C, Burlacchini G, Bianchi F, Cavalleri G, Canepari P. Differences in microbiological composition of saliva and dental plaque in subjects with different drinking habits. New Microbiol. 2006;29:293–302. [PubMed] [Google Scholar]
- Song WO, Chun OK. Tea is the major source of flavan-3-ol and flavonol in the U.S. diet. J Nutr. 2008;138:1543S–1547S. doi: 10.1093/jn/138.8.1543S. [DOI] [PubMed] [Google Scholar]
- Steinberg D, Feldman M, Ofek I, Weiss EI. Effect of a high-molecular-weight component of cranberry on constituents of dental biofilm. J Antimicrob Chemother. 2004;54:86–89. doi: 10.1093/jac/dkh254. [DOI] [PubMed] [Google Scholar]
- Tapiero H, Tew KD, Ba GN, Mathe G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed Pharmacother. 2002;56:200–207. doi: 10.1016/s0753-3322(02)00178-6. [DOI] [PubMed] [Google Scholar]
- Tavares L, Fortalezas S, Carrilho C, McDougall GJ, Stewart D, Ferreira RB, Santos CN. Antioxidant and antiproliferative properties of strawberry tree tissues. J Berry Res. 2010;1:3–12. [Google Scholar]
- Taylor PW, Hamilton-Miller JM, Stapleton PD. Antimicrobial properties of green tea catechins. Food Sci Technol Bull. 2005;2:71–81. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca-Smith AM, Venkitaraman AR, Schilling KM, Bowen WH. Characterization of glucosyltransferase of human saliva adsorbed onto hydroxyapatite surfaces. Caries Res. 1996;30:354–360. doi: 10.1159/000262342. [DOI] [PubMed] [Google Scholar]
- Vauzour D, Vafeiadou K, Rendeiro C, Corona G, Spencer JPE. The inhibitory effects of berry-derived flavonoids against neurodegenerative processes. J Berry Res. 2010;1:45–52. [Google Scholar]
- Yam TS, Shah S, Hamilton-Miller JM. Microbiological activity of whole and fractionated crude extracts of tea (Camellia sinensis), and of tea components. FEMS Microbiol Lett. 1997;152:169–174. doi: 10.1111/j.1574-6968.1997.tb10424.x. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Kimizuka R, Kato T, Okuda K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol Immunol. 2004;19:150–154. doi: 10.1111/j.0902-0055.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]