Abstract
Extramedullary plasmacytomas are tumors of monoclonal plasma cells arising within soft tissue that uncommonly occur in multiple myeloma patients. While sporadic development of these tumors at cutaneous trauma sites, including venous catheter access sites, has been reported, interventional radiologists seldom encounter this disease. Herein, we describe a case of metastatic subcutaneous plasmacytoma precipitated by tunneled central venous catheter insertion in a male patient undergoing stem cell therapy for treatment of multiple myeloma. In addition, we review the identification, diagnostic pitfalls, pathogenesis, and treatment of this rare entity.
Key Words: Central venous catheter, Multiple myeloma, Extramedullary plasmacytoma, Subcutaneous plasmacytoma metastasis
Introduction
Extramedullary plasmacytomas, which are tumors of monoclonal plasma cells arising within soft tissue, represent an uncommon manifestation of multiple myeloma [1, 2]. These lesions are present in approximately 7% of multiple myeloma patients at the time of diagnosis, and occur in an additional 6-7% of patients over the course of their disease [1, 2]. The primary site of involvement is the upper aerodigestive tract [3], and dermatologic manifestations of multiple myeloma are rare [4]. Cutaneous plasmacytomas generally result from direct spread from underlying bone or hematogenous spread in patients with high primary disease burden [5, 6]. While sporadic occurrence at venous catheter access sites has been described [7, 8], medical oncologists and interventional radiologists rarely encounter this disease. As such, identification may be challenging, and lack of recognition may lead to misdiagnosis and unnecessary interventions. Herein, we describe a case of metastatic subcutaneous plasmacytoma that developed as a result of tunneled central venous catheter insertion in a man undergoing stem cell therapy for treatment of multiple myeloma, and highlight the recognition, diagnostic pitfalls, pathogenesis, and management of this entity.
Case Report
A 50-year-old Caucasian man with a past medical history notable for hypertension, high cholesterol, peripheral vascular disease, and coronary artery disease presented to medical attention in November 2009 with progressive neck pain. A CT scan of his cervical spine revealed a lytic lesion in the C2 vertebral body. Further diagnostic workup included a skeletal survey, which showed additional scattered lytic lesions in the right femur, skull, and multiple ribs. Subsequent bone marrow biopsy revealed 90% plasma cells with hyperdiploid cytogenetics, compatible with a diagnosis of multiple myeloma, staged as 3a. Initial treatment consisted of external beam radiation therapy for the C2 vertebral lesion, followed by systemic chemotherapy using bortezomib (Velcade; Millennium Pharmaceuticals, Cambridge, Mass., USA) and dexamethasone. The patient completed 3 chemotherapeutic cycles by the end of December 2009. Lenalidomide (Revlimid; Celgene Corporation, Summit, N.J., USA) was then added to the chemotherapy regimen in January 2010, and the patient completed 4 cycles by late May 2010. Of note, a left orbital plasmacytoma that had developed during the patient's chemotherapy course was also treated with radiation therapy.
Although response to therapy was initially favorable, the patient developed a florid systemic relapse in July 2010 and became transfusion dependent. Vincristine, doxorubicin, and dexamethasone chemotherapy was restarted in early August 2010, with cyclophosphamide initiated in early September 2010 due to progressive disease and persistent anemia. The patient responded favorably to cyclophosphamide and received 2 cycles of cyclophosphamide, dexamethasone, etoposide, and cisplatin in October 2010. Repeat bone marrow biopsy in late October 2010 revealed no evidence of myeloma.
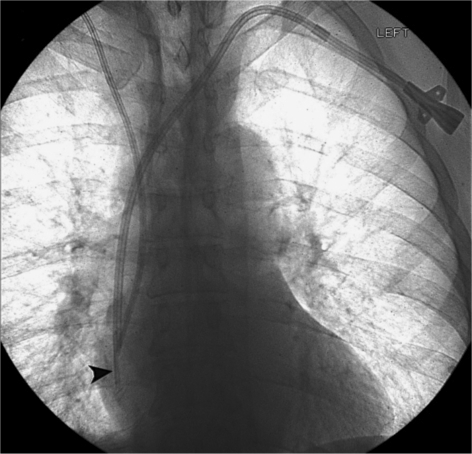
At this point, given the patient's young age and the extent of his disease, autologous stem cell transplantation was recommended. The patient agreed for this therapy and was referred to interventional radiology in early November 2010 for tunneled central venous catheter insertion for transplant therapy. Catheter insertion was performed using standard techniques, and no prophylactic antibiotics were administered. Direct ultrasound guidance was used to guide access into the left internal jugular vein using a 21-gauge needle (Micropuncture Introducer Set; Cook Medical, Bloomington, Ind., USA). The percutaneous access was dilated to accept a 5-French introducer (Micropuncture Introducer Set; Cook Medical), through which a 0.035-inch stiff guide wire was advanced into the inferior vena cava under fluoroscopic guidance. Attention was then turned to the left chest wall, where a 5-mm incision was made. A triple-lumen catheter (TriFusion; C. R. Bard, Murray Hill, N.J., USA) was tunneled from this location to the venotomy site. After serial tract dilation, the catheter was then introduced into the central venous system (fig. 1) and was secured to the skin with suture.
Fig. 1.
Fluoroscopic spot image after tunneled central venous catheter insertion for stem cell therapy shows the newly placed left-sided triple-lumen catheter with the tip (arrowhead) appropriately positioned in the upper right atrium. Note that a right-sided central venous catheter was also present.
The patient subsequently underwent melphalan conditioning, followed by stem cell transplantation in early December 2010. He tolerated the treatment well, and his hospital course was complicated only by neutropenic fever treated with intravenous antibiotics. The patient showed response to the stem cell transplant, and the tunneled central venous catheter was removed without incident in mid-December 2010 by standard local anesthesia, blunt dissection, and gentle traction. The skin at the catheter entry site was approximated using adhesive strips (Steri-Strips; 3M, Maplewood, Minn., USA). The patient's disease course was then unremarkable until he returned to the interventional radiology clinic in mid-February 2011 with complaint of a painful and enlarging subcutaneous nodule located at the exact site of the previous central venous catheter insertion in the left chest.
Clinical examination and ultrasound of the mass were performed, and a diagnosis of mature hematoma was initially entertained. The patient was therefore reassured and instructed to return to clinic 1 week later for reassessment. Upon returning to clinic, however, the mass had enlarged to 2 × 2 cm (fig. 2), and the decision was made to pursue incision and drainage in order to evacuate any hematoma and to ligate any potentially oozing subcutaneous vessels. In the angiography suite, the left chest was prepped and draped. After local anesthesia was applied, a 2-cm incision was made along the inferior aspect of the mass. Open visual assessment of the lesion was not consistent with hematoma, and as such, after consultation with the patient's medical oncologist, two open biopsy samples were obtained. The incision was then closed in two layers using 3-0 and 5-0 Vicryl sutures (Ethicon, Inc., Somerville, N.J., USA). The final pathology report indicated that the mass was an extramedullary plasmacytoma (fig. 3). The patient subsequently received palliative radiation to the chest lesion and blood transfusions for treatment of ongoing anemia. He is alive and doing well at the time of this report. Institutional review board approval is not required for single retrospective case studies at the authors' institutions.
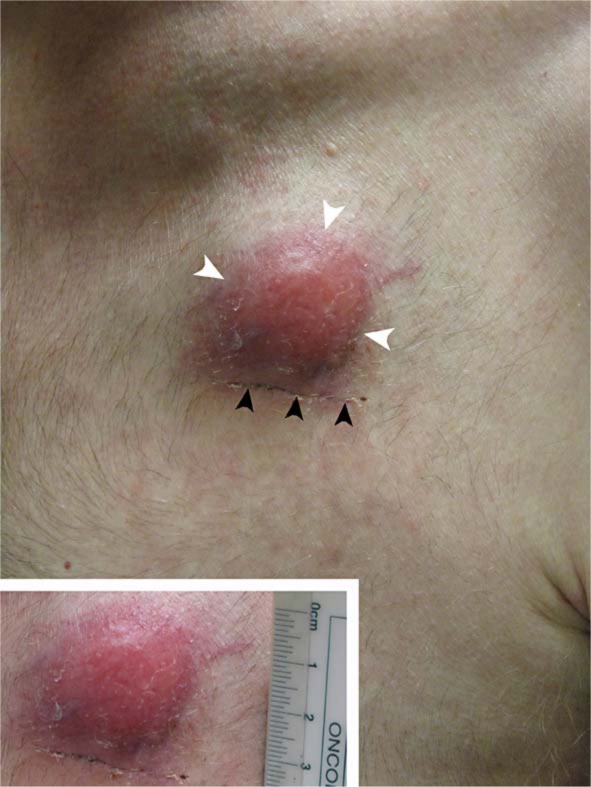
Fig. 2.
Photograph of the patient's chest reveals a subcutaneous mass (demarcated by white arrowheads) with overlying erythema located at the skin entry site of the previously tunneled central venous catheter. Note the healing incision from the subsequent open biopsy (demarcated by black arrowheads). Inset shows that the nodule measures 2 × 2 cm.
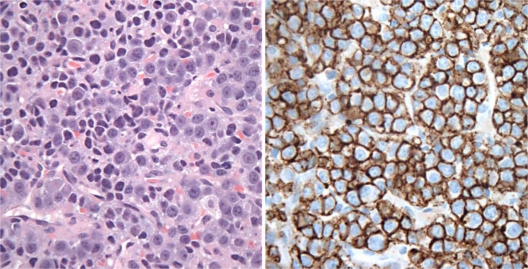
Fig. 3.
High magnification (×40) of a HE-stained slide (left) showing sheets of malignant plasma cells with eccentrically located nuclei and prominent nucleoli. An immunoperoxidase-stained slide (right) which is CD138 positive in malignant plasma cells.
Discussion
Multiple myeloma is a disease resulting from the clonal proliferation of malignant plasma cells [9]. This neoplasm accounts for 13% of hematologic cancers [9] and typically involves the skeletal marrow. Extramedullary plasmacytoma is a manifestation of multiple myeloma in which a discrete mass of neoplastic monoclonal plasma cells forms in soft tissue. The diagnosis of extramedullary plasmacytoma is established on the basis of tissue biopsies, although some patients may have an elevated serum or urine monoclonal protein level.
Extramedullary relapse of multiple myeloma is uncommon, occurring with an incidence of 6-7% [1, 2]. The rate of recurrence is increased after autologous bone marrow transplantation as compared to conventional chemotherapy [10]. The oral cavity and upper respiratory tract are the most common sites of development, accounting for 80% of cases [3]. Few cases of cutaneous extramedullary plasmacytoma have been reported, and growth at sites of trauma, including central venous access sites, as in our case, is very rare. Kerob et al. [7] described the identification of cutaneous lesions in 2 patients who received chemotherapy through a central venous catheter, and Rosenblum et al. [8] reported multiple subcutaneous plasmacytomas localized to areas of previous trauma, including two central venous catheter access sites.
Systemic tumor spread is a complex multistep process. The occurrence of tumor implants at surgical wound sites is related to both local and systemic etiologic factors and may be predisposed to by direct wound contamination, tumor manipulation and aerosolization, and changes in host immune response, among other causes [11]. Multiple theories have been proposed to explain the pathophysiology of plasmacytoma development at trauma sites. A dominant theory relates to survival and clonal selection of myeloma cells expressing the CXCR-4 chemokine receptor after high-dose induction chemotherapy [2]. The ligand for this receptor, stromal cell-derived factor-1 (SDF-1), is a highly potent immune cell (mononuclear cell, mast cell, and T-cell) attractant [12, 13, 14] expressed in areas of trauma and inflammation. When a traumatic insult to the cutaneous or subcutaneous tissues results in increased local SDF-1 levels, preferential trafficking of CXCR-4-positive cells to these areas may occur [12]. Subsequently, the release of various inflammatory cytokines may enable the proliferation of recruited plasma cells, resulting in focal tumor formation [15]. To the best of our knowledge, there is no association between needle or vascular access device caliber and the formation of local tumor implantation.
Although an infrequent occurrence, medical oncologists and interventional radiologists should be aware of the potential for plasmacytoma formation at central venous access sites or sites of other percutaneous intervention in order to avoid misdiagnosis and unnecessary procedures. Multiple myeloma patients are periodically referred to interventional radiology for catheter access prior to stem cell therapy or other procedures, and intermittent interaction with these patients is likely in routine interventional radiology practice. In our case, the metastatic tumor was initially mistaken to represent a mature hematoma, which may occur after catheter removal in a stem cell transplant patient. However, clues that may have led to the correct diagnosis sooner were delayed lesion development after catheter removal and considerable focal pain at the abnormal site. Authors reporting cases of port site breast cancer and leukemic implants have advocated prompt biopsy of persistent chest wall masses [16, 17]. Although a percutaneous biopsy would have still been appropriate if plasmacytoma had been an earlier diagnostic consideration, a 2-3-cm incision may have been avoided in lieu of a small needle puncture. For these reasons, it is important to consider extramedullary plasmacytoma in the differential diagnosis of superficial masses in high-risk patients with a history of multiple myeloma.
While cutaneous plasmacytomas are typically associated with favorable 5-year survival rates [18], some reported cases of cutaneous extramedullary plasmacytoma relapse indicate a rapid decline in the patients' medical condition and eventual expiration [7, 8]. Therapy often includes a combination of chemotherapy, local radiation therapy and/or surgical resection [19]. Because there are so few case reports involving the treatment of cutaneous plasmacytomas, however, there are currently no strong treatment recommendations. Future reports will continue to shed light on this rare disease.
Disclosure Statement
The authors have no conflicts of interest to declare.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Annesley TM, Burritt MF, Kyle RA. Artifactual hypercalcemia in multiple myeloma. Mayo Clin Proc. 1982;57:572–575. [PubMed] [Google Scholar]
- 2.Moreau P, Bataille R, Mahe B, Milpied N, Harousseau JL. High-dose melphalan is not associated with extramedullary relapses in high-risk multiple myeloma. J Clin Oncol. 1993;11:1832. doi: 10.1200/JCO.1993.11.9.1832. [DOI] [PubMed] [Google Scholar]
- 3.Soesan M, Paccagnella A, Chiarion-Sileni V, Salvagno L, Fornasiero A, Sotti G, Zorat PL, Favaretto A, Fiorentino M. Extramedullary plasmacytoma: clinical behaviour and response to treatment. Ann Oncol. 1992;3:51–57. doi: 10.1093/oxfordjournals.annonc.a058070. [DOI] [PubMed] [Google Scholar]
- 4.Kato N, Kimura K, Yasukawa K, Aikawa K. Metastatic cutaneous plasmacytoma: a case report associated with IgA lambda multiple myeloma and a review of the literature of metastatic cutaneous plasmacytomas associated with multiple myeloma and primary cutaneous plasmacytomas. J Dermatol. 1999;26:587–594. doi: 10.1111/j.1346-8138.1999.tb02053.x. [DOI] [PubMed] [Google Scholar]
- 5.Jorizzo JL, Gammon WR, Briggaman RA. Cutaneous plasmacytomas. A review and presentation of an unusual case. J Am Acad Dermatol. 1979;1:59–66. doi: 10.1016/s0190-9622(79)70006-5. [DOI] [PubMed] [Google Scholar]
- 6.Bayer-Garner IB, Smoller BR. The spectrum of cutaneous disease in multiple myeloma. J Am Acad Dermatol. 2003;48:497–507. doi: 10.1067/mjd.2003.180. [DOI] [PubMed] [Google Scholar]
- 7.Kerob D, Vantelon JM, Ribrag V, Bosq J, Desruennes E, Bourhis JH, Avril MF. Cutaneous localization of multiple myeloma on the tract of a central venous catheter. Ann Dermatol Venereol. 2002;129:311–314. [PubMed] [Google Scholar]
- 8.Rosenblum MD, Bredeson CN, Chang CC, Rizzo JD. Subcutaneous plasmacytomas with tropism to sites of previous trauma in a multiple myeloma patient treated with autologous bone marrow transplant. Am J Hematol. 2003;72:274–277. doi: 10.1002/ajh.10296. [DOI] [PubMed] [Google Scholar]
- 9.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 10.Trullemans F, Schots R, Storme G, Camp BV. Late and localized extramedullary relapse of a light chain kappa myeloma after syngeneic bone marrow transplantation. Bone Marrow Transplant. 2000;25:115–117. doi: 10.1038/sj.bmt.1702092. [DOI] [PubMed] [Google Scholar]
- 11.Curet MJ. Port site metastases. Am J Surg. 2004;187:705–712. doi: 10.1016/j.amjsurg.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin TJ, Issekutz TB, Marshall JS. SDF-1 induces IL-8 production and transendothelial migration of human cord blood-derived mast cells. Int Arch Allergy Immunol. 2001;124:142–145. doi: 10.1159/000053693. [DOI] [PubMed] [Google Scholar]
- 14.Nanki T, Hayashida K, El-Gabalawy HS, Suson S, Shi K, Girschick HJ, Yavuz S, Lipsky PE. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol. 2000;165:6590–6598. doi: 10.4049/jimmunol.165.11.6590. [DOI] [PubMed] [Google Scholar]
- 15.Hallek M, Bergsagel PL, Anderson KC. Multiple myeloma: increasing evidence for a multistep transformation process. Blood. 1998;91:3–21. [PMC free article] [PubMed] [Google Scholar]
- 16.Moya Horno I, Fernández Morales LA, Dalmau Portulas E, Sequí Palmer MS, Font Renom J, Aparicio Roríguez O, Falcó Fages J, Querol Niñerola R, Pampols Felip M, Saigí Grau E. Infiltrating ductal carcinoma: infiltrates at the exit site of a central venous access port device. Ann Oncol. 2010;21:1377–1379. doi: 10.1093/annonc/mdq074. [DOI] [PubMed] [Google Scholar]
- 17.Niazi Z, Molt P, Mittleman A, Arlin ZA, Ahmed T. Leukemic dermal infiltrates at permanent indwelling central venous catheter insertion sites. Cancer. 1991;68:2281–2283. doi: 10.1002/1097-0142(19911115)68:10<2281::aid-cncr2820681029>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 18.Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992–2004. Br J Haematol. 2009;144:86–94. doi: 10.1111/j.1365-2141.2008.07421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart AK, Richardson PG, San-Miguel JF. How I treat multiple myeloma in younger patients. Blood. 2009;114:5436–5443. doi: 10.1182/blood-2009-07-204651. [DOI] [PubMed] [Google Scholar]