Abstract
This brief review discusses some aspects of hypertensive damage to the kidneys and cardiovascular system. A comparison of renal and cardiac manifestations of hypertensive disease between results of clinical and experimental studies was made, with a major focus on the possible role of salt and the renin-angiotensin system (RAS) in inducing target organ damage. Thus, some degree of renal impairment is often present in patients with essential hypertension, varying from microalbuminuria to end-stage renal disease, whereas in rats with spontaneous hypertension only slight renal damage is seen in old rats with little evidence of renal failure. Since renal damage in hypertensive rats is induced when they are exposed to increased salt intake, we suggested that salt may also account for kidney injury in hypertensive patients. Similarly, cardiac damage is aggravated in hypertensive human beings and rats when given salt excess. We further presented evidence that the RAS may mediate adverse cardiac and renal effects of excessive salt intake. Finally, we also discussed some aspects of the cardiovascular physiology in the giraffe, the only mammal that in comparison with the human being has extremely high pressure at the level of the heart and kidneys but no target organ damage.
Key Words: Arterial pressure, Cardiac/renal damage, Giraffe, Hypertension, Salt intake, SHR
Introduction
Some 30 years ago Circulation Research published a series of articles entitled ‘Controversies in Cardiovascular Research’. Among those, there were reports by McGiff and Quilley and our group related to spontaneously hypertensive rats (SHR) as a model of essential hypertension in humans [1,2,3]. By title, our papers were exactly opposites: ‘Similarities of genetic (spontaneous) hypertension: man and rat’ in ours and ‘The rat with spontaneous hypertension is not a suitable model …’ [1,2]. However, as stated in our response to that review, we did agree on many other aspects related to the use of genetically hypertensive rats in studying the pathophysiological mechanisms underlying essential hypertension [3]. Actually, the first strain of genetically hypertensive rats, the New Zealand strain, had been introduced 5 years earlier than Okamoto's SHRs [4,5]. Several other strains, including the Milan strain, Dahl salt-sensitive and -resistant rats, as well as the Sabra and Lyon strains had also been developed within a span of several years [6,7,8,9]. The pathogenesis of hypertension in some of these strains (e.g., Milan and Dahl strains) was more definitive and was related to renal sodium handling and sodium intake. In others, the mechanism of pressure increase was, like in SHRs, more elusive. Yet, the SHR strain has continued to be employed far more frequently in experimental studies than any other strain at the time of ‘controversial’ articles and has remained the most often used strain to this day. It is true that one reason for this preferential use of SHRs might be in their widespread availability, which may be traced back to the donation of SHR breeders to the National Institutes of Health in 1966. However, the SHR model still remains very similar to essential hypertension pathophysiologically in today's literature.
In this brief review, we present some of the recent results demonstrating the usefulness of animal models in studying cardiovascular and renal diseases. It is not a comprehensive review; we have chosen only a few interesting topics and, whenever possible, we have focused on the results of our own studies.
Hypertensive Disease and the Kidney
Some degree of renal impairment is often present in patients with essential hypertension, varying from microalbuminuria to end-stage renal disease [10].
This is supported by the finding that hypertension, after diabetes mellitus, is the second most common cause of end-stage renal disease in the United States, accounting for about 23% of cases between 1996 and 2000 [10]. However, even in patients with diabetes, hypertensive disease is, almost universally, a major complicating co-morbid disease. Furthermore, elevated blood pressure is considered an important modifiable risk factor for progressive chronic kidney disease regardless of the initial cause of kidney injury. Thus, observational studies have established that patients with increased arterial pressure are at much greater risk of progressive renal insufficiency than normotensive patients [11,12]. Indirect support to the notion that increased arterial pressure is an important risk factor for the development of renal disease is also provided by the results of clinical studies showing that arterial pressure reduction reduces the rate of loss of renal function and progression to renal failure, and this information has been incorporated into current clinical practice guidelines [13,14]. These studies have demonstrated a strong and graded association between blood pressure reduction and the rate of decline in glomerular filtration rate that persists to blood pressure levels of 130/80 mm Hg [13,14,15]. This observation has established a threshold by NIH and AHA/ACC for long-term management of hypertensive patients with chronic renal disease [13,14,15].
Role of Salt
On the other hand, the association between arterial pressure and renal damage is not at all totally clear in the SHR. Proteinuria only becomes apparent in old SHR (after about 70 weeks of age) but, even at this age, no evidence oft renal failure could be demonstrated [16,17]. These findings do not appear to coincide with findings in patients where a clear association between arterial pressure and renal damage has been observed. These observations also raise the question as to whether another factor exists that serves to protect kidneys in SHRs or whether there is another issue that predisposes patients with hypertension to renal injury? In fact, both experimental and clinical studies suggest that prolonged dietary increased salt (i.e., sodium) intake may be the link that connects increased pressure and renal damage, although this link is very often neglected.
In addition to increased arterial pressure, excessive salt intake has also been linked to cardiovascular and renal injury. Since salt excess may exacerbate arterial pressure and hypertensive disease, it is often assumed that salt-induced cardiovascular and renal injuries are related to the concomitant increase in pressure. However, there is a plethora of evidence from experimental and some clinical studies that have demonstrated that, in addition to this arterial pressure-mediated effect, there are other direct adverse effects of salt excess on cardiovascular and renal function and structure [18,19,20].
To begin, a number of rat strainshave not been shown to develop hypertension when exposed to dietary salt excess but, nevertheless, they developed adverse structural and functional cardiovascular and renal effects [20,21,22,23]. Thus, arterial pressure did not increase in normotensive Wistar or Wistar-Kyoto rats exposed to salt overload but, nevertheless, they developed increased left ventricular (LV) mass, LV remodeling, proteinuria and cardiac and renal fibrosis [21,22,23]. More recently, we explored further salt-induced renal injury in greater detail and also examined further the role of salt overload on renal structure and function [24,25,26]. To this end, we investigated the effects of salt loading on renal function, systemic and renal hemodynamics, and glomerular dynamics (determined by renal micropuncture). Thus, at 8 weeks of age, the SHR rats were given either a regular diet or that regular chow contained 4, 6, or 8% salt for the ensuing 8 weeks [24]. Proteinuria and albuminuria increased significantly in proportion to the magnitude of salt loading, beginning in the 2nd week of salt loading. In response, renal plasma flow decreased, and renal vascular resistance and glomerular hydrostatic pressure increased as did the serum creatinine concentration in those rats receiving 6 and 8% salt diets [24]. Moreover, the micropuncture studies revealed diminished single-nephron plasma flow, and increased afferent and efferent glomerular arteriolar resistance [24]. These findings gave further support to the notion of a strong causal relationship between salt excess, and renal and cardiovascular injury. Furthermore, our subsequent studies clearly demonstrated that angiotensin II may be involved in mediating salt-related renal injury, since each of two structurally different angiotensin II receptor blocking agents (losartan and candesartan) prevented the salt-induced renal injury, without affecting arterial pressure [25]. Finally, we demonstrated that, after 4 weeks of excessive salt intake, the activity of the systemic as well as local kidney renin-angiotensin-system (RAS) was either not suppressed or was even augmented despite high salt intake and increased arterial pressure [26]. This inappropriately ‘normal’ or increased activity may then mediate structural and functional cardiovascular and renal injury of salt overload [26]. These studies in experimental rats strongly suggest a causal relationship between salt excess and target organ injury. It is independent of the increase in arterial pressure, although it is sometimes coincidental and may be additive when pressure is already increased.
There are very few clinical studies that have examined the relationship between salt intake and renal injury, although potentially deleterious effects of salt excess that are independent of arterial pressure had been repeatedly suggested [27,28]. Of interest, one study examined renal hemodynamics after a change from a low-salt to a high-salt diet in hypertensive individuals classified as ‘salt sensitive’ or ‘salt resistant’ [29]. Salt-sensitive subjects changing from a low-salt to a high-salt diet showed a dramatic increase in renal vascular resistance and decreased renal blood flow. In conjunction with the reduction in renal blood flow was an increased glomerular arterial resistance, with efferent arterial flow decreasing greater than afferent arterial flow, and a net increase in glomerular pressure and filtration fraction [29]. This also suggested that changes in renal microcirculation favor progression of renal injury [30]. Furthermore, it has been shown that the intrarenal RAS system is more active in African Americans than whites when they switch from a low-salt diet (10 mmol of sodium a day) to a typical diet (200 mmol of sodium per day) [31]. It was also suggested that this difference in the activity of the RAS reflects incomplete renal tissue renin suppression by an increase in salt intake and that this mechanism may contribute to increased susceptibility to renal injury in African Americans [31]. This increased susceptibility to renal injury may also account for their greater representation in end-stage renal disease programs compared to white patients [32]. Additionally, we have also suggested that sodium excess may stimulate local RASs in the kidney as well as heart and vessels independent of the classical system [18,20].
The results of the above-mentioned clinical studies are clearly in agreement with results of our studies on the effects of salt excess on renal function and structure and the activity of RAS in SHR. Together, these results suggest that incomplete suppression of renal tissue RAS may, through alterations in the renal microcirculation or increased oxidative stress for example, mediate adverse renal effects of salt excess. Increased sodium intake also attenuates the beneficial effects of antihypertensive drugs, such as the antiproteinuric effect of angiotensin-converting enzyme inhibitors [33]. Consequently, a reduction in salt intake should be considered as a valid therapeutic choice for the prevention and/or hindrance of progression of already existing renal injury.
Hypertensive Disease and the Cardiovascular System
Hypertension is primarily a disease of the cardiovascular system – blood vessels (arterioles) and the heart. Brain and kidneys are also target organs of hypertension but blood vessels and the heart are affected earlier [34]. Pressure overload imposes a hemodynamic burden on the left ventricle which, in turn, initiates a series of events leading to ventricular remodeling, hypertrophy, and, eventually, to heart failure. Hypertrophy is a response to the increased LV afterload (elevated vascular resistance and arterial pressure) and it is considered an adaptive and protective process up to a certain point. Beyond that, various types of cardiac dysfunction occur, including alterations in ventricular wall mechanics and function. A characteristic of pressure overload LV hypertrophy is the existence of the so-called isolated diastolic dysfunction or diastolic dysfunction with normal ejection fraction [35]. Similar functional alterations are also seen in SHRs and we studied the progression of LV dysfunction over time using echocardiography in SHRs and normotensive rats [36]. To this end, we have conducted echocardiographic examinations in hypertensive and normotensive rats at 8, 15, 20, 35, and 80 weeks of age. The results clearly demonstrated that LV weight progressively increased from weeks 20 to 80 in both strains and was always greater in SHR. Ventricular relaxation was impaired early in the life of SHR, as determined by prolonged isovolumic relaxation time, increased myocardial index, and decreased velocity of early mitral flow propagation. This impairment progressed with aging. Furthermore, ventricular compliance was altered, but systolic function remained unchanged in old SHR. In contrast, relaxation and systolic function were only slightly altered in the old WKY rats, suggesting that pressure-related changes in ventricular function were the dominant features in the SHR [36].
Role of Salt
There is also bountiful evidence that, similar to that with renal injury, excessive salt intake may also play an important role in cardiovascular injury in hypertensive subjects [18,19,20,21,22,23]. Furthermore, we have demonstrated recently that salt excess increased arterial pressure, ventricular mass, and myocardial and perivascular collagen deposition in SHR [37,38]. In addition, diastolic function demonstrated impaired LV relaxation as manifested by prolonged isovolumic relaxation time, decreased early and atrial filling velocity ratio, and slower propagation of E wave in all SHRs [37]. These findings demonstrated that sodium sensitivity in SHRs was manifested by increased arterial pressure and by significant LV functional impairment that was most likely associated with ventricular remodeling promoted by enhanced myocardial fibrosis. We have also examined the effects of salt excess on the function of both ventricles and on coronary hemodynamics in SHRs [38]. Importantly, salt overload increased myocardial collagen content, diminished coronary flow reserve, and impaired diastolic function in both ventricles of the SHR thereby strongly suggesting a pressure-independent role [38]. Finally, our findings suggest that participation of the local cardiac RAS may be mediating the adverse cardiovascular effects of salt overload. Thus, angiotensin II receptor blockade failed to prevent the salt-induced rise in arterial pressure in the salt-loaded SHRs, but it attenuated ventricular remodeling, myocardial fibrosis, and the development of diastolic dysfunction [39]. Moreover, the angiotensin receptor blockade also prevented salt-induced deterioration of coronary and renal hemodynamics, but not large-arterial stiffening (as determined by pulse wave velocity) [39]. On the other hand, there are no clinical studies that directly relate salt intake to cardiovascular end-points, although the results of some studies clearly demonstrate an association between salt, and cardiovascular morbidity and mortality [40,41,42]. It should be noted that similar pathologic changes are seen in experimental desoxycorticosterone salt hypertension which may imply similar pathophysiological mechanisms, although there are no available data on the activity of systemic and local RASs during the progression of the disease.
Giraffe Phenomenon
The differences in the ‘physiological’ or ‘normal’ level of arterial pressure between mammals, ranging from mice to elephants including human beings, are small [43,44]. Furthermore, elevated arterial pressure in the human being is considered to be a major independent risk factor for the cardiovascular and renal morbidity and mortality, including stroke, coronary heart disease, heart failure, sudden cardiac death, chronic kidney disease, and end-stage renal failure [45,46,47]. We are not aware of any naturally occurring disorder in animals that resembles hypertensive disease in humans. However, experimentally induced hypertension in different animals has similar cardiovascular and renal outcomes as in man. Thus, it may be assumed that, in mammals, increased arterial pressure exerts adverse cardiac, vascular, and renal effects. Apparently, the only exception to that rule are long-necked animals, e.g., llama and giraffe.
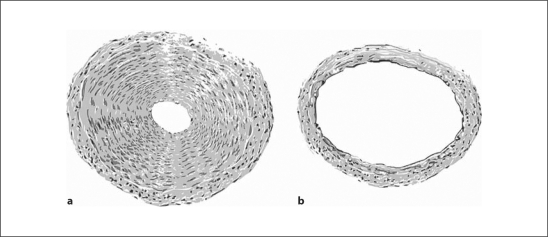
Mean arterial pressure at the cardiac level determined in 4 conscious standing giraffes ranged from 210 to 325 mm Hg, and the pressure at the base of the head was from 60 to 125 mm Hg [48]. Values for cardiac output normalized for body weight correspond to values determined in domestic cattle [48]. Thus, high arterial pressure in the giraffe is a consequence of increased total peripheral vascular resistance. Another interesting detail about the cardiovascular system in the giraffe is that, in addition to arterioles, peripheral arteries significantly contribute toward total peripheral resistance, whereas, in most mammals, peripheral resistance is determined by the resistance in peripheral arterioles [49] (fig. 1). In addition to vascular smooth muscle hypertrophy in arteries and arterioles, the giraffe also exhibits signs of heart hypertrophy [50,51]. When normalized for body weight, the giraffe's heart is 2–4 times greater than in other ruminants [50]. However, no signs of other structural or functional renal or cardiac damages could be found [50,51]. Consistent with this is the fact that life expectancy in the giraffe is about 25 years, whereas in captivity they live much longer due to the absence of natural predators [50].
Fig. 1.
Comparison of arteries with approximately the same diameter in the leg (branch of the posterior tibial artery; a) and head (branch of the carotid artery; b) in the giraffe. The wall thickness of the tibial artery is about 7 times greater than in the carotid artery and the lumen is about 6 times smaller. Blood flow through both vessels is the same with roughly estimated arterial pressure values of 500 (a) and 100 mm Hg (b) [adapted from ref. [48]].
From an evolutionary point of view, the absence of cardiac and renal damage in the giraffe is simple to explain and involves natural selection. The giraffe is born with normal blood pressure, but after standing erect, arterial pressure becomes progressively increased with growth. Individuals that cannot withstand the resulting hypertension do not live to reproductive age, and only ‘resistant’ individuals survive and propagate [50,51]. However, from a medical standpoint, not so simple intricate physiological and biochemical mechanisms that protect the vasculature in the giraffe from hypertensive damage would be far more interesting. Is it that salt is involved again?
To paraphrase Burton [52] instead of the conclusion: ‘Giraffe is an excellent example of the fact that it is not more difficult for the heart to pump blood uphill than downhill, but high hydrostatic pressure is needed to keep blood vessels in the head open’.
Disclosure Statement
The authors have no conflict to disclose.
References
- 1.Trippodo NC, Frohlich ED. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ Res. 1981;48:309–319. doi: 10.1161/01.res.48.3.309. [DOI] [PubMed] [Google Scholar]
- 2.McGiff JC, Quilley CP. The rat with spontaneous genetic hypertension is not a suitable model of human essential hypertension. Circ Res. 1981;48:455–464. doi: 10.1161/01.res.48.4.455. [DOI] [PubMed] [Google Scholar]
- 3.Frohlich ED, Trippodo NC. Response to ‘The rat with spontaneous genetic hypertension is not a suitable model of human essential hypertension’. Circ Res. 1981;48:464. doi: 10.1161/01.res.48.4.455. [DOI] [PubMed] [Google Scholar]
- 4.Smirk FH, Hall WH. Inherited hypertension in rats. Nature. 1958;182:727–728. doi: 10.1038/182727a0. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi G, Fox U, Imbasciati E. The development of a new strain of spontaneously hypertensive rats. Life Sci. 1974;14:339–347. doi: 10.1016/0024-3205(74)90064-2. [DOI] [PubMed] [Google Scholar]
- 7.Dahl LK, Heine M, Tassinari L. Role of genetic factors in susceptibility to experimental hypertension due to chronic excess salt ingestion. Nature. 1962;194:480–482. doi: 10.1038/194480b0. [DOI] [PubMed] [Google Scholar]
- 8.Zamir N, Gutman Y, Ben-Ishay D. Hypertension and brain catecholamine distribution in the Hebrew University Sabra, H and N rats. Clin Sci Mol Med. 1978;55(suppl 4):105s–107s. doi: 10.1042/cs055105s. [DOI] [PubMed] [Google Scholar]
- 9.Vincent M, Bornet H, Berthezene F, Dupont J, Sassard J. Thyroid function and blood pressure in two new strains of spontaneously hypertensive and normotensive rats. Clin Sci Mol Med. 1978;54:391–395. doi: 10.1042/cs0540391. [DOI] [PubMed] [Google Scholar]
- 10.McClellan WM. Epidemiology and risk factors for chronic kidney disease. Med Clin North Am. 2005;89:419–445. doi: 10.1016/j.mcna.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41:1341–1345. doi: 10.1161/01.HYP.0000069699.92349.8C. [DOI] [PubMed] [Google Scholar]
- 12.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington county, Maryland. J Am Soc Nephrol. 2003;14:2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 13.Bakris GL, Williams M, Dworkin L, Elliot WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hseuh W, Sowers J. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 15.Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek DA, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Rhillips RA, Toto RD, Middleton JP, Rostand SG, African American Study of Kidney Disease, Hypertension Study Group Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu K, Frohlich ED, Ono H, Ono Y, Numabe A, Willis GW. Glomerular dynamics and morphology of aged spontaneously hypertensive rats. Effects of angiotensin-converting enzyme inhibition. Hypertension. 1995;25:207–213. doi: 10.1161/01.hyp.25.2.207. [DOI] [PubMed] [Google Scholar]
- 17.Bakoush O, Tencer J, Torffvit O, Tenstad O, Skogvall I, Rippe B. Increased glomerular albumin permeability in old spontaneously hypertensive rats. Nephrol Dial Transplant. 2004;19:1724–1731. doi: 10.1093/ndt/gfh276. [DOI] [PubMed] [Google Scholar]
- 18.Frohlich ED. The salt conundrum: a hypothesis. Hypertension. 2007;50:161–166. doi: 10.1161/HYPERTENSIONAHA.107.088328. [DOI] [PubMed] [Google Scholar]
- 19.He FJ, MacGregor GA. Salt, blood pressure and cardiovascular disease. Curr Opin Cardiol. 2007;22:298–305. doi: 10.1097/HCO.0b013e32814f1d8c. [DOI] [PubMed] [Google Scholar]
- 20.Susic D, Fares H, Frohlich ED. Salt, arterial pressure, and cardiovascular and renal damage. Ochsner J. 2009;9:197–203. [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan BX, Leenen FH. Dietary sodium intake and left ventricular hypertrophy in normotensive rats. Am J Physiol. 1991;261:H1397–H1401. doi: 10.1152/ajpheart.1991.261.5.H1397. [DOI] [PubMed] [Google Scholar]
- 22.Leenen FH, Yuan B. Dietary sodium-induced cardiac remodeling in spontaneously hypertensive rats versus Wistar-Kyoto rats. J Hypertens. 1998;16:885–892. doi: 10.1097/00004872-199816060-00020. [DOI] [PubMed] [Google Scholar]
- 23.Yu HC, Burrell, Black MJ, Wu LL, Dilley RJ, Johnston CI. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1999;98:2621–2628. doi: 10.1161/01.cir.98.23.2621. [DOI] [PubMed] [Google Scholar]
- 24.Matavelli LC, Zhou X, Varagic J, Susic D, Frohlich ED. Salt-loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H814–H819. doi: 10.1152/ajpheart.00671.2006. [DOI] [PubMed] [Google Scholar]
- 25.Susic D, Zhou X, Frohlich ED. Angiotensin blockade prevents salt-induced injury of the renal circulation in spontaneously hypertensive rats. Am J Nephrol. 2009;29:639–645. doi: 10.1159/000195633. [DOI] [PubMed] [Google Scholar]
- 26.Susic D, Frohlich ED, Kobori H, Shao W, Seth D, Navar G. Renin-angiotensin system as a mediator of salt-induced cardiovascular and renal injury. J Hypertens. 2011;29:716–723. doi: 10.1097/HJH.0b013e3283440683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonios TF, MacGregor GA. Salt intake: potential deleterious effects excluding blood pressure. J Hum Hypertens. 1995;9:511–515. [PubMed] [Google Scholar]
- 28.Weir MR. Salt-intake and hypertensive renal injury in African-Americans. A therapeutic perspective. Am J Hypertens. 1995;8:635–644. doi: 10.1016/0895-7061(95)00048-T. [DOI] [PubMed] [Google Scholar]
- 29.Campese VM, Parise M, Karubian F, Bigazzi R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension. 1991;18:805–812. doi: 10.1161/01.hyp.18.6.805. [DOI] [PubMed] [Google Scholar]
- 30.Weir MR, Fink JC. Salt intake and progression of chronic kidney disease: an overlooked modifiable exposure? A commentary. Am J Kidney Dis. 2005;45:176–188. doi: 10.1053/j.ajkd.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 31.Price DA, Fisher ND, Lansang MC, Stevanovic R, Williams GH, Hollenberg NK. Renal perfusion in blacks: alterations caused by insuppressibility of intrarenal renin with salt. Hypertension. 2002;40:186–189. doi: 10.1161/01.hyp.0000024349.85680.87. [DOI] [PubMed] [Google Scholar]
- 32.Rostand SG. US minority groups and end stage renal disease: a disproportionate share. Am J Kidney Dis. 1992;19:411–413. doi: 10.1016/s0272-6386(12)80946-2. [DOI] [PubMed] [Google Scholar]
- 33.Heeg JE, de Jong PE, van der Hem GK, de Zeeuw D. Efficacy and variability of the antiproteinuric effect of ACE inhibition by lisinopril. Kidney Int. 1989;36:272–279. doi: 10.1038/ki.1989.190. [DOI] [PubMed] [Google Scholar]
- 34.Kobrin I, Frohlich ED, Ventura HO, Messerli FH. Renal involvement follows cardiac enlargement in essential hypertension. Arch Intern Med. 1986;146:272–276. [PubMed] [Google Scholar]
- 35.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis and measurements of diastolic function. Circulation. 2002;105:1503–1508. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 36.Slama M, Ahn J, Varagic J, Susic D, Frohlich ED. Long-term left ventricular echocardiographic follow-up of SHR and WKY rats: effects of hypertension and age. Am J Physiol Heart Circ Physiol. 2004;286:H181–H185. doi: 10.1152/ajpheart.00642.2003. [DOI] [PubMed] [Google Scholar]
- 37.Ahn I, Varagic J, Slama M, Susic D, Frohlich ED. Cardiac structural and functional responses to salt loading in SHR. Am J Physiol Heart Circ Physiol. 2004;287:H767–H772. doi: 10.1152/ajpheart.00047.2004. [DOI] [PubMed] [Google Scholar]
- 38.Varagic J, Frohlich ED, Díez J, Susic D, Ahn J, Gonzalez A, Lopez B. Myocardial fibrosis, impaired coronary hemodynamics, and biventricular dysfunction in salt-loaded SHR. Am J Physiol Heart Circ Physiol. 2006;290:H1503–H1509. doi: 10.1152/ajpheart.00970.2005. [DOI] [PubMed] [Google Scholar]
- 39.Varagic J, Frohlich ED, Susic D, et al. AT1 receptor antagonism attenuates target organ effects of salt excess in SHRs without affecting pressure. Am J Physiol Heart Circ Physiol. 2008;294:H853–H858. doi: 10.1152/ajpheart.00737.2007. [DOI] [PubMed] [Google Scholar]
- 40.Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, Nissinen A. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet. 2001;357:848–851. doi: 10.1016/S0140-6736(00)04199-4. [DOI] [PubMed] [Google Scholar]
- 41.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long-term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention. BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis. 2006;49:59–75. doi: 10.1016/j.pcad.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Seymour RS, Blaylock AJ. The principle of Laplace and scaling of ventricular wall stress and blood pressure in mammals and birds. Physiol Biochem Zoo. 2000;73:389–405. doi: 10.1086/317741. [DOI] [PubMed] [Google Scholar]
- 44.Honeyman VL, Pettifer GR, Dyson DH. Arterial blood pressure and blood gas values in normal standing and laterally recumbent African (Loxodonta africana) and Asian (Elephas maximus) elephants. J Zoo Wildlife Med. 1992;23:205–210. [Google Scholar]
- 45.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275:1571–1576. [PubMed] [Google Scholar]
- 46.Frohlich ED. Fibrosis and ischemia: the real risks in hypertensive heart disease. Am J Hypertens. 2001;14:194S–199S. doi: 10.1016/s0895-7061(01)02088-x. [DOI] [PubMed] [Google Scholar]
- 47.Frohlich ED. Recent advances in hypertension, stroke, and cardiorenal disease. Curr Opin Cardiol. 2005;20:258–263. doi: 10.1097/01.hco.0000166594.06594.fe. [DOI] [PubMed] [Google Scholar]
- 48.Goetz RH, Warren JV, Gauer OH, Patterson JL, Doyle JT, Keen EN, McGregor M. Circulation of the giraffe. Circ Res. 1960;8:1049–1058. doi: 10.1161/01.res.8.5.1049. [DOI] [PubMed] [Google Scholar]
- 49.Goetz RH, Keen EN. Some aspects of the cardiovascular system in the giraffe. Angiology. 1957;8:542–564. doi: 10.1177/000331975700800609. [DOI] [PubMed] [Google Scholar]
- 50.Zhang QG. Hypertension and counter-hypertension mechanisms in giraffes. Cardiovasc Hematol Disord Drug Targets. 2006;6:63–67. doi: 10.2174/187152906776092640. [DOI] [PubMed] [Google Scholar]
- 51.Paton JFR, Dickinson CJ, Mitchell G. Harvey Cushing and the regulation of blood pressure in giraffe, rat and man: introducing ‘Cushing mechanism’. Exp Physiol. 2009;94:11–17. doi: 10.1113/expphysiol.2008.043455. [DOI] [PubMed] [Google Scholar]
- 52.Burton AC. Physiology and Biophysics of Circulation. ed 2. Chicago: Year Book Medical Publisher; 1972. [Google Scholar]