Abstract
Objective
Previous melanoma studies evaluating prognostic factors of survival at recurrence have focused on primary tumor characteristics and clinical variables at first recurrence. We examined the prognostic relevance of recurrent tumor proliferation.
Methods
114 melanoma patients with available recurrent tissues who were prospectively enrolled at New York University Medical Center were studied. Standard of care prognostic variables (e.g. stage at initial diagnosis and lactate dehydrogenase level) and recurrent tissue expression of proliferative marker Ki-67 were evaluated for their association with overall survival.
Results
High Ki-67 expression was observed in 57 (50%) of the 114 recurrent melanomas. On univariate analysis, the median overall survival of patients whose recurrent tumors overexpressed Ki-67 was significantly shorter than that of patients whose recurrent tumors had low Ki-67 expression (3.6 vs. 9.5 years, p = 0.03). On multivariate analysis, a high proliferative index of the recurrent melanoma remained an independent predictor of worse overall survival, controlling for stage at initial diagnosis, disease-free survival, and stage at first recurrence [HR = 2.09 (95% CI 1.24–3.54), p = 0.006].
Conclusions
Our results demonstrate the prognostic relevance of tumor proliferation in recurrent melanoma patients. Data also support restratification of risk assessment upon recurrence that considers tumor biology in addition to clinical variables evaluated as part of the standard of care.
Key Words: Melanoma, Prognosis, Recurrence, Ki-67 antigen, Disease-free survival
Introduction
Most melanoma patients are diagnosed with thin (≤1.00 mm) primaries [1], and their 10-year survival rate is 93% [2]. The 10-year survival rate, however, decreases to 9% in patients who develop a recurrence [3]. Twenty percent of patients with localized melanoma ultimately recur [4]; therefore, it is important to identify prognostic determinants of survival upon recurrence that might influence treatment decisions.
Previous studies evaluating prognostic factors of survival at recurrence have focused on primary tumor characteristics and first recurrence clinical variables [4,5,6,7,8,9,10,11,12,13,14]. While the prognostic relevance of primary tumor characteristics in recurrent melanoma patients remains unclear [4,6,7,10,12,13], the general consensus is that clinical variables present at recurrence have prognostic value [4,5,6,7,8,10,11,12,13,14]. Few studies, however, have investigated the prognostic relevance of tumor characteristics at recurrence [15,16] despite their key role in defining prognostic subgroups at primary diagnosis. The importance of examining tumor proliferation has been underscored by the addition of mitotic rate to the 7th edition of the American Joint Committee on Cancer (AJCC) melanoma staging system [2].
Compared to mitotic rate, the expression level of nuclear antigen Ki-67 may be a better assessment of proliferation since Ki-67 is expressed in mitosis as well as during the G1, S, and G2 phases of the cell cycle in proliferating cells [17]. Ki-67 expression has also been shown to have prognostic value in other cancers (most notably in breast cancer) [18,19,20,21,22,23]. In breast cancer, Ki-67 expression is a prognostic factor of overall survival, but like HER2 receptor status it has not been universally applied to the clinical management of patients pending further validation [18]. In the current study, we investigated the prognostic relevance of the proliferative index of the recurrent melanoma in a cohort of patients with prospective clinical follow-up.
Methods
Study Population
Recurrent melanoma tissues were obtained from patients prospectively enrolled in the Interdisciplinary Melanoma Cooperative Group (IMCG) at New York University (NYU) Medical Center between August 2002 and June 2008 [24]. Demographic and clinicopathologic data were recorded prospectively, and all patients were followed through February 2010. This study was approved by the Institutional Review Board at the NYU School of Medicine (IRB No. 10362), and informed consent was obtained from all patients at the time of enrollment.
Clinicopathologic Variables
Baseline variables, including age at primary diagnosis, gender, and stage at initial diagnosis, were collected. Stage at initial diagnosis was included in the analysis to control for the effect of primary tumor characteristics. The following clinical variables at recurrence were analyzed: disease-free survival (date of initial diagnosis to date of first recurrence), stage at first recurrence, total number of recurrences, lactate dehydrogenase (LDH) level at first recurrence, and Eastern Cooperative Oncology Group (ECOG) performance status at first recurrence. To ensure uniform collection of LDH levels, only lab results from NYU were utilized. Values above 618 U/l (normal reference range 313–618 U/l) were considered high.
Assessment of Ki-67 Expression
Immunohistochemistry was performed using mouse anti-human Ki-67 clone 30-9 (Ventana Medical Systems, Tucson, Ariz., USA) on formalin fixed, paraffin-embedded tissue specimens from patients undergoing metastasectomy for surgically accessible recurrent lesions or from patients who had already undergone metastasectomy within the past 6 months for recurrence with no systemic intervention prior to surgery. Recurrent tissue was obtained from the site of first recurrence when possible. In brief, sections were deparaffinized in xylene (3 changes), rehydrated through graded alcohols (3 changes, 100% ethanol; 3 changes, 95% ethanol), and rinsed in distilled water. Heat-induced epitope retrieval was performed in 10 mM citrate buffer, pH 6.0, for Ki-67 for 20 min in a 1,200-watt microwave oven at 90% power. Sections were allowed to cool for 30 min and then rinsed in distilled water. Antibody incubations and detection were carried out at 37°C on a NexES instrument (Ventana Medical Systems) using Ventana's reagent buffer and detection kits unless otherwise noted. Ki-67 was applied neatly and incubated for 30 min. Primary antibodies were detected with Ventana's biotinylated goat anti-mouse secondary antibody followed by streptavidin-horseradish-peroxidase conjugate. The complex was visualized with Naphthol-AS-MX phosphatase and Fast Red complex. Slides were washed in distilled water, counterstained with hematoxylin, dehydrated, and mounted with permanent media. Appropriate positive and negative controls were included with the study sections.
Blinded to patients’ clinical data, an attending pathologist (H.Y.) scored Ki-67 expression by the percentage of positively-stained tumor cells on a continuous scale of 1–100. In tumors with focal regions of immunoreactivity, representative averages of positively-stained melanoma cells were recorded. After the scoring of Ki-67 expression in the recurrent tissue, values were categorized as ≤25% (low expression) or >25% (overexpression). Since there is no validated cutoff value, ours was set at 25% as it was previously shown to identify rapidly proliferating tumors [25] and as it was both the mean and the median percentage of Ki-67 expression in this study.
Statistical Analysis
Complete-case univariate analysis evaluating overall survival was performed for each variable, excluding unavailable data. For binary variables, the estimates of the survival curves were computed using the Kaplan-Meier method, and the differences of these curves were tested with a logrank (Mantel-Haenszel) test. A multivariate Cox proportional hazards regression model was then fitted using the regressors significant on univariate analysis with the most clinical applicability. Hazard ratios (HR) with corresponding 95% confidence intervals (CI) are reported. All p values reported are 2-sided, with statistical significance evaluated at the 0.05 alpha level. A percentage agreement test in 2 variables calculated the coefficient of concordance. All analyses were performed in R, a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria).
Results
264 recurrent melanoma patients were identified in the IMCG database. Fifteen (6%) patients with incomplete follow-up were excluded. Of the remaining 249 patients, 114 had recurrent tumor specimens available for analysis, and 108 of these (95%) were from the site of first recurrence. There was no difference in baseline characteristics (age at primary diagnosis, gender, primary tumor thickness, ulceration, mitotic rate, histological type, and anatomic site) between the patients with available recurrent tissue and those who did not have accessible recurrent tissue (table 1). It is important to note that 18 patient-matched pairs of primary tumors and first recurrence tissues were obtained to examine concordance of Ki-67 expression, but the small sample size prohibited further analysis.
Table 1.
Baseline characteristics of recurrent melanoma patients (n = 249)
| Variable | Recurrent tissue not available (n = 135) | Recurrent tissue available (n = 114) |
|---|---|---|
| Age at primary diagnosis (years) | ||
| Median (range) | 62 (19–91) | 57 (15–92) |
| <60 | 60 (44%) | 61 (54%) |
| ≥60 | 75 (56%) | 53 (46%) |
| Gender | ||
| Male | 80 (59%) | 63 (55%) |
| Female | 55 (41%) | 51 (45%) |
| Primary tumor thickness (mm) | ||
| ≤1.0 | 22 (16%) | 11 (10%) |
| 1.01–4.0 | 65 (48%) | 65 (57%) |
| >4.0 | 29 (21%) | 17 (15%) |
| Unclassified | 3 (2%) | 0 (0%) |
| Unknown primary | 16 (12%) | 21 (18%) |
| Primary tumor ulceration status | ||
| Absent | 64 (47%) | 60 (53%) |
| Present | 53 (39%) | 32 (28%) |
| Unclassified | 2 (1%) | 1 (1%) |
| Unknown primary | 16 (12%) | 21 (18%) |
| Primary tumor mitotic rate (mitoses/mm2) | ||
| 0 | 11 (8%) | 13 (11%) |
| ≥1 | 98 (73%) | 74 (65%) |
| Unclassified | 10 (7%) | 6 (5%) |
| Unknown primary | 16 (12%) | 21 (18%) |
| Primary tumor histological type | ||
| Superficial spreading melanoma | 38 (28%) | 26 (23%) |
| Nodular melanoma | 55 (41%) | 46 (40%) |
| Acral lentiginous melanoma | 4 (3%) | 6 (5%) |
| Lentigo maligna melanoma | 2 (1%) | 2 (2%) |
| Desmoplastic melanoma | 4 (3%) | 5 (4%) |
| Other | 2 (1%) | 4 (4%) |
| Unclassified | 14 (10%) | 4 (4%) |
| Unknown primary | 16 (12%) | 21 (18%) |
| Primary tumor anatomic site | ||
| Extremity | 48 (36%) | 36 (32%) |
| Axial | 71 (53%) | 57 (50%) |
| Unknown primary | 16 (12%) | 21 (18%) |
Percentages may not sum to 100 due to rounding numbers.
The median age at recurrence in the group of 114 patients with available recurrent tissue was 62. There were 63 males and 51 females. The majority of patients recurred at stage III (n = 80, 70%). Twenty-one (18%) patients had an unknown primary. The median follow-up time from the date of initial diagnosis to the date of last follow-up was 3.9 years. The median follow-up time was 1.6 years from the date of first recurrence to the date of last follow-up. Forty-six (40%) patients were alive at last follow-up, and this group had a median overall survival of 5.7 years. The remaining 68 (60%) patients died during the follow-up period, and 66 of these patients died of melanoma. The median overall survival of the whole cohort was 3.9 years, with a 5-year survival rate of 38%.
Patients Whose Recurrent Tumors Overexpressed Ki-67 Had a Shorter Median Overall Survival
All clinicopathologic variables assessed in this study were predictive of overall survival in our cohort of recurrent melanoma patients (table 2). The median overall survival was significantly reduced in patients with advanced-stage melanoma at initial diagnosis (stage IV vs. stage I, 0.7 and 9.7 years, respectively; p < 0.001), in patients with a shorter disease-free survival (<1 year vs. >3 years, 2.5 and 10.8 years, respectively; p < 0.001), in patients with stage IV disease at recurrence (stage IV vs. stage III, 2.9 and 7.9 years, respectively; p = 0.001), in patients with ≥3 recurrences (≥3 vs. 1, 3.0 years vs. insufficient number of deaths to determine median survival, respectively; p < 0.001), in patients with a high LDH level at first recurrence (high vs. normal, 1.5 and 6.9 years, respectively; p = 0.03), and in patients with a worse ECOG status at first recurrence (1–2 vs. 0, 1.7 and 10.2 years, respectively; p < 0.001).
Table 2.
Univariate analysis of clinicopathologic characteristics associated with overall survival in recurrent melanoma patients (n = 114)
| Variable | n = 114a | Median survival (years) | p valueb |
|---|---|---|---|
| Stage at initial diagnosis | <0.001 | ||
| I | 31 (27%) | 9.7 | |
| II | 34 (30%) | 7.1 | |
| III | 42 (37%) | 2.7 | |
| IV | 7 (6%) | 0.7 | |
| Disease-free survival (years) | <0.001 | ||
| <1 | 48 (42%) | 2.5 | |
| 1–3 | 36 (32%) | 4.8 | |
| >3 | 30 (26%) | 10.8 | |
| Stage at first recurrence | 0.001 | ||
| III | 80 (70%) | 7.9 | |
| IV | 34 (30%) | 2.9 | |
| Total number of recurrences | <0.001 | ||
| 1 | 25 (22%) | NAc | |
| 2 | 19 (17%) | 9.5 | |
| ≥3 | 70 (61%) | 3.0 | |
| LDH level at first recurrence | 0.03 | ||
| Normal | 50 (82%) | 6.9 | |
| High | 11 (18%) | 1.5 | |
| ECOG performance status at first recurrence | <0.001 | ||
| 0 | 49 (82%) | 10.2 | |
| 1 or 2 | 11 (18%) | 1.7 | |
| Ki-67 expression level in recurrent tissue | 0.03 | ||
| Low (≤25%) | 57 (50%) | 9.5 | |
| High (>25%) | 57 (50%) | 3.6 |
Number of patients may not sum to 114 due to unavailable data (unknown values).
By logrank test.
Insufficient deaths to determine median survival.
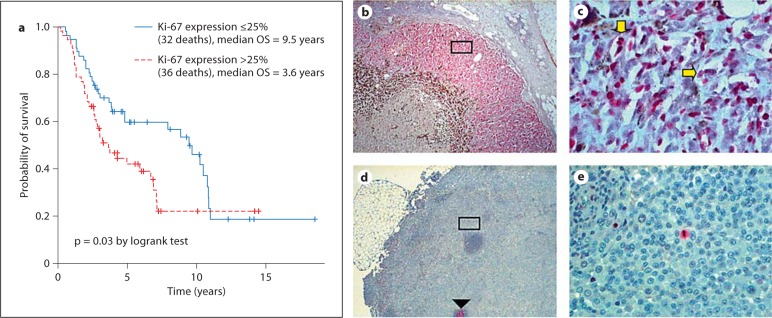
Patients whose recurrent tumors overexpressed Ki-67 also had a shorter median overall survival (high vs. low Ki-67 expression, 3.6 and 9.5 years, respectively; p = 0.03) (fig. 1a). High Ki-67 expression was seen in 57 (50%) of the 114 recurrent tissues. Fifty-three (93%) of the 57 cases were from the site of first recurrence (39 locoregional and 14 distant). Recurrent tissues with low Ki-67 expression were similarly distributed (table 3). A representative case of Ki-67 overexpression is displayed in figure 1b, c. A negative metastatic lymph node specimen illustrates a representative case of low Ki-67 expression in which a mitotic figure and a germinal center serve as internal positive controls (fig. 1d, e).
Fig. 1.
Evaluation of Ki-67 expression in recurrent melanoma tissue. a Kaplan-Meier curve showing overall survival stratified by expression of Ki-67 in the recurrent melanoma tissue. b A skin metastasis overexpressing Ki-67 demonstrates strong nuclear staining of cells in all phases of the cell cycle (except G0 and early G1). 40×. c High-power (400×) view of the inset. Cells in the S phase (horizontal arrow) and M phase (vertical arrow) are prominent. d Ki-67 positively stains a germinal center (arrowhead) and a few mitotic figures (inset), serving as internal positive controls in a recurrent lymph node with low Ki-67 expression. 40×. e High-power (400×) view of the inset.
Table 3.
Recurrent tissue type by anatomic site (n = 108)
| Anatomic site | Low Ki-67 expression (n = 55) | High Ki-67 expression (n = 53) |
|---|---|---|
| Locoregional | ||
| Local recurrence | 2 (4%) | 3 (6%) |
| Regional skin | 10 (18%) | 16 (30%) |
| Regional lymph node | 27 (49%) | 20 (38%) |
| Distant | ||
| Distant skin | 2 (4%) | 3 (6%) |
| Distant lymph node | 1 (2%) | 1 (2%) |
| Distant visceral | 13 (24%) | 10 (19%) |
| Brain | 4 | 3 |
| Lung | 5 | 2 |
| Other | 4 | 5 |
Percentages do not sum to 100 due to rounding numbers. Includes patients with recurrent tissue from the site of first recurrence only.
A High Proliferative Index in Recurrent Melanoma Is Independently Associated with Worse Survival
Stage IV at initial diagnosis, a shorter disease-free survival, stage IV at first recurrence, and a high proliferative index in the recurrent tumor were independently associated with worse overall survival (table 4). Compared to patients with stage I melanoma at initial diagnosis, those with stage IV melanoma had a shorter overall survival (HR = 15.64, p < 0.001). Both a disease-free survival greater than 3 years and one lasting 1–3 years were significantly associated with improved overall survival when compared to a disease-free survival of less than 1 year (HR = 0.15, p < 0.001; HR = 0.45, p = 0.02, respectively). Patients with stage IV melanoma at first recurrence had a much worse prognosis than those who recurred at stage III (HR = 2.81, p < 0.001). High Ki-67 expression in the recurrent tissue was an independent predictor of reduced overall survival (HR = 2.09, p = 0.006).
Table 4.
Multivariate analysis of clinicopathologic variables associated with overall survival in recurrent melanoma patients (n = 114)
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Stage at initial diagnosis | |||
| II vs. I | 1.34 | 0.67–2.66 | 0.41 |
| III vs. I | 1.97 | 0.98–3.96 | 0.06 |
| IV vs. I | 15.64 | 4.11–59.53 | <0.001 |
| Disease-free survival (years) | |||
| >3 vs. <1 | 0.15 | 0.07–0.34 | <0.001 |
| 1–3 vs. <1 | 0.45 | 0.23–0.86 | 0.02 |
| Stage at first recurrence | |||
| IV vs. III | 2.81 | 1.55–5.09 | <0.001 |
| Ki-67 expression level in recurrent tissue | |||
| >25% vs. ≤25% | 2.09 | 1.24–3.54 | 0.006 |
Discussion
Our results underscore the importance of obtaining recurrent melanoma tissue to assess survival probability in recurrent patients. The evolution of tumor biology may underlie the predictive value of a longer disease-free survival in recurrent melanoma patients. First recurrences within 1 year of the primary diagnosis may be more likely to reflect the biological characteristics of the original lesion, whereas the phenotype of a recurrent tumor appearing after an extended period may diverge. Phenotypic changes between the primary and the recurrent tumor have been noted in breast cancer [26,27], emphasizing the importance of investigating both clinical and pathologic variables at recurrence. In the current melanoma paradigm, however, staging at recurrence is based on clinical and radiologic evaluations, such as the LDH level and findings on CT/MRI/PET imaging. Recurrent melanomas are therefore not routinely biopsied, limiting the amount of tissue available for evaluation.
Our data support the prognostic relevance of recurrent tumor proliferation in melanoma patients. Two studies have previously evaluated the prognostic value of Ki-67 expression in recurrent melanoma [28,29]. The small sample sizes in these 2 studies (n = 12 [28] and n = 60 [29]) and the lack of control for first recurrence clinical variables, however, limit the applicability of the results. The limited availability of recurrent tissue likely contributes to the paucity of melanoma studies investigating the prognostic role of Ki-67 expression at recurrence. Instead, the focus has been on the predictive value of primary tumor Ki-67 expression [30,31], and studies of both thin and thick melanomas have shown a high Ki-67 score to be an independent predictor of reduced overall survival controlling for mitotic rate [30,31]. Studies of Ki-67 expression in other cancers have similarly concentrated on evaluating its prognostic value at primary diagnosis [19,20,21,22,23]. Two studies in colorectal cancer, however, investigated the prognostic relevance of Ki-67 expression in metastatic tissue from the liver, and both demonstrated that a high Ki-67 score independently predicted worse overall survival [32,33].
An examination of the tissue is especially relevant in melanoma patients with an unknown primary as the prognosis of this group remains unclear. Some studies report improved survival while others find no difference or even decreased survival compared to matched counterparts with known primaries [34,35,36,37,38]. A more robust immune response is thought to underlie the better prognosis of patients with melanoma from an unknown primary since these melanomas may represent metastases from regressed lesions and regression is mediated by host immunity. Of the patients in this study with an unknown primary, half of those alive at last follow-up had received immunotherapy (data not shown). In this regard, a recent study by our group [15] demonstrated that an assessment of immune response in the recurrent tissue significantly improved the ability of the current staging system to predict survival in recurrent patients. In particular, a high CD3 count, increased tumor infiltrating leukocytes, and a specific immune response gene signature were all predictive of prolonged survival. The predictive value of the recurrent melanoma mitotic index was also evaluated in this study, and a high mitotic index of the recurrent melanoma was shown to be predictive of reduced survival. Moreover, compared to the 3 aforementioned parameters, the mitotic index of the recurrent tumor was the most significant predictor of survival [15].
Recurrent tumor proliferation has therapeutic implications as well. The Ki-67 index has been shown to be predictive of the immunochemotherapy response in metastatic renal cell carcinoma [39]; therefore, Ki-67 expression in recurrent melanoma may also prove to be simi-larly predictive. Rapidly proliferating cells are the most frequent target of anti-melanoma treatments as uncontrollable growth is one of the hallmarks of cancer. Melanoma, however, is notoriously chemoresistant [40], and currently available immunotherapies have limited clinical efficacy. A subpopulation of slowly proliferating tumor cells may serve as a continual source of renewal and play a role in non-responders. Recurrent melanomas with a low proliferative index may have an expanded population of these slowly cycling cells, which were recently identified in melanoma cell lines in culture as being characterized by the expression of JARID1B [41]. JARID1B+ melanoma cells not only cycle more slowly but they are also more tumorigenic. Novel therapeutic approaches are therefore needed, combining anti-melanoma drugs which target this subset of slowly proliferating cells and traditional regimens which debulk the tumor of rapidly cycling cells. Chemoresistance in melanoma may be mediated by one of many intrinsic anti-apoptotic mechanisms as well, which in turn affects the relative contribution of proliferation and apoptosis to melanoma progression. NF-κB inhibits both the intrinsic and the extrinsic apoptotic pathways [40], and it is a downstream effector of the RAS/RAF/MAPK cascade, which is constitutively activated in the majority of melanomas [42,43]. Constitutive signaling through this pathway results from activating mutations of the oncogene NRAS or BRAF, both of which are common in melanoma [42,43]. Mutations in the tumor suppressor gene p53 occur less often, but a downstream target of p53, namely apoptotic activator 1 (Apaf-1), is frequently inactivated in melanoma, impairing p53-induced apoptosis and thus the chemotherapy response [40,44].
Conclusions
High Ki-67 expression in recurrent melanoma is predictive of worse overall survival. Our data therefore demonstrate the clinical relevance of assessing tumor biology at recurrence in addition to standard of care variables.
Acknowledgements
This work was supported by a National Cancer Institute Cancer Center Support Grant (Grant No. 5 P30 CA 016087-27) and the Marc Jacobs Campaign to support the IMCG. The study sponsors had no role in the design of the study; in the collection, analysis, and interpretation of data; in the writing of the manuscript, or in the decision to submit the manuscript for publication.
References
- 1.Gimotty PA, Guerry D, Ming ME, Elenitsas R, Xu X, Czerniecki B, Spitz F, Schuchter L, Elder D. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J Clin Oncol. 2004;22:3668–3676. doi: 10.1200/JCO.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch CM, Soong SJ, Smith T, Ross MI, Urist MM, Karakousis CP, Temple WJ, Mihm MC, Barnhill RL, Jewell WR, Wanebo HJ, Desmond R, Investigators from the Intergroup Melanoma Surgical Trial Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann Surg Oncol. 2001;8:101–108. doi: 10.1007/s10434-001-0101-x. [DOI] [PubMed] [Google Scholar]
- 4.Francken AB, Accortt NA, Shaw HM, Wiener M, Soong SJ, Hoekstra HJ, Thompson JF. Prognosis and determinants of outcome following locoregional or distant recurrence in patients with cutaneous melanoma. Ann Surg Oncol. 2008;15:1476–1484. doi: 10.1245/s10434-007-9717-9. [DOI] [PubMed] [Google Scholar]
- 5.Dalal KM, Patel A, Brady MS, Jaques DP, Coit DG. Patterns of first-recurrence and post-recurrence survival in patients with primary cutaneous melanoma after sentinel lymph node biopsy. Ann Surg Oncol. 2007;14:1934–1942. doi: 10.1245/s10434-007-9357-0. [DOI] [PubMed] [Google Scholar]
- 6.Cohn-Cedermark G, Mansson-Brahme E, Rutqvist LE, Larsson O, Singnomklao T, Ringborg U. Metastatic patterns, clinical outcome, and malignant phenotype in malignant cutaneous melanoma. Acta Oncol. 1999;38:549–557. doi: 10.1080/028418699431122. [DOI] [PubMed] [Google Scholar]
- 7.Soong SJ, Harrison RA, McCarthy WH, Urist MM, Balch CM. Factors affecting survival following local, regional, or distant recurrence from localized melanoma. J Surg Oncol. 1998;67:228–233. doi: 10.1002/(sici)1096-9098(199804)67:4<228::aid-jso4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Fusi S, Ariyan S, Sternlicht A. Data on first recurrence after treatment for malignant melanoma in a large patient population. Plast Reconstr Surg. 1993;91:94–98. doi: 10.1097/00006534-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Crowley NJ, Seigler HF. Relationship between disease-free interval and survival in patients with recurrent melanoma. Arch Surg. 1992;127:1303–1308. doi: 10.1001/archsurg.1992.01420110045011. [DOI] [PubMed] [Google Scholar]
- 10.Reintgen DS, Cox C, Slingluff CL, Jr, Seigler HF. Recurrent malignant melanoma: the identification of prognostic factors to predict survival. Ann Plast Surg. 1992;28:45–49. doi: 10.1097/00000637-199201000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz JS, Cosimi LA, Carey RW, Kang S, Padyk C, Sober AJ, Cosimi AB. Prognosis after initial recurrence of cutaneous melanoma. Arch Surg. 1991;126:703–708. doi: 10.1001/archsurg.1991.01410300045006. [DOI] [PubMed] [Google Scholar]
- 12.Hoyt DJ, Fisher SR. Survival following recurrent malignant melanoma of the head and neck. Laryngoscope. 1989;99:586–589. doi: 10.1288/00005537-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Shaw HM, Beattie CW, McCarthy WH, Milton GW. Late relapse from cutaneous stage I malignant melanoma. Arch Surg. 1985;120:1155–1159. doi: 10.1001/archsurg.1985.01390340053010. [DOI] [PubMed] [Google Scholar]
- 14.Karakousis CP, Temple DF, Moore R, Ambrus JL. Prognostic parameters in recurrent malignant melanoma. Cancer. 1983;52:575–579. doi: 10.1002/1097-0142(19830801)52:3<575::aid-cncr2820520333>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Bogunovic D, O'Neill DW, Belitskaya-Levy I, Vacic V, Yu YL, Adams S, Darvishian F, Berman R, Shapiro R, Pavlick AC, Lonardi S, Zavadil J, Osman I, Bhardwaj N. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci USA. 2009;106:20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reintgen DS, Vollmer R, Tso CY, Seigler HF. Prognosis for recurrent stage I malignant melanoma. Arch Surg. 1987;122:1338–1342. doi: 10.1001/archsurg.1987.01400230126022. [DOI] [PubMed] [Google Scholar]
- 17.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, Ruby SG, O'Malley F, Simpson JF, Connolly JL, Hayes DF, Edge SB, Lichter A, Schnitt SJ. Prognostic factors in breast cancer: College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 19.Keshgegian AA, Cnaan A. Proliferation markers in breast carcinoma: mitotic figure count, S-phase fraction, proliferating cell nuclear antigen, Ki-67 and MIB-1. Am J Clin Pathol. 1995;104:42–49. doi: 10.1093/ajcp/104.1.42. [DOI] [PubMed] [Google Scholar]
- 20.Gaglia P, Bernardi A, Venesio T, Caldarola B, Lauro D, Cappa AP, Calderini P, Liscia DS. Cell proliferation of breast cancer evaluated by anti-BrdU and anti-Ki-67 antibodies: its prognostic value on short-term recurrences. Eur J Cancer. 1993;29A:1509–1513. doi: 10.1016/0959-8049(93)90284-m. [DOI] [PubMed] [Google Scholar]
- 21.Railo M, Nordling S, von Boguslawsky K, Leivonen M, Kyllonen L, von Smitten K. Prognostic value of Ki-67 immunolabelling in primary operable breast cancer. Br J Cancer. 1993;68:579–583. doi: 10.1038/bjc.1993.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mucci NR, Rubin MA, Strawderman MS, Montie JE, Smith DC, Pienta KJ. Expression of nuclear antigen Ki-67 in prostate cancer needle biopsy and radical prostatectomy specimens. J Natl Cancer Inst. 2000;92:1941–1942. doi: 10.1093/jnci/92.23.1941. [DOI] [PubMed] [Google Scholar]
- 23.Bettencourt MC, Bauer JJ, Sesterhenn IA, Mostofi FK, McLeod DG, Moul JW. Ki-67 expression is a prognostic marker of prostate cancer recurrence after radical prostatectomy. J Urol. 1996;156:1064–1068. [PubMed] [Google Scholar]
- 24.Wich LG, Hamilton HK, Shapiro RL, Pavlick A, Berman RS, Polsky D, Goldberg JD, Hernando E, Manga P, Krogsgaard M, Kamino H, Darvishian F, Lee P, Orlow SJ, Ostrer H, Bhardwaj N, Osman I. Developing a multidisciplinary prospective melanoma biospecimen repository to advance translational research. Am J Transl Res. 2009;1:35–43. [PMC free article] [PubMed] [Google Scholar]
- 25.Spyratos F, Ferrero-Pous M, Trassard M, Hacene K, Phillips E, Tubiana-Hulin M, Le Doussal V. Correlation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff value. Cancer. 2002;94:2151–2159. doi: 10.1002/cncr.10458. [DOI] [PubMed] [Google Scholar]
- 26.MacFarlane R, Speers C, Masoudi H, Chia S. Molecular changes in the primary breast cancer versus the relapsed/metastatic lesion from a large population based database and tissue microarray series (abstract) J Clin Oncol. 2008;26:15s. [Google Scholar]
- 27.Broglio K, Moulder SL, Hsu L, Kau S, Pusztai L, Symmans WF, Hortobagyi GN, Gonzalez-Angulo AM, Liedtke C. Prognostic impact of discordance/concordance of triple-receptor expression between primary tumor and metastasis in patients with metastatic breast cancer (abstract) J Clin Oncol. 2008;26:15s. [Google Scholar]
- 28.Hernberg M, Turunen JP, von Boguslawsky K, Muhonen T, Pyrhönen S. Prognostic value of biomarkers in malignant melanoma. Melanoma Res. 1998;8:283–291. doi: 10.1097/00008390-199806000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Vlaykova T, Talve L, Hahka-Kemppinen M, Hernberg M, Muhonen T, Franssila K, Collan Y, Pyrhönen S. MIB-1 immunoreactivity correlates with blood vessel density and survival in disseminated malignant melanoma. Oncology. 1999;57:242–252. doi: 10.1159/000012038. [DOI] [PubMed] [Google Scholar]
- 30.Gimotty PA, Van Belle P, Elder DE, Murry T, Montone KT, Xu X, Hotz S, Raines S, Ming ME, Wahl P, Guerry D. Biologic and prognostic significance of dermal Ki67 expression, mitoses, and tumorigenicity in thin invasive cutaneous melanoma. J Clin Oncol. 2005;23:8048–8056. doi: 10.1200/JCO.2005.02.0735. [DOI] [PubMed] [Google Scholar]
- 31.Ladstein RG, Bachmann IM, Straume O, Akslen LA. Ki-67 expression is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. BMC Cancer. 2010;10:140. doi: 10.1186/1471-2407-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrowsky H, Sturm I, Graubitz O, Kooby DA, Staib-Sebler E, Gog C, Kohne CH, Hillebrand T, Daniel PT, Fong Y, Lorenz M. Relevance of Ki-67 antigen expression and K-ras mutation in colorectal liver metastases. Eur J Surg Oncol. 2001;27:80–87. doi: 10.1053/ejso.2000.1029. [DOI] [PubMed] [Google Scholar]
- 33.Smith DL, Soria JC, Morat L, Yang Q, Sabatier L, Liu DD, Nemr RA, Rashid A, Vauthey JN. Human telomerase reverse transcriptase (hTERT) and Ki-67 are better predictors of survival than established clinical indicators in patients undergoing curative hepatic resection for colorectal metastases. Ann Surg Oncol. 2004;11:45–51. doi: 10.1007/BF02524345. [DOI] [PubMed] [Google Scholar]
- 34.Lee CC, Faries MB, Wanek LA, Morton DL. Improved survival for stage IV melanoma from an unknown primary site. J Clin Oncol. 2009;27:3489–3495. doi: 10.1200/JCO.2008.18.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CC, Faries MB, Wanek LA, Morton DL. Improved survival after lymphadenectomy for nodal metastasis from an unknown primary melanoma. J Clin Oncol. 2008;26:535–541. doi: 10.1200/JCO.2007.14.0285. [DOI] [PubMed] [Google Scholar]
- 36.Anbari KK, Schuchter LM, Bucky LP, Mick R, Synnestvedt M, Guerry D, 4th, Hamilton R, Halpern AC. Melanoma of unknown primary site: presentation, treatment, and prognosis – a single institution study. Cancer. 1997;79:1816–1821. doi: 10.1002/(sici)1097-0142(19970501)79:9<1816::aid-cncr26>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Schlagenhauff B, Stroebel W, Ellwanger U, Meier F, Zimmermann C, Breuninger H, Rassner G, Garbe C. Metastatic melanoma of unknown primary origin shows prognostic similarities to regional metastatic melanoma: recommendations for initial staging examinations. Cancer. 1997;80:60–65. doi: 10.1002/(sici)1097-0142(19970701)80:1<60::aid-cncr8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 38.Nambisan RN, Alexiou G, Reese PA, Karakousis CP. Early metastatic patterns and survival in malignant melanoma. J Surg Oncol. 1987;34:248–252. doi: 10.1002/jso.2930340407. [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulos I, Rudolph P, Weichert-Jacobsen K, Thiemann O, Papadopoulou D. Prognostic indicators for response to therapy and survival in patients with metastatic renal cell cancer treated with interferon alpha-2 beta and vinblastine. Urology. 1996;48:373–378. doi: 10.1016/S0090-4295(96)00168-9. [DOI] [PubMed] [Google Scholar]
- 40.Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 41.Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker JC, Kirkwood JM, Agarwala SS, Dummer R, Schrama D, Hauschild A. Molecularly targeted therapy for melanoma: current reality and future options. Cancer. 2006;107:2317–2327. doi: 10.1002/cncr.22273. [DOI] [PubMed] [Google Scholar]
- 43.Lorigan P, Eisen T, Hauschild A. Systemic therapy for metastatic malignant melanoma – from deeply disappointing to bright future? Exp Dermatol. 2008;17:383–394. doi: 10.1111/j.1600-0625.2007.00673.x. [DOI] [PubMed] [Google Scholar]
- 44.Dai DL, Martinka M, Bush JA, Li G. Reduced Apaf-1 expression in human cutaneous melanomas. Br J Cancer. 2004;91:1089–1095. doi: 10.1038/sj.bjc.6602092. [DOI] [PMC free article] [PubMed] [Google Scholar]