Abstract
2′-O-(2-methoxyethyl) (2′-MOE) RNA possesses favorable pharmocokinetic properties that make it a promising option for the design of oligonucleotide drugs. Telomerase is a ribonucleoprotein that is up-regulated in many types of cancer, but its potential as a target for chemotherapy awaits the development of potent and selective inhibitors. Here we report inhibition of human telomerase by 2′-MOE RNA oligomers that are complementary to the RNA template region. Fully complementary oligomers inhibited telomerase in a cell extract with IC50 values of 5–10 nM at 37°C. IC50 values for mismatch-containing oligomers varied with length and phosphorothioate substitution. After introduction into DU 145 prostate cancer cells inhibition of telomerase activity persisted for up to 7 days, equivalent to six population doublings. Inside cells discrimination between complementary and mismatch-containing oligomers increased over time. Our results reveal two oligomers as especially promising candidates for initiation of in vivo preclinical trials and emphasize that conclusions regarding oligonucleotide efficacy and specificity in cell extracts do not necessarily offer accurate predictions of activity inside cells.
INTRODUCTION
Backbone phosphorothioate (PS) linkages in which a sulfur atom replaces one of the non-bridging oxygen atoms are an important consideration when planning in vivo applications of oligonucleotides (1,2). PS linkages confer enhanced stability against nuclease degradation and improve pharmacokinetic properties by increasing binding to serum proteins and in vivo half-life (3). One PS oligonucleotide is an approved drug and several others are in clinical trials (4). However, because PS linkages increase the propensity for oligonucleotides to bind to proteins, there is an increased likelihood that the biological effects attributed to an oligonucleotide might not be due to Watson–Crick base pairing to its intended target (5,6). It is critical, therefore, to develop rules correlating PS substitution with specificity and potency of oligonucleotides directed towards cellular targets. One such target is human telomerase, a ribonucleoprotein that offers important opportunities for the design of oligonucleotide drugs (7).
Human telomerase is a ribonucleoprotein that adds repeated units of sequence TTAGGG to the ends of telomeres. Telomerase activity has been found in germ cells, stem cells and most types of human tumors, but is absent in non-cancerous cells adjacent to the tumor (8,9). This observation has led to the hypothesis that activation of telomerase is necessary for sustained tumor growth and that telomerase inhibitors might be a new option for the treatment of minimal residual disease in a wide range of cancers. The arguments for and against telomerase as a target for chemotherapy have been extensively reviewed (7,10,11) and to resolve the debate it will be necessary to identify telomerase inhibitors that are highly active when administered in vivo.
Telomeres in human tumor cells range in length from several hundred to several thousand bases. In the absence of telomerase activity, telomeres erode at a rate of 50–100 bases per population doubling (12,13). These basic facts of telomere biology present a severe challenge to drug design, because it is reasonable to assume that there will be a delay between the start of treatment and observation of decreased cell proliferation (7). Experiments that aim to investigate the effects of inhibitors in vivo will last longer than similar studies for most other anti-proliferative agents. As a result, it is important to identify telomerase inhibitors that possess optimal properties prior to commencing lengthy studies in animals or human clinical trials. Characteristics to optimize include potency, specificity, the toxicology profile and pharmacokinetics.
The RNA domain of human telomerase, hTR, contains an 11 base region (nucleotides +46 to + 56) that acts as a template for binding and extending telomeres (14). This critical role requires that hTR be predominantly single-stranded, making it exceptionally accessible and an ideal target for inhibition by oligonucleotides. We have previously shown that peptide nucleic acid and 2′-O-methyl RNA oligomers inhibit telomerase, causing telomeres to shorten and cell proliferation to decrease (15,16). As a practical starting point for studies in animals with xenograft tumors we chose 2′-O-(2-methoxyethyl) (2′-MOE) RNA (Fig. 1) because the pharmacological and toxicological properties of 2′-MOE RNA have already been well characterized. 2′-MOE RNA has demonstrated superior properties relative to other oligonucleotide modifications, including decreased immune stimulation and increased hybridization strength, oral bioavailability and antisense efficacy (17–20). A practical advantage is that large-scale synthesis of MOE oligomers has been developed, allowing gram synthesis for animal studies and kilogram synthesis if testing progresses to human trials.
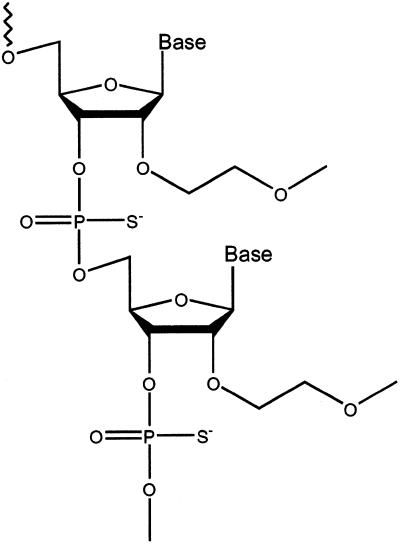
Figure 1.
Structure of PS 2′-MOE RNA.
Here we report on the ability of partially and fully PS-substituted 13 and 20 nt 2′-MOE RNA oligomers to effectively inhibit hTR. We suggest two inhibitors as excellent candidates for in vivo preclinical studies.
MATERIALS AND METHODS
Oligomer synthesis
2′-MOE RNA oligonucleotides were synthesized by ISIS Pharmaceuticals Inc. (Carlsbad, CA) as described (21) and purified by reversed phase HPLC. The RNA oligonucleotide used for melting temperature determinations was purchased from Oligos Etc. (Wilsonville, OR). The absorbance of each oligonucleotide solution was determined at 260 nm using a Hewlett Packard 8452 diode array spectrophotometer (Palo Alto, CA) or a Beckman Coulter DU7500 spectrophotometer (Beckman Instruments, Fullerton, CA). Concentrations were determined using the absorbance at 260 nm and the extinction coefficient for each oligonucleotide was calculated as described (22).
DU 145 cells
To ensure that experiments were performed using cells capable of forming tumors, 5 million DU 145 cells were injected into a Harlan nude athymic mouse, which was irradiated with 400 rad γ-irradiation 24 h prior to injection. Tumors were harvested when they reached a size of ∼400 mm3. Tumors were minced and placed back into tissue culture. Cells were passaged in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 20 U/ml penicillin, 0.02 mg/ml streptomycin and 1× Anti-PPLO (anti-mycoplasma agent, 6 mg/ml Tylosin; Life Technologies, Gaithersburg, MD).
Telomerase assays
Telomerase activity from immortal human prostate DU 145 cells was determined with the telomere repeat amplification protocol (TRAP) using the TRAPeze telomerase detection kit (Intergen Co., Purchase, NY) (23). The oligomer being tested for inhibition was prepared at a concentration range of 100 µM to 1 nM in logarithmic steps. Each concentration of oligomer was incubated with 200 cell equivalents of DU 145 cell lysate for 30 min at 25 or 37°C. The TRAPeze reaction mixture was added to each sample and then incubated for 30 min at 25 or 37°C to allow extension of the radiolabeled primer by telomerase. Once extended the products were amplified by PCR with a two-step cycle of 30 s at 94°C followed by 30 s at 60°C, repeated 27 times. The following controls were included in every experiment. A sample containing buffer and amplification reagents to which no cell lysate was added was used to ensure that false products were not being amplified by PCR. Cell lysate in the absence of oligomer inhibitor was tested to determine the maximum level of telomerase activity. An internal amplification standard was included to monitor the success of the PCR. As a final control the oligonucleotides being tested as inhibitors were added at a concentration of 3.3 µM prior to PCR amplification to confirm that the observed inhibition was due to binding of telomerase rather than interference with the template during PCR. No inhibition of the PCR step was observed for any of the oligomers tested.
Reaction products were subjected to non-denaturing PAGE analysis, followed by PhosphorImager analysis (Molecular Dynamics, Piscataway, NJ), which provided quantitative data on the extent of telomerase inhibition. The internal standard also served as a control for amplification efficiency in each reaction and was used for quantitative analysis of the TRAP products. The lanes were divided into one region encompassing the telomerase products and another including the internal standard signal. The radioisotope density was integrated for each area and the ratio of telomerase product to internal standard was determined. The extent of inhibition as a function of concentration of added inhibitor was plotted and these graphs were used to derive IC50 values. All assays were performed in triplicate and the reported values for inhibition are averages of these triplicate determinations.
Transfections
Prostate tumor-derived DU 145 cells were plated at 25 000 cells/well in a 24-well plate in DMEM supplemented with 10% fetal calf serum, 20 U/ml penicillin, 0.02 mg/ml streptomycin and 1× Anti-PPLO. After cells were allowed to adhere overnight they were transfected with 2 µl of LipofectAMINE (Life Technologies) and 500 or 100 nM oligomer in 200 µl of OPTI-MEM (Life Technologies) according to the manufacturer’s directions. After 6 h at 37°C the transfection mixture was removed and growth medium (containing serum, penicillin, streptomycin and Anti-PPLO) was added. Cells were harvested 18 (day 1 post-transfection), 90 (day 4 post-transfection) and 138 h (day 7 post-transfection) later. Harvesting consisted of washing the cells with 1× phosphate-buffered saline (PBS), followed by trypsinization with 100 µl/well trypsin and inactivation of the trypsin with 400 µl of growth medium/well. Cells were counted using a Coulter Z Series cell counter (Beckman Coulter, Fullerton, CA). Lysates were prepared using 1× CHAPS lysis buffer (Intergen) and samples were assayed for inhibition of telomerase.
RESULTS AND DISCUSSION
TRAP assay for telomerase inhibition
We designed a series of 2′-MOE RNA oligonucleotides complementary to the template region of hTR. To determine the ability of these oligonucleotides to inhibit telomerase, we added them to a lysate of prostate tumor-derived DU 145 cells and monitored telomerase activity using the PCR-based TRAP (22). Levels of telomerase activity and inhibition were evaluated relative to positive controls to which no 2′-MOE RNA was added. An internal amplification standard was included in every experiment to allow quantification. Levels of telomerase activity were within the linear range of the TRAP assay.
Inhibition of telomerase by 13 base 2′-MOE RNA oligomers
We initiated testing using 13 base phosphodiester 2′-MOE RNA oligonucleotides I and II. Oligomer I was fully complementary to hTR and inhibited telomerase activity with an IC50 of 30 nM at 25°C and 10 nM at 37°C (Table 1). In contrast, the IC50 of II, which contained two mismatch bases, was 300- to 1000-fold higher. Fully phosphodiester-substituted oligomers are relatively susceptible to nuclease digestion, so to improve stability towards exonucleases we introduced two PS linkages at the 3′- and 5′-termini to afford 13 base inhibitor III and analogous mismatch-containing oligomer IV. Oligomer III was a potent inhibitor, with an IC50 value of 2 nM when measured at 25°C and 9 nM at 37°C. The corresponding mismatch-containing oligomer IV possessed an IC50 of 3.3 µM at 25°C and 7 µM at 37°C, again demonstrating a match versus mismatch discrimination of >100-fold.
Table 1. Inhibition of telomerase by 2′-MOE RNA and 2′-MOE RNA/DNA chimeric oligomers.
| Compound | Chemistry | No. | Sequence | IC50 (µM) | |
| |
|
|
|
25°C |
37°C |
| 3′-GAAGAGUCAAUCCCAAUCUG-5′ (RNA template) | |||||
| ISIS24690 | 2′-MOE RNA | I | CAGUUAGGGUUAG | 0.03 | 0.01 |
| ISIS125626 | 2′-MOE RNA | II | CAGUUAGAAUUAG (mismatch) | >10 | >10 |
| ISIS125625 | 2′-MOE RNA | III | CAGUUAGGGUUAG | 0.002 | 0.009 |
| ISIS125627 | 2′-MOE RNA | IV | CAGUUAGAAUUAG (mismatch) | 3.3 | 7 |
| ISIS24691 | 2′-MOE RNA | V | CAGUUAGGGUUAG | 0.003 | 0.007 |
| ISIS125628 | 2′-MOE RNA | VI | CAGUUAGAAUUAG (mismatch) | 0.7 | 1.1 |
| ISIS113753 | 2′-MOE RNA/DNA | VII | CUUCUCAGTTAGGGTUAGAC | 0.025 | 0.02 |
| ISIS113754 | 2′-MOE RNA/DNA | VIII | CUUCUCAGTTAGAATUAUAC (mismatch) | 1.0 | >5.5 |
| ISIS113751 | 2′-MOE RNA/DNA | IX | CUUCUCAGTTAGGGTUAGAC | 0.002 | 0.005 |
| ISIS113752 | 2′-MOE RNA/DNA | X | CUUCUCAGTTAGAATUAUAC (mismatch) | 0.004 | 0.010 |
| ISIS113749 | 2′-MOE RNA/DNA | XI | CUUCUCAGTTAGGGTUAGAC | 0.006 | 0.009 |
| ISIS113750 | 2′-MOE RNA/DNA | XII | CUUCUCAGTTAGAATUAUAC (mismatch) | 0.008 | 0.009 |
Underlined nucleotides have PS linkages. The italicized bases in the 2′-MOE/DNA chimera are DNA.
To improve stability and pharmacokinetic properties further we tested oligomers V and VI that contained PS substitutions at every phosphate. Fully complementary oligomer V had an IC50 of 3 nM at 25°C and 7 nM at 37°C (Table 1). Similarly, potent inhibition was also observed in extracts of prostate tumor-derived cell lines LNCaP, C4 and C4-2 (data not shown). We had expected that mismatch-containing oligomer VI might also inhibit telomerase potently because previous studies reported that PS DNA oligonucleotides blocked telomerase activity through non-sequence-specific interactions between the PS backbone and the reverse transcriptase component of telomerase, hTERT (7,24–26). Surprisingly, in spite of its extensive PS substitution, VI was a poor inhibitor, with IC50 values of 700 nM at 25°C and 1.1 µM at 37°C. Melting temperature (Tm) values of match oligonucleotides I, III and V for complementary RNA oligomers were 58, 53 and 52°C, respectively. Tm values for the mismatch-containing oligomers II, IV and VI were 35, 33 and 30°C, respectively.
Our results suggest that PS substitution does not greatly affect the ability of fully complementary 13 base 2′-MOE oligomers to potently and sequence-selectively inhibit telomerase in cell extract. This result suggests that anti-telomerase oligomers can exploit the benefits of PS substitution, improved stability and pharmacokinetics, while retaining the ability to be potent and sequence-selective inhibitors.
Inhibition of telomerase by 20 base 2′-MOE RNA/DNA chimeras
2′-MOE RNA is used in combination with DNA to create optimized chimeric oligomers to act as potent antisense agents. Chimeric 2′-MOE DNA antisense oligomers are usually ∼20 bases long and contain a central DNA region to allow RNase H to be recruited upon binding to mRNA. This DNA region is necessary because RNase H cannot recognize and cleave hybrids between RNA and 2′-MOE RNA. Flanking 2′-MOE RNA sequences increase hybridization affinity. As demonstrated by the potent inhibition of 2′-MOE RNA oligomers I, III and V, RNase H activation by anti-telomerase oligomers is not necessary for inhibition. However, the pharmacokinetic and toxicological properties of 20 base mixed backbone oligomers are better characterized than the properties of 13 base 2′-MOE RNA, knowledge that would be invaluable for the design of in vivo trials. Recognizing this, we decided to test a series of similar 20 base oligomers for their ability to inhibit telomerase (Table 1).
We synthesized 2′-MOE RNA/DNA chimeras consisting of a nested 10 base sequence of DNA with five flanking 2′-MOE bases. Fully complementary oligomer VII contained no PS linkages and possessed an IC50 value of 20 nM at 37°C. The IC50 value for the analogous mismatch sequence was >5.5 µM at 37°C. To enhance the stability towards nuclease degradation we then synthesized 20 base chimeras IX and X to contain PS substitution throughout the DNA portion. The IC50 values for the complementary oligonucleotide IX were 2 nM at 25°C and 5 nM at 37°C. The mismatch-containing oligomer possessed IC50 values that were very similar, 4 nM at 25°C and 10 nM at 37°C. Finally, because PS substitution has been demonstrated to improve the pharmacokinetic properties of oligonucleotides, we also synthesized 20 base oligomers XI and XII that contained PS substitutions at every position. Fully complementary oligomer XI had IC50 values of 6 nM at 25°C and 9 nM at 37°C (Fig. 2A). Inhibition by the mismatch-containing oligomer XII was similar, with values of 8 nM at 25°C and 9 nM at 37°C (Fig. 2B).
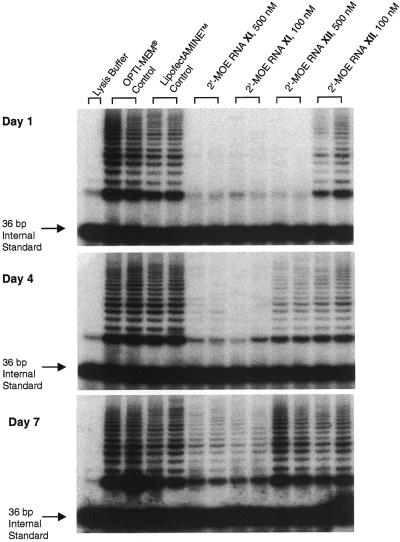
Figure 2.
TRAP assay of inhibition of telomerase activity upon addition of 20 base 2′-MOE RNA/DNA chimeras XI and XII to cell extract at 37°C. (A) Triplicate assay of match oligomer XI. (B) Triplicate assay of mismatch-containing oligomer XII. The internal amplification standard (ITAS) is noted. At the highest concentration ITAS DISAPPEARS because of inhibition of Taq polymerase by PS-substituted oligomers.
These results demonstrate that 20 base 2′-MOE RNA oligomers are potent inhibitors of telomerase. However, mismatch discrimination in cell extracts can be negligible if PS substitutions are present. The poor mismatch discrimination for the match oligomers IX and XI relative to mismatched oligomers X and XII is likely due to two reasons. The first is that their PS linkages probably promote non-sequence-specific interactions with the protein component of telomerase. This cannot be the entire explanation, however, as 20 base oligomers IX and X, which have only 10 PS substitutions, show much less mismatch discrimination than 13 base oligomer V, which has 13 PS linkages. Therefore, it is also likely that the seven additional bases possessed by X and XII form additional interactions that compensate for the presence of mismatched bases. These interactions could be base pairing between the seven bases and the RNA component of telomerase hTR. Alternatively, the interactions could be with the protein component hTERT.
Inhibition of telomerase within cells
Our studies of inhibition of telomerase by 2′-MOE RNA in cell extracts showed that both 13 and 20 base oligomers were potent inhibitors, but that 20 base oligomers containing PS substitution showed little or no discrimination against mismatched bases. To determine whether these results would also be observed inside living cells, we transfected fully complementary oligomers III, V and XI and analogous mismatch-containing oligomers IV, VI and XII into DU 145 cells using the cationic lipid LipofectAMINE. We monitored telomerase activity 1, 4 and 7 days after transfection by lysing the cells and evaluation using the PCR-based TRAP assay.
We observed that the IC50 values for inhibition of telomerase in cell extracts by 13 base oligomers accurately predicted the ability of these inhibitors to block telomerase activity inside cells (Table 2). We found that fully complementary oligomers III and V were efficient inhibitors when transfected at 100 or 500 nM. Even after 7 days incubation in cells, during which six population doublings occurred, >50% of telomerase activity was inhibited, demonstrating a long cellular half-life. The analogous mismatch-containing oligomer was not an effective inhibitor, with <30% inhibition observed after 1 day and little or no inhibition observed subsequently.
Table 2. Inhibition of intracellular telomerase by 2′-MOE RNA and 2′-MOE RNA/DNA chimeric oligomers 1, 4 and 7 days after transfection.
| Compound | Chemistry | No. | Sequence | Conc. | Inhibition (%) | ||
| |
|
|
|
|
Day 1 |
Day 4 |
Day 7 |
| 3′-GAGUCAAUCCCAAUCUG-5′ (RNA template) | |||||||
| ISIS125625 | 2′-MOE RNA | III | CAGUUAGGGUUAG | 500 | 82 | 67 | 76 |
| 100 | 77 | 87 | 76 | ||||
| ISIS125627 | 2′-MOE RNA | IV | CAGUUAGAAUUAG (mismatch) | 500 | 24 | nd | nd |
| 100 | 23 | 20 | nd | ||||
| ISIS24691 | 2′-MOE RNA | V | CAGUUAGGGUUAG | 500 | 68 | 87 | 50 |
| 100 | 80 | 78 | 52 | ||||
| ISIS125628 | 2′-MOE RNA | VI | CAGUUAGAAUUAG (mismatch) | 500 | 30 | nd | nd |
| 100 | 32 | nd | nd | ||||
| ISIS113749 | 2′-MOE RNA/DNA | XI | CUUCUCAGTTAGGGTUAGAC | 500 | 54 | 93 70 | |
| 100 | 68 | 91 | 60 | ||||
| ISIS113750 | 2′-MOE RNA/DNA | XII | CUUCUCAGTTAGAATUAUAC (mismatch) | 500 | 66 | 65 | nd |
| 100 | 18 | 42 | nd |
Underlined nucleotides have PS linkages. The italicized bases in the 2′-MOE/DNA chimera are DNA. Bases that are mismatched relative to the RNA template of hTR are in bold. Nd, no significant inhibition detected. Individual experiments were performed in duplicate and all experiments were repeated at least once. Reported values are an average of the percent inhibition values for all determinations.
The IC50 values for inhibition of telomerase in cell extracts by 20 base oligomers XI and XII, in contrast, proved less accurate guides to their relative inhibition inside cells. Fully complementary oligomer XI was a highly effective inhibitor 1, 4 and 7 days post-transfection (Table 2 and Fig. 3), blocking >70% of telomerase activity. The mismatch-containing oligomer XII showed some inhibition at 1 and 4 days post-transfection, but no inhibition was observed when telomerase activity was assayed 7 days after transfection. The important conclusion from these experiments is that inhibition of telomerase in cells by oligomer XI appears to be significantly more sequence selective than similar inhibition observed in cell extract.
Figure 3.
TRAP assay of inhibition of telomerase activity 1, 4 and 7 days after transfection of 100 and 500 nM concentrations of 20 base chimeras XI and XII into DU 145 cells. Assays were performed in duplicate. The ITAS is noted.
Dose–response characteristics of inhibition of cellular telomerase
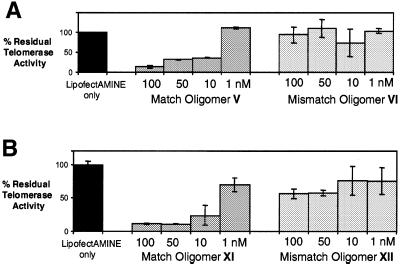
To further analyze the intracellular effectiveness of oligonucleotides we assayed inhibition of cellular telomerase by oligomers V and XI as a function of concentration (1, 10, 50 and 100 nM) and compared the dose–response curve to that produced by analogous mismatch-containing oligomers VI and XII. We used LipofectAMINE to introduce the oligomers and telomerase activity was measured 4 days after transfection. We observed that by day 4 oligomer V inhibited telomerase at concentrations as low as 10 nM, while the analogous mismatched oligomer did not significantly inhibit telomerase at any of the tested concentrations (Fig. 4A). We also observed that by day 4 oligomer XI strongly inhibited telomerase at concentrations as low as 10 nM, while the analogous mismatch oligomer XII was appreciably less active (Fig. 4B). The match versus mismatch discrimination displayed by comparison of the effects of oligomers XI and XII is in striking contrast to their IC50 values when measured in cell extracts and corroborates the observation made above that the sequence specificity of inhibition inside cells appears to increase significantly.
Figure 4.
Dose–response characteristics for inhibition of cellular telomerase by (A) 13 base match oligomer V and analogous mismatch oligomer VI and (B) 20 base match oligomer XI and mismatch oligomer XII. Inhibitors were assayed at 1, 10, 50 and 100 nM, 4 days after transfection into cells.
2′-MOE RNA oligonucleotides as anti-telomerase agents
Of the telomerase inhibitors tested in our studies our data suggest that oligomers V and XI share several advantages that make them stand out as candidates for in vivo investigation. Oligomers V and XI are potent inhibitors, possessing IC50 values of 7 and 9 nM, respectively (Table 1), meeting the most obvious criterion for telomerase inhibition. Both oligomers continue to inhibit >50% of telomerase activity 7 days after transfection (Table 2), suggesting that they are reasonably stable inside cells, and both inhibit intracellular telomerase at concentrations as low as 10 nM (Fig. 4). Both oligomers also contain PS linkages, which have been noted to improve serum half-life and in vivo pharmacokinetics. Finally, both contain nucleic acid chemistries for which kilogram scale production of clinical grade oligomer has been optimized.
Oligomers V and XI also each possess unique advantages. An advantage possessed by oligomer XI is that it is the same length as antisense oligomers that have been the subject of extensive clinical trials, allowing pharmacokinetic properties to be more accurately predicted. In contrast, oligomer V is shorter than typical antisense oligomers and its pharmacokinetic properties are less predictable. However, the shorter length of V offers the advantage of much less expensive synthesis and possible improvements in cell uptake and oral bioavailability.
We find that 13 base oligomer V displays >100-fold match versus mismatch discrimination when assayed at 37°C (Table 1). Oligomer XI, in contrast, inhibits telomerase no better than does analogous mismatch-containing oligomer XII when inhibition is measured in cell lysates. The availability of a much less active control oligomer may be an advantage when using oligomer V for cell culture and preclinical animal studies because comparison of the effects of match and mismatch oligomers will help support the conclusion that the oligomers act by inhibiting telomerase. The low match versus mismatch discrimination for the fully substituted PS 20 base oligomer is likely due to interactions with the reverse transcriptase domain of telomerase (hTERT). Such interactions have already been postulated for hTERT and PS DNA oligomers (7,24–26). It is also possible that the 20 base oligomers may form additional base pairs with the telomerase template and that these may also contribute to reduced match versus mismatch discrimination.
While clearly a complicating factor, the lack of match versus mismatch discrimination for oligomer XI versus XII that we observed in short-term assays may not prove to be a severe disadvantage for drug development. Our primary reason for believing this derives from our observation that fully complementary 20 base oligomer XI is a much more effective inhibitor than its mismatch-containing analog XII after extended incubation inside cells and at lower concentrations (Table 2 and Figs 3 and 4). It is reasonable to speculate that the increase in specificity during long incubations occurs because the mismatch-containing oligomer binds less tightly and is degraded more rapidly than the match oligomer. Similar 20 base chimeric 2′-MOE DNA oligomers show low toxicity in vivo, so even if 20 base oligomer XI does bind to non-target proteins, this binding is not likely to lead to severe side effects. Indeed, initial animal studies with nude mice have not shown any toxicity at dosages as high as 50 mg/kg/day (L.White, unpublished results). Thus, while quantitative comparisons of the sequence specificity of inhibition of telomerase in cell extracts may seem to greatly favor 13 base oligomer V, it is not obvious whether such considerations will have in vivo relevance.
CONCLUSIONS
The telomerase inhibitors that we describe here are potent, selective and belong to a class of molecules possessing favorable pharmacokinetic properties and the potential for large-scale synthesis. These molecules are well suited for use in animals to determine the effect of telomerase inhibitors on telomere length and tumor growth and also to study the synergistic effects of combining telomerase inhibition with existing cancer therapies. The 13 base oligomer V and 20 base oligomer XI are both potent inhibitors. Oligomer V shows better match versus mismatch discrimination over the short term and may be more useful for controlled preclinical studies aimed at validating telomerase as a therapeutic target. Oligomer V is also relatively small, reducing the expense of large-scale production. Oligomer XI is similar to oligomers now in clinical trials, facilitating rapid transition to clinical testing. Since the two oligomers possess different strengths, further testing of both in animals is justified.
Acknowledgments
ACKNOWLEDGEMENTS
The authors wish to thank Henri Sasmor and the oligonucleotide group at ISIS Pharmaceuticals for their excellent assistance in oligonucleotide supply and Dr Laura White (UT Southwestern) for the DU 145 cells. We also thank Dr Edward Sausville and his colleagues at the Development Therapeutics Program of the National Cancer Institute for emphasizing the importance of identifying one or two optimal lead compounds prior to initiating preclinical trials in animals. This work was supported by a grant from the National Institutes of Health (CA 74908) to D.R.C.
References
- 1.Levin A.A. (1999) A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim. Biophys. Acta, 1489, 69–84. [DOI] [PubMed] [Google Scholar]
- 2.Eckstein F. (2000) Phosphorothioate oligonucleotides: what is their origin and what is unique about them. Antisense Nucleic Acid Drug Dev., 10, 117–121. [DOI] [PubMed] [Google Scholar]
- 3.Crooke S.T., Graham,M.J., Zuckermann,J.E., Brooks,D., Conklin,B.S., Cummins,L.L., Greig,M.J., Guinosso,C.J., Kornbrust,D., Manoharan,M., Sasmor,H.M., Schleich,T., Tivel,K.T. and Griffey,R.H. (1996) Pharmacokinetic properties of several oligonucleotide analogs in mice. J. Pharmacol. Exp. Ther., 277, 923–937. [PubMed] [Google Scholar]
- 4.Hogrefe R.I. (1999) An antisense oligonucleotide primer. Antisense Nucleic Acid Drug Dev., 9, 351–357. [DOI] [PubMed] [Google Scholar]
- 5.Barton C.M. and Lemoine,N.R. (1995) Antisense oligonucleotides directed against p53 have antiproliferative effects unrelated to effects on p53 expression. Br. J. Cancer, 71, 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee M., Simon,A.D., Stein,C.A. and Rabbani,L.E. (1999) Antisense strategies to inhibit restenosis. Antisense Nucleic Acid Drug Dev., 9, 487–492. [DOI] [PubMed] [Google Scholar]
- 7.Pitts A.E. and Corey,D.R. (1999) The telomerase challenge. Drug Discov. Today, 4, 155–161. [DOI] [PubMed] [Google Scholar]
- 8.Kim N.W., Piatyszek,M.A., Prowse,K.R., Harley,C.B., West,M.D., Ho,P.L.C., Coviello,G.M., Wright,W.E., Weinrich,S.L. and Shay,J.W. (1994) Specific association of human telomerase with immortal cells and cancer. Science, 266, 2011–2015. [DOI] [PubMed] [Google Scholar]
- 9.Counter C.M., Hirte,H.W., Bachetti,S. and Harley,C.B. (1994) Telomerase activity in human ovarian carcinoma. Proc. Natl Acad. Sci. USA, 91, 2900–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Autexier C. (1999) Telomerase as a possible target for anticancer therapy. Chem. Biol., 6, R299–R303. [DOI] [PubMed] [Google Scholar]
- 11.McKenzie K.E., Umbricht,C.B. and Sukumar,S. (1999) Applications of telomerase research in the fight against cancer. Mol. Med. Today, 5, 114–122. [DOI] [PubMed] [Google Scholar]
- 12.Harley C.B., Futcher,A.B. and Greider,C.W. (1990) Telomeres shorten during ageing of human fibroblasts. Nature, 345, 458–460. [DOI] [PubMed] [Google Scholar]
- 13.Hastie N.D., Dempster, M., Dunlop,M.G., Thompson,A.M., Green,D.K. and Allshire,R.C. (1990) Telomere reduction in human colorectal carcinoma and with ageing. Nature, 346, 866–868. [DOI] [PubMed] [Google Scholar]
- 14.Feng J., Funk,W.D., Wang,S.-S., Weinrich,S.L., Avilion,A.A., Chiu,C.-P., Adams,R.R., Chang,E., Allsopp,R.C., Yu,J., Le,S., West,M.D., Harley,C.B., Andrews,W.H., Greider,C.W. and Villeponteau,B. (1995) The RNA component of human telomerase. Science, 269, 1236–1241. [DOI] [PubMed] [Google Scholar]
- 15.Shammas M.A., Simmons,C.G., Corey,D.R. and Shmookler-Reis,R.J. (1999) Inhibition of telomerase reverses immortality of transformed cells. Oncogene, 18, 6191–6200. [DOI] [PubMed] [Google Scholar]
- 16.Herbert B.-S., Pitts,A.E., Baker,S.I., Hamilton,S.E., Wright,W.E., Shay,J.W. and Corey,D.R. (1999) Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc. Natl Acad. Sci. USA, 96, 14726–14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry S., Steker,K., Brooks,D., Monteith,D., Conklin,B. and Bennett,C.F. (2000) Chemically modified oligonucleotides exhibit decreased immune stimulation in mice. J. Pharmacol. Exp. Ther., 292, 468–479. [PubMed] [Google Scholar]
- 18.Khatsenko O., Morgan,R., Truong,L., York-Defalco,C., Sasmor,H., Conklin,B. and Geary,R.S. (2000) Absorption of antisense oligonucleotides in rat intestine: effect of chemistry and length. Antisense Nucleic Acid Drug Dev., 10, 35–44. [DOI] [PubMed] [Google Scholar]
- 19.Baker B.F., Lot,S.S., Condon,T.P., Cheng-Flournoy,S., Lesnik,E.A., Sasmor,H.M. and Bennett,C.F. (1997) 2′-O-(2-methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J. Biol. Chem., 272, 11994–12000. [DOI] [PubMed] [Google Scholar]
- 20.Stepowski S.M., Wang,M.E., Tian,L., Chen,W.H., Wancewicz,E.V., Johnston,J.F., Bennett,C.F. and Monia,B.P. (2000) Inhibition of C-raf expression by antisense oligonucleotides extends heart allograft survival in rats. Transplantation, 70, 656–661. [DOI] [PubMed] [Google Scholar]
- 21.Martin P. (1995) Ein neuer zugan zu 2′-O-alkylribonucleosiden und eigenschaften deren oligonucleotide. Helv. Chim. Acta, 78, 486–504. [Google Scholar]
- 22.Fasman G. (1975) Handbook of Biochemistry and Molecular Biology, Nucleic Acids, 3rd Edn. CRC Press, Boca Raton, FL. Vol. 1.
- 23.Holt S.E., Norton,J.C., Wright,W.E. and Shay,J.W. (1996) Enhanced detection of human telomerase activity. Methods Cell Sci., 18, 237–248. [DOI] [PubMed] [Google Scholar]
- 24.Norton J.C., Piatyszek,M.A., Wright,W.E., Shay,J.W. and Corey,D.R. (1996) Inhibition of human telomerase by peptide nucleic acids. Nat. Biotechnol., 14, 615–620. [DOI] [PubMed] [Google Scholar]
- 25.Sharma H.W., Hsiao,R. and Narayan,R. (1996) Telomerase as a potential molecular target to study G-quartet phosphorothioates. Antisense Nucleic Acid Drug Dev ., 6, 3–7. [DOI] [PubMed] [Google Scholar]
- 26.Matthes E. and Lehmann,C. (1999) Telomerase protein, rather than its RNA is the target of phosphorothioate oligonucleotides. Nucleic Acids Res., 27, 1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]