Abstract
Background
Isocyanates, a leading cause of occupational asthma, are known to induce adaptive immune responses; however, innate immune responses, which generally precede and regulate adaptive immunity, remain largely uncharacterized.
Objective
Identify and characterize cellular, molecular and systemic innate immune responses induced by hexamethylene diisocyanate (HDI).
Methods
Human peripheral blood mononuclear cells (PBMCs) were stimulated in vitro with HDI-albumin conjugates or control antigen, and changes in phenotype, gene, and protein expression were characterized by flow cytometry, microarray, Western blot and ELISA. Cell uptake of isocyanate was visualized microscopically using HDI-albumin conjugates prepared with fluorescently-labeled albumin. In vivo, human HDI exposure was performed via specific inhalation challenge, and subsequent changes in PBMCs and serum proteins were measured by flow cytometry and ELISA. Genotypes were determined by PCR.
Results
Human monocytes take-up HDI-albumin conjugates and undergo marked changes in morphology and gene/protein expression in vitro. The most significant (p 0.007 – 0.05) changes in mircoarray gene expression were noted in lysosomal genes, especially peptidases and proton pumps involved in antigen processing. Chemokines that regulate monocyte/macrophage trafficking (MIF, MCP-1), and pattern recognition receptors that bind chitin (chitinases) and oxidized low-density lipoprotein (CD68) were also increased following isocyanate-albumin exposure. In vivo, HDI exposed subjects exhibited an acute increase in the percentage of PBMCs with the same HDI-albumin responsive phenotype characterized in vitro (HLA-DR+/CD11c+ with altered light scatter properties). An exposure-dependent decrease (46±11%; p<0.015) in serum concentrations of chitinase-3-like-1 was also observed, in individuals that lack the major (type 1) human chitinase (due to genetic polymorphism), but not in individuals possessing at least one functional chitinase-1 allele.
Conclusions
Previously unrecognized innate immune responses to HDI and HDI-albumin conjugates could influence the clinical spectrum of exposure reactions.
Keywords: Isocyanate, Innate, Monocyte, Macrophage, Chitinase, CD68, Albumin, MIF, Cathepsin, Exposure, Asthma
INTRODUCTION
Isocyanates, highly reactive chemicals used to make polyurethane, are a leading cause of occupational asthma world-wide.1 The natural history, clinical symptoms, and pathology of isocyanate asthma are similar to common environmental asthma, suggesting the disease is caused by a similar immune-mediated process.2, 3 However, prototypic pathologic indices of common environmental asthma differ from isocyanate asthma. T-helper type 2 (TH2) cells, which are crucial to common environmental asthma and proliferate vigorously in response to causative allergens in vitro, exhibit comparatively minimal responses to isocyanate.3–6 Allergen-specific IgE, atopy, and elevated total serum IgE, which are strongly associated with common environmental asthma, are not generally associated with isocyanate asthma.7, 8 Thus, in contrast to common allergens, the cell types and effector molecules stimulated by isocyanate exposure remain unclear, including those that promote the development of pathogenic hypersensitivity responses vs. apparent “tolerance”. The characteristic differences between isocyanate and common environmental asthma, have lead to the hypothesis that (low molecular weight) isocyanate chemicals elicit immune responses fundamentally distinct from those induced by common environmental allergens, which are generally (high molecular weight) proteins.2, 8, 9
Uncertainty regarding the immunologic basis of isocyanate asthma, is due in part, to isocyanate’s unique chemical reactivity, which presents a theoretical and technical obstacle to mechanistic studies.10 Isocyanates react rapidly with proteins in vitro and in vivo, and isocyanate-protein conjugates are believed to represent the major immunogenic form of the chemical.6, 9, 11, 12 In exposed humans, albumin in the airway fluid is a major target for isocyanate and isocyanate-albumin conjugates are the only known “isocyanate antigen” specifically recognized by rearranged antigen receptors of the adaptive immune system (i.e. IgG).9, 13, 14 However, as mentioned above, antigenic isocyanate-albumin conjugates do not stimulate vigorous in vitro TH2 cellular responses.4, 7 Instead, limited recent studies suggest that human monocyte responses may play an important role in clinical isocyanate exposure outcome.15, 16
Monocytes are crucial cells of the innate arm of the immune system that circulate in an immature form, and differentiate into “tissue macrophages”, dendritic cells and alveolar macrophages.17–20 Monocytes possess the capacity to modulate immune responses by secreting chemokines and processing/presenting exogenous and “self” antigens.21–23 Functional responses of monocytes (and other cells of the innate immune system) are also governed, in part, via germline-encoded pattern-recognition receptors, evolved to detect specific molecular motifs.24
The present study investigates human monocyte responses to a major commercial isocyanate, hexamethylene diisocyanate (HDI), primarily used in protective spray coatings (e.g. automobiles, aircraft).10 Data were derived from in vitro experiments on human PBMCs, and clinical studies of human subjects before and after specific inhalation challenge. The findings are discussed in the context of immune mechanisms that may modulate pathologic responses to exposure.
MATERIALS AND METHODS
Human subjects and samples
The study was approved by the Human Investigations Committee of Yale University, and written informed consent was obtained from all participants. Thirty-one subjects were recruited prospectively from the SPRAY study and Yale’s Occupational and Environmental Medicine Clinic in New Haven CT.25 Peripheral blood was obtained by venipuncture and mononuclear cells were purified by density gradient centrifugation.
Preparation of isocyanate antigens
HDI (Sigma; St. Louis, MO) was conjugated to endotoxin-free human albumin (albØ) (Sigma) as previously described, with slight modifications to minimize processing steps that might introduce contaminants (i.e. endotoxin, trace metals, glycerol etc.).5 Briefly, 100 µl of HDI was added to 25 ml of a 0.5% (w/v) solution of albØ in tissue-culture grade phosphate buffered saline (PBS) pH 7.2 (Gibco; Grand Island, NY), and mixed end-over-end for 2 hrs. The isocyanate-albumin conjugated reaction product (HDI-albØ) was immediately sterile filtered (0.2 µm) for use in culture. FITC-labeled human albumin (albFITC) (Sigma) was used to generate fluorescently labeled isocyanate conjugates (HDI-albFITC), using an abbreviated reaction time (20 min) to prevent quenching of the fluorochrome signal. Adipoyl chloride (Sigma) exposed, timellitic anhydride (TMA) (TCI America; Portland, OR) and DNCB conjugated albumin were prepared according to previously published methods, except using endotoxin-free albumin.26–28 For HDI-albØ and TMA-albØ conjugates, the molar ratios of chemical:albumin were 36:1 and 38:1 respectively, based on chemical substitution analysis, performed as previously described.29 Heat denatured/aggregated albumin was prepared by placing samples of 0.5% albØ in PBS, pH 7.2, into a rapidly boiling water bath for 10 min. Control antigen for all studies was “mock exposed” albØ, which had been processed the same as the HDI- albØ (equal concentration, mixing, filtration etc.), except omitting the HDI.
In vitro culture and processing
Human peripheral blood mononuclear cells (PBMCs) were cultured at 2 × 106 cells/ml in RPMI 1640 media (Gibco) supplemented with 10% autologous serum and stimulated with 500 µg/ml of HDI-albØ or control antigen or 5 µg/ml concanavalin A (ConA) (Sigma). An Olympus microscope (Nashua, NH) was used to photograph cultures with a Pentax camera under automatic exposure settings. Cells were cytospun on slides with a Shandon Cytospin (Cheshire, UK) and stained with Diff-Quick (VWR; Bridgeport, NJ), or RNA was isolated using RNA-easy columns from Qiagen (Valencia, CA) and DNA was removed with a kit from Ambion (Austin, TX). In other experiments, cell proteins were solubilized with Cell Lytic-M™ lysis buffer (Sigma) supplemented with a protease inhibitor cocktail (Sigma).
Flow cytometry
Cells were incubated with fluorochrome-conjugated monoclonal antibodies (mAbs) to HLA-DR, CD11c, CD3 and CD19 and data were acquired on a FACscan and analyzed with Cell Quest software, from Becton-Dickinson (San Jose, CA).
Immunofluorescence analysis (IFA)
PBMCs were incubated with 50 µg/ml of albFITC or HDI-albFITC for varying amounts of time, washed, fixed with paraformaldehyde (Sigma) and settled onto poly-L-lysine (Sigma) coated coverslips. In some experiments, cells were incubated with phycoerythrin-conjugated mAbs to CD3 or CD14 (Becton-Dickinson), or a lysosomal stain, Lysotracker-Red DND-99 (Molecular Probes; Eugene, OR) before fixation. Coverslips were mounted with anti-fade mounting media and viewed under a Nikon Microphot-FXA microscope (Melville, NY).
Microarray studies
DNA-free, total RNA, from 5-day PBMC cultures of individual subjects was transcribed to labeled cDNA with the the MICROMAX™ TSA labeling and detection kit (PerkinElmer; Shelton, CT), according to the manufacturers instructions. Labeled cDNA was hybridized to a broad spectrum 4.6K cDNA microarray (Gene Expression Omnibus repository platform GPL993). Fluorescence hybridization data were acquired on an Axon GenePix 4000A scanner and processed using GenePix software (Molecular Devices Corp; Sunnyvale, CA). The fold increase in gene expression was calculated based on the ratio of the average median fluorescence intensity of duplicate spots after subtraction of background signal. A 2-fold change in expression was statistically significant using a Student’s t-test. Hierarchical cluster analysis and gene ontology (GO) groupings were completed using GeneSpring software (Agilent Technologies; Palo Alto, CA). Data underwent Lowess normalization before clustering. Significance of enrichment for genes in GO groups was determined using a Fischer Exact Test.
Protein studies
Cell proteins were Western blotted as previously described, using mAbs specific for CD68, cathepsin B, and Annexin, (Santa Cruz; Santa Cruz, CA).26 Chitinase-1 enzyme activity levels in culture supernatants were determined as previously reported, based on spectrophotomtric measurements of cleavage of a fluorescent chitin analogue.30 Chitinase 3 like-1, monocyte chemoattractant protein (MCP)-1 and macrophage migration inhibitory factor (MIF) concentrations, in serum or culture supernatants, were quantitated by ELISA, using kits respectively from Quidel Corp (San Diego, CA), BD Biosciences-Pharmingen (San Diego, CA) and R&D Systems (Minneapolis, MN). Statistical significance of changes in chemokine concentrations or enzyme activity were determined using Student t-test or ANOVA.
In vivo respiratory tract isocyanate exposure
Human subjects were exposed to 15–20 parts per billion (ppb) of isocyanate for up to 2 hrs, in an isocyanate exposure chamber as previously described.31
Genotyping
Chit-1 genotype was determined by PCR using primers that span the polymorphic region, a 24-bp duplication in exon 10.32 PCR with the forward primer 5’ CTC CCT GCA CAG TCA GCT AT 3’ and the reverse primer 5’ TAG GAT GTT TGG CTC CTT GG 3’ amplify an 86 bp fragment corresponding to the normal allele and an 110 bp fragment corresponding to the polymorphic allele.
RESULTS
Characterization of isocyanate-albumin conjugates
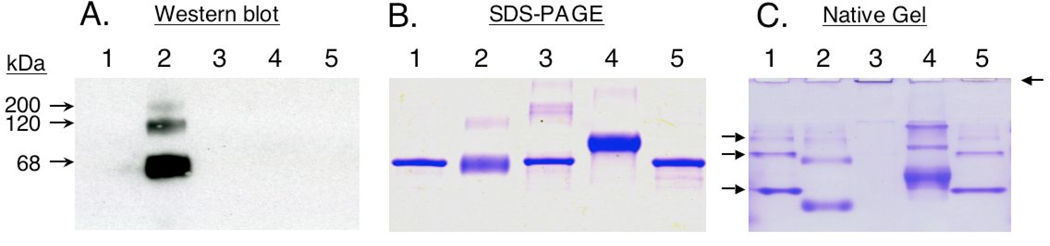
The antigenicity of hexamethylene diisocyanate (HDI)-albumin conjugates used in the present study (HDI-albØ) was confirmed by Western blot with serum from exposed workers (Fig. 1A). Serum IgG binding to HDI-albØ is associated with chemical conjugation, cross-linking, and conformational changes in albumin, highlighted by reducing SDS-PAGE, native gel electrophoresis (Fig. 1B and C) and previously published studies.33 [Fig. 1 also shows other “modified” albumins used in later experiments (see below)].
Figure 1.
Characterization of isocyanate-albumin conjugates. Pooled serum IgG from autobody shop workers was blotted (panel A) against control “mock exposed” endotoxin-free albumin (albØ) (lane 1), HDI-albØ (lane 2), heated/aggregated albØ (lane 3), TMA-albØ (lane 4), and adipoyl chloride-albØ (lane 5). Parallel protein stains of SDS-PAGE (panel B) and native gels (panel C) highlight changes in albumin’s conformation, as reflected by gel migration. The major band observed under SDS-PAGE, is monomeric albumin, while fainter upper bands are likely “multimers”. Albumin multimers that occur spontaneously (see arrows in lane 1- panel C), but not those chemically/physically-induced (lanes 2–4, panel B, and lane 2 panel A), are dissociated under disulfide-reducing conditions (lanes 2–4, panel B). *Note: Western blots with serum from unexposed human subjects were uniformly negative against the conjugates shown above.
Isocyanate-albumin induced changes in cell morphology
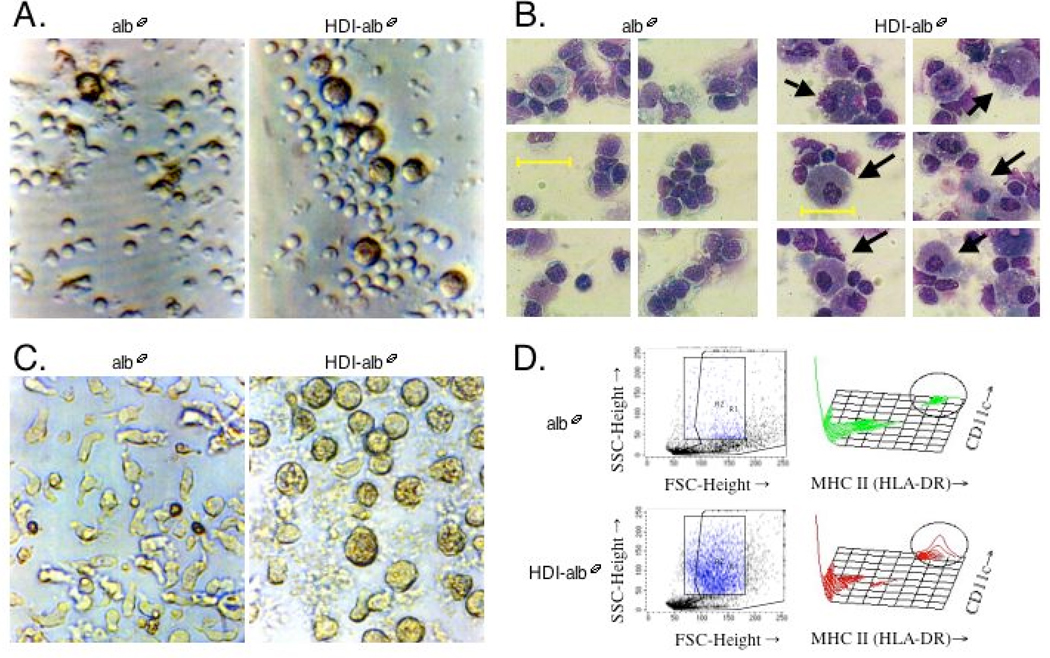
HDI-albØ induced cellular immune responses were studied in vitro using PBMCs from individuals participating in a study on HDI exposure health effects. Microscopic examination of cultures (Fig. 2A & B) revealed that HDI-albØ, but not control “mock exposed” albØ, induced striking morphologic changes in PBMCs from all study subjects (N=31). The HDI- albØ responsive cells could be purified by plastic adherence (Fig. 2C), and co-expressed high levels of monocyte/macrophage-associated cell membrane proteins (HLA-DR and CD11c) (Fig. 2D), but not T- or B-cell specific markers CD3 (Fig. 3C) or CD19 (not shown). Morphologic changes observed under the light microscope were accompanied by a marked shift in side and forward light scatter in flow cytometry studies, consistent with changes in cell shape and complexity (Fig. 2D).
Figure 2.
Isocyanate-albumin induced phenotypic changes in PBMCs. Cells were cultured with 500 µg/ml HDI-albØ or control antigen (albØ) for 3 days and photographed under the light microscope at 400X. Panel A- live cultures, Panel B- cytospins, Panel C- live cultures initiated with plastic adherent PBMCs. Panel D- Flow cytometry light scatter and two-dimensional histogram staining for HLA-DR and CD11c of PBMCs. Cells with increased light side scatter are highlighted by boxed region and correspond to circled area of histogram plot. SSC= side scatter, FSC= forward scatter.
Figure 3.
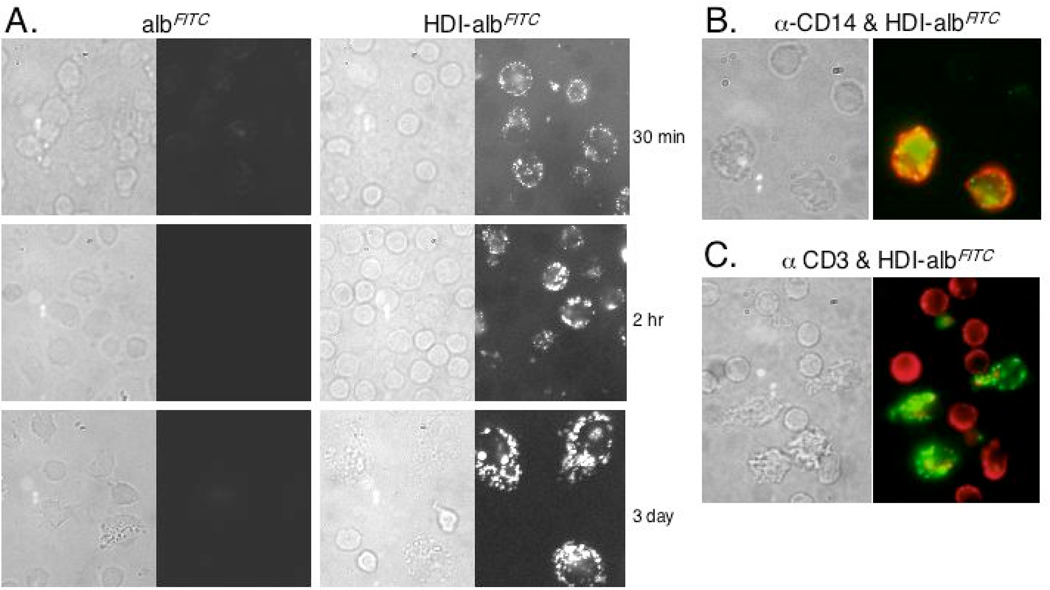
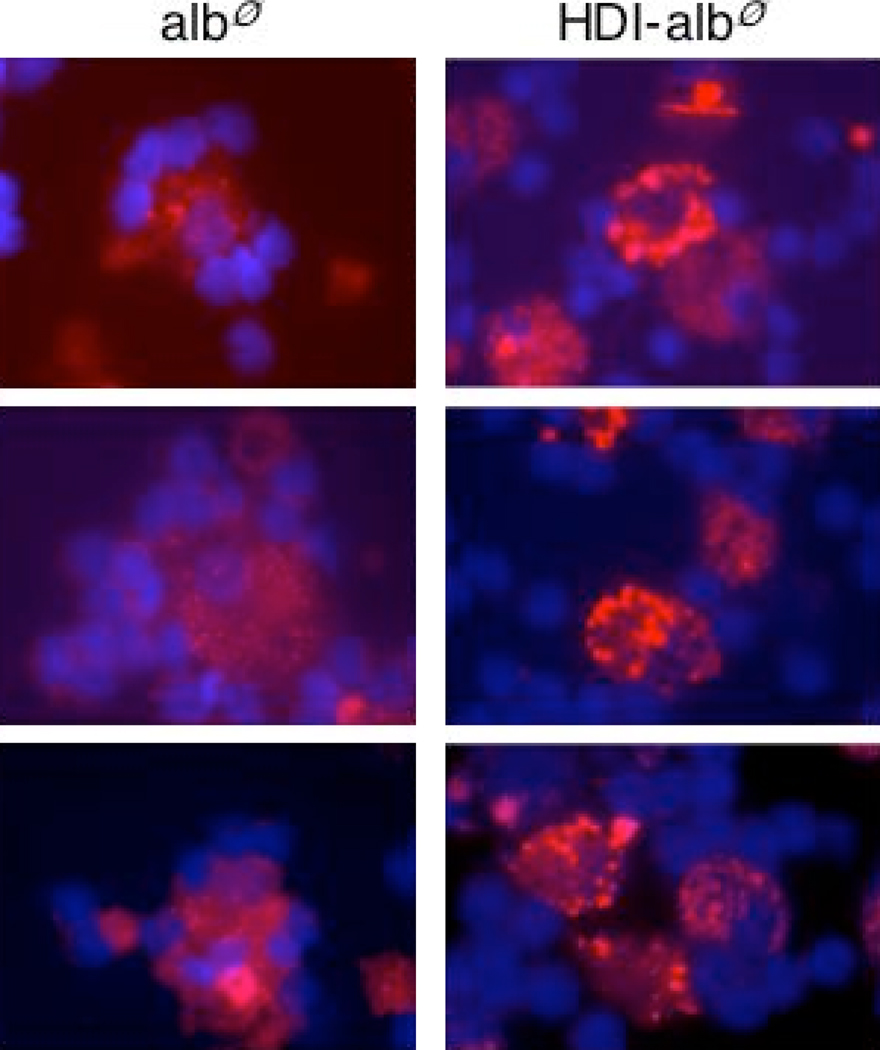
Isocyanate-albumin is taken-up and accumulates in cells of monocyte lineage. PBMCs were cultured with fluorescently labeled control “mock exposed” albumin (albFITC) or HDI-albFITC, and analyzed under phase contrast (left frame) and fluorescence (right frame) microscopy at different time points shown (panel A). HDI-albFITC, but not albFITC is taken-up along the outer cell membrane in a subset of cells, and accumulates over time. Monocyte-derived cells (panel B), but not T cells (panel C) are the major cell type that take up HDI-albFITC (green), as shown by dual staining with PE (red)-labeled α-CD14 or α-CD3, at 2hr and 3 day time points respectively.
Isocyanate-albumin uptake
To better understand the marked cell morphology changes induced by isocyanate-albumin, we performed additional studies that trace the chemical-protein conjugate’s cell uptake and subcellular distribution. HDI conjugates were prepared with fluorescently-labeled albumin (albFITC), which could be directly visualized through fluorescence microscopy. As shown in Fig. 3A, HDI-albFITC uptake occurred within 30 minutes of culture, at which time it was localized around the cell membrane. Over time (72 hrs), HDI-albFITC accumulated deeper inside the cytoplasm, with a staining pattern suggestive of localized sequestration. In contrast, little or no staining was observed in cultures incubated with control antigen, “mock exposed” albFITC. The cells that take-up high levels of HDI-albFITC were further phenotyped as CD14+/CD3−, in double stain experiments (Fig. 3B & C).
Isocyanate-albumin induced changes in gene expression
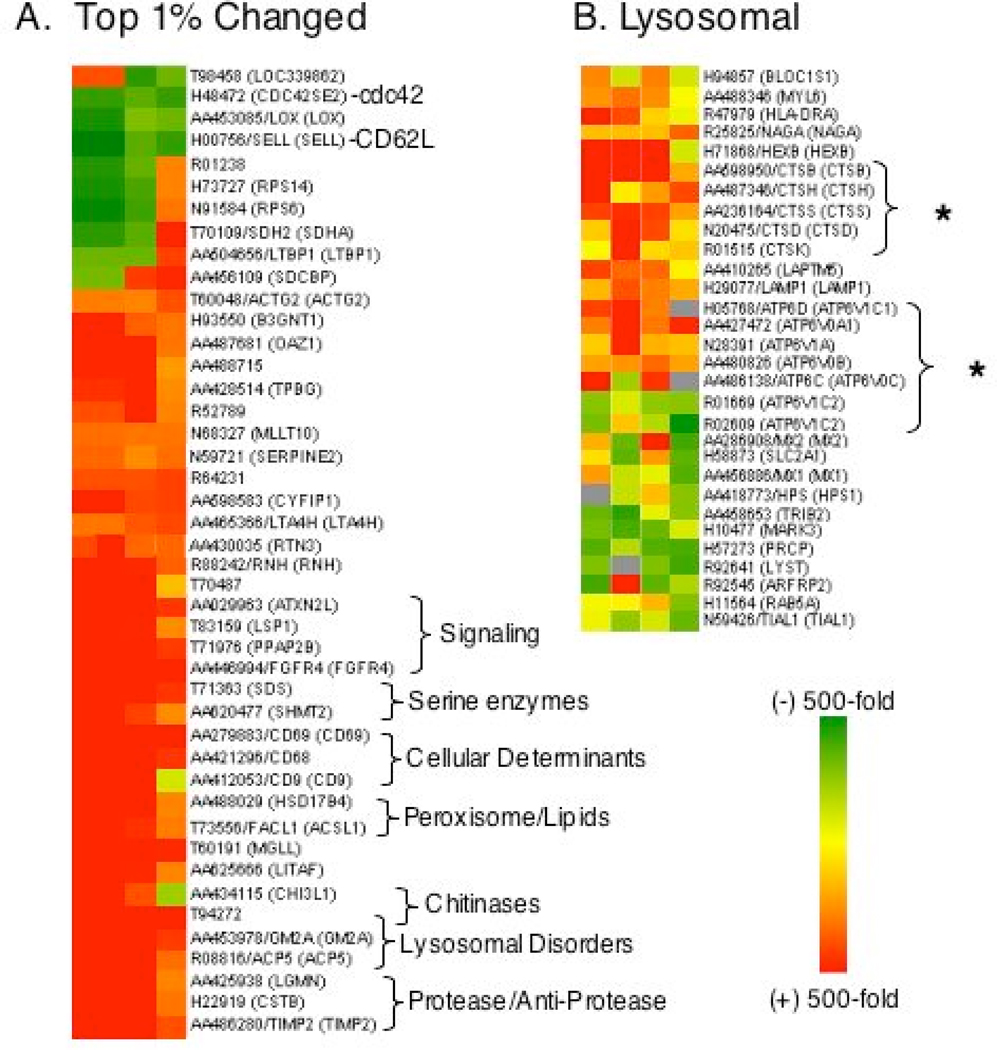
To help understand the molecular changes underlying the human PBMC response to HDI- albØ, we profiled gene expression changes using a broad spectrum, 4.6k cDNA microarray. HDI-albØ-induced genes were identified relative to control cultures stimulated with “mock exposed” albØ, and further stratified through hierarchical clustering and gene ontology (GO). As shown (Fig. 4), HDI-albØ induced substantial changes in gene expression of PBMCs from each of 4 different subjects tested. The most significant changes (p values .007 to .05) were consistently observed in lysosomal genes, especially proteases and proton pump subunits associated with antigen processing (Fig. 4B). Several of the HDI-albØ induced genes are also known to be highly dysregulated in genetic lysosomal storage diseases, including chitinase-1 (T94272), tartrate-resistant acid phosphatase (ACP5), and GM2 ganglioside-activating protein (GM2A).34–36 Consistent with the microarray data, cellular lysosomal content was also elevated in cultures of PBCMs stimulated with HDI-albØ (Fig 5).
Figure 4.
Isocyanate-albumin induced changes in gene expression. Cluster analysis of genes most up/down regulated in PBMCs cultured with HDI-albumin for 5 days. Color and intensity represents the fold change in cDNA microarrays (4.6k) comparing cultures stimulated with HDI-albØ vs. control “mock exposed” albØ. Panel A- Top 1% of affected genes based on fold change in expression. Panel B- Lysosome gene ontology subset; GO:0005764- Functional Cluster-Cellular Component: cell: intracellular: cytoplasm: vacuole: lysosme; one third (29/95) of genes (those >2-fold change in expression) are shown.
Figure 5.
Isocyanate-albumin induced increases in lysosomal content. PBMCs cultured with 500 µg/ml HDI-albØ or control antigen (albØ) for 5 days were incubated with a lysosomal membrane stain (red/pink) and counterstained with DAPI (blue) before settling onto poly-lysine coated coverslips. Representative fields were photomicrographed at 200X.
Isocyanate-albumin induced changes in cellular proteins
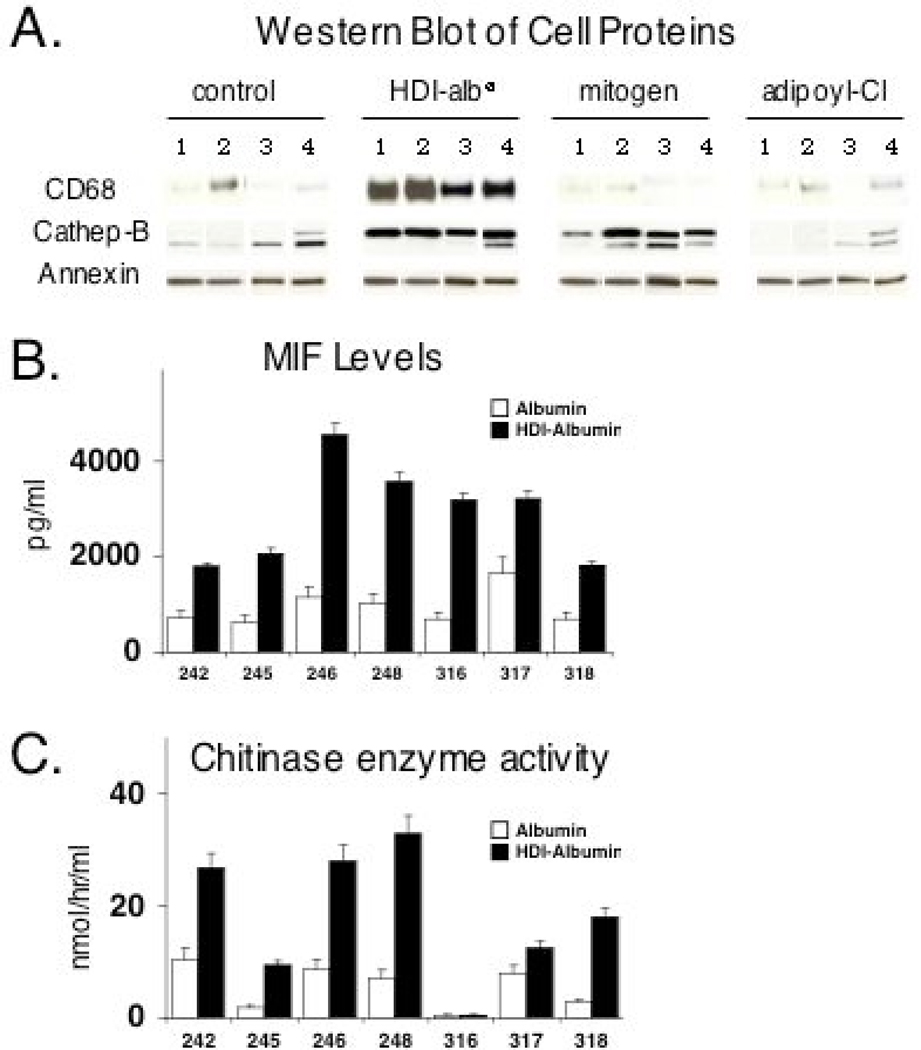
To determine if HDI-albØ-induced gene expression profiles extend to the protein level, to validate specific microarray findings, and to begin evaluating the specificity of the response for isocyanate, we performed additional Western blots experiments. Protein levels for two of the genes whose expression was most consistently elevated in microarray experiments, CD68 and cathepsin B (CTSB), were substantially increased by HDI-albØ. CD68 was not induced by other stimuli tested, including mitogen or albumin exposed to adipoyl chloride, another reactive chemical of similar size and bi-functionality (Fig. 6A). The HDI-albØ induced cathepsin B protein differed in size from the lysosomally-processed fully mature form37 found in control cultures (Fig. 6A), supporting the gene expression and cytology data (above) suggestive of altered lysosomal activity.
Figure 6.
Isocyanate-albumin induced changes in intracellular and secreted proteins. Panel A- is a composite of representative Western blots of cultures of PBMCs from four different subjects (lanes 1–4). Equivalent amounts of cell protein from PBMCs cultured with 500 µg/ml of “mock-exposed” albØ (control), HDI-albØ, mitogen, or adipoyl-chloride for 5 days, were blotted with mAb against CD68, cathepsin B, and annexin 1. Panel B- MIF levels (pg/ml) in the culture media of PBMCs from 7 additional human subjects (identification numbers 242–318 shown on X-axis) similarly stimulated with 500 µg/ml of “mock-exposed” albØ (open bars) or HDI-albØ (filled bars) for 72 hrs. Panel C- Chitotriosidase enzyme activity (nmol/hr/ml) on day 5 of the same cultures.
Isocyanate-albumin induced changes in secreted proteins
We also evaluated isocyanate-albumin induced changes in monocyte secreted proteins of particular interest to asthma. The chemokine MIF, an important modulator of asthma severity,38 rapidly accumulated in the culture media of PBMCs stimulated with isocyanate-albumin. Compared to control cultures, MIF levels were increased >2–fold by 6 hours (not shown) and continued to increase for at least 72 hrs (Fig. 6B). Another chemokine, MCP-1, which acts as an autocrine factor for monocytes (and induces histamine release from mast cells)39 was variably increased at the protein level in culture supernatants; however was consistently upregulated at the message level based by RT-PCR (not shown). Chitinase-1, a soluble secreted pattern-recognition receptor, was significantly increased by isocyanate-albumin, based on enzyme activity in PBMC culture supernatants (Fig. 6C), consistent with the microarray data, and morphologic changes (Figs. 1 & 2), reminiscent of Gauchers’ cells, which are known to be a major source of chitinase-1 in vivo.34, 40
Specificity of the in vitro human response to isocyanate-albumin conjugates
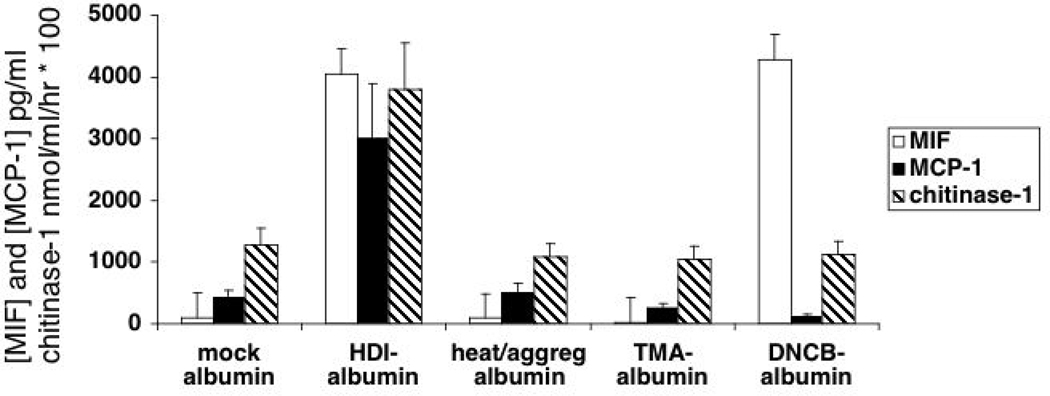
Additional experiments were performed to determine if in vitro responses to HDI-albØ were HDI-dependent, or merely reflected biophysical/biochemical changes in albumin (solubility, conformation, aggregation), known to influence any proteins’ immunogenicity.41 Heat denatured/aggregated albumin, as well as albumin conjugated with other reactive, asthma-causing chemicals (see Fig. 1), were compared with HDI-albØ for their ability to induce chemokine and chitinase-1 secretion. As shown in Fig. 7, HDI-albØ, but not heat denatured/aggregated albØ or TMA-albØ, induced marked MIF, MCP-1 and chitinase-1 production. HDI-albØ was also distinct from DNCB-albØ, which induced MIF (consistent with previous reports)28 but not chitinase-1 or MCP-1. Thus, the human cellular response to HDI-albumin exhibits HDI-specificity and does not reflect nonspecific consequences of biochemical/biophysical changes, or chemical conjugation.
Figure 7.
Specificity of the human innate immune response to isocyanate-albumin. PBMCs from non-isocyanate exposed individuals (N=4) were cultured with 500 µg/ml of control “mock exposed” albØ, HDI-albØ, heated/aggregated albØ, TMA-albØ, DNCB-albØ. The concentrations of MIF (open bars) and MCP-1 (filled bars) in the culture supernatants at 48 hours were measured by ELISA, and chitinase-1 enzyme activity (stripped bars) on day 5 was measured fluorometrically as described in the methods.
Monocyte activation in vivo in exposed subjects
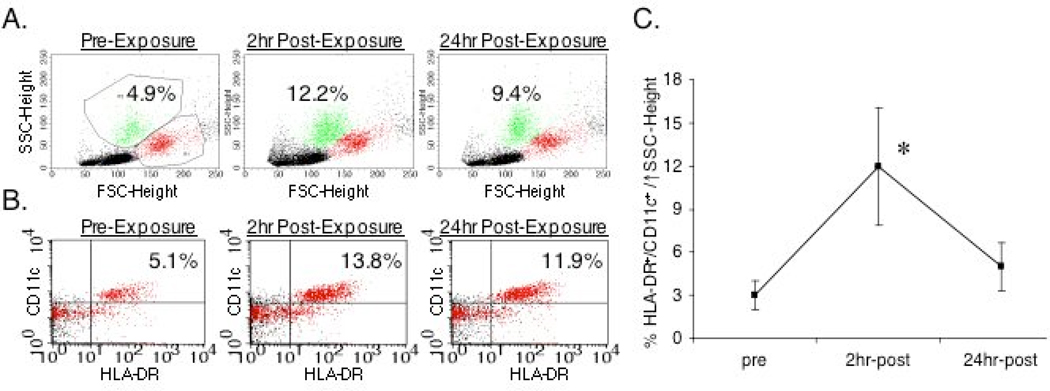
To begin to determine if isocyanate exposure, in vivo, has effects on human monocytes, similar to those observed in vitro, we studied human subjects exposed to HDI under controlled laboratory settings. Human volunteers breathed for 2 hours from a chamber, conditioned with 15–20 parts per/billion of HDI, the short-term exposure limit recommended by the NIOSH. Following the exposure period, cells with the HDI responsive phenotype defined in vitro (Fig. 1D) were quantitated in the peripheral blood by flow cytometry. As shown (Fig. 8A–C), HDI exposure induced an acute, 3 to 5-fold increase in the percentage of HLA-DR+/CD11c+ cells with altered light scatter in the peripheral blood of exposed individuals (N=5), which returned to near baseline levels by 24 hours post exposure.
Figure 8.
In vivo monocyte/macrophage response to isocyanate exposure. The peripheral blood cells of human subjects exposed to 20 ppb isocyanate (HDI) for 2 hrs, were analyzed by flow cytometry. Panel A shows light scatter and Panel B shows HLA-DR/CD11c dot plots from a representative subject pre-exposure, 2hr and 24 hr post exposure. The percentages of cells in the gated region or quadrant are shown. Panel C summarizes the percentage of HLA-DR+/CD11c+ cells with shifted light scatter (as gated) in the peripheral blood of five different subjects at each time point (mean +/− S.E.). Pre-exposure and 2 hr post-exposure values are significantly different (p<0.01).
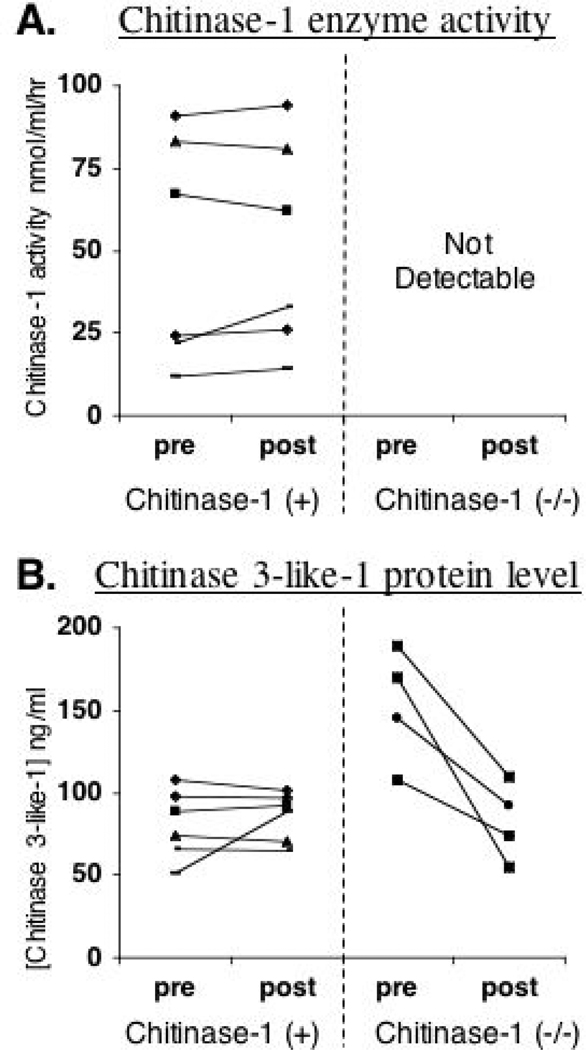
In vivo chitinase response to isocyanate exposure
Based upon our microarray findings, we further explored the potential in vivo effect of isocyanate exposure on chitinases, a family of innate immune proteins that bind chitin, a polysaccharide found in fungi, parasites, and nematodes, but not in mammals.40, 42 We focused on two different chitinases, chitinase-1, the major human chitinase, which possesses true chitin-degrading capabilities,40, 43 and chitinase 3-like-1 (HC-gp39, YKL-40), which binds, but cannot cleave chitin.44, 45 An “inactivating” polymorphism exists in chitinase-1 and individuals with 2 polymorphic alleles (~6% of the population) are deficient in the enzyme’s activity throughout their body.32 Chitinase 3-like-1 is thought to play a role in tissue remodeling and serum levels have recently been associated with human asthma severity.46
In the present study, chitinase-1 enzyme activity and chitinase 3-like-1 protein concentrations were measured in serum before, and 24-hours after specific inhalation challenge with HDI. In the majority of subjects (N=6), there was no significant change in chitinase-1 or chitinase 3-like-1 following exposure (Fig. 9A & B). However, in a subset of subjects who were chitinase-1 deficient due to genetic polymorphism (N=4), isocyanate exposure caused a 46±11% (p < 0.015) decrease in serum concentrations of chitinase 3-like-1 (Fig. 9B).
Figure 9.
In vivo effect of isocyanate exposure on chitinases. Chitinase-1 enzyme activity levels (panel A) and chitinase 3-like-1 protein levels (panel B) in study subjects’ serum were measured pre- and (24 hrs) post- respiratory tract exposure. Each line represents measurements from a single individual, with at least one active (+) chitinase allele (N=6), or two copies (−/−) of the non-active (polymorphic) allele (N=4, the only 4 subjects in our study population with this genotype.)
DISCUSSION
The present studies demonstrate that human monocytes, important cells of the innate arm of the immune system, respond to hexamethylene diisocyanate in a manner that could modulate exposure outcome. In vitro, HDI-albumin conjugates were shown to be taken-up by monocytes, and stimulate marked changes in morphology, phenotype and gene/protein expression. In clinical studies, respiratory tract exposure to HDI vapors increased the percentage of circulating HLA-DR+/CD11c+ blood cells with the HDI-albumin responsive phenotype observed in vitro. Furthermore, a multi gene (chitinase) / environment (isocyanate exposure) interaction was observed in vivo, which affects the serum concentrations of chitinase 3-like-1, a soluble pattern-recognition receptor recently associated with human asthma.46 Together, the data describe previously unrecognized effects of HDI on the innate arm of the human immune system, which may provide novel insights into HDI’s immunogenicity and help explain unusual features of exposure-induced asthma.
The present findings are consistent with limited reports on human cellular responses to isocyanate published to date, including pronounced effects of cells and cytokines/chemokines other than the prototypic, adaptive, TH2-type driven by common environmental allergens. Lummus et. al. have shown that isocyanate-albumin conjugates stimulate PBMCs to produce chemokines in vitro, with profiles typical of monocyte-dominated responses (MCP-1, IL-8, TNF-a),16 while Bernstein et. al. further demonstrated that isocyanate-albumin induced in vitro MCP-1 production by PBMCs was associated with clinical responses to inhalation challenge tests.15 The present data suggest chemokine responses represent part of a much broader cellular reaction triggered by isocyanate, which may contribute to the chemical’s potent (hyper)sensitizing capability.
Innate immune responses to isocyanate, as described in this study, could influence exposure outcomes through several different, though not necessarily independent, pathways. Isocyanate-induced chemokine production may create a microenvironment that favors further development of pathologic immune responses. Two isocyanate-antigen induced chemokines, MIF and MCP-1, have well characterized pro-inflammatory properties, and contribute to the airway pathology observed in common environmental asthma.38, 39 Isocyanate-induced up-regulation of germ-line encoded (innate immune) pattern recognition receptors, may also alter secondary “signals” that influence antigenic responsiveness.24 Two isocyanate-antigen induced pattern-recognition receptors (CD68 and chitinases) bind ligands (oxidized LDL, and chitin respectively) that evoke potent immunostimlatory responses.40, 47, 48 Isocyanate’s effects on lysosomes, the cellular compartment where exogenous antigens are processed, might further modulate adaptive responses.23 General disruption of lysososmal equilibrium underlies and/or contributes to the activity of some well-known immunomodulatory drugs/agents (e.g. lysosomotropic amines), as well as the classical concept of lysosomal stabilization/labilization as a mechanism of immune regulation.49, 50 Recent studies have identified the specific lysosomal proteases and proton pumps induced by isocyanate antigen (e.g. cathepsin B, legumaine a.k.a. AEP) as important determinants of “antigen fate”, differentially expressed in functionally distinct antigen-presenting cells (macrophage vs. dendritic cells).51, 52 The present data suggest lysosomal effects may represent a previously unappreciated mechanism by which isocyanates might stimulate the human immune system. Thus, innate immune responses to isocyanate could influence isocyanatye’s antigenicity in multiple ways that differ from other common allergens.
Isocyanate’s affects on the innate arm of the human immune system may at first seem surprising, given the modern history (1930s) of isocyanate usage, and the prevailing theory that the innate immune system evolved to specifically detect pathogen-associated molecular patterns (PAMPs).10, 53 However, the data fit well with recent hypotheses suggesting overlap between PAMPs and damage associated molecular patterns (DAMPs), based on shared hydrophobic moitifs, as well as what is known about isocyanate’s chemistry.53, 54 Commercially used isocyanates are highly hydrophobic, comprised almost entirely of carbon and hydrogen.10 Their bivalent nature and intra-molecular cross-linking ability are also known to alter the “normal” structure of albumin, perhaps exposing stimulatory hydrophobic regions (“hypos”) or creating other “damage” moieties that might act as innate immune signals.33, 54 Enzymatic or hydrolytic metabolism of isocyanate might yield derivatives similar to other immunomodulatory agents described above (lysosomotropic amines).10, 50 Additionally, isocyanate conjugation raises the isoelectric point of albumin, and increases its sensitivity to (acid) precipitation at lysosomal pH (unpublished observations). Thus, multiple characteristics of isocyanates could contribute individually or coordinately towards their ability to stimulate innate immune responses
A major strength of the present study is the use of human subjects (PBMCs, serum, albumin), rather than laboratory animals, given well-recognized species-specific differences in gene polymorphisms/mutations and expression, as well as isocyanate responsiveness. For example, in contrast to humans, murine macrophages do not express chitinase-1 and lack the inactivating polymorphism of the gene. Furthermore, chitinase-1 over-producing cells, observed in Gauchers disease, a genetic human lysosomal storage disorder, exhibit striking morphologic similarities with HDI-responsive cells, but are not known to occur in other species. Chitinase 3-like-1, which has recently been linked to human asthma, possess several polymorphisms in humans; however its expression, activity, disease association and genetics in other species are less understood.46 Another strength of the present study was use of specific inhalation challenge for in vivo exposures, which allowed precise dosing and timing of sample acquisition in relation to exposure. The use of human subjects for the present study did, however, impose certain limitations, particularly for in vivo studies, which required intensive safety precautions, specialized well-calibrated equipment, physician and industrial hygienist oversight.55, 56 Safety and ethical concerns precluded human subjects from repeated exposures, and limited the dosage, timing and available tissue samples/subjects. Thus, potential differences in human monocyte responses between subjects with different levels of exposure and/or isocyanate sensitivity, could not be deduced from the present data. Future studies will address this provocative possibility, but will require substantial subject characterization and selective recruitment, beyond the scope of the present study.
In summary, we have identified and characterized effects of HDI and HDI-albumin conjugates on the innate arm of the human immune system. The data highlight lysosomal responses, known to affect antigen processing, as well as a family of pattern-recognition receptors, (chitinases) recently linked to asthma. These newly defined HDI-induced responses provide unique perspectives on the chemical’s immunogenicity and suggest several mechanisms by which innate immune responses to exposure could modulate the development of allergy/asthma. Future studies will address whether qualitative individual variation in the innate immune response to HDI influences the outcome of occupational exposure and/or helps explain potential differences between isocyanate asthma and prototypical “atopic”/IgE-dependent asthma.
ACKNOWLEDGMENTS
The work was supported by grants from the National Institutes of Health (HL62622, M01RR00125), National Institute of Occupational Safety and Health (OH3457) and National Institute of Environmental Health Sciences (ES00355). The authors acknowledge Carole Holm, RN, Frank Walsh and Dr. You-cheng Liu for help with patient recruitment and isocyanate chamber operation.
ABBREVIATIONS
- PAMP
pathogen-associated molecular pattern
- DAMP
damage-associated molecular pattern
- MIF
migration Inhibitory Factor
- MCP
monocyte chemoattractant protein
- PBMC
peripheral blood mononuclear cells
- HDI
hexamethylene diisocyanate
- TMA
trimellitic anhydride
- DNCB
1,4-dinitro-1-chlorobenzene
- FITC
fluorescein isothiocyanate
REFERENCES
- 1.Tarlo SM, Liss GM. Diisocyanate-induced asthma: diagnosis, prognosis, and effects of medical surveillance measures. Appl Occup Environ Hyg. 2002;17:902–908. doi: 10.1080/10473220290107101. [DOI] [PubMed] [Google Scholar]
- 2.Mapp C, Boschetto P, Miotto D, De Rosa E, Fabbri LM. Mechanisms of occupational asthma. Ann Allergy Asthma Immunol. 1999;83:645–664. doi: 10.1016/S1081-1206(10)62888-8. [DOI] [PubMed] [Google Scholar]
- 3.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Ann Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein JA, Munson J, Lummus ZL, Balakrishnan K, Leikauf G. T-cell receptor V beta gene segment expression in diisocyanate-induced occupational asthma. J Allergy Clin Immunol. 1997;99:245–250. doi: 10.1016/s0091-6749(97)70104-0. [DOI] [PubMed] [Google Scholar]
- 5.Redlich CA, Stowe MH, Wisnewski AV, Eisen EA, Karol MH, Lemus R, Holm CT, Chung JS, Sparer J, Liu Y, Woskie SR, Appiah-Pippim J, Gore R, Cullen MR. Subclinical immunologic and physiologic responses in hexamethylene diisocyanate-exposed auto body shop workers. Am J Ind Med. 2001;39:587–597. doi: 10.1002/ajim.1058. [DOI] [PubMed] [Google Scholar]
- 6.Wisnewski AV, Redlich CA. Recent developments in diisocyanate asthma. Curr Opin Allergy Clin Immunol. 2001;1:169–175. doi: 10.1097/01.all.0000011003.36723.d8. [DOI] [PubMed] [Google Scholar]
- 7.Tee RD, Cullinan P, Welch J, Burge PS, Newman-Taylor AJ. Specific IgE to isocyanates: a useful diagnostic role in occupational asthma. Journal of Allergy & Clinical Immunology. 1998;101:709–715. doi: 10.1016/S0091-6749(98)70181-2. [DOI] [PubMed] [Google Scholar]
- 8.Jones MG, Floyd A, Nouri-Aria KT, Jacobson MR, Durham SR, Taylor AN, Cullinan P. Is occupational asthma to diisocyanates a non-IgE-mediated disease? J Allergy Clin Immunol. 2006;117:663–669. doi: 10.1016/j.jaci.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Wisnewski AV. Recent developments in diisocyanate asthma. Ann Allergy Asthma Immunol. 2003;90:35–41. doi: 10.1016/s1081-1206(10)61647-x. [DOI] [PubMed] [Google Scholar]
- 10.Urlich H. Chemistry and Technology of Isocyanates. West Sussex, England: John Wiley & Sons. Ltd; 1996. [Google Scholar]
- 11.Tse CS, Pesce AJ. Chemical characterization of isocyanate-protein conjugates. Toxicol Appl Pharmacol. 1979;51:39–46. doi: 10.1016/0041-008x(79)90006-1. [DOI] [PubMed] [Google Scholar]
- 12.Wisnewski A, Redlich C, Mapp C, Bernstein D. Polyisocyanates. 3rd edition. Informa Healthcare; 2006. [Google Scholar]
- 13.Wass U, Belin L. Immunologic specificity of isocyanate-induced IgE antibodies in serum from 10 sensitized workers. J Allergy Clin Immunol. 1989;83:126–135. doi: 10.1016/0091-6749(89)90487-9. [DOI] [PubMed] [Google Scholar]
- 14.Wisnewski AV, Srivastava R, Herick C, Xu L, Lemus R, Cain H, Magoski NM, Karol MH, Bottomly K, Redlich CA. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am J Respir Crit Care Med. 2000;162:2330–2336. doi: 10.1164/ajrccm.162.6.2002086. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein DI, Cartier A, Côté J, Malo JL, Boulet LP, Wanner M, Milot J, L'Archevéque J, Trudeau C, Lummus Z. Diisocyanate antigen-stimulated monocyte chemoattractant protein-1 synthesis has greater test efficiency than specific antibodies for identification of diisocyanate asthma. Am J Respir Crit Care Med. 2002;166:445–450. doi: 10.1164/rccm.2109018. [DOI] [PubMed] [Google Scholar]
- 16.Lummus ZL, Alam R, Bernstein JA, Bernstein DI. Diisocyanate antigen-enhanced production of monocyte chemoattractant protein-1, IL-8, and tumor necrosis factor-alpha by peripheral mononuclear cells of workers with occupational asthma. J Allergy Clin Immunol. 1998;102:265–274. doi: 10.1016/s0091-6749(98)70095-8. [DOI] [PubMed] [Google Scholar]
- 17.Fels AO, Cohn ZA. The alveolar macrophage. J Appl Physiol. 1986;60:353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- 18.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci U S A. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cushing SD, Fogelman AM. Monocytes may amplify their recruitment into inflammatory lesions by inducing monocyte chemotactic protein. Arterioscler Thromb. 1992;12:78–82. doi: 10.1161/01.atv.12.1.78. [DOI] [PubMed] [Google Scholar]
- 23.Unanue ER. Perspective on antigen processing and presentation. Immunol Rev. 2002;185:86–102. doi: 10.1034/j.1600-065x.2002.18510.x. [DOI] [PubMed] [Google Scholar]
- 24.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 25.Sparer J, Stowe MH, Bello D, Liu Y, Gore RJ, Youngs F, Cullen MR, Redlich CA, Woskie SR. Isocyanate exposures in autobody shop work: the SPRAY study. J Occup Environ Hyg. 2004;1:570–581. doi: 10.1080/15459620490485909. [DOI] [PubMed] [Google Scholar]
- 26.Wisnewski AV, Lemus R, Karol MH, Redlich CA. Isocyanate-conjugated human lung epithelial cell proteins: A link between exposure and asthma? J Allergy Clin Immunol. 1999;104:341–347. doi: 10.1016/s0091-6749(99)70377-5. [DOI] [PubMed] [Google Scholar]
- 27.Valstar DL, Schijf MA, Stelekati E, Nijkamp FP, Bloksma N, Henricks PA. Trimellitic anhydride-conjugated serum albumin activates rat alveolar macrophages in vitro. J Occup Med Toxicol. 2006;1:13. doi: 10.1186/1745-6673-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordqvist BC, Rosenthal SA. Studies on contact sensitivity to DNCB in guinea pigs by the macrophage migration test. Int Arch Allergy Appl Immunol. 1978;56:73–78. doi: 10.1159/000232005. [DOI] [PubMed] [Google Scholar]
- 29.Snyder SL, Sobocinski PZ. An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines. Anal Biochem. 1975;64:284–288. doi: 10.1016/0003-2697(75)90431-5. [DOI] [PubMed] [Google Scholar]
- 30.Artieda M, Cenarro A, Ganan A, Jerico I, Gonzalvo C, Casado JM, Vitoria I, Puzo J, Pocovi M, Civeira F. Serum chitotriosidase activity is increased in subjects with atherosclerosis disease. Arterioscler Thromb Vasc Biol. 2003;23:1645–1652. doi: 10.1161/01.ATV.0000089329.09061.07. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Berode M, Stowe MH, Holm CT, Walsh FX, Slade MD, Boeniger MF, Redlich CA. Urinary hexane diamine to assess respiratory exposure to hexamethylene diisocyanate aerosol: a human inhalation study. Int J Occup Environ Health. 2004;10:262–271. doi: 10.1179/oeh.2004.10.3.262. [DOI] [PubMed] [Google Scholar]
- 32.Boot RG, Renkema GH, Verhoek M, Strijland A, Bliek J, de Meulemeester TM, Mannens MM, Aerts JM. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J Biol Chem. 1998;273:25680–25685. doi: 10.1074/jbc.273.40.25680. [DOI] [PubMed] [Google Scholar]
- 33.Jin RZ, Karol MH. Intra- and intermolecular reactions of 4,4'-diisocyanatodiphenylmethane with human serum albumin. Chem Res Toxicol. 1988;1:281–287. doi: 10.1021/tx00005a005. [DOI] [PubMed] [Google Scholar]
- 34.Hollak CE, van Weely S, van Oers MH, Aerts JM. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93:1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troy K, Cuttner J, Reilly M, Grabowski G, Desnick R. Tartrate-resistant acid phosphatase staining of monocytes in Gaucher disease. Am J Hematol. 1985;19:237–244. doi: 10.1002/ajh.2830190305. [DOI] [PubMed] [Google Scholar]
- 36.Mahuran DJ. Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim Biophys Acta. 1999;1455:105–138. doi: 10.1016/s0925-4439(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 37.Yan S, Sloane BF. Molecular regulation of human cathepsin B: implication in pathologies. Biol Chem. 2003;384:845–854. doi: 10.1515/BC.2003.095. [DOI] [PubMed] [Google Scholar]
- 38.Mizue Y, Ghani S, Leng L, McDonald C, Kong P, Baugh J, Lane SJ, Craft J, Nishihira J, Donnelly SC, Zhu Z, Bucala R. Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci U S A. 2005;102:14410–14415. doi: 10.1073/pnas.0507189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TN, Proudfoot A, Martinez AC, Dorf M, Bjerke T, Coyle AJ, Gutierrez-Ramos JC. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Eijk M, van Roomen CP, Renkema GH, Bussink AP, Andrews L, Blommaart EF, Sugar A, Verhoeven AJ, Boot RG, Aerts JM. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol. 2005;17:1505–1512. doi: 10.1093/intimm/dxh328. [DOI] [PubMed] [Google Scholar]
- 41.Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant. 2005;20 Suppl 6:vi3–vi9. doi: 10.1093/ndt/gfh1092. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 43.Boot RG, Bussink AP, Verhoek M, de Boer PA, Moorman AF, Aerts JM. Marked differences in tissue-specific expression of chitinases in mouse and man. J Histochem Cytochem. 2005;53:1283–1292. doi: 10.1369/jhc.4A6547.2005. [DOI] [PubMed] [Google Scholar]
- 44.Renkema GH, Boot RG, Au FL, Donker-Koopman WE, Strijland A, Muijsers AO, Hrebicek M, Aerts JM. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251:504–509. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- 45.Boot RG, van Achterberg TA, van Aken BE, Renkema GH, Jacobs MJ, Aerts JM, de Vries CJ. Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol. 1999;19:687–694. doi: 10.1161/01.atv.19.3.687. [DOI] [PubMed] [Google Scholar]
- 46.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 47.de Villiers WJ, Smart EJ. Macrophage scavenger receptors and foam cell formation. J Leukoc Biol. 1999;66:740–746. doi: 10.1002/jlb.66.5.740. [DOI] [PubMed] [Google Scholar]
- 48.Pearson AM. Scavenger receptors in innate immunity. Curr Opin Immunol. 1996;8:20–28. doi: 10.1016/s0952-7915(96)80100-2. [DOI] [PubMed] [Google Scholar]
- 49.de Duve C, de Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 50.Arai K, Yasuda N, Isohashi F, Okamoto K, Ohkuma S. Inhibition of weak-base amine-induced lysis of lysosomes by cytosol. J Biochem. 2002;132:529–534. doi: 10.1093/oxfordjournals.jbchem.a003253. [DOI] [PubMed] [Google Scholar]
- 51.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 52.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 53.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 54.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 55.Vandenplas O, Cartier A, Ghezzo H, Cloutier Y, Malo JL. Response to isocyanates: effect of concentration, duration of exposure, and dose. Am Rev Respir Dis. 1993;147:1287–1290. doi: 10.1164/ajrccm/147.5.1287. [DOI] [PubMed] [Google Scholar]
- 56.Vandenplas O, Malo JL, Cartier A, Perreault G, Cloutier Y. Closed-circuit methodology for inhalation challenge tests with isocyanates. Am Rev Respir Dis. 1992;145:582–587. doi: 10.1164/ajrccm/145.3.582. [DOI] [PubMed] [Google Scholar]