Abstract
Background
Brain natriuretic peptide (BNP) is elevated in patients with end-stage renal disease and could reflect left ventricular dysfunction. Aim: To evaluate the plasma levels of BNP in two groups of asymptomatic patients on different dialysis programs and to correlate their variations with echocardiographic parameters.
Methods
Group A consisted of 36 patients on chronic hemodialysis (HD), and group B included 38 patients on continuous ambulatory peritoneal dialysis (CAPD). ECG and echocardiography were performed, and concomitantly plasma BNP levels were determined before and after a regular 4-hour session in HD patients and before performing a dialysate exchange in patients on CAPD.
Results
BNP values in group A were found to be higher than in group B (419 ± 76 vs. 193 ± 56 pg/ml; p < 0.03). The cutoff point which discriminated both groups was 194 pg/ml (sensitivity: 64% and specificity: 76%; p = 0.001). Significant differences were found with respect to the following echocardiographic data (group A vs. group B): left atrial (LA) size (40 ± 13 vs. 34 ± 1 mm), LA volume (59 ± 16 vs. 41 ± 32 ml), transmitral flow E/A (1.17 ± 0.01 vs. 0.9 ± 0.06), the movement of the mitral valve annulus e/a (tissue Doppler imaging; 1.19 ± 0.15 vs. 1.05 ± 0.13) and left ventricular mass index (133 ± 10 vs. 108 ± 11).
Conclusion
Patients on CAPD had lower levels of BNP, and echocardiographic findings indicated decreased volume overload. In asymptomatic patients, marked increases in BNP levels may reflect early stages of pathological processes that precede the development of apparent cardiac manifestations (left ventricular hypertrophy). Only echocardiographic parameters of cardiac dysfunction should be used as diagnostic criteria.
Key Words: Brain natriuretic peptide, Cardiac function, Continuous ambulatory peritoneal dialysis, Hemodialysis, Left ventricular function, Renal failure
Introduction
The principal trigger for the secretion of brain natriuretic peptide (BNP) is the stretching of myocardial fibers, which is initially produced as pre-proBNP and later on as a molecule of active BNP. Both BNP and the inactive molecule Nt-pro-BNP have been used as indicators of cardiac insufficiency and to assist in the differential diagnosis of dyspnea in the emergency department [1]. Moreover, in patients treated for acute cardiac failure, BNP concentrations decrease concomitant with pulmonary capillary wedge pressure during a 24-hour period.
Plasma BNP levels are also elevated in patients with chronic renal failure on hemodialysis (HD). Volume overload is the most frequently mentioned along with cardiovascular disease or ventricular dysfunction detected by echocardiography, since the majority of patients suffer from structural cardiomyopathy [2]. One of the main clinical differences between patients on HD and those on continuous ambulatory peritoneal dialysis (CAPD) is the different pattern observed in their volume plasma oscillation, which cannot only induce changes in BNP levels but also in echocardiographic parameters [3,4].
The primary objectives of this study were to evaluate the plasma levels of BNP in two groups of asymptomatic patients on different dialysis programs and to correlate their variations with echocardiographic parameters.
Patients and Methods
Patient Selection
This study was carried out in a single dialysis center between January 2007 and December 2009. In addition to the patients’ demographic data, information on their clinical variables, HD time, dialyzer membrane, causes of nephropathy, cardiovascular risk factors and concomitant pathological diseases was obtained.
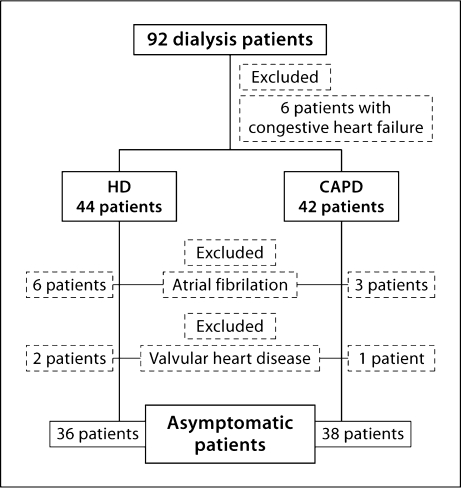
Figure 1 depicts a flow chart of the study design. Patients with valvular heart disease, persistent atrial fibrillation in ECG, symptoms and signs of congestive heart failure and/or NYHA class >2 were excluded from the study. Two groups of asymptomatic patients were studied: group A consisted of 36 patients on chronic HD and group B included 38 patients on CAPD (table 1). Informed consent was obtained from all patients and the study protocol was approved by the institutional ethical committee of the hospital.
Fig. 1.
Flow chart of the original study.
Table 1.
Clinical characteristics of the study patients
| HD (n = 36) | CAPD (n = 38) | p value | |
|---|---|---|---|
| Demographic parameters | |||
| Age, years | 61 ± 14 | 57 ± 17 | NS |
| Male sex | 22 (61%) | 19 (50%) | NS |
| Body mass index | 27.6 ± 5 | 27 ± 5 | NS |
| New York Heart Association class >1 | 18 (50%) | 17 (45%) | NS |
| Significant diuresis | 26 (72%) | 26 (68%) | NS |
| Time on dialysis, months | 45 ± 51 | 26 ± 19 | 0.04 |
| Residual renal function, ml/min | 10.6 ± 4.5 | 10.5 ± 5.3 | NS |
| Nephropathy | |||
| Diabetes mellitus | 5 (14%) | 11 (29%) | NS |
| Vascular disease | 10 (28%) | 9 (24%) | NS |
| Chronic glomerulonephritis | 5 (14%) | 5 (13%) | NS |
| Polycystic kidney disease | 4 (11%) | 1 (3%) | NS |
| Collagenopathy | 4 (11%) | 2 (5%) | NS |
| Unknown | 8 (22%) | 10 (26%) | NS |
| Cardiovascular risk factors | |||
| Hypercholesterolemia | 18 (50%) | 25 (66%) | NS |
| Smoking | 15 (30%) | 10 (26%) | NS |
| Diabetes mellitus | 12 (33%) | 12 (32%) | NS |
| Hypertension | 30 (83%) | 32 (84%) | NS |
| Peripheral vascular disease | 1 (3%) | 2 (5%) | NS |
| Coronary artery disease | 9 (25%) | 6 (16%) | NS |
NS = Nonsignificant. Means ± SD and numbers (%) are shown.
Patients on HD were dialyzed on average for 4 h, three times a week, using low- or high-flux dialyzer membranes. The concentrations of sodium, potassium and bicarbonate were 138, 1.5 and 30 mEq/l, respectively. Blood flow varied between 300 and 350 ml/min and the dialysate flow was 700 ml/min. The ultrafiltration volume varied between 1.5 and 3.5 liters per HD session. Patient weight was checked before and after dialysis using an electronic scale. Patients on CAPD were dialyzed using a conventional solution of lactate and glucose. The study was not carried out on any particular day of the week, but avoiding Mondays for patients on HD. Vital constants were determined in all patients, and an echocardiographic study was performed before and after HD. Heart rate and blood pressure were monitored every hour during the procedure.
BNP Measurements
Plasma BNP levels were assessed in venous blood before and 10 min after HD. In patients on CAPD, blood was sampled after abdominal emptying with patients in the supine position. The Triage BNP test kit (Biosite Diagnostics, San Diego, Calif., USA) combined with AxSYM System was employed to assess the concentration of BNP in whole blood. This immunoassay consists of a disposable device to which EDTA is added at a concentration of 1 mg/ml. The test procedure includes the addition of various drops of a blood sample on the orifice of a card. Thereafter, the card is introduced in the Triage Meter Reader, which automatically determines BNP levels between 5 and 5,000 pg/ml.
Assessment of Residual Renal Function and Left Ventricular Function
In patients with decreased renal function, tubular secretion of creatinine causes on average a 15% overestimate of creatinine clearance and tubular reabsorption of urea on average a 15% underestimate of urea clearance; therefore, the mean of these two measurements is believed to represent the most accurate non-invasive test of glomerular filtration rate (GFR) in patients with decreased GFR. Combined urea and creatinine clearance was the arithmetic mean of the 24- (CAPD) or 48-hour (inter-HD) urea and creatinine clearance rates corrected for body surface area. We used this measurement as a surrogate for estimated GFR, and GFR <15 ml/min/1.73 m2 was regarded as the theoretic threshold for starting dialysis [5].
Before and after HD, a bedside transthoracic echocardiography study was carried out in sinus rhythm, using a cardiac ultrasound unit equipped with a multifrequency transducer and tissue Doppler imaging (TDI; Aloka, Tokyo, Japan). Once the presence of valvular cardiopathy is ruled out, the size of cardiac cavities, cardiac wall thickness, the variations in transmitral flow, and the movement of the mitral valve annulus in the lateral wall by TDI are all assessed according to the recommendations of scientific societies [6,7]. Left ventricular mass (LVM) was calculated using the regression equation of Devereux and Reichek [8]. Based on the echocardiographic observation of LVM adjusted for the body surface area, LVM indices (LVMI) >130 g/m2 for men and >110 g/m2 for women were chosen as upper limits of normal [9].
Left ventricular ejection fraction was calculated using end-diastolic and end-systolic volume measurements from 4- and 2-chamber views calculated by the Simpson method. A decrease in left ejection fraction <50% by echocardiography was considered diagnostic of systolic left ventricular dysfunction. Doppler techniques evaluating mitral inflow and TDI of the mitral valve annulus have been used to categorize diastolic dysfunction in delayed or abnormal relaxation and pseudonormal patterns (moderate, marked and irreversible).
Statistical Analysis
Continuous variables with an abnormal distribution [Kolmogorov-Smirnov test] were transformed by neperian logarithm before use and are expressed as means ± SEM; differences in mean group values were assessed by Student's t test. The χ2 test was employed to analyze differences in categorical variables. ANOVA was used to analyze differences in parameters between groups. The relationships between continuous variables were analyzed by the Pearson correlation. To determine changes in BNP levels after HD, the following equation was used:
.
Using receiver-operating characteristic (ROC) curves, the cutoff point and maximum sensitivity and specificity were determined. For group differences, multivariate analysis (logistic regression) was applied to obtain independent parameters. A value of p ≤ 0.05 was considered statistically significant.
Results
General Findings
The baseline clinical features of the patients are summarized in table 1. Time on dialysis was longer for HD than CAPD patients (45 ± 51 vs. 26 ± 19 months; p = 0.04). Regarding treatment, angiotensin-converting enzyme inhibitors were more frequently administered in patients on CAPD (22 vs. 50%; p = 0.03). The most significant cardiovascular risk factor was arterial hypertension, being present in >80% of patients in both groups. Twenty-six patients were on diuretic medication in each group (72% in the HD group vs. 68% in the CAPD group).
There was no significant difference in residual renal function between both groups: CAPD = 10.6 ± 4.5 ml/min versus HD = 10.5 ± 5.3 ml/min (p = 0.89).
Echocardiographic Characteristics
Table 2 shows the main echocardiographic parameters in both groups. The most significant differences were noted in left atrial (LA) diameter and volume, transmitral flow with TDI and LVMI. After the HD session, the parameters were lower, reaching similar values to the ones observed in patients on CAPD. Using logistic regression, LA size (AI ≥40 mm) [b = 1.9; Exp (b) = 7.14 (1.3–37; p = 0.019)] and the transmitral flow (E/A >1.2) [b = 1.5; Exp. (B) = 4.6 (1.27–17)] were independent predictive variables in the differentiation between HD and CAPD patients.
Table 2.
Echocardiographic characteristics
| Before HD | After HD | CAPD | p value | |
|---|---|---|---|---|
| LA volume, ml | 59 ± 36 | 46 ± 17 | 41 ± 17 | 0.05 |
| LA size, cm | 4 ± 0.5 | 3.6 ± 0.5 | 3.4 ± 0.5 | <0.001 |
| LVDD | 4.7 ± 0.8 | 4.6 ± 0.9 | 4.7 ± 0.6 | NS |
| LVSD | 3.5 ± 0.9 | 3.4 ± 0.8 | 3.3 ± 0.5 | NS |
| LVEF | 54 ± 13 | 54 ± 13 | 55 ± 12 | NS |
| E/A (transmitral flow) | 1.17 ± 0.4 | 1.13 ± 0.6 | 0.9 ± 0.3 | 0.009 |
| e/a (TDI) | 1.14 ± 0.7 | 1 ± 0.6 | 0.78 ± 0.4 | 0.01 |
| E/e | 8.5 ± 4 | 8.6 ± 4 | 9.1 ± 4 | NS |
| LVMI, g/m2 | 133 ± 51 | 124 ± 62 | 108 ± 40 | 0.04 |
| Systolic dysfunction | 12 (33%) | 9 (24%) | NS | |
| Diastolic dysfunction | 19 (53%) | 27 (71%) | NS |
LVDD = Left ventricular diastolic diameter; LVSD = left ventricular systolic diameter; LVEF = left ventricular ejection fraction; NS = nonsignificant. p values: CAPD vs. before HD.
Twenty-one patients (29% of 74 patients) had systolic dysfunction: 12 patients (33%) in the HD group and 9 patients (24%) in the CAPD group (nonsignificant difference).
Diastolic dysfunction was observed in 46 of the 74 patients (62%), being more frequent in patients on CAPD (27 of 38; 71%) than in the HD group (19 of 36; 53%; nonsignificant). Twelve of the 74 patients (16%) had systolic and diastolic dysfunction concomitantly.
BNP Level and Its Relationship with Other Parameters
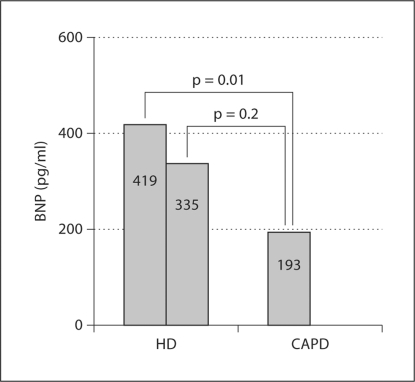
BNP concentrations before dialysis were clearly higher in patients of group A (mean ± SEM: 419 ± 76 vs. 193 ± 56 pg/ml; p = 0.01). Plasma BNP levels 4 h after HD decreased to 335 ± 80 pg/ml (decrement of 20%), being still higher than those found in CAPD patients, although the difference did not reach statistical significance (fig. 2).
Fig. 2.
BNP levels in HD and CAPD patients.
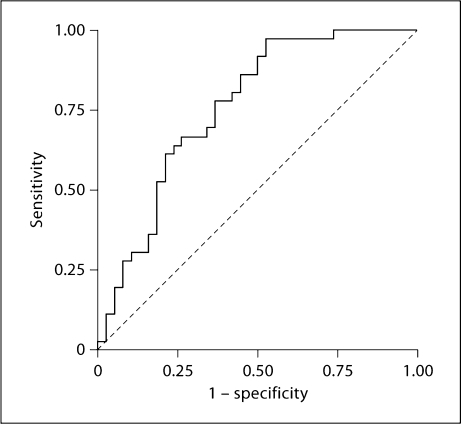
Figure 3 shows the ROC curve with the best cutoff value of baseline BNP which helped to differentiate HD from CAPD patients. The cutoff level was set at 194 pg/ml, resulting in a sensitivity of 64% and a specificity of 76%; the positive predictive value was 72% and the negative predictive value was 69%. The global accuracy of the test was 48%. The area under the curve was 0.752 (0.640–0.864; p < 0.001).
Fig. 3.
ROC curve of plasma BNP levels in dialysis patients. A plasma BNP level of 194 ng/ml is the best cutoff to differentiate HD and CAPD patients (sensitivity of 64% and specificity of 76%). Area = 0.759 (0.649–0.869; p ≤ 0.001).
In table 3, associations between BNP levels (≥194 pg/ml) and echocardiographic parameters, and corresponding odds ratios are listed. Of note, in HD patients, LA size was higher (≥40 mm) and left ventricular hypertrophy was more frequent (LVMI ≥125 g/m2). No correlation was found between BNP levels and systolic or diastolic dysfunction.
Table 3.
Association of BNP levels (≥194 pg/ml) with echocardiographic parameters
| OR | 95% CI | p value | |
|---|---|---|---|
| Systolic dysfunction (EF <50%) | 1.8 | 0.8–3.8 | 0.1 |
| Diastolic dysfunction | 0.8 | 0.6–1.3 | 0.2 |
| E/A (transmitral flow) | 1.5 | 0.7–3 | 0.2 |
| LA size >4 cm | 2.7 | 1–7.3 | 0.037 |
| LVMI ≥125 g/m2 | 2.9 | 1.4–6 | 0.002 |
EF = Ejection fraction; OR = odds ratio; CI = confidence interval.
Discussion
A significant rise in the BNP concentration has been observed in patients on chronic dialysis. Renal insufficiency by itself does not appear to explain the plasma BNP levels. General consensus exists that BNP is related to the stretching of myocardial fibers following volume overload [2,3]. However, since BNP levels remain elevated after significant fluid loss after each dialysis session in both HD as well as CAPD patients, other factors must also be involved.
Because all patients included in this study were asymptomatic, and signs of cardiac failure were absent, it could be inferred that both echocardiographic parameters and BNP levels could be used to detect preclinical ventricular dysfunction. LVM, myocardial ischemia and fibrosis are the major factors affecting systolic and/or diastolic function. The majority of patients on dialysis suffers from hypertension and often shows echocardiographic signs of myocardial hypertrophy and ventricular dysfunction (systolic and/or diastolic). In our experience, hypertensive cardiomyopathy is present in >80% of patients in both study groups. In agreement with other authors, a good correlation between BNP and LVMI was found [4], with LVMI being the most significant factor indicating the BNP increase.
In a multicenter Canadian study by Foley et al. [10], the incidence of ventricular dysfunction was 74%, compared to 88% in the study by Lopez-Gomes et al. [11]. Similar to our results, in many patients with left ventricular dysfunction, parameters indicating ventricular diastolic dysfunction were increased, rising to 86% in the series reported by Kalfallah et al. [12], and clinical symptoms of ventricular failure were often absent. In these dialysis outpatients, echocardiography was a very useful marker to assess volume overload [13,14] using the diameter of the inferior vena cava. Because it is difficult to obtain the diameter of the inferior vena cava in these patients, and in accord with other authors [15], we think that LA diameter and volume may improve the morphophysiologic expression of volume overload and may reduce the probability of assessment errors. According to our results, LA measurements have been the most important parameters differentiating HD patients from CAPD patients.
Various studies have attempted to clarify the role of BNP in the diagnosis of ventricular dysfunction in asymptomatic patients with renal failure. According to some investigators, continuous increases in BNP levels may be a marker of preclinical ventricular dysfunction associated with ventricular hypertrophy and coronary artery disease, with a high positive predictive value. In the study by Cataliotti et al. [16], BNP concentrations in HD patients without cardiovascular anomalies, hypertensive cardiopathy or ventricular dysfunction did not differ from those obtained from healthy subjects without cardiovascular or renal pathology. Similar results were found by Akiba et al. [2], who did not find differences in BNP levels between asymptomatic patients with and without renal insufficiency; consequently, renal insufficiency by itself does not appear to explain the plasma BNP levels. Also, the suitable cutoff point of BNP which helps to differentiate patients on different dialysis programs (e.g. HD vs. CAPD) more clearly remains to be determined. According to our results, the use of 194 pg/ml as a cutoff point of BNP values greatly increases the number of false-negative results, and the global accuracy of the test of 48% could be considered low in the differentiation between both patient groups in clinical practice.
Some studies have attempted to clarify the value of BNP in diastolic dysfunction [17]. In our study, a correlation between the type of ventricular dysfunction and BNP levels was not found. In the study by De Keulenaer and Brutsaert [18], both types of ventricular dysfunction probably represent different phenotypes or clinical presentations of the same cardiovascular event.
In patients on intermittent dialysis, there is controversy regarding BNP levels during HD. Some studies do not show variations in the plasma BNP levels after HD [19], while other authors reported marked decreases in BNP levels after dialysis [20] (mean BNP decrease: 13–14%), in accord with our results. For Ishizaka et al. [21], the most important mechanism is a decrease in the secretion of BNP associated with a reduction in the plasma volume. In contrast to this observation, we have not found a correlation of BNP levels either with loss of volume after HD or with the presence of diuresis. Wahl et al. [22] and Racek et al. [23] observed that BNP mainly decreases after HD in the group of patients treated with the high-flux membrane. Nevertheless, it must be stressed that in patients receiving daily HD with the same weekly hours of dialysis, basal levels of BNP did not differ significantly from those obtained after dialysis, being apparently similar to those of patients on CAPD. Recently, a randomized controlled trial confirmed that frequent nocturnal hemodialysis decreased blood pressure and resulted in a higher decrease in LV hypertrophy compared with conventional HD [24].
Limitation
This is a single-center observational study; therefore, caution should be applied when hypothesizing about the mechanisms involved and in extrapolating our results to other populations. Due to the observational design of this study, the possibility of selection bias and/or residual confounding from unknown or unmeasured covariates cannot be excluded.
Our main limitation is that we did not use an exogenous marker to estimate GFR because it would have been impractical to infuse an exogenous marker during the dialysis session in our patients. The 4-variable MDRD formula has not been tested very often in this patient population, and overestimation of residual renal function may occur in patients with significantly impaired function in up to 36% of cases [25]. Against this background, residual renal function was assessed combined with urea and creatinine clearance in our patients, as this is widely believed to be the most accurate non-invasive test for GFR in patients with decreased GFR.
Conclusion
CAPD is a more physiological form of extrarenal clearance than HD since patients on CAPD present with decreased BNP levels and lower volume overload by echocardiography.
In asymptomatic patients, marked increases in BNP levels may reflect very early stages of pathological processes that precede the development of apparent cardiac signs (such as measurable left ventricular hypertrophy) in patients on extrarenal dialysis. Only echocardiographic parameters of cardiac dysfunction should be used as diagnostic criteria.
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 2.Akiba T, Tachibana K, Togashi K, Hiroe M, Marumo F. Plasma human brain natriuretic peptide in chronic renal failure. Clin Nephrol. 1995;44(suppl 1):S61–S64. [PubMed] [Google Scholar]
- 3.Odar-Cederlöf I, Bjellerup P, Williams A, Blagg CR, Twardowski Z, Ting G, et al. Daily dialyses decrease plasma levels of brain natriuretic peptide (BNP), a biomarker of left ventricular dysfunction. Hemodial Int. 2006;10:394–398. doi: 10.1111/j.1542-4758.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 4.Bavbek N, Akay H, Altay M, Uz E, Turgut F, Uyar ME, et al. Serum BNP concentration and left ventricular mass in CAPD and automated peritoneal dialysis patients. Perit Dial Int. 2007;27:663–668. [PubMed] [Google Scholar]
- 5.Traynor J, Mactier R, Geddes CC, Fox JG. How to measure renal function in clinical practice. BMJ. 2006;333:733–737. doi: 10.1136/bmj.38975.390370.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barberato SH, Mantilla DE, Misocami MA, Gonçalves SM, Bignelli AT, Riella MC, et al. Effect of preload reduction by haemodialysis on left atrial volume and echocardiographic Doppler parameters in patients with end-stage renal disease. Am J Cardiol. 2004;94:1208–1210. doi: 10.1016/j.amjcard.2004.07.100. [DOI] [PubMed] [Google Scholar]
- 7.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al: ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography. www.acc.org/clinical/guidelines/echo/index.pdf [DOI] [PubMed]
- 8.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 9.Feigenbaum H, Armstrong WF, Ryan T, editors. Feigenbaum's Echocardiography. ed 6. Philadelphia: Lippincott, William & Wilkins; 2005. [Google Scholar]
- 10.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Gomez JM, Verde E, Perez-Garcia R. Blood pressure, left ventricular hypertrophy and long-term prognosis in hemodialysis patients. Kidney Int Suppl. 1998;68:S92–S98. doi: 10.1046/j.1523-1755.1998.06820.x. [DOI] [PubMed] [Google Scholar]
- 12.Kalfallah AB, Ghodhbane L, Tili R, Annabi N. Doppler echocardiographic study of left ventricular diastolic function in hemodialysis patients. Arch Mal Coeur Vaiss. 2005;98:31–38. [PubMed] [Google Scholar]
- 13.van de Pol AC, Frenken LA, Moret K, Baumgarten R, van der Sande FM, Beerenhout CM, et al. An evaluation of blood volumen changes during ultrafiltration pulses and natriuretic peptides in the assessment of dry weight in hemodialysis patients. Hemodial Int. 2007;11:51–61. doi: 10.1111/j.1542-4758.2007.00154.x. [DOI] [PubMed] [Google Scholar]
- 14.Brennan JM, Ronan A, Goonewardena S, Blair JE, Hammes M, Shah D, et al. Hand carried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749–753. doi: 10.2215/CJN.00310106. [DOI] [PubMed] [Google Scholar]
- 15.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 16.Cataliotti A, Malatino LS, Jougasaki M, Zoccali C, Castellino P, Giacone G, et al. Circulating natriuretic peptide concentrations in patients with end-stage renal disease: role of brain natriuretic peptide as a biomarker for ventricular remodeling. Mayo Clin Proc. 2001;76:1111–1119. doi: 10.4065/76.11.1111. [DOI] [PubMed] [Google Scholar]
- 17.Lubien E, DeMaria A, Krishnaswamy P, Clopton P, Koon J, Kazanegra R, et al. Utility of B-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation. 2002;105:595–601. doi: 10.1161/hc0502.103010. [DOI] [PubMed] [Google Scholar]
- 18.De Keulenaer GW, Brutsaert DL. Systolic and diastolic heart failure: different phenotypes of the same disease? Eur J Heart Fail. 2007;9:136–143. doi: 10.1016/j.ejheart.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Sheen V, Bhalla V, Tulua-Tata A, Bhalla MA, Weiss D, Chiu A, et al. The use of B-type natriuretic peptide to assess volume status in patients with end-stage renal disease. Am Heart J. 2007;153:244.e1–e5. doi: 10.1016/j.ahj.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Odar-Cederlöf I, Bjellerup P, Williams A, Blagg CR, Twardowski Z, Ting G, Kjellstrand CM. Daily dialyses decrease plasma levels of brain natriuretic peptide (BNP), a biomarker of left ventricular dysfunction. Hemodial Int. 2006;10:394–398. doi: 10.1111/j.1542-4758.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishizaka Y, Yamamoto Y, Fukunaga T, Yolota N, Kida O, Kitamura K, et al. Plasma concentration of human brain natriuretic peptide in patients on haemodialysis. Am J Kidney Dis. 1994;24:461–472. doi: 10.1016/s0272-6386(12)80903-6. [DOI] [PubMed] [Google Scholar]
- 22.Wahl HG, Graf S, Renz H, Fassbinder W. Elimination of the cardiac natriuretic peptides B-type natriuretic peptide (BNP) and N-terminal proBNP by hemodialysis. Clin Chem. 2004;50:1071–1074. doi: 10.1373/clinchem.2003.030692. [DOI] [PubMed] [Google Scholar]
- 23.Racek J, Kralova H, Trefil L, Rajdl D, Eiselt J. Brain natriuretic peptide and N-terminal proBNP in chronic haemodialysis patients. Nephron Clin Pract. 2006;103:162–172. doi: 10.1159/000092914. [DOI] [PubMed] [Google Scholar]
- 24.FHN Trial Group, Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;263:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almond A, Siddiqui S, Robertson S, Norrie J, Isles C. Comparison of combined urea and creatinine clearance and prediction equations as measures of residual renal function when GFR is low. QJ Med. 2008;101:619–624. doi: 10.1093/qjmed/hcn032. [DOI] [PubMed] [Google Scholar]