Abstract
Purpose of review
Isocyanates, reactive chemicals used to generate polyurethane, are a leading cause of occupational asthma worldwide. Workplace exposure is the best-recognized risk factor for disease development, but is challenging to monitor. Clinical diagnosis and differentiation of isocyanates as the cause of asthma can be difficult. The gold-standard test, specific inhalation challenge, is technically and economically demanding, and is thus only available in a few specialized centers in the world. With the increasing use of isocyanates, efficient laboratory tests for isocyanate asthma and exposure are urgently needed.
Recent findings
The review focuses on literature published in 2005 and 2006. Over 150 articles, identified by searching PubMed using keywords ‘diphenylmethane’, ‘toluene’ or ‘hexamethylene diisocyanate’, were screened for relevance to isocyanate asthma diagnostics. New advances in understanding isocyanate asthma pathogenesis are described, which help improve conventional radioallergosorbent and enzyme-linked immunosorbent assay approaches for measuring isocyanate-specific IgE and IgG. Newer immunoassays, based on cellular responses and discovery science readouts are also in development.
Summary
Contemporary laboratory tests that measure isocyanate-specific human IgE and IgG are of utility in diagnosing a subset of workers with isocyanate asthma, and may serve as a biomarker of exposure in a larger proportion of occupationally exposed workers.
Keywords: antibody, asthma, diagnosis, exposure, isocyanate
Introduction
Diisocyanates (toluene diisocyanate, TDI; hexamethylene diisocyanate, HDI; and diphenylmethane diisocyanate, MDI) or functionally similar polymeric isocyanates are the obligate cross-linking agent for the commercial production of polyurethane, a polymer upon which modern society has become dependent. Millions of tons of isocyanate are produced and consumed annually throughout the world in a wide variety of end-use work environments [1,2–5,6•,7•]. Workplace exposure remains the best-recognized risk factor for isocyanate asthma, but is complicated to quantitate, involving mixtures of isomers and ‘prepolymers’ diluted in solvents, in aerosol and vapor phases. In certain occupational settings, exposure can cause isocyanate asthma and long-lasting bronchial hyperreactivity [1,8,9,10•,11•]. Early recognition of isocyanate asthma and prompt removal from isocyanate exposure improves the long-term prognosis for sensitive individuals [9]. There thus exists the need for practical screening/diagnostic tests for isocyanate asthma as well as tests that can monitor personal exposure.
The clinical presentation of isocyanate asthma is strikingly similar to common environmental asthma, prompting the hypothesis that the disease has an immunological basis, although subtle differences have been noted [9,10•,12•]. Animal models support this hypothesis, and are beginning to dissect the potential role of individual genes with transgenic strains [13••,14••,15,16••,17,18]. Allergists and immunologists have overcome substantial challenges working with reactive isocyanates to develop serology assays for isocyanate-specific antibodies [19–21]. Such assays have provided evidence to support allergic asthma to isocyanate in a small percentage of workers, but cannot detect isocyanate-specific IgE in the majority of sensitive individuals. These results have left great uncertainty in the field. Does isocyanate asthma involve mechanisms of pathogenesis (e.g. non-IgE) distinct from those in common atopic asthma or are specific IgE antibodies present, but our detection assay for them is flawed? Are we using the wrong antigenic form of isocyanate in our serology tests, or testing workers at the wrong time (after removal from exposure)? Does isocyanate asthma, as presently defined, possibly represent a spectrum of diseases, which only in some cases is associated with an antibody response [3,9,10•]?
The present review summarizes the rationale and use of clinical laboratory tests for immune responses that reflect isocyanate exposure and asthma, with emphasis on data generated within the past year. The potential utility of ‘isocyanate-specific’ serum IgE and IgG as biomarkers and the isocyanate antigen recognized by these immunoglobulins are described [22••,23]. Clinical usage and limits of contemporary assays for isocyanate asthma and exposure are discussed along with promising future assays [20,24,25••].
Immunological tests as markers of isocyanate asthma
The natural history of isocyanate asthma generally resembles that of a type I hypersensitivity response, including an early lag time (during which systemic sensitization is thought to occur) and increasing sensitization to extremely low exposure doses, resulting in early, late and dual phase asthmatic responses. Allergen-specific IgE is a key aspect of diseases that involves type I hypersensitivity (e.g. asthma) and also serves as a biomarker of allergic sensitization to many common asthmogens (e.g. cat dander, dust mite allergens), and a target of immunotherapy [26]. Therefore, by analogy, isocyanate-specific IgE may indicate the potential for IgE-mediated inflammatory responses in the airways upon respiratory tract isocyanate exposure. When present, isocyanate-specific serum IgE is strongly predictive of isocyanate asthma [21,27]. An improvement of traditional isocyanate serology as a diagnostic aid can probably be achieved with specific attention to exposure (see below) and the isocyanate antigen (see ‘Isocyanate antigen’ section below).
Isocyanate exposure and half-life of IgE
The short half-life of unbound serum IgE (approximately 2 days) is of unique importance to occupational illnesses such as isocyanate asthma, in which exposure to the causative agent occurs exclusively in the workplace [26,28,29]. Brief periods (weeks) away from the workplace (i.e. exposure) may result in a decrease in serum IgE levels, to levels undetectable by conventional radioallergosorbent test (RAST) [27]. In some cases, the socioeconomics of isocyanate asthma may impede an individual from seeking medical attention until the condition becomes severe enough to prevent them from working [8]. In addition, isocyanate usage in the workplace may be sporadic, adding further time variability between exposure and serology [30••]. This situation differs considerably from common environmental aeroallergens, in which exposure is often more ubiquitous (i.e. dust mite). A clearer definition of isocyanate-specific IgE responses, in relation to exposure, will probably require prospective studies, because this information is usually unavailable in retrospect, and cross-sectional studies may be limited by ‘healthy worker’ effects [4,22••,30••,31–34]. Without accurate exposure information, negative isocyanate-specific IgE assays may lead to misdiagnosis and false conclusions about pathogenic mechanisms.
Immunological tests as markers of isocyanate exposure
The best-recognized risk factor for isocyanate asthma described to date is exposure, but airborne exposure is practically and technically difficult to quantify [4,6•,7•,8, 30••,32,35••]. Furthermore, the internal exposure dose of isocyanate may differ substantially from that measured using external monitors, especially in workers using personal protective equipment [36,37]. Respiratory tract exposure has been the focus of most industrial hygiene efforts to date, although skin exposure also occurs and may be immunologically important (see ‘Immunological sensitization through skin exposure’ section).
Immunological responses to isocyanates may serve as surrogate biological indicators of isocyanate exposure. Immunoglobulins of the IgG subclass have a half-life of approximately 30 days, and have been suggested to represent a potentially useful, integrated, biomarker of long-term exposure [38•,39]. IgG that recognizes isocyanate-albumin conjugates is almost never observed in unexposed individuals [20,22••,39,40,41•]. Among exposed workers, the serum titer of isocyanate-specific IgG has been documented to correlate closely with occupational exposure levels [39]. Elevated levels of IgG among individuals with isocyanate asthma, reported in some studies, may reflect increased exposure of these individuals, which may partly explain their disease development [21,22••,39].
In workers who develop isocyanate-specific IgG responses, these levels might provide a useful indicator of industrial hygiene and personal protection from exposure. Such individuals may serve as sentinels for a given work environment, and act as biologically responsive indicators of engineering efforts to reduce exposure.
The biological role of isocyanate-specific IgG in response to exposure remains unclear. A pathogenic role seems unlikely, given its occurrence in asymptomatic individuals, and some have proposed a protective role. Potential differences in isocyanate-specific IgG between individuals with isocyanate asthma and asymptomatic individuals (i.e. epitope specificity, affinity, IgG subclass) have yet to be studied.
Immunological sensitization through skin exposure
Accumulating evidence suggests that skin may have become a more dominant route of occupational isocyanate exposure in recent years, with the common use of less volatile isocyanate formulations (see ‘Isocyanate isomers and prepolymers’ section), and better respiratory hygiene. Isocyanates can cause allergic contact dermatitis in rare cases, and commercial patch tests for dermatological sensitivity have recently been re-evaluated [42,43]. More importantly, however, animal studies have suggested that isocyanates penetrate skin tissue and induce systemic sensitization, which under appropriate conditions can elicit ‘asthma’ (e.g. airway inflammation and physiological responses) upon subsequent respiratory tract exposure [13••,14••,44,45,46•,47•,48]. The dosage and frequency of skin exposure appears to exert substantial effects upon the quality of the elicited immune response [13••,14••]. Heightened awareness of skin exposure in the workplace appears warranted on the basis of these data.
The contribution of skin exposure to the systemic isocyanate-specific humoral immune response in humans remains unclear. Studies in workplaces with extremely low levels of airborne isocyanates have suggested that skin exposure alone may induce systemic sensitization [49]. The potential differences between immune responses generated via skin compared with airway exposure are, however, challenging to dissect in clinical studies, because exposure on-the-job rarely occurs exclusively at one site [4,30••,50•]. Future clinical–epidemiological investigations are needed to clarify the potential contribution of skin exposure to human systemic immune sensitization, including isocyanate-specific IgE and IgG [50•,51•].
Isocyanate antigen
The basis of allergen serology is the specific three-dimensional structure of the allergen, which remains incompletely defined for isocyanates, and has been a major obstacle to research and clinical studies. Isocyanates change their structure upon reacting with self-molecules (and create neo-epitopes described below), and are thus more challenging to standardize than common aeroallergens that can be molecularly defined by DNA sequence (dust, cat allergens). A theoretical understanding of, and technical methods for, generating biologically relevant isocyanate antigens are crucial to isocyanate immunoassays.
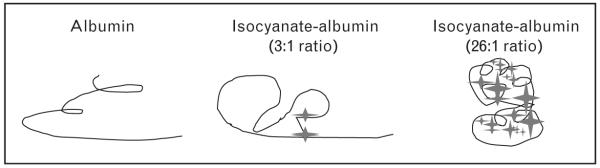
Early investigators assumed that isocyanates act as haptens, which is true, but only partly accounts for their immunogenicity. Perhaps equally important, isocyanates create new (neo) epitopes, through intra and intermolecular cross-links that change the charge and conformation of (self) carrier proteins [52,53]. This fundamental concept, which has evolved slowly over the past 40 years, has an important impact on the development of ‘isocyanate antigens’ used for immunological assays. For haptens, increased conjugation to the carrier protein increases epitope density and generally improves sensitivity for detecting specific immunoglobulins. If the critical epitope is a unique conformation induced by a specific isocyanate cross-linkage, ‘over-reactivity’, or use of the wrong carrier protein may lead to biologically irrelevant isocyanate-protein conjugates (antigens), as depicted in Fig. 1 [22••,54,55]. Isocyanate ‘antigens’ used for clinical studies should reflect those in vivo, in the airway microenvironment where epithelial fluid is exposed to low (occupational) levels of isocyanate [33].
Figure 1. Neo-epitopes induced by isocyanate conjugation to human albumin.
A two-dimensional model in which albumin is shown by a line and isocyanate is shown by a star, depicts new conformations formed by isocyanate cross-linking, which may be recognized as foreign by the host immune system.
Isocyanate-albumin conjugate(s)
To date, the only protein shown to act as an effective isocyanate ‘carrier’ (for inducing antibodies), in occupationally exposed individuals, is albumin [22••,39,54,55]. Isocyanate conjugated to proteins other than albumin have not been extensively evaluated, but the few that have (see below) are not recognized by human antibodies.
Reaction products between isocyanate and albumin (and their antigenicity) are highly dependent upon reaction conditions. Exposure of fluid phase albumin (i.e. in the epithelial lining fluid) to occupational (approximately 20 ppb) levels of isocyanate vapors results in limited, highly specific chemical conjugates [23,39]. Recent studies have suggested that serum IgE, which recognizes such isocyanate vapor-exposed albumin can be found in 40–50% of TDI and HDI asthma sufferers, but only 1–4% of exposed asymptomatic individuals [23,39]. In addition, vapor isocyanate-albumin-specific IgG strongly correlates with occupational exposure levels in asymptomatic individuals. In contrast, isocyanate-albumin conjugates made by reacting fluid phase albumin with more concentrated liquid phase isocyanate (the method used in most studies before 2004) are less sensitive in detecting IgE and are more likely to be recognized by IgG from unexposed individuals [22••,39].
Two isocyanate conjugation sites to human albumin have recently been identified for HDI vapor (Fig. 2) [39]. One site, Lys414, is part of a di-lysine motif unique to human albumin, which is noteworthy given that isocyanates conjugated with albumin from cow, mouse or chicken (ovalbumin) are not recognized by human antibodies [39,54,55]. The other potential HDI conjugation site is His247, which is exposed on the surface of albumin, and under physiological conditions possesses a secondary amine with a favorable pKa for nucleophilic addition with isocyanate. It appears likely that TDI may also conjugate with His247 (personal observations) [22••].
Figure 2. Structural three-dimensional model of the hexamethylene diisocyanate conjugation sites of human albumin.
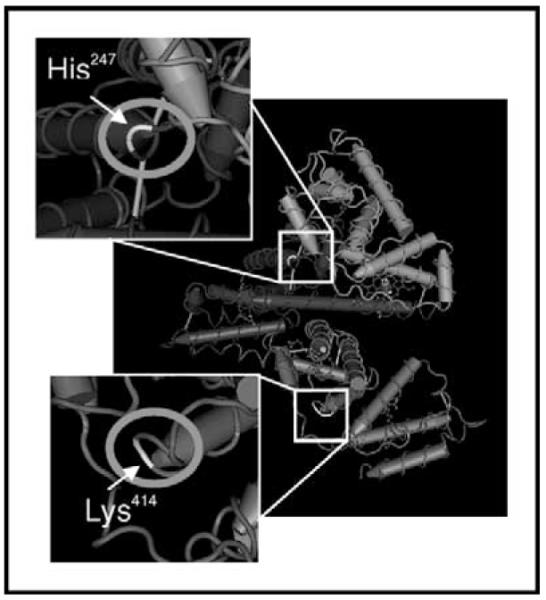
Major hexamethylene diisocyanate conjugation sites after vapor exposure, His-247 and Lys-414 are enlarged and ringed.
Therefore, a major isocyanate antigen in exposed humans is isocyanate conjugated with albumin. Under physiological conditions of occupational exposure, isocyanate vapors react with human albumin in a highly specific manner, which in turn produces a highly specific antigen. Albumin conjugates, formed with isocyanate under reaction conditions that deviate substantially from those in vivo (pH, concentration, etc.) may be immunologically different, and could lead to artifacts in serology studies.
Isocyanate ‘prepolymers’ and isomers
As mentioned in the introduction, occupational isocyanate exposure is complex, involving mixtures of different isocyanate isomers and ‘prepolymers’ diluted in solvents, in aerosol and vapor phases. For example, industrial blends of TDI isomers (e.g. 2,4 versus 2,6-TDI) may be used in 80 : 20 or 65 : 35 ratios, whereas crude MDI contains a mixture of multiple isomers as well as polymers (see Table 1) [40,56]. For HDI, biuret and isocyanurate ‘prepolymers’ are predominately used in autobody finishes, but contain trace amounts of more volatile HDI ‘monomer’.
Table 1.
Isocyanate isomers and ‘pre-polymers’
| Isomer | Ratio |
|---|---|
| Toluene diisocyanate | 80 : 20 |
| Toluene diisocyanate (2,4 : 2,6) | 65 : 35 |
| Diphenylmethane diisocyanate | Mixture |
| Diphenylmethane diisocyanate (4,4′, 2,4′, 2,2′) | |
| Monomer | Polymer |
| Hexamethylene diisocyanate | Biuret isocyanurate |
Recent studies have documented differences in the antigenicity of albumin conjugates prepared with different isomers or polymers of the same isocyanate [22••,40]. Immunoassays for isocyanate-specific antibodies should thus ideally use antigens prepared with the same isocyanate polymer/isomer used ‘on-the-job’. Given the high cross-reactivity of different isocyanates, it is possible that a limited number of isomers or polymeric formulations may be universally applicable, but will require empirical testing to define.
Using current isocyanate laboratory tests in clinical practice
Contemporary isocyanate laboratory tests have improved considerably since their initial description, and are probably of greater utility than past publications may suggest. For the clinician, the presence of isocyanate-specificIgEor IgG in a patient’s serum is a helpful piece of evidence in differentiating isocyanates as a potential cause of asthma, although this information alone is not sensitive enough to serve as an exclusive diagnostic. The major utility of isocyanate laboratory tests are: (1) to confirm the presence of an isocyanate-specific type I hypersensitivity response in individuals with suspected disease; or (2) to document exposure on the basis of isocyanate-specific IgG titers.
Isocyanate-specific serum IgE can be found in a substantial fraction of isocyanate asthma sufferers, up to 50% as suggested by recent studies, depending upon the isocyanate-albumin conjugates used as antigens [22••,27,39]. The most sensitive assay currently performed is the ‘conventional’ (paper disk-125I) isocyanate-RAST, although newer non-isotopic enzyme-linked immunosorbent assay approaches perform similarly [22••,27,39]. A limited number of commercial vendors offer isocyanate-specific IgE assays (or measure isocyanate-specific IgE); however; the antigenic basis for such tests is unclear [57]. The utility of isocyanate-specific IgE testing is primarily limited to actively employed individuals, because, as mentioned above, isocyanate-specific IgE levels can decreasebelow detection limits after brief periods away from exposure.
‘Isocyanate’-specific IgE should always be measured in parallel with total IgE levels and a ‘control’ sample (i.e. ‘mock’ exposed albumin), because serum samples with high IgE show elevated non-specific binding in vitro [19]. Inhibition tests are helpful to confirm specificity [19]. The presence of isocyanate-specific IgE in workers’ serum is a strong indicator of isocyanate asthma, and is rarely (< 5%) observed in non-sensitized workers, especially after controlling for total IgE and non-specific binding [22••,27,39].
Serum levels of isocyanate-specific IgG are helpful to confirm that an individual has experienced previous exposure to isocyanate. Serial measurements over time may help monitor subsequent exposures and probably represent an integrated marker of the past several months, rather than hours to days.
Like all immunoassays, those for isocyanate should be performed with well-characterized ‘antigens’, and should be interpreted only in the context of clinically defined positive (isocyanate asthmatic individuals) and negative control samples (exposed and unexposed). In performing and assessing the significance of isocyanate serology tests, advice from experienced investigators can be extremely helpful, because important practical details of isocyanate serology, developed over decades of research, often through trial and error, commonly remain unpublished, or are under-recognized.
Future assays for isocyanate sensitivity
Future assays for isocyanate sensitivity will probably fall into two different categories, those aimed at improving current serology approaches and those not focused on antibody responses to exposure, but rather based on novel immune ‘readouts’ (see below).
Isocyanate-specific IgE detection can probably be improved by incorporating newer vapor and ‘prepolymer’ isocyanate-albumin conjugates into existing methodologies. The most commonly used matrix for clinical IgE measurements is the capsulated polymer, Immuno-CAP [58]. Until recently, coupling allergens to Immuno-CAP was performed exclusively by the manufacturer, which offers assays for TDI, MDI and HDI, but provides little information on these ‘antigens’ [57,59]. The recent availability of Streptavidin-ImmunoCAP (to which any biotin labeled allergen may be coupled) should allow individual researchers to explore the potential utility of the ImmunoCAP matrix with antigenically distinct isocyanate antigens [59].
Allergen microarrays are a relatively new approach under development, and are especially effective for simultaneously analysing IgE binding to thousands of different proteins or epitopes. Using this approach, protein allergens are spotted onto glass slides and serum IgE binding is detected with a fluorescent labeled anti-IgE [60•]. For limited allergens tested to date, serum IgE binding patterns differ with clinical status. The technique has a sensitivity comparable to CAP methods, and can be performed with very little serum. ‘Allergen’ microarray approaches have yet to be applied to isocyanate-exposed workers, but are ideally suited to analysing a wide range of different isocyanate-albumin conjugates and potential epitopes.
Cell-based immunoassays with novel immunological readouts may also be useful in the diagnosis of isocyanate asthma, especially if the disease involves predominately IgE/IgG-independent mechanisms as many have suggested [23,61•]. Bernstein and colleagues [20] have recently reported an in-vitro assay of isocyanate antigen-induced monocyte/macrophage chemoattractant protein (MCP) 1 production by peripheral blood mononuclear cells (PBMCs), which in preliminary studies differentiates isocyanate asthmatic individuals. MCP-1 is the major histamine-releasing factor (other than IgE) produced by human PBMCs, and is probably one of several mediators differentially induced by isocyanate in sensitive individuals [62].
Future cell-based assays using discovery science approaches such as expression profiling, proteomics and metabolomics may identify patterns of immunological responses that define isocyanate sensitivity and differentiate susceptible from tolerized or unsensitized workers. An important determinant of all such future studies will probably be the form of isocyanate (antigen) used as the immunological stimulus.
Conclusion
Insights into the immune responses and pathology induced by isocyanate exposure continue to emerge through sustained research efforts. Characterization of isocyanate antigenicity has reached unprecedented levels by combining technologies such as matrix-assisted laser desorption/ionization mass spectrometry and in-vitro exposure systems designed to mimic the biochemistry and biophysics of the airway microenvironment. These advances are beginning to optimize immunological tests for isocyanate-specific immune responses.
A major advance has been theoretical and technical changes in our perception of isocyanate antigens, and how these reaction products form, which has evolved with empirical guidance. Novel methods for generating isocyanate-albumin conjugates via exposure to vapor (rather than liquid) isocyanate produces ‘antigens’ of potentially greater biological relevance than classic methods, which are prone to generating ‘over’-reacted and potentially artifactual ‘antigens’. The isomer and polymer composition of isocyanates also contributes substantially to antibody recognition. Further optimization of isocyanate antigens may increase the sensitivity/specificity of isocyanate-specific IgE tests to the point at which it becomes a viable method of isocyanate-induced disease control. Even if isocyanate IgE testing only identifies a fraction of isocyanate asthmatic individuals, widespread screening may still be cost-effective, given the long-lasting decrements in lung function that sensitized workers may face if their disease goes unrecognized.
Despite recent advances in understanding isocyanate immunogenicity, the appropriateness of IgE testing for isocyanate sensitization continues to be questioned, based partly on past failures/low sensitivity and partly on alternative (IgE-independent) hypotheses of pathogenesis. Newer immunoassays, based on the hypothesis that isocyanate-induced cellular (versus humoral) responses are more important in isocyanate asthma pathogenesis, are beginning to be developed. Using PBMCs, such assays may focus on specific readouts (MCP-1 protein), or patterns of gene/protein/metabolite expression, which may prove to correlate with or define clinical status.
In summary, serology studies on isocyanates have evolved over recent years and improvements make measurements of isocyanate-specific IgE and IgG useful information in the clinical workup of occupationally exposed individuals. Although possibly restricted to a subset of workers, isocyanate-specific IgE, when present, is strongly predictive of isocyanate asthma, and specific IgG may provide sentinel host exposure information, which may help guide industrial hygiene efforts. Newer tests for isocyanate sensitivity, based on cellular responses, may provide greater diagnostic sensitivity in the future.
Acknowledgments
Sponsorship: This work was partly supported by the National Institutes of Health (R01 HL-62622) and the American Lung Association.
Abbreviations
- HDI
hexamethylene diisocyanate
- MCP
monocyte/macrophage chemoattractant protein
- MDI
diphenylmethane diisocyanate
- PBMC
peripheral blood mononuclear cell
- RAST
radioallergosorbent test
- TDI
toluene diisocyanate
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Bonauto DK, Sumner AD, Curwick CC, et al. Work-related asthma in the spray-on truck bed lining industry. J Occup Environ Med. 2005;47:514–517. doi: 10.1097/01.jom.0000161735.29805.45. [DOI] [PubMed] [Google Scholar]
- 2.Taramian A, Doosthoseini K, Mirshokraii SA, Faezipour M. Particleboard manufacturing: an innovative way to recycle paper sludge. Waste Manag. 2007 doi: 10.1016/j.wasman.2006.09.009. In press. [DOI] [PubMed] [Google Scholar]
- 3.Berode M, Jost M, Ruegger M, Savolainen H. Host factors in occupational diisocyanate asthma: a Swiss longitudinal study. Int Arch Occup Environ Health. 2005;78:158–163. doi: 10.1007/s00420-004-0568-4. [DOI] [PubMed] [Google Scholar]
- 4.Robert A, Ducos P, Francin JM, Marsan P. Biological monitoring of workers exposed to 4,4′-methylenediphenyl diisocyanate (MDI) in 19 French polyurethane industries. Int Arch Occup Environ Health. 2007 doi: 10.1007/s00420-006-0150-3. In press. [DOI] [PubMed] [Google Scholar]
- 5.Sennbro CJ, Littorin M, Tinnerberg H, Jonsson BA. Upper reference limits for biomarkers of exposure to aromatic diisocyanates. Int Arch Occup Environ Health. 2005;78:541–546. doi: 10.1007/s00420-005-0619-5. [DOI] [PubMed] [Google Scholar]
- 6•.Sennbro CJ, Lindh CH, Mattsson C, et al. Biological monitoring of exposure to 1,5-naphthalene diisocyanate and 4,4′-methylenediphenyl diisocyanate. Int Arch Occup Environ Health. 2006;79:647–653. doi: 10.1007/s00420-006-0096-5. Biomarkers of isocyanate exposure are detectable by gas chromatography mass spectrometry methods after derivitization of hyrolyzed urine and plasma, documenting internal exposure on the job. There was a wide range of individual variability, which questions the utility of the biomarker as a surrogate for exposure when assessing a large population of workers.
- 7•.Boutin M, Dufresne A, Ostiguy C, Lesage J. Determination of airborne isocyanates generated during the thermal degradation of car paint in body repair shops. Ann Occup Hyg. 2006;50:385–393. doi: 10.1093/annhyg/mei075. A poorly recognized exposure risk occurs when isocyanates are released by thermal degredation that occurs with cutting, grinding and orbital sanding operations in autobody shops.
- 8.Tarlo SM, Liss GM. Prevention of occupational asthma – practical implications for occupational physicians. Occup Med (Lond) 2005;55:588–594. doi: 10.1093/occmed/kqi182. [DOI] [PubMed] [Google Scholar]
- 9.Pisati G, Baruffini A, Bernabeo F, et al. Rechallenging subjects with occupational asthma due to toluene diisocyanate (TDI), after long-term removal from exposure. Int Arch Occup Environ Health. 2007 doi: 10.1007/s00420-006-0134-3. In press. [DOI] [PubMed] [Google Scholar]
- 10•.Jones MG, Floyd A, Nouri-Aria KT, et al. Is occupational asthma to diisocyanates a non-IgE-mediated disease? J Allergy Clin Immunol. 2006;117:663–669. doi: 10.1016/j.jaci.2005.09.053. The data extend the lack of isocyanate-induced IgE to the airway submucosa via bronchoscopy and in-situ hybridization. The subjects were recruited from a specialist clinic after referal by their general practitioner or occupational health physician. Before the study, the majority of subjects had previously been away from exposure for more than 30 days, which may have effected their results.
- 11•.Rudbeck MG, Omland O. Work-related asthma caused by IgE-verified allergy to methylene di-isocynate. Ugeskr Laeger. 2006;168:1345–1346. A case report from a foreign journal documenting MDI asthma with MDI-specific IgE.
- 12•.Kim SH, Oh HB, Lee KW, et al. HLA DRB1 15-DPB1 05 haplotype: a susceptible gene marker for isocyanate-induced occupational asthma? Allergy. 2006;61:891–894. doi: 10.1111/j.1398-9995.2006.01023.x. The association of human leukocyte antigen haplotypes with isocyanate asthma support the role of the dendritic/T cell responses in isocyanate asthma. The effect is weak, however, individual human leukocyte antigen alleles were not significantly associated, and differed from those previously associated with isocyanate asthma in earlier studies by other authors.
- 13••.Tarkowski M, Vanoirbeek JA, Vanhooren HM, et al. Immunological determinants of ventilatory changes induced in mice by dermal sensitization and respiratory challenge with toluene diisocyanate. Am J Physiol Lung Cell Mol Physiol. 2007;292:L207–L214. doi: 10.1152/ajplung.00157.2005. An extension of previous work by the same authors, further exploring the mechanisms of dermal exposure leading to systemic sensitization and asthma upon subsequent airway exposure. The study highlights the potential for skin to serve as an effective route of sensitization and exposure-induced pathology that involves a mixture of T helper type 1 and 2 responses.
- 14••.Ban M, Morel G, Langonne I, et al. TDI can induce respiratory allergy with Th2-dominated response in mice. Toxicology. 2006;218:39–47. doi: 10.1016/j.tox.2005.09.013. This study highlights the possible contribution of skin exposure to asthma development, and suggests that the skin may be the most effective exposure route for inducing allergic (T helper type 2) responses to isocyanate.
- 15.Furusho S, Myou S, Fujimura M, et al. Role of intercellular adhesion molecule-1 in a murine model of toluene diisocyanate-induced asthma. Clin Exp Allergy. 2006;36:1294–1302. doi: 10.1111/j.1365-2222.2006.02568.x. [DOI] [PubMed] [Google Scholar]
- 16••.Lee KS, Park SJ, Kim SR, et al. Modulation of airway remodeling and airway inflammation by peroxisome proliferator-activated receptor gamma in a murine model of toluene diisocyanate-induced asthma. J Immunol. 2006;177:5248–5257. doi: 10.4049/jimmunol.177.8.5248. The peroxisome proliferator-activated receptor gamma agonist, rosiglitazone, or pioglitazone reduce pathology in a mouse model of TDI asthma. Could these drugs help ameliorate isocyanate asthma in humans?
- 17.Matheson JM, Johnson VJ, Luster MI. Immune mediators in a murine model for occupational asthma: studies with toluene diisocyanate. Toxicol Sci. 2005;84:99–109. doi: 10.1093/toxsci/kfi051. [DOI] [PubMed] [Google Scholar]
- 18.Matheson JM, Johnson VJ, Vallyathan V, Luster MI. Exposure and immunological determinants in a murine model for toluene diisocyanate (TDI) asthma. Toxicol Sci. 2005;84:88–98. doi: 10.1093/toxsci/kfi050. [DOI] [PubMed] [Google Scholar]
- 19.Karol MH, Kramarik JA, Ferguson J. Methods to assess RAST results in patients exposed to chemical allergens. Allergy. 1995;50:48–54. doi: 10.1111/j.1398-9995.1995.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein DI, Cartier A, Côté J, et al. Diisocyanate antigen-stimulated monocyte chemoattractant protein-1 synthesis has greater test efficiency than specific antibodies for identification of diisocyanate asthma. Am J Respir Crit Care Med. 2002;166:445–450. doi: 10.1164/rccm.2109018. [DOI] [PubMed] [Google Scholar]
- 21.Cartier A, Grammer L, Malo JL, et al. Specific serum antibodies against isocyanates: association with occupational asthma. J Allergy Clin Immunol. 1989;84:507–514. doi: 10.1016/0091-6749(89)90364-3. [DOI] [PubMed] [Google Scholar]
- 22••.Ye YM, Kim CW, Kim HR, et al. Biophysical determinants of toluene diisocyanate antigenicity associated with exposure and asthma. J Allergy Clin Immunol. 2006;118:885–891. doi: 10.1016/j.jaci.2006.06.026. Promising improvements of TDI–RAST for the diagnosis of TDI asthma achieved through a multidisciplinary approach. Matrix-assisted laser desorption ionization mass spectrometry defines differences between vapor versus liquid TDI-albumin conjugates, which correlate with antigenicity. Vapor TDI-albumin-specific IgE was found in 29 out of 66 TDI asthmatic sufferers (44%), but only seven out of 167 of exposed control (4%) and none out of 64 atopic (non-isocyanate) asthmatic individuals.
- 23.Wisnewski AV, Liu Q, Liu J, Redlich CA. Glutathione protects human airway proteins and epithelial cells from isocyanates. Clin Exp Allergy. 2005;35:352–357. doi: 10.1111/j.1365-2222.2005.02185.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee CT, Ylostalo J, Friedman M, Hoyle GW. Gene expression profiling in mouse lung following polymeric hexamethylene diisocyanate exposure. Toxicol Appl Pharmacol. 2005;205:53–64. doi: 10.1016/j.taap.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 25••.Izuhara K, Saito H. Microarray-based identification of novel biomarkers in asthma. Allergol Int. 2006;55:361–367. doi: 10.2332/allergolint.55.361. An excellent review of applying gene chip technology to identify asthma biomarkers.
- 26.Infuhr D, Crameri R, Lamers R, Achatz G. Molecular and cellular targets of anti-IgE antibodies. Allergy. 2005;60:977–985. doi: 10.1111/j.1398-9995.2005.00832.x. [DOI] [PubMed] [Google Scholar]
- 27.Tee RD, Cullinan P, Welch J, et al. Specific IgE to isocyanates: a useful diagnostic role in occupational asthma. J Allergy Clin Immunol. 1998;101:709–715. doi: 10.1016/S0091-6749(98)70181-2. [DOI] [PubMed] [Google Scholar]
- 28.Hellman L. Regulation of IgE homeostasis, and the identification of potential targets for therapeutic intervention. Biomed Pharmacother. 2007 doi: 10.1016/j.biopha.2006.10.001. In press. [DOI] [PubMed] [Google Scholar]
- 29.Patterson R, Oh SH, Roberts M, Hsu CC. Massive polyclonal hyperimmunoglobulinemia E, eosinophilia and increased IgE-bearing lymphocytes. Am J Med. 1975;58:553–558. doi: 10.1016/0002-9343(75)90130-8. [DOI] [PubMed] [Google Scholar]
- 30••.Pronk A, Yu F, Vlaanderen J, et al. Dermal, inhalation, and internal exposure to 1,6-HDI and its oligomers in car body repair shop workers and industrial spray painters. Occup Environ Med. 2006;63:624–631. doi: 10.1136/oem.2005.023226. Liquid chromatography–mass spectrometry/mass spectrometry analysis of hydrolyzed, derivatized urine prove that internal isocyanate exposure occurs in painting industry, in bystanders as well as spray painters themselves. The analytical equipment required for analysis is substantial, which may limit the practical application of exposure monitoring.
- 31.Redlich CA, Stowe MH, Coren BA, et al. Diisocyanate-exposed auto body shop workers: a one-year follow-up. Am J Ind Med. 2002;42:511–518. doi: 10.1002/ajim.10143. [DOI] [PubMed] [Google Scholar]
- 32.Sakai T, Morita Y, Roh J, et al. Improvement in the GC-MS method for determining urinary toluene-diamine and its application to the biological monitoring of workers exposed to toluene-diisocyanate. Int Arch Occup Environ Health. 2005;78:459–466. doi: 10.1007/s00420-004-0571-9. [DOI] [PubMed] [Google Scholar]
- 33.Gagne S, Lesage J, Ostiguy C, et al. Quantitative determination of hexamethylene diisocyanate (HDI), 2,4-toluene diisocyanate (2,4-TDI) and 2,6-toluene diisocyanate (2,6-TDI) monomers at ppt levels in air by alkaline adduct coordination ionspray tandem mass spectrometry. J Environ Monit. 2005;7:145–150. doi: 10.1039/b412078g. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa M, Oyama T, Isse T, et al. Hemoglobin adducts as a marker of exposure to chemical substances, especially PRTR class I designated chemical substances. J Occup Health. 2006;48:314–328. doi: 10.1539/joh.48.314. [DOI] [PubMed] [Google Scholar]
- 35•.Creely KS, Hughson GW, Cocker J, Jones K. Assessing isocyanate exposures in polyurethane industry sectors using biological and air monitoring methods. Ann Occup Hyg. 2006;50:609–621. doi: 10.1093/annhyg/mel024. Despite low airborne isocyanate concentrations, it is possible to demonstrate biological uptake based on urine metabolites, with the highest inhalation exposures during truck spray painting (0.066 mg/m3) and sprayed polyurethane foam insulation (0.023 mg/m3). Additional high-exposure activities, mixing/casting polyurethane products, semi-automatic moulding, and resin applications have been suggested to involve skin exposure.
- 36.Hogberg J, Larsson K, Albin M, et al. Comments on ‘Respiratory effects of toluene diisocyanate in the workplace: a discussion of exposure–response relationships’. Crit Rev Toxicol. 2005;35:459–460. doi: 10.1080/10408440590944503. author reply 461–452. [DOI] [PubMed] [Google Scholar]
- 37.Isocyanate exposure in an autobody repair and collision center. J Occup Environ Hyg. 2006;3:D24–D27. doi: 10.1080/15459620500506935. [DOI] [PubMed] [Google Scholar]
- 38•.Church JA, Leibl H, Stein MR, et al. Efficacy, safety and tolerability of a new 10% liquid intravenous immune globulin [IGIV 10%] in patients with primary immunodeficiency. J Clin Immunol. 2006;26:388–395. doi: 10.1007/s10875-006-9025-3. Typical serum IgG half-life approximately 35 days.
- 39.Wisnewski AV, Stowe MH, Cartier A, et al. Isocyanate vapor-induced anti-genicity of human albumin. J Allergy Clin Immunol. 2004;113:1178–1184. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Aul DJ, Bhaumik A, Kennedy AL, et al. Specific IgG response to monomeric and polymeric diphenylmethane diisocyanate conjugates in subjects with respiratory reactions to isocyanates. J Allergy Clin Immunol. 1999;103:749–755. doi: 10.1016/s0091-6749(99)70415-x. [DOI] [PubMed] [Google Scholar]
- 41•.Bernstein DI, Ott MG, Woolhiser M, et al. Evaluation of antibody binding to diisocyanate protein conjugates in a general population. Ann Allergy Asthma Immunol. 2006;97:357–364. doi: 10.1016/S1081-1206(10)60801-0. The data suggest that isocyanate-specificIgG maybeabiomarkerofexposure, and is not found in the general population when the testing (enzyme-linked immunosorbent assay) is based on isocyanate-albumin conjugates with low substitution ratios (3 : 1). IgG enzyme-linked immunosorbent assay test results with serum dilutions less than 1 : 10, or isocyanate-albumin conjugates with high substitution ratios (> 19 : 1), should be interpreted cautiously. Non-occupational sources of exposure (e.g. hobbies, home improvement) should also be considered.
- 42.Frick-Engfeldt M, Zimerson E, Karlsson D, et al. Chemical analysis of 2,4-toluene diisocyanate,16-hexamethylene diisocyanate and isophorone diisocyanate in petrolatum patch-test preparations. Dermatitis. 2005;16:130–135. [PubMed] [Google Scholar]
- 43.Hannu T, Estlander T, Jolanki R. Allergic contact dermatitis due to MDI and MDA from accidental occupational exposure. Contact Dermatitis. 2005;52:108–109. doi: 10.1111/j.0105-1873.2005.00498b.x. [DOI] [PubMed] [Google Scholar]
- 44.Pauluhn J. Brown Norway rat asthma model of diphenylmethane 4,4′-diisocyanate. Inhal Toxicol. 2005;17:729–739. doi: 10.1080/08958370500224631. [DOI] [PubMed] [Google Scholar]
- 45.Pauluhn J, Woolhiser MR, Bloemen L. Repeated inhalation challenge with diphenylmethane-4,4′-diisocyanate in brown Norway rats leads to a time-related increase of neutrophils in bronchoalveolar lavage after topical induction. Inhal Toxicol. 2005;17:67–78. doi: 10.1080/08958370590898434. [DOI] [PubMed] [Google Scholar]
- 46•.Bello D, Smith TJ, Woskie SR, et al. An FTIR investigation of isocyanate skin absorption using in vitro guinea pig skin. J Environ Monit. 2006;8:523–529. doi: 10.1039/b517948c. Novel application of Fourier transform infrared spectrometry to isocyanate research that warrants further exploration of detection limits and practicality.
- 47•.Selgrade M, Boykin EH, Haykal-Coates N, et al. Inconsistencies between cytokine profiles, antibody responses, and respiratory hyperresponsiveness following dermal exposure to isocyanates. Toxicol Sci. 2006;94:108–117. doi: 10.1093/toxsci/kfl094. Skin exposure to isocyanates increases serum IgE and cytokine production, highlighting the potential role of the skin in the development of asthma.
- 48.Nabe T, Yamauchi K, Shinjo Y, et al. Delayed-type asthmatic response induced by repeated intratracheal exposure to toluene-2,4-diisocyanate in guinea pigs. Int Arch Allergy Immunol. 2005;137:115–124. doi: 10.1159/000085466. [DOI] [PubMed] [Google Scholar]
- 49.Petsonk EL, Wang ML, Lewis DM, et al. Asthma-like symptoms in wood product plant workers exposed to methylene diphenyl diisocyanate. Chest. 2000;118:1183–1193. doi: 10.1378/chest.118.4.1183. [DOI] [PubMed] [Google Scholar]
- 50•.Fent KW, Jayaraj K, Gold A, et al. Tape-strip sampling for measuring dermal exposure to 1,6-hexamethylene diisocyanate. Scand J Work Environ Health. 2006;32:225–240. doi: 10.5271/sjweh.1003. A novel non-invasive method for detecting isocyanate on the human skin surface represents a potentially important approach to future studies on the role of skin in developing isocyanate sensitization.
- 51•.Flynn MR, Koto Y, Fent K, Nylander-French LA. Modeling dermal exposure – an illustration for spray painting applications. J Occup Environ Hyg. 2006;3:475–480. doi: 10.1080/15459620600854367. A mathmatical model of skin exposure calls attention to the potential role of hair in the skin microenvironment to increase isocyanate deposition. Similar modeling of disrupted or wounded skin would be of interest.
- 52.Chen SE, Bernstein IL. The guinea pig model of diisocyanate sensitization. I. Immunologic studies. J Allergy Clin Immunol. 1982;70:383–392. doi: 10.1016/0091-6749(82)90029-x. [DOI] [PubMed] [Google Scholar]
- 53.Jin RZ, Karol MH. Intra- and intermolecular reactions of 4,4′-diisocyanatodi-phenylmethane with human serum albumin. Chem Res Toxicol. 1988;1:281–287. doi: 10.1021/tx00005a005. [DOI] [PubMed] [Google Scholar]
- 54.Baur X. Immunologic cross-reactivity between different albumin-bound isocyanates. J Allergy Clin Immunol. 1983;71:197–205. doi: 10.1016/0091-6749(83)90100-8. [DOI] [PubMed] [Google Scholar]
- 55.Wass U, Belin L. Immunologic specificity of isocyanate-induced IgE anti-bodies in serum from 10 sensitized workers. J Allergy Clin Immunol. 1989;83:126–135. doi: 10.1016/0091-6749(89)90487-9. [DOI] [PubMed] [Google Scholar]
- 56.Cummings BJ, Booth KS. Industrial hygiene sampling for airborne TDI in six flexible slabstock foam manufacturing facilities in the United States: a comparison of the short-term and long-term sampling data. Appl Occup Environ Hyg. 2002;17:863–871. doi: 10.1080/10473220290107066. [DOI] [PubMed] [Google Scholar]
- 57.Baur X, Chen Z, Flagge A, et al. EAST and CAP specificity for the evaluation of IgE and IgG antibodies to diisocyanate-HSA conjugates. Int Arch Allergy Immunol. 1996;110:332–338. doi: 10.1159/000237325. [DOI] [PubMed] [Google Scholar]
- 58.Bousquet J, Chanez P, Chanal I, Michel FB. Comparison between RAST and Pharmacia CAP system: a new automated specific IgE assay. J Allergy Clin Immunol. 1990;85:1039–1043. doi: 10.1016/0091-6749(90)90048-9. [DOI] [PubMed] [Google Scholar]
- 59.Sander I, Kespohl S, Merget R, et al. A new method to bind allergens for the measurement of specific IgE antibodies. Int Arch Allergy Immunol. 2005;136:39–44. doi: 10.1159/000082583. [DOI] [PubMed] [Google Scholar]
- 60•.Mothes N, Valenta R, Spitzauer S. Allergy testing: the role of recombinant allergens. Clin Chem Lab Med. 2006;44:125–132. doi: 10.1515/CCLM.2006.024. A review of allergen microarrays.
- 61•.Ogawa H, Inoue S, Ogushi F, et al. Toluene diisocyanate (TDI) induces production of inflammatory cytokines and chemokines by bronchial epithelial cells via the epidermal growth factor receptor and p38 mitogen-activated protein kinase pathways. Exp Lung Res. 2006;32:245–262. doi: 10.1080/01902140600817515. The data further indicate active involvement of the epithelium in asthma pathogenesis by suggesting that airway epithelial cells respond to isocyanate conjugated albumin conjugates (antigens) with increased activity of signalling pathways that trigger inflammatory gene expression.
- 62.Kuna P, Reddigari SR, Rucinski D, et al. Monocyte chemotactic and activating factor is a potent histamine-releasing factor for human basophils. J Exp Med. 1992;175:489–493. doi: 10.1084/jem.175.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]