Abstract
Miriplatin, a cisplatin derivative with a high affinity for iodized ethyl esters of fatty acids from poppy seed oil, is a novel chemotherapeutic agent designed for use in the transarterial treatment of hepatocellular carcinoma (HCC). Here, we describe transcatheter arterial infusion (TAI) using miriplatin to treat a case of advanced HCC with portal vein tumor thrombus (PVTT) refractory to TAI with epirubicin. A 66-year-old man with advanced hepatitis C virus-related HCC with PVTT in the right lobe of the liver was treated with TAI with epirubicin suspended in iodized oil; however, tumor marker levels (alpha-fetoprotein and des-gamma-carboxy protein) did not decrease. Next, he was treated twice with TAI with miriplatin suspended in iodized oil. The tumor marker levels markedly decreased to a nearly normal range and the size of the main tumor was markedly reduced according to dynamic computed tomography. No serious adverse events occurred during the course of treatment with TAI and miriplatin. Therefore, we suggest that TAI with miriplatin is a safe and effective treatment option for advanced HCCs refractory to TAI with epirubicin.
Key Words: Miriplatin, Tumor marker, Hepatocellular carcinoma, Epirubicin
Introduction
Miriplatin, a cisplatin derivative with a high affinity for iodized ethyl esters of fatty acids from poppy seed oil [1], is a novel chemotherapeutic agent designed for use in the transarterial treatment of hepatocellular carcinoma (HCC). Miriplatin (1) inhibits cell proliferation in a similar manner as cisplatin and has superior solubility in ethyl esters of iodized fatty acids from poppy seed oil; (2) releases its platinum constituent continuously by remaining at local tumor sites together with ethyl esters of iodized fatty acids from poppy seed oil (sustained release); and (3) alleviates adverse effects as it maintains an antitumor effect owing to its sustained release as well as its minimal presence in the general circulation [1, 2, 3, 4]. Despite such characteristics, however, many aspects of its antitumor effects are not clear.
We experienced a case of advanced HCC that showed marked decrease in tumor markers following treatment with transcatheter arterial infusion (TAI) with miriplatin, despite being refractory to TAI with epirubicin.
Case Report
The patient, a 66-year-old man, had been diagnosed with chronic hepatitis C and had been followed-up at another hospital for the past 5 years. He had never smoked cigarettes and occasionally drank alcohol. During follow-up for chronic hepatitis C at the other hospital, the levels of tumor markers were found to be elevated, and he was referred to our hospital. An advanced HCC with portal vein tumor thrombus (PVTT) in the right lobe was observed on the dynamic computed tomography (CT) scan, and he was admitted to our hospital for medical treatment in July 2010.
The patient's height was 157 cm, body weight was 46 kg, blood pressure was 134/74 mm Hg, body temperature was 36.6°C, heart rate was 84 bpm, and no significant findings were observed upon physical examination. Laboratory data are shown in table 1. A marked increase in the levels of tumor markers was observed. The patient's Child-Pugh score was 6 points (grade A).
Table 1.
Laboratory data on admission
| Hematology | |
| White blood cells, /μl | 2,720 |
| Red blood cells, /μl | 354 ×104 |
| Hemoglobin, g/dl | 10.5 |
| Platelets, /μl | 8.9 ×104 |
| Coagulation | |
| Prothrombin time, % | 87 |
| APTT, s | 36 |
| Virus marker | |
| HBs antigen | negative. |
| HCV antibody | positive. |
| Tumor marker | |
| AFP, ng/ml | 2,116 |
| AFP(L3), % | 67.4 |
| DCP, mAU/ml | 373 |
| Chemistry | |
| Total protein, g/dl | 7.5 |
| Albumin, g/dl | 3.4 |
| Total bilirubin, mg/dl | 0.4 |
| AST, IU/l | 52 |
| ALT, IU/l | 38 |
| ALP, IU/l | 288 |
| γGTP, IU/l | 169 |
| Choline esterase, IU/l | 103 |
| LDH, IU/l | 200 |
| Na, mEq/l | 139 |
| K, mEq/l | 4.4 |
| Cl, mEq/l | 106 |
| BUN, mg/dl | 23.6 |
| Cr, mg/dl | 0.93 |
APTT = Activated partial thromboplastin time; HBs = hepatitis B surface; HCV = hepatitis C virus; AST = aspartate aminotransferase; ALT = alanine aminotransferase; ALP = alkaline phosphatase; γGTP = γ-glutamyl transpeptidase; LDH = lactate dehydrogenase; Na = sodium; K = potassium; Cl = chlorine; BUN = blood urea nitrogen; Cr = creatinine.
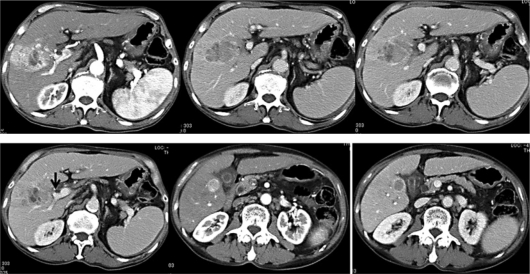
Dynamic CT showed an irregularly shaped tumor, 5.6 × 3.8 cm in size, which was enhanced in the early phase (not enhanced in some areas) and indicated a region of deficiency in the delayed phase, in liver segment 8/7. The tumor had invaded the right anterior branch of the portal vein, and was cut off at the portion indicated by the arrow in fig. 1 (i.e. vp2). In segment 5, adjacent to the gallbladder, a classical HCC 2 cm in size was observed, which was enhanced in the early phase and indicated a region of deficiency in the delayed phase.
Fig. 1.
Dynamic CT findings before treatment. In liver segment 8/7, an irregularly shaped tumor was found, 5.6 cm × 3.8 cm in size, which was irregularly enhanced in the early phase (top left) and indicated a region of deficiency in the delayed phase (top middle, top right, and bottom left). The tumor invaded the right anterior branch of the portal vein (top right and bottom left) and was cut off at the portion indicated by the arrow (i.e. vp2) (bottom left). In segment 5, which is adjacent to the gallbladder, a classical HCC was found, 2 cm in size, which was enhanced in the early phase (bottom middle) and indicated a region of deficiency in the delayed phase (bottom right).
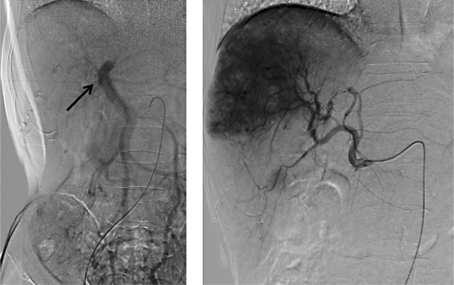
As an initial clinical course beginning in late July 2010, abdominal angiography (fig. 2) was performed. Digital subtraction angiography (DSA) of the superior mesenteric artery showed disruption of the right anterior branch of the portal vein. The lumen of the posterior branch of the postal vein was narrowed. In the DSA of the common hepatic artery, a large irregular tumor stain in the right lobe of the liver and a nodular tumor stain, 2 cm in size, were found in segment 5. An emulsion of epirubicin (50 mg) and lipiodol (8 ml) was infused primarily from the right hepatic artery. After TAI, a transient fever over 38°C was observed; however, the fever was easily managed using only antipyretics, and there were no other adverse events. The patient was discharged in early August 2010.
Fig. 2.
Abdominal angiography results. Left: trans-superior mesenteric arterial portography (superior mesenteric artery-DSA). The right anterior branch of the portal vein was not enhanced, suggesting the presence of a PVTT. The lumen of the right posterior branch of the portal vein was narrowed (arrow). Right: DSA of the common hepatic artery. A large irregular tumor stain in the right lobe and a small nodular tumor stain were observed.
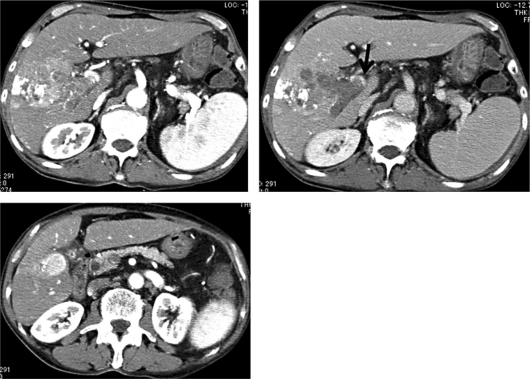
A blood test conducted in late September revealed a marked increase in tumor marker levels (alpha-fetoprotein [AFP] from 2,116 ng/ml to 4,622 ng/ml, and des-gamma-carboxy protein [DCP] from 373 mAU/ml to 8,394 mAU/ml). Dynamic CT confirmed tumor progression (fig. 3). Therefore, he was admitted for retreatment in early October 2010.
Fig. 3.
Dynamic CT findings of tumor progression. In the main tumor, lipiodol accumulation was observed in some areas; however, tumor enhancement indicated progression in size (top left). The PVTT invaded into the main stem of the portal vein (top right; arrow). The tumor in segment 5 also increased in size (from 2 to 2.4 cm).
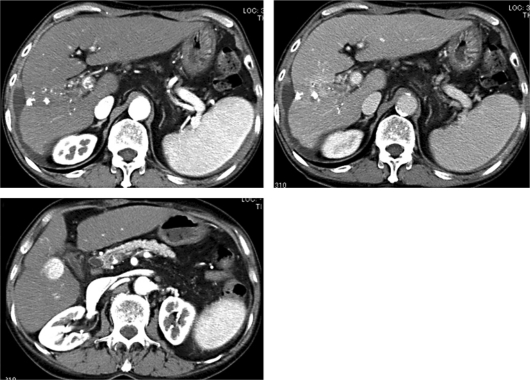
The second clinical course was begun in early October 2010, during which abdominal angiography was performed. Next, an emulsion of miriplatin (80 mg) and lipiodol (8 ml) was infused primarily from the right hepatic artery because the tumors were refractory to TAI with epirubicin. One month after TAI with miriplatin, the AFP level was 1,507 ng/ml and the DCP level was 9,457 mAU/ml, indicating that the therapy was not effective. However, 2 months after TAI, the AFP level was 63.9 ng/ml and the DCP level was 64 mAU/ml. A marked decrease in the levels of the tumor markers was observed. Because of the good response to TAI with miriplatin, an emulsion of miriplatin (100 mg) and lipiodol (5 ml) was again infused primarily from the right hepatic artery in early January 2011. One month after the 2nd TAI with miriplatin, the levels of the tumor markers improved to a nearly normal range (AFP, 16.3 ng/ml and DCP, 34 mAU/ml). Dynamic CT performed 3 months after the 2nd TAI with miriplatin showed that the size of the main tumor in segment 8/7 had markedly decreased, resulting in less obstruction of the right branch of the portal vein (fig. 4). Because the tumor in segment 5 exhibited poor response to the treatment, percutaneous therapy to the nodule is under consideration. The transition of the tumor markers is shown in fig. 5.
Fig. 4.
Dynamic CT 3 months after the 2nd TAI with miriplatin (April 2011). Enhancement of the main tumor was markedly reduced in the remaining area of portal obstruction and most of the administered lipiodol was washed out (top right). The tumor in segment 5 did not change in size.
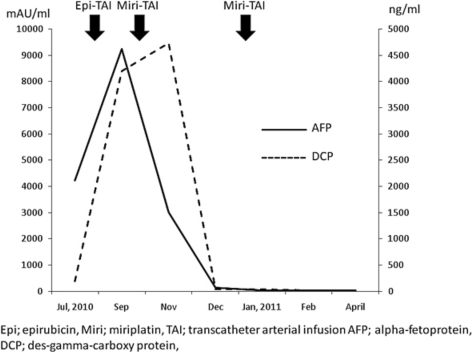
Fig. 5.
Transition of tumor markers. After TAI with epirubicin, elevation of the tumor marker levels continued; however, after TAI with miriplatin, tumor marker levels markedly decreased and improved to nearly the normal range.
Discussion
Several intra-arterial chemotherapy regimens using epirubicin [5], mitomycin [6], adriamycin [7], fluorouracil [8], fluorodeoxyuridine [9], mitoxantrone [10], and cisplatin [11] as treatment for HCC have been reported. However, the optimal regimen for intra-arterial chemotherapy for HCC is still unknown.
In the case described, the patient obtained a marked decrease in tumor marker levels following treatment with TAI and miriplatin, despite being refractory to TAI with epirubicin. There have been no case reports describing this treatment regimen. Considering the clinical courses used, it was thought that the main tumor caused an elevation in the tumor marker levels.
The tumor enhancement pattern seen on the dynamic CT indicated that the main tumor contained an undifferentiated region (irregular enhancement with deficit regions in certain areas) in the early phase. The tumor in segment 5 exhibited classical features of HCC. Considering the effects of the applied treatment (i.e. remarkably effective for the former tumor and ineffective for the latter tumor), TAI with miriplatin may be effective for treating undifferentiated tumors.
It is noteworthy that in the ineffective treatment of the tumor in segment 5 as well as in the markedly effective treatment of the main tumor, most of the administered lipiodol was washed out after treatment with TAI and miriplatin. TAI treatment typically results in tumor necrosis, which is normally evaluated by lipiodol accumulation and CT because lipiodol accumulation in the area of the tumor corresponds to the necrotic area of the tumor [12, 13]. The Liver Cancer Study Group of Japan recommends that the area of lipiodol accumulation should be calculated when evaluating the effects of TAI [14]. However, 1 case has been reported in which sufficient treatment effects were obtained despite washing out of most of the infused lipiodol as was observed for the main tumor in this case. The relationship between the degree of lipiodol accumulation and tumor necrosis should be verified.
In conclusion, TAI with miriplatin is a safe and effective treatment option for advanced HCCs refractory to TAI with epirubicin. Miriplatin can be used as a first-line chemotherapeutic agent for HCC.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Maeda M, Uchida NA, Sasaki T. Liposoluble platinum (II) complexes with antitumor activity. Jpn J Cancer Res. 1986;77:523–525. [PubMed] [Google Scholar]
- 2.Kishimoto S, Noguchi T, Yamaoka T, Fukushima S, Takeuchi Y. Antitumor effects of a novel lipophilic platinum complex (SM-11355) against a slowly growing rat hepatic tumor after intra-hepatic arterial administration. Biol Pharm Bull. 2000;23:344–348. doi: 10.1248/bpb.23.344. [DOI] [PubMed] [Google Scholar]
- 3.Hanada M, Baba A, Tsutsumishita Y, Noguchi T, Yamaoka T, Chiba N, et al. Intra-arterial administration with miriplatin suspended in an oily lymphographic agent inhibits the growth of tumors implanted in rat livers by inducing platinum-DNA adducts to form and massive apoptosis. Cancer Chemother Pharmacol. 2009;64:473–483. doi: 10.1007/s00280-008-0895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanada M, Baba A, Tsutsumishita Y, Noguchi T, Yamaoka T. Intra-arterial administration with miriplatin suspended in an oily lymphographic agent inhibits the growth of human hepatoma cells orthotopically implanted in nude rats. Cancer Sci. 2009;100:189–194. doi: 10.1111/j.1349-7006.2008.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung WT, Shiu WC, Leung N, Chan M, Tao M, Li AK, et al. Treatment of inoperable hepatocellular carcinoma by intra-arterial lipiodol and 4′-epidoxorubicin. Cancer Chemother Pharmacol. 1992;29:401–404. doi: 10.1007/BF00686011. [DOI] [PubMed] [Google Scholar]
- 6.Kinami Y, Miyazaki Y. The superselective and selective one shot methods for treating inoperable cancer of the liver. Cancer. 1978;41:1720–1727. doi: 10.1002/1097-0142(197805)41:5<1720::aid-cncr2820410511>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Kraybill WG, Harrison M, Sasaki T, Fletcher WS. Regional intra-arterial infusion of adriamycin in the treatment of cancer. Surg Gynecol Obstet. 1977;144:335–538. [PubMed] [Google Scholar]
- 8.Ansfield FJ, Ramirez G, Davis HL, Jr, Wirtanen GW, Johnson RO, Bryan GT, et al. Further clinical studies with intrahepatic arterial infusion with 5-fluorouracil. Cancer. 1975;36:2413–2417. doi: 10.1002/1097-0142(197512)36:6<2413::aid-cncr2820360621>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Grage TB, Vassilopoulos PP, Rohde TD, Varco RL, Blackshear PJ. Intraarterial infusion chemotherapy for hepatic carcinoma using a totally implantable infusion pump. Cancer. 1980;45:866–869. doi: 10.1002/1097-0142(19800301)45:5<866::aid-cncr2820450507>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa W, Togo S, Osanai T, Miyanaga T, Sawai S, Yamashita T, et al. Clinical study on the effect of intra-arterial injection of mitoxantrone-lipiodol emulsion for hepatocellular carcinoma. Gan To Kagaku Ryoho. 1994;21:2212–2214. [PubMed] [Google Scholar]
- 11.Shibata J, Fujiyama S, Sato T, Kishimoto S, Fukushima S, Nakano M. Hepatic arterial injection chemotherapy with cisplatin suspended in an oily lymphographic agent for hepatocellular carcinoma. Cancer. 1989;64:1586–1594. doi: 10.1002/1097-0142(19891015)64:8<1586::aid-cncr2820640805>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Okusaka T, Okada S, Ueno H, Ikeda M, Yishimori M, Shimada K, et al. Evaluation of the therapeutic effect of transcatheter arterial embolization for hepatocellular carcinoma. Oncology. 2000;58:293–299. doi: 10.1159/000012115. [DOI] [PubMed] [Google Scholar]
- 13.Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. Am J Roentgenol. 2000;175:699–704. doi: 10.2214/ajr.175.3.1750699. [DOI] [PubMed] [Google Scholar]
- 14.Kudo M, Kubo S, Takayasu K, Sakamoto M, Tanaka M, Ikai I, et al. Response Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 Revised Version) Hepatol Res. 2010;40:686–692. doi: 10.1111/j.1872-034X.2010.00674.x. [DOI] [PubMed] [Google Scholar]