Abstract
Central hyperosmotic stimulation (HS) evokes increases in sympathetic nerve activity mediated by activation of angiotensin type 1 receptors in the hypothalamic paraventricular nucleus (PVN). Macrophage inhibitory migration factor (MIF) is an intracellular inhibitory regulator of angiotensin type 1 receptor–mediated actions of angiotensin II within neurons of the PVN. MIF mediates its actions via its intrinsic thiol-protein oxidoreductase activity. We demonstrate that intracerebroventricular injection of hypertonic saline into Sprague-Dawley rats elicits a significant (≈112%) increase in MIF mRNA expression in the PVN. Next, we evaluated the effect of viral-mediated expression of either MIF or [C60S]-MIF (which lacks thiol-protein oxidoreductase activity) in the PVN on the sympathoexcitation evoked by HS. We used a decorticate, arterially perfused in situ preparation of male Wistar rats (60 to 80 g). HS was induced by raising perfusate osmolality from 290 to 380 milliosmoles for 40 seconds. Seven to 10 days before experiments, rats were injected bilaterally (500 nL per side) with 0.9% saline (control) or with adenoassociated virus to express MIF, [C60S]-MIF, or enhanced green fluorescent protein in the PVN. HS produced sympathoexcitation in both the 0.9% saline and enhanced green fluorescent protein groups (sympathetic nerve activity increase of +27±4% and +25±4%, respectively; P<0.05), an effect that was not observed in the MIF group (+4±5%). Conversely, the HS-induced increase in sympathetic nerve activity was potentiated in the [C60S]-MIF group (+45±6%; P<0.05). We propose that MIF acting within the PVN is a major counterregulator of HS-induced sympathoexcitation, an effect that depends on thiol-protein oxidoreductase activity.
Keywords: hypothalamus, gene transfer, sympathetic nerve activity, angiotensin type 1 receptors, macrophage migration inhibitory factor
The paraventricular nucleus (PVN) of the hypothalamus plays a crucial role in the regulation of cardiovascular function1 and body fluid homeostasis.2,3 Studies have shown that activation of PVN neurons increases blood pressure and sympathetic nerve activity in response to a hyperosmotic challenge, which is driven by the afferent inputs from the lamina terminalis in the forebrain.4–7 Two key structures have been well accepted as osmosensors in the central nervous system, the subfornical organ and organum vasculosum laminae terminalis (OVLT) located along the dorsal and ventral portions of the lamina terminalis, respectively. In respect to OVLT, it has been shown to possess osmosensitivity.4,5,8–10 Lesion or chemical inhibition of OVLT neurons abolishes hyperosmolality-induced drinking,11–13 vasopressin release,6,11,14 and sympathoexcitation.9 Activated OVLT neurons project to PVN neurons via monosynaptic input or via a relay in the median preoptic nucleus.10,15–18
The PVN integrates multiple inputs from forebrain (eg, OVLT)2,10,19 and hindbrain (eg, nucleus tractus solitarii)3 during osmotic perturbations and then modulates vasopressin release and sympathetic discharge appropriately. PVN hyperosmolality-induced pressor responses and sympathetic activation involve activation of the angiotensin type 1 receptor (AT1R)20,21 and distinct descending pathways,22–24 including glutamatergic25 and vasopressinergic pathways.19,26
Considering that hyperosmolality, angiotensin II (Ang II) and AT1R in the PVN exert profound stimulatory influences on sympathetic outflow and arterial blood pressure, it is, therefore, important to understand the mechanisms within this nucleus that mediate these responses. Our attempts to understand regulatory mechanisms led to the discovery of macrophage migration inhibitory factor (MIF) as a novel intracellular inhibitory regulator of AT1R-mediated actions of Ang II within PVN neurons. We demonstrated that MIF acts intracellularly to counterregulate the firing responses evoked by Ang II in PVN neurons cultured from normotensive rats.27,28 MIF elicits this inhibitory effect via its intrinsic thiol-protein oxidoreductase (TPOR) activity that is exerted by a C-A-L-C motif that exists at residues 57 to 60 of the MIF molecule.27,28 MIF is expressed in neurons in the PVN of normotensive rats, and intracerebroventricular (ICV) injection of Ang II increases MIF expression in this cardiovascular control center.28,29 In addition, transient viral-mediated transduction of MIF into the PVN of normotensive rats reduces the pressor response to ICV-injected Ang II.28 Collectively, this evidence indicates that MIF is a counterregulator of Ang II–induced cardiovascular effects mediated via the PVN in normotensive rats.
Because the PVN mechanism for hyperosmolality-induced increases in sympathetic nervous system activity is Ang II/AT1R–dependent, we investigated the role of MIF and its TPOR moiety in PVN neurons. Our novel findings reported herein indicate that exposure of rats to a hyperosmotic challenge increases MIF mRNA expression in the PVN. In addition, viral-mediated increases in MIF levels in PVN neurons produce complete inhibition of the increase in sympathetic nerve activity elicited by hyperosmolar (high-salt) conditions. This inhibitory action of MIF involves its TPOR activity. These data identify MIF in the PVN as a major regulator of salt-induced increases in sympathetic outflow.
Materials and Methods
Ethical Approval
For the experiments that generated the data shown in Figures 1 and 2, we used a total of 15 male Sprague-Dawley rats (200 to 250 g), purchased from Charles River Farms (Wilmington, MA). All of these procedures were approved by the institutional animal care and use committee of the University of Florida. For the experiments that generated the data shown in Figures 3 to 6, we used a total of 42 male Wistar rats (60 to 120 g; postnatal age: 28 to 38 days), purchased from B&K. All of the procedures conformed to the United Kingdom Animals (Scientific Procedures) Act 1986 and were approved by the University of Bristol Ethical Review Committee.
Figure 1.
Colocalization of AVP and MIF in the PVN. Top, Low-power representative fluorescence images of the PVN showing endogenous MIF (green) and AVP (red) immunoreactivities and their colocalization (orange) at 3 different levels (−1.60, −1.88, and −2.12 mm relative to the bregma). Bars=100 μm. Middle, Representative fluorescence micrographs are a higher-power view (from the inset shown in the top left) showing MIF (green) and AVP (red) immunofluorescence at PVN level −1.60 mm relative to bregma. Examples of AVP and MIF colocalization are indicated by white arrows. Bar=20 μm. Bottom left, Bar graph showing the number of immunoreactive AVP and MIF cells counted at each of the 3 PVN levels and also the number of overlapping AVP- and MIF-positive cells. Data are mean±SEM of numbers of AVP-, MIF-, or AVP/MIF-positive cells (n=3 rats). Bottom right, Bar graph showing the colocalization of immunoreactive AVP and MIF in various subregions of the PVN at each of the levels investigated. dp indicates dorsal parvocellular; mp, medial parvocellular; pm, posterior magnocellular; vp, ventrolateral parvocellular; lp, lateral parvocellular. Data are mean±SEM of numbers of AVP/MIF-positive cells (n=3 rats).
Figure 2.
Hyperosmotic stimulation induces MIF mRNA expression in the PVN via an AT1R-dependent process. Rats were injected ICV with either 2 μL of losartan (Los; 2.1 nmol/μL) or 2 μL of isoosmotic (0.9%) saline. Fifteen minutes later, half of the rats from each group were injected ICV with 2 μL of isoosmotic (0.9%) saline, and the others were injected ICV with 2 μL of 2.0-mol/L NaCl. Three hours later brains were removed for analysis of MIF mRNA in the PVN. Data are mean±SEM of MIF mRNA levels normalized against 18S rRNA (n=5 to 10 per treatment condition). *P<0.05 (Student t test).
Figure 3.
Increased MIF expression in the PVN decreases HS-evoked sympathoexcitation. Representative tracings showing changes in raw and integrated SNA during isosmotic (290 mosmol/kg · water−1) and hyperosmotic stimuli (HS; 380 mosmol/kg · water−1; 40 seconds) in rats that had received bilateral injections of AAV2-CBA-eGFP, AAV2-CBA-MIF, or AAV2-CBA-[C60S]-MIF into the PVN 10 days earlier.
Figure 6.
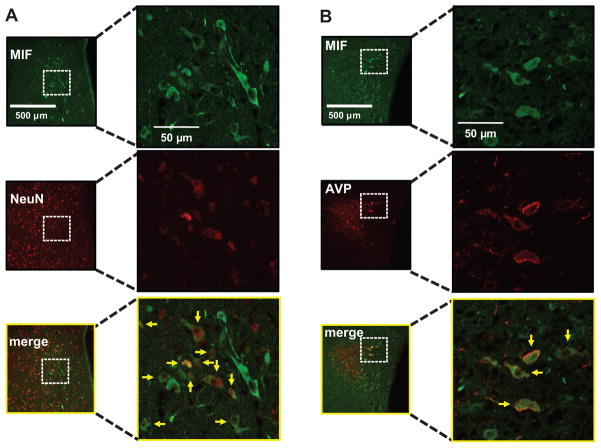
AAV2-CBA-MIF–induced expression of MIF in AVP neurons in the PVN. Wistar rats were injected bilaterally into the PVN with AAV2-CBA-MIF, as detailed in the Methods section. A, Micrographs of MIF and NeuN immunostaining from the same field of the PVN 10 days after microinjection of AAV2-CBA-MIF. Merge: MIF+NeuN. Yellow arrows indicate coexpression of MIF and NeuN. B, Representative fluorescence micrographs of showing MIF and AVP immunostaining from the same field of the PVN 10 days after microinjection of AAV2-CBA-MIF. Merge: MIF+AVP. Yellow arrows indicate coexpression of MIF and AVP.
Adenoassociated Viral Vector 2 Constructs
Three adenoassociated viral vectors (AAVs), AAV2-chicken β-actin promoter (CBA)-MIF, AAV2-CBA-[C60S]-MIF, and AAV2-CBA-enhanced green fluorescent protein (eGFP), were constructed and prepared exactly as detailed previously.29 These constructs contained expression cassettes flanked by the rAAV2 terminal repeats. Expressions of eGFP, MIF, and [C60S]-MIF were driven by a CBA with a human cytomegalovirus enhancer. Vector doses were expressed as genome copies.
Gene Transfer Into the PVN In Vivo
Juvenile (28- to 38-day–old) rats were anesthetized with ketamine (60 mg/kg) and medetomidine (250 mg/kg) via intramuscular injection and placed in a stereotaxic frame (David Kopf Instruments). The skull was leveled between bregma and lambda and bilateral injections (500 nL) of the recombinant AAV2-CBA-MIF (1.0×108 genome copies per microliter), AAV2-CBA-C60S-MIF-eGFP (1.0×108 genome copies per microliter), AAV2-CBA-eGFP (8.3×108 genome copies per microliter), or control solution (0.9% saline) were performed into the PVN using the following stereotaxic coordinates: 0.8 mm caudal to bregma, 0.3 mm lateral to bregma, and 7.2 mm ventral to the dura mater. (Note that, in preliminary experiments, the appropriate PVN coordinates for this size of rat were determined.) Each injection was made over 1 minute. After surgery, anesthesia was reversed with an intramuscular injection of atipamezole (1 mg/kg). Animals were allowed to recover for 7 to 10 days before being studied using the decorticate-perfused in situ preparation.
Decorticate, Unanaesthetized, Arterially Perfused In Situ Rat Preparation
Experiments were performed at the University of Bristol. Male Wistar rats were prepared as described previously.19,30 Rats were placed under deep halothane anesthesia (5%) and assessed by a failure to respond to a noxious pinch of either a paw or the tail. Anesthetized rats were transected below the diaphragm, then submerged in ice-cooled Ringer solution (see below), and the cerebral hemispheres, hippocampus and thalamic areas removed by gentle aspiration. The preoptic area and adjacent septal nuclei and hypothalamic areas remained intact. The preparation was skinned, transferred to a recording chamber, and the left phrenic nerve isolated. A double-lumen catheter was inserted into the descending aorta. One lumen was used to delivery perfusate pumped using a roller pump (Watson Marlow 505S). The perfusate was an isosmotic Ringer solution (containing, in mM: NaCl 120.00, NaHCO3 24.00, KCl 3.00, CaCl2 2.50, MgSO4 1.25, KH2PO4 1.25, and glucose 10.00) containing an oncotic agent (polyethylene glycol: 1.5%; Sigma United Kingdom), gassed with carbogen (95% O2 and 5% CO2), warmed to 32°C (pH 7.3) after carbogenation, and filtered using a nylon screen (pore size: 25 μm). After respiratory-related movements commenced, a neuromuscular blocker (vecuronium bromide, 40 μg · mL−1 Norcuron Organon Teknika) was added to the perfusate to mechanically stabilize the preparation. The second lumen of the catheter was used to monitor aortic perfusion pressure. Phrenic nerve activity was recorded from its cut central end using a glass suction bipolar electrode held in a 3D micromanipulator. Rhythmic ramping phrenic nerve activity gave a continuous physiological index of preparation viability. Sympathetic nerve activity (SNA) was recorded from the thoracic sympathetic chain using a bipolar glass suction electrode. Signals were AC amplified (Neurolog NL104), band-pass filtered (8 Hz to 3 kHz), rectified, and integrated. Noise levels were subtracted from all of the SNA recordings by application of lidocaine to the chain, which was subtracted from the integrated signal during analysis.
Osmotic Stimulus
The osmolalities of iso-osmotic and hyperosmotic solutions were, respectively, 290 and 380 milliosmol (mosmol; kilograms · water−1), as measured by a freezing point depression osmometer (Camlab, Roebling Micro-osmometer). Hyperosmotic ionic Ringer solution was prepared by adjusting the final concentration of NaCl. The in situ preparation was perfused with isosmotic Ringer solution, and the osmotic stimulus was performed by perfusing the preparation for 40 seconds with hyperosmotic Ringer solution, contained in a separate reservoir and connected to the perfusion system via a 3-way tap.
ICV Injections of Saline and Analysis of MIF mRNA
Rats were anesthetized with a mixture of 4% isoflurane in pure O2 (1 L/min) and placed in a Kopf stereotaxic frame. Anesthesia was maintained using an O2/isoflurane (2%) mixture delivered through a specialized nose cone for the duration of the injection procedure. Rats were injected into the right lateral cerebroventricle (ICV) with either 2 μL of losartan (2.1 nmoL/μL) or isoosmotic (0.9%) saline, followed 15 minutes later by ICV injections of 2 μL of isoosmotic (0.9%) saline or 2.0 mol/L of NaCl at an infusion rate of 1 μL/min. All of the stereotaxic ICV injection procedures were as detailed previously.28 An analgesic agent (buprenorphine; 0.05 mg/kg SC) was administered to the rats before waking. Three hours later, rats were euthanized, brains were removed, and the PVN was isolated from each as detailed previously.31 Endogenous levels of MIF mRNA in the PVN were analyzed by real-time RT-PCR, as detailed previously.29 MIF mRNA data were normalized to 18S rRNA.
Immunocytochemical Detection of Endogenous MIF in PVN Vasopressinergic Neurons
Please see http://hyper.ahajournals.org for the online Data Supplement for text and Figures S2 and S3.
Immunohistochemistry for Arginine Vasopressin, Neuron-Specific Nuclear Protein, and Virally Expressed MIF
Please see the online Data Supplement for text and Figures S2 and S3 (http://hyper.ahajournals.org).
Data Analysis
All of the values are expressed as the mean±SEM. One-way ANOVA followed by Student-Newman-Keuls post hoc or Student t test were used to assess differences between individual means. Differences were taken as significant at P<0.05.
Results
Colocalization of MIF and Arginine Vasopressin in the PVN
In a previous study, we showed that endogenous MIF staining in the PVN of normotensive rats was localized to neurons primarily with lesser amounts in glia.29 Because of the importance of vasopressin and vasopressinergic neurons in cardiovascular control, we wished to determine whether endogenous MIF resided within this neuronal phenotype. The lower power fluorescence micrographs shown in Figure 1 (top) depict MIF (green) and arginine vasopressin (AVP) (red) immunofluorescence at 3 different levels of the PVN (−1.60 mm, −1.88 mm, and −2.12 mm relative to the bregma) and also where MIF and AVP overlap. The fluorescence micrographs shown in Figure 1 (middle) are a higher power view (from the inset shown at the top left) depicting MIF (green) and AVP (red) immunofluorescence at PVN level −1.60 mm relative to bregma. MIF and AVP colocalizations are shown in orange. Quantification of the colocalization of MIF and AVP in different parts of the PVN is shown in the bar graphs in Figure 1. The data in the left bar graph show the number of immunoreactive AVP cells counted at each of the 3 PVN levels and also the number that overlap with MIF-positive cells. The data in the right bar graph show the colocalization of AVP and MIF in various subregions of the PVN at each of the levels investigated. Collectively, these data indicate that the degree of colocalization is low within each of the PVN levels, and when it occurs it is in both magnocellular and parvocellular regions of the PVN.
Induction of MIF mRNA Expression in the PVN by Central Hyperosmotic Stimulation
Because the PVN mechanism for hyperosmolality-induced increases in sympathetic nervous system activity is Ang II/AT1R–dependent and MIF is a regulator of Ang II/AT1R actions in the PVN,27,28 we investigated whether hyperosmotic challenge could induce changes in endogenous MIF mRNA levels at this hypothalamic nucleus. The data in Figure 2 indicate that rats injected ICV with hypertonic NaCl (2 μL of 2.0 mol/L) displayed significantly greater levels of MIF mRNA in the PVN when compared with rats that underwent ICV injections of 0.9% saline. This stimulatory effect of hypertonic saline on MIF mRNA levels in the PVN was abolished by pretreatment of rats with losartan (2.1 nmol/μL; ICV; Figure 2).
Increased Expression of MIF in the PVN Blunts the HS-Induced Sympathoexcitation
As an initial step, we performed studies to confirm the dependency of HS-induced increases in SNA on AT1R activation in the in situ preparation. Baseline SNA was recorded in rats perfused intra-arterially with isosmotic solution (290 mosmol/kg · water−1). After this, rats exposed to HS by perfusion for 40 seconds with a hyperosmotic solution (380 mosmol/kg · water−1) demonstrated a 23±3% increase in SNA. Addition of the AT1R antagonist losartan (20 μmol/L) to the perfusate for 10 minutes severely reduced the HS-induced sympathoexcitation to 5±5% (P=0.002). Considering that the HS-induced sympathoexcitation depends on the PVN19 and is AT1R mediated20 (please see Figure S1 at http://hyper.ahajournals.org), hyperosmotic challenge increases MIF expression in the PVN (Figure 2), and MIF counterregulates AT1R actions with the PVN,27,28 we determined whether increased expression of MIF in this hypothalamic nucleus would modify the increase in SNA produced by acute HS. The left and right PVNs of rats were microinjected with 0.9% saline, AAV2-CBA-MIF, or AAV2-CBA-eGFP, as described in the Methods section. AAV2-CBA-MIF and AAV2-CBA-eGFP produce expression of MIF and eGFP, respectively, in the PVN within 7 days. Seven to 10 days after the PVN injections, the decorticate-perfused in situ rat preparation was made. Baseline SNA in each group was established after intra-arterial perfusion of isosmotic Ringer solution (290 mosmol/kg · water−1). Intra-arterial infusion of hyperosmotic solution (Ringer solution at 380 mosmol/kg · water−1) for 40 seconds elicited sympathoexcitation in both the 0.9% saline and eGFP groups (increases of 27±4% and 25±4%, respectively; Figures 3 and 4). In contrast, HS for 40 seconds did not produce sympathoexcitation in the rats overexpressing MIF in the PVN (increased SNA of 4±5%; Figures 3 and 4A; P=0.008 versus saline). The area under the curve and the duration of the sympathoexcitation were reduced in the MIF-treated rats compared with the 0.9% saline (respectively, P=0.012 and P<0.001) and the eGFP groups (P=0.019 and P<0.001; Figure 4B and 4C). When MIF injections were located outside of the PVN (0.5 mm dorsal; see Figure 5A), there was no inhibition of the HS-induced sympathoexcitation, and a response similar to control was observed (data not shown), indicating a specific role of the PVN in this response. Finally, the sympathoexcitatory response to stimulating the peripheral chemoreflex (NaCN: 0.03%; 25 to 75 μL) was similar between control and MIF-expressing rats indicating relative specificity of MIF in the PVN for HS-induced sympathoexcitation (data not shown).
Figure 4.
HS-evoked sympathoexcitation is reduced by MIF and augmented by [C60S]-MIF expression in the PVN. Sympathoexcitation was measured as the percentage increase (peak response) from baseline over the 40-second period of HS (A), the total increase above baseline (B), and the duration of the response (C). Values in bar graphs are mean±SEM from rats that had received bilateral injections of saline, AAV2-CBA-eGFP, AAV2-CBA-MIF, or AAV2-CBA-[C60S]-MIF into the PVN 10 days earlier. (*=P<0.05, 1-way ANOVA; A: F(3,20)=11.432; B: F(3,20)=24.891; C: F(3,20)=77.750; n=6 rats per group).
Figure 5.
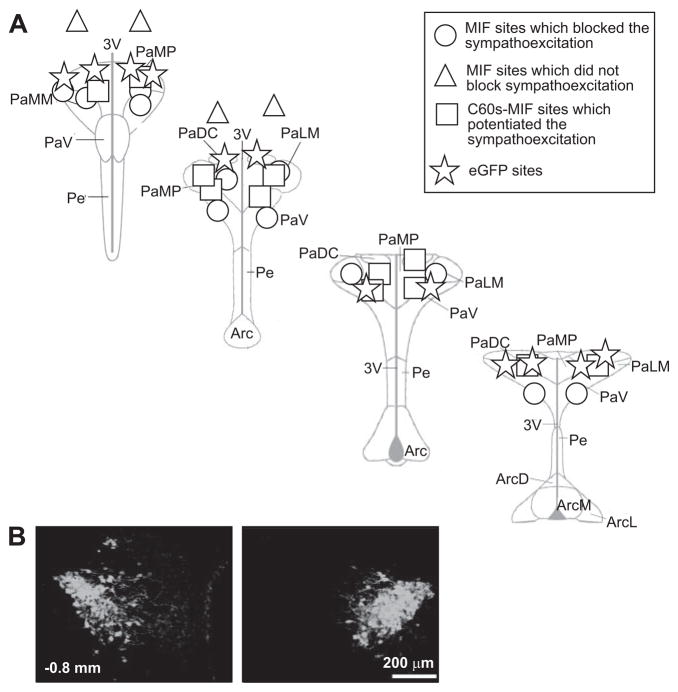
Localization of MIF transduction in the PVN. A, The center of the injection sites for eGFP, MIF, and C60S-MIF within the PVN are indicated. Spread of transduction was between 400 to 500 μm in diameter. Injections of MIF that fell outside of the PVN were not effective in blocking the hyperosmolality-induced sympathoexcitation. Section levels are relative to the bregma. Abbreviations: PaDC indicates paraventricular hypothalamic nucleus, dorsal cap; PaLM, paraventricular hypothalamic nucleus, lateral magnocellular part; PaMP, paraventricular hypothalamic nucleus, medial parvicellular part; PaV, paraventricular hypothalamic nucleus, ventral part; PaMM, paraventricular hypothalamic nucleus, medial magnocellular part; Pe, periventricular hypothalamic nucleus; Arc, arcuate hypothalamic nucleus; 3V, third ventricle. B, A representative example of MIF immunostaining in PVN induced by AAV2-CBA-MIF in vivo gene transfer.
The inhibitory effects of MIF on Ang II actions in the PVN are mediated by its intrinsic TPOR moiety.27,29 Because the sympathetic response to hyperosmotic stimulation was AT1R dependent, we next tested whether the inhibitory effect of MIF in the PVN on HS-induced sympathoexcitation was also attributed to its TPOR activity. An additional group of rats received injections of AAV2-CBA-[C60S]-MIF bilaterally into the PVN for expression of [C60S]-MIF as before.29 [C60S]-MIF, in which the cysteine at position 60 is replaced with serine, lacks TPOR activity.32 In contrast to MIF, the HS-induced sympathoexcitation was potentiated (45±6%) in rats that expressed [C60S]-MIF (Figures 3 and 4A; P=0.023 versus saline). The area under the curve and the duration of the sympathoexcitation were both augmented significantly in the [C60S]-MIF group (Figure 4B and 4C; P<0.001 versus saline).
Localization of MIF Expression in the PVN
Schematic reconstruction of injection sites at different rostral-caudal levels of the PVN are shown in Figure 5A. As can be seen, the sites of injection of AAV2-CBA-MIF, AAV2-CBA-[C60S]-MIF, and AAV2-CBA-eGFP were, in most part, within the PVN (maximal spread was between 400 to 500 μm in diameter). We never saw transduction of circumventricular organs or ependymal cells. We recognize the difficulty of distinguishing between endogenous and virally expressed MIF, which is a limitation of our study. However, we did use a lower concentration of antibody to detect virally induced MIF that rarely detected endogenous protein (1:500 versus 1:200 dilution, respectively; please see Figure S2 at http://hyper.ahajournals.org). Figure 5 also shows sites of AAV2-CBA-MIF injection outside the PVN, which were ineffective. Figure 5B shows a fluorescence micrograph depicting bilateral expression of MIF immunoreactivity in the PVN, typically obtained after AAV2-CBA-MIF injection. Virally expressed MIF is largely found in neurons, as demonstrated by its coexpression with the specific neuronal marker neuron-specific nuclear protein (NeuN) (Figure 6A). Interestingly, the neuronal populations in which MIF is transduced in the PVN include vasopressinergic neurons (Figure 6B).
Discussion
The major findings of this study are as follows: (1) MIF is present in PVN vasopressinergic neurons; (2) hyperosmotic challenge increases MIF expression in the PVN, which is AT1R dependent; (3) increased MIF expression within the PVN of normotensive rats abolishes the sympathoexcitation produced by hyperosmotic stimulation; and (4) the actions of MIF appear to be mediated by its intrinsic TPOR activity. Collectively, these data provide the first indication that MIF, present within neurons in the PVN, serves as a negative regulator of HS-induced sympathoexcitation. The demonstration that HS conditions also produce an increase in MIF mRNA expression in the PVN opens up the possibility that MIF is a major central nervous system regulator of sympathetic outflow in conditions of high salt load.
The osmotic stimulus used in our in situ experiments is higher than that used previously (see Reference 19). Here we used 380 mosmol/kg · water−1. This produced a robust and reproducible sympathoexcitation that was important for assessing accurately the magnitude by which MIF expression in PVN could sequester this evoked response. We acknowledge that the stimulus is high. However, this level of osmolality is unlikely to be seen by the preparation because of the transient nature of the stimulus and significant dilution that occurs within the perfusion circuit and preparation; this is discussed further in the online Data Supplement at http://hyper.ahajournals.org.
The mechanisms for hyperosmolality-induced increases in SNA from the PVN are Ang II/AT1R dependent,28 and the negative regulatory action of MIF quenches Ang II/AT1R-stimulated increases in neuronal discharge and elevations in arterial pressure.28 Findings from the latter study were consistent with the earlier in vitro observation that the Ang II-increased firing response of PVN neurons is blunted by MIF, which is likely to be activated by continual AT1R stimulation.28 Thus, an Ang II activity-dependent feedback mechanism may exist to protect against overstimulation by Ang II, which we presume operates in vivo. However, crucial further experiments should include determining whether HS-induced induction of endogenous MIF in the PVN can be prevented by AT1R blockade and provide tempering over HS-induced sympathoexcitation. Also, it now becomes important to determine the role that MIF plays in the PVN for chronic elevations of sympathetic nerve activity and arterial pressure observed during dehydration, for example.
We acknowledge that our study does not allow us to differentiate whether the MIF mechanism is sodium or osmotically sensitive. However, if endogenous MIF does serve as a “salt-sensitive” endogenous regulator of HS-induced sympathoexcitation, then one primary question concerns the nature of the mechanisms that mediate hyperosmolality-induced increases in MIF expression in the PVN. Osmolality-induced changes in SNA are mediated through osmosensitive neurons in the OVLT and subfornical organ,3 which subsequently transmit information concerning the osmotic status of the animal via efferent pathways to the PVN.2,19 Ang II, released either from these efferent pathways or generated locally within the PVN itself increases, via presynaptic and/or postsynaptic AT1R,33,34 the activity of PVN presympathetic neurons projecting to the intermediolateral nucleus or rostral ventrolateral medulla.1 Our current data confirm that the HS-induced sympathoexcitation is AT1R dependent. Considering that ICV injection of Ang II increases MIF expression in the PVN of normotensive rats,29 it is likely that the HS-induced MIF expression in the PVN is also Ang II/AT1R dependent. However, overexpression of MIF does not affect basal blood pressure in both normotensive (this study) and spontaneously hypertensive rats,29 suggesting a specific functional role related to salt-mediated sympathoexcitation.
The inhibitory action of MIF on HS-induced sympathoexcitation appears to involve its TPOR activity. This is because increased expression of the mutant protein [C60S]-MIF in the PVN failed to blunt the HS-induced increased in SNA (Figures 3 and 4). However, the [C60S]-MIF rats displayed an unexpected higher increase in sympathoexcitation in response to HS challenge compared with the eGFP group (Figures 3 and 4). The reasons for this action of [C60S]-MIF are not obvious but may involve a dominant-negative action of this mutant protein over endogenous MIF within the PVN; however, this remains speculative. If so, this would argue that PVN parvocellular neurons are under tonic control by MIF, which would be entirely consistent with the specific role as a mediator of increased sympathetic activity in response to salt loading.
The finding that a vasopressinergic descending pathway from PVN to intermediolateral nucleus contributes to sympathoexcitation elicited by hyperosmolality19 suggests that a possible location of MIF’s inhibitory actions in the PVN include the vasopressinergic neurons. Indeed, our data (Figure 1) illustrate that PVN vasopressinergic neurons express MIF endogenously. Moreover, Figure 6B demonstrates that the AAV2-CBA-MIF–injected rats display MIF transduction into AVP neurons in the PVN. Thus, it is likely that the inhibitory actions of MIF in the PVN on HS-induced sympathoexcitation involve, in part, the vasopressinergic neurons. However, whereas the CBA promoter that is used in the present study produces MIF expression primarily within neurons in the PVN,29 it does not discriminate between different neuronal phenotypes. Further development of promoters that would allow for transduction of MIF into specific neuron populations (eg, AVP) would help to tease out whether the actions of MIF in controlling sympathoexcitation in response to HS are limited to a specific neuronal phenotype, such as glutamatergic PVN neurons known to be activated by osmotic stimuli that project to the rostral ventrolateral medulla.26 Also, we cannot comment on whether AT1Rs involved are localized to the vasopressinergic neurons or nonvasopressinergic interneurons within PVN.35
Perspectives
Our new findings indicate that MIF operates as a novel inhibitor within the PVN of osmolality-induced increases in sympathetic outflow and substantiates the developing notion that MIF is an important regulator of Ang II-AT1R–induced excitability within the PVN, especially to salt loading. Further phenotyping of the MIF containing neurons in the PVN in terms of their neurochemical content and connectivity is now needed. Subsequently, chronic studies to assess whether MIF expression in the PVN can prevent the hypertension associated with salt loading and dehydration in conscious animals would further validate the importance of this protein. Indeed, whether MIF plays a role in controlling other forms of hypertension, such as stress-related hypertension, would add further credence as to its wider applicability to hypertension. It also needs to be established where else in the brain MIF may exist to keep sympathetic nerve activity in check: the nucleus tractus solitarii and rostral ventrolateral medulla are both likely sites. The significant finding described herein that the salt-induced sympathetic overdrive depended on the TPOR activity of MIF leads way to proof-of-principle studies testing whether the AAV2-CBA-C60S-MIF-eGFP vector can prevent hypertension development in a salt-sensitive rat model such as the Dahl. Accordingly, we propose that MIF might be exploitable as a novel target to control (salt-related) hypertension in humans.
Supplementary Material
Acknowledgments
Sources of Funding
E.C. was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, Coordenaçao de Aperfeiçoamento de Pessoal de Nivel Superior, and Faculdade de Medicina ABC from Brazil and a Benjamin Meaker Fellowship awarded from the University of Bristol. D.S.A.C. was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico and a Wellcome Trust Value in People Award (University of Bristol). This work was supported by the British Heart Foundation and National Institutes of Health grants 1R01HL-076803 and HL33610. J.F.R.P. was in receipt of a Royal Society Wolfson Research Merit Award.
Footnotes
Disclosures
None.
References
- 1.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 2.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177:43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- 3.Bourque CW. Central mechanisms of osmosensation and systemic osmo-regulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 4.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 5.McKinley MJ, Allen AM, May CN, McAllen RM, Oldfield BJ, Sly D, Mendelsohn FA. Neural pathways from the lamina terminalis influencing cardiovascular and body fluid homeostasis. Clin Exp Pharmacol Physiol. 2001;28:990–992. doi: 10.1046/j.1440-1681.2001.03592.x. [DOI] [PubMed] [Google Scholar]
- 6.McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci. 2004;19:1–6. doi: 10.1152/nips.01470.2003. [DOI] [PubMed] [Google Scholar]
- 7.Stocker SD, Osborn JL, Carmichael SP. Forebrain osmotic regulation of the sympathetic nervous system. Clin Exp Pharmacol Physiol. 2008;35:695–700. doi: 10.1111/j.1440-1681.2007.04835.x. [DOI] [PubMed] [Google Scholar]
- 8.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci. 2006;26:9069–9075. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2279–R2289. doi: 10.1152/ajpregu.00160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi P, Martinez MA, Calderon AS, Chen Q, Cunningham JT, Toney GM. Intra-carotid hyperosmotic stimulation increases fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus. J Physiol. 2008;586:5231–5245. doi: 10.1113/jphysiol.2008.159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thrasher TN, Keil LC, Ramsay DJ. Lesions of the organum vasculosum of the lamina terminalis (OVLT) attenuate osmotically-induced drinking and vasopressin secretion in the dog. Endocrinology. 1982;110:1837–1839. doi: 10.1210/endo-110-5-1837. [DOI] [PubMed] [Google Scholar]
- 12.Ho JM, Zierath DK, Savos AV, Femiano DJ, Bassett JE, McKinley MJ, Fitts DA. Differential effects of intravenous hyperosmotic solutes on drinking latency and c-fos expression in the circumventricular organs and hypothalamus of the rat. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1690–R1698. doi: 10.1152/ajpregu.00547.2006. [DOI] [PubMed] [Google Scholar]
- 13.McKinley MJ, Denton DA, Leksell LG, Mouw DR, Scoggins BA, Smith MH, Weisinger RS, Wright RD. Osmoregulatory thirst in sheep is disrupted by ablation of the anterior wall of the optic recess. Brain Res. 1982;236:210–215. doi: 10.1016/0006-8993(82)90048-8. [DOI] [PubMed] [Google Scholar]
- 14.Morris M, Rocha MJ, Sim LJ, Johnson AK, Callahan MF. Dissociation between vasopressin and oxytocin mRNA and peptide secretion after AV3V lesions. Am J Physiol. 1994;267:R1640–R1645. doi: 10.1152/ajpregu.1994.267.6.R1640. [DOI] [PubMed] [Google Scholar]
- 15.Saper CB, Levisohn D. Afferent connections of the median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res. 1983;288:21–31. doi: 10.1016/0006-8993(83)90078-1. [DOI] [PubMed] [Google Scholar]
- 16.Camacho A, Phillips MI. Horseradish peroxidase study in rat of the neural connections of the organum vasculosum of the lamina terminalis. Neurosci Lett. 1981;25:201–204. doi: 10.1016/0304-3940(81)90391-8. [DOI] [PubMed] [Google Scholar]
- 17.Stocker SD, Toney GM. Vagal afferent input alters the discharge of osmotic and ANG II-responsive median preoptic neurons projecting to the hypothalamic paraventricular nucleus. Brain Res. 2007;1131:118–128. doi: 10.1016/j.brainres.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol. 2005;563:249–263. doi: 10.1113/jphysiol.2004.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antunes VR, Yao ST, Pickering AE, Murphy D, Paton JF. A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. J Physiol. 2006;576:569–583. doi: 10.1113/jphysiol.2006.115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen QH, Toney GM. AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1844–R1853. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- 21.Freeman KL, Brooks VL. AT(1) and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1675–R1682. doi: 10.1152/ajpregu.00623.2006. [DOI] [PubMed] [Google Scholar]
- 22.Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol. 1998;25:461–463. doi: 10.1111/j.1440-1681.1998.tb02235.x. [DOI] [PubMed] [Google Scholar]
- 23.Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- 24.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Bertram D, Coote JH. The role of glutamate and vasopressin in the excitation of RVL neurones by paraventricular neurones. Brain Res. 2001;908:99–103. doi: 10.1016/s0006-8993(01)02593-8. [DOI] [PubMed] [Google Scholar]
- 26.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun C, Li H, Leng L, Raizada MK, Bucala R, Sumners C. Macrophage migration inhibitory factor: an intracellular inhibitor of angiotensin II-induced increases in neuronal activity. J Neurosci. 2004;24:9944–9952. doi: 10.1523/JNEUROSCI.2856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Gao Y, Freire CD, Raizada MK, Toney GM, Sumners C. Macrophage migration inhibitory factor in the PVN attenuates the central pressor and dipsogenic actions of angiotensin II. FASEB J. 2006;20:1748–1750. doi: 10.1096/fj.06-5836fje. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Gao Y, Qi Y, Katovich MJ, Jiang N, Braseth LN, Scheuer DA, Shi P, Sumners C. Macrophage migration inhibitory factor in hypothalamic paraventricular nucleus neurons decreases blood pressure in spontaneously hypertensive rats. FASEB J. 2008;22:3175–3185. doi: 10.1096/fj.08-108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 31.Jiang N, Shi P, Li H, Lu S, Braseth L, Cuadra AE, Raizada MK, Sumners C. Phosphate-activated glutaminase-containing neurons in the rat para-ventricular nucleus express angiotensin type 1 receptors. Hypertension. 2009;54:845–851. doi: 10.1161/HYPERTENSIONAHA.109.134684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleemann R, Kapurniotu A, Frank RW, Gessner A, Mischke R, Flieger O, Juttner S, Brunner H, Bernhagen J. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol. 1998;280:85–102. doi: 10.1006/jmbi.1998.1864. [DOI] [PubMed] [Google Scholar]
- 33.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol. 2005;93:403–413. doi: 10.1152/jn.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qadri F, Edling O, Wolf A, Gohlke P, Culman J, Unger T. Release of angiotensin in the paraventricular nucleus in response to hyperosmotic stimulation in conscious rats: a microdialysis study. Brain Res. 1994;637:45–49. doi: 10.1016/0006-8993(94)91215-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.