Abstract
In the Syrian hamster (Mesocricetus auratus) glutamate activity has been implicated in the modulation of adolescent anabolic-androgenic steroid (AAS)-induced aggression. The current study investigated the time course of adolescent AAS-induced neurodevelopmental and withdrawal effects on the glutamatergic system and examined whether these changes paralleled those of adolescent AAS-induced aggression. Glutamate activity in brain areas comprising the aggression circuit in hamsters and aggression were examined following 1, 2, 3 and 4 weeks of AAS treatment or 1, 2, 3 and 4 weeks following the cessation of AAS exposure. In these studies glutamate activity was examined using vesicular glutamate transporter 2 (VGLUT2). The onset of aggression was observed following 2 weeks exposure to AAS and continued to increase showing maximal aggression levels after 4 weeks of AAS treatment. This aggressive phenotype was detected after 2 weeks of withdrawal from AAS. The time-course of AAS-induced changes in latero anterior hypothalamus (LAH)-VGLUT2 closely paralleled increases in aggression. Increases in LAH-VGLUT2 were first detected in animals exposed to AAS for 2 weeks and were maintained up to 3 weeks following the cessation of AAS treatment. AAS treatment also produced developmental and long-term alterations in VGLUT2 expression within other aggression areas. However, AAS-induced changes in glutamate activity within these regions did not coincide with changes in aggression. Together, these data indicate that adolescent AAS treatment leads to alterations in the glutamatergic system in brain areas implicated in aggression control, yet only alterations in LAH-glutamate parallel the time course of AAS-induced changes in the aggressive phenotype.
Keywords: Syrian hamster, aggression, glutamate, AAS, development, withdrawal
1. INTRODUCTION
During the past decades, the naturally occurring hormone testosterone and its synthetic derivates (collectively termed anabolic androgenic steroids [AAS]) have been used by athletes and bodybuilders to enhance athletic performance and improve physical appearance (Yesalis, Kennedy, Kopstein, & Bahrke, 1993). Of particular concern is the increased use of AAS by the adolescent population, with more than a half million 8th-10th graders in the United States reporting AAS use each year and a lifetime use in males of almost 2% in 2007 (NIDACapsules, 2007). This pattern of abuse is important since AAS is highly associated with increases in anger, hostility, antisocial behaviors and violent acts in adolescents (Dabbs, Jurkovic, & Frady, 1991; Finkelstein et al., 1997; Mattsson, Schalling, Olweus, Low, & Svensson, 1980; Scerbo & Kolko, 1994). In fact, recent results showed that 7th to 12th graders exposed to AAS were more likely to be involved in serious violent acts (Beaver, Vaughn, Delisi, & Wright, 2008). Moreover, early onset of AAS use is associated with more frequent and heavier use later in life despite its negative psychological and physiological consequences, including aggressive behavior (Pope & Katz, 1994; Su et al., 1993). Thus, this population of AAS users may comprise a considerable portion of the stable, long-term AAS abusing population, increasing the risk for the numerous behavioral and psychiatric effects such as increased aggression and hostility. To date, however, little is known about the time course of developmental neuroplastic events underlying the emergence of the aggressive phenotype and how these changes may be associated with the long-term negative consequences of adolescent AAS use.
In our laboratory we have used subadult male Syrian hamsters (Mesocricetus auratus) as an adolescent animal model to investigate the link between developmental AAS exposure and the behavioral neurobiology of offensive aggression (Melloni & Ricci, 2009). In this animal model, exposure to AAS during a relatively short period (i.e., 2 weeks) or the majority (i.e., 4 weeks) of adolescence results in animals with elevated aggression levels (Grimes, Ricci, & Melloni, 2007; Ricci, Grimes, & Melloni, 2007). In addition, Syrian hamsters administered AAS during 4 weeks of adolescence maintain this heightened aggressive phenotype for up to 2 weeks after the cessation of AAS treatment (Grimes & Melloni, 2006; Grimes, Ricci, & Melloni, 2006). These findings that animals administered AAS throughout adolescence display escalated aggression in the absence of prior social interactions and dominant cues and that the aggressive phenotype is observed after cessation from AAS suggests that: 1) AAS may stimulate aggression by altering the normal development or activity of the neural circuit regulating aggression and 2) AAS may induce persistent increases in aggression levels by producing lasting changes in the circuit involved in this response.
In hamsters, the anterior hypothalamus (AH) appears to be at the center of a neural network modulating offensive aggression (Delville, De Vries, & Ferris, 2000). The AH shares reciprocal connections with various brain regions implicated in aggression control including, the lateral septum (LS), bed nucleus of the stria terminalis (BNST), medial amygdala (MeA), central amygdala (CeA) and ventrolateral hypothalamus (VLH) (Delville et al., 2000). The activity of this neural network is, regulated at least partially, by the activity of the centrally released neuropeptide vasopressin (AVP) in the AH (Ferris & Delville, 1994; Grimes et al., 2006, 2007; Harrison, Connor, Nowak, Nash, & Melloni, 2000). Within this brain region, AVP facilitates aggression and the activity of this neuropeptide correlates with the expression of the aggressive phenotype (Harrison et al., 2000). In fact, the time course of AAS-induced neurodevelopmental and long-term changes in aggression coincide with alterations in the AVPergic system (Grimes et al., 2006, 2007). For example, AAS-induced increases in AVP fiber density and peptide production are first observed in adolescent animals treated with AAS for 2 weeks (i.e., time when aggression emerges). Moreover, similar to AAS-induced long-term changes in aggression, AH-AVP remains elevated up to 2 weeks after cessation from AAS and return to baseline levels following 3 weeks of AAS withdrawal. These data indicate that AAS-induced changes in AH-AVP play a key role on the behavioral phenotype of adolescent AAS-treated hamsters.
The facilitatory role AH-AVP has on aggression has been hypothesized to occur through modulation of the AH-aggression output system, likely glutamate (Ferris et al., 1997; Ricci et al., 2007). In the AH, glutamate is the predominant excitatory amino acid neurotransmitter showing intense immunoreactivity as well as wide receptor expression (i.e., α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate [AMPA] and N-methyl-D-aspartate [NMDA]) (Eyigor, Centers, & Jennes, 2001; Meeker, Greenwood, & Hayward, 1994; Meeker, Swanson, Greenwood, & Hayward, 1993; van den Pol, 1991; van den Pol, Hermans-Borgmeyer, Hofer, Ghosh, & Heinemann, 1994; van den Pol & Trombley, 1993). In addition, within this region, glutamate modulates the majority of excitatory postsynaptic potentials (EPSPs) (Brann & Mahesh, 1995; van den Pol & Trombley, 1993). Previous reports from our laboratory have shown AAS-mediated alterations in hypothalamic glutamate activity (Carrillo, Ricci, & Melloni, 2009; Fischer, Ricci, & Melloni, 2007). For instance, a significant increase in phosphate activated glutaminase (PAG; the rate limiting enzyme for glutamate production) levels was detected within the AH of highly aggressive AAS-treated animals. Adolescent AAS-treated animals also exhibited a significant increase in the number of glutamate cells expressing FOS (i.e., a marker of neuronal activation), suggesting that AAS treatment not only stimulates glutamate production but also enhances the activity of glutamate producing cells (Carrillo et al., 2009). Interestingly, increases in PAG and in PAG cells expressing FOS were primarily observed in the latero-anterior portion of the AH (LAH), localized between the medial supraoptic nucleus (mSON) and nucleus circularis (NC) (Paxinos & Watson, 1997). These findings indicate that glutamate activity within the LAH is particularly sensitive to adolescent AAS exposure. Notably, AAS-induced modifications in glutamate activity were also detected in various brain regions sharing reciprocal connections with the AH and part of the neural circuit regulating aggression including, the LS, BNST, MeA, CeA and VLH (Fischer et al., 2007). For example, aggressive AAS-treated hamsters have increased glutamate production within the LS and MeA. Further, animals exposed to AAS throughout adolescence exhibit elevated expression of the glutamate receptor 1 subunit of the AMPA receptor within the VLH and BNST. Together, these data suggest that AAS-mediated changes in the glutamatergic system within the aggression neural circuitry might underlie the aggressive phenotype observed in hamsters administered AAS during adolescence. To date, however, the temporal relationship between aggression levels and glutamate activity during development and following the cessation of AAS exposure is unknown.
Given these data we questioned whether the developmental and long term time course of AAS-induced changes in aggression parallel alterations in glutamate activity in the aggression neural circuitry. In previous studies we have used PAG as a marker for glutamate cells (Carrillo et al., 2009; Fischer et al., 2007). However, immunohistochemical results have indicated a potential confound for selective identification of glutamate, as the substrate for PAG has also been localized to □-aminobutyric acid (GABA) cells (Kaneko & Fujiyama, 2002). Thus, the first study validates previous findings using vesicular glutamate transporter 2 (VGLUT2; a more specific immunohistochemical marker for identification of glutamate producing cells). Then to examine the time course of AAS-induced neurodevelopmental changes in glutamate activity within brain regions modulating aggression expression, and more specifically to investigate whether changes in glutamate parallel the time course of AAS-induced increases in aggression, aggression and glutamate activity were examined following 1, 2, 3 and 4 weeks of AAS treatment, using immunohistochemistry. Lastly, to investigate whether changes in glutamate activity parallel the long-term effects chronic AAS exposure has on aggression, aggression and glutamate activity was examined following 1, 2, 3 and 4 weeks after cessation from AAS exposure.
2. MATERIALS AND METHODS
2.1. Animals
Male Syrian hamsters (Mesocricetus auratus) postnatal day 21 (P21) were obtained from Charles River Laboratories (Wilmington, MA), individually housed in polycarbonate cages and maintained at ambient room temperature (22-24 °C, with 55% relative humidity) on a reverse light dark cycle (14:10D; lights off at 08:00). Food and water were provided ad libitum. For aggression testing stimulus (intruder) male hamsters of equal size and weight to experimental animals were obtained from Charles River Laboratories one week prior to the behavioral test, group housed at five animals per cage in large polycarbonate cages and maintained as above to acclimate to the animal facility. One day prior to aggression test, all intruders were prescreened for low social interest (i.e., disengage and evade) and submission (i.e., tail-up freeze, flee and fly-away) to control for behavioral differences between stimulus animals. Stimulus animals displaying no social interest and/or submissive postures (2-3%) were excluded from use in the behavioral assay (Ferris et al., 1997; Grimes & Melloni, 2006; Melloni, Connor, Hang, Harrison, & Ferris, 1997). All studies using live animals were preapproved by the Northeastern University Institutional Animal Care and Use Committee (NU-IACUC) and all methods used were consistent with guidelines provided by the National Institute of Health for the scientific treatment of animals.
2.2. Experimental procedures
2.2.1. AAS treatment
For Experiment 1, Syrian hamsters (P28) were weighed and randomly distributed into two groups (n = 7 animals/group). One group of animals received daily subcutaneous (SC) injections of an AAS cocktail composed of 2mg/kg testosterone cypionate, 2mg/kg nandrolone decanoate and 1mg/kg boldenone undecylenate (Steraloids, Newport, RI) for 30 consecutive days (P28-P57) as previously described (Carrillo et al., 2009). This daily treatment of AAS was designed to mimic a chronic regimen (Pope & Katz, 1994). The second group of hamsters received SC injections of sesame oil (SO) vehicle. One day following the last injection (P58), animals were tested for offensive aggression (as detailed below). Animals were then sacrificed and brains removed and processed for immunohistochemical analysis.
For Experiment 2 (developmental study), hamsters (P28) were weighed and randomly distributed into 4 groups (Groups 1-4) corresponding to the time at which animals would be tested for offensive aggression and sacrificed for immunohistochemical analysis. Each group was divided into two drug treatment subgroups; those administered the AAS cocktail (i.e., same as in Experiment 1) and those administered SO (vehicle control). Hamsters (n = 5-7 animals/drug treatment/group) received daily SC injections of the AAS mixture or SO for 7 (Group 1), 14 (Group 2), 21 (Group 3) or 28 (Group 4) consecutive days. One day following the last injection animals were tested for offensive aggression and then sacrificed 24 hours later for immunohistochemical analysis. Specifically, hamsters in Group 1 were tested for aggression after 7 days of exposure to AAS (P35; 1 week of AAS/SO treatment) and sacrificed the following day (P36), while hamsters in Group2, Group 3 and Group 4 were tested for aggression following 14 (P42; 2 weeks of AAS treatment), 21 (P49; 3 weeks of AAS treatment) and 28(P56; 4 weeks of AAS treatment) days of exposure to AAS and sacrificed on P43, P50 and P57.
The experimental design for Experiment 3 (withdrawal study) was similar to that of Experiment 2. At P28 animals were weighted and randomly distributed into four groups (Groups 5-8) corresponding to the time at which animals would be tested for offensive aggression and sacrificed for immunohistochemical analysis. Each group was divided into AAS and SO treatment groups (i.e., as in Experiment 1). Hamsters (n = 5-7 animals/drug treatment/group) received daily SC injections of the AAS mixture or SO for 30 consecutive days. Following the last injection day, animals were tested for offensive aggression and then 24 hours later sacrificed for immunohistochemical analysis at varied time points of AAS withdrawal. Specifically, hamsters in Group 5 were tested for aggression 6 days after the last AAS/SO injection (P63-1 week withdrawal) and then sacrificed 24 hours later (P64) while hamsters in Group 6, 7, and 8 were tested for aggression following 12 (P70-2 weeks withdrawal), 20 (P77-3 weeks withdrawal), and 27 (P84; 4 weeks withdrawal) days of AAS/SO injections and sacrificed on P71, P78 and P85.
2.2.2. Aggression testing
Experimental animals were tested for offensive aggression using the resident-intruder paradigm, a well characterized and ethologically valid model of offensive aggression in Syrian hamsters (Floody & Pfaff, 1977; Lerwill, 1971). Briefly, an intruder of similar size and weight was introduced into the home cage of the experimental animal (resident) and the resident was scored for specific and targeted aggressive responses including, upright offensive postures, lateral attacks, chases, and flank and rump bites as previously described (Grimes & Melloni, 2006). An attack was scored each time the resident animal would pursue and then either: (1) lunge toward and/or (2) confine the intruder by upright and sideways threat; each generally followed by a direct attempt to bite the intruder’s dorsal rump and/or flank target area. Composite Aggression Score (Grimes et al., 2006), used as a general measure of offensive aggression, was defined as the total number of attacks (i.e., upright offensives and lateral attacks) and bites (i.e., head and neck bites and flank and rump bites) during the behavioral test period. Each aggression test lasted for 10 minutes and was scored by two independent observers blind to experimental treatment. No stimulus animal was used for more than one behavioral test and all animals were tested during the first 4 hours of the dark cycle under dim-red illumination to control for circadian influence on behavioral responding.
2.2.3. Immunohistochemistry
One day following behavioral test for aggression (P59), hamsters were anesthetized with sodium pentobarbital (90mg/kg) and transcardially perfused with a 21°C saline rinse followed by 4% paraformaldehyde. Brains were removed, post-fixed for 90 minutes in perfusion fixative and cryoprotected in 30% sucrose solution at 4°C overnight Brains were cut at 35μm using a freezing microtome in serial, coronal sections and all subsequent immunohistochemical procedures were conducted at 21°C unless otherwise specified. Sections were washed 3 times for 5 minutes in 0.1M phosphate buffer saline (PBS) and pre-incubated in a solution containing 5% bovine serum albumin (BSA) and 5% normal horse serum (NHS) in 0.1M PBS for 60 minutes. Sections were then incubated in goat anti-VGLUT2 primary antiserum (Santa Cruz Biotechnology, CA, USA) diluted 1:3000 and left overnight at 4°C. Subsequently sections were incubated in secondary horse anti-goat antiserum (1:200) for 60 minutes followed by incubation with avidin-biotin complex (Vectastain ABC elite, Burlingame, CA). The peroxidase reaction was revealed using 0.5% 3,3′-diaminobenzidine in distilled water, per manufacturer’s recommendations (DAB, Vector Labs, Burlingame, CA). Sections were mounted on gelatin-coated slides, allowed to air-dry and dehydrated through a series of ethanol and xylene solutions. Then, slides were coverslipped using cytoseal-60 mounting medium (VWR scientific, West Chester, PA, USA). Together with the above immunohistochemical assay, brain sections omitting the primary and secondary antibodies were processed for control purposes.
2.2.4. Image analysis
In Experiments 1, 2 and 3, VGLUT2-ir was examined within aggression brain regions using BIOQUANT NOVA 5.0 computer assisted microscopic analysis software package as previously described (Carrillo et al., 2009). The areas analyzed were selected based on previous data showing these regions as part of the neural circuit regulating AAS-induced aggressive behavior (Carrillo et al., 2009; Delville et al., 2000; Ricci et al., 2007). The areas analyzed included the LAH in Experiment 1 and the LAH, LS, MeA, CeA, VLH and BNST in Experiments 2 and 3 (Figure 1). In Experiments 1, 2 and 3, glutamate activity within these brain regions was examined by quantifying the number of VLGUT2-ir cell bodies. In addition, in Experiments 2 and 3, the number of VLGUT2-ir fibers was examined. BIOQUANT NOVA 5.0 image analysis software running on a Pentium III CSI open PC computer (R&M Biometrics, Nashville, TN, USA) was utilized to identify the brain regions of interest (ROI) at low power (4X) using a Nikon E600 microscope. A standard computer-generated box was drawn to fit within a particular ROI at 4X. Positively stained cells or fibers were identified using a mouse driven cursor for quantification. Following identification of the ROI at 4X, manual measurements were done at 20X (Experiments 2 and 3) and 40X (Experiment 1) until all cells bodies and/or fibers were counted throughout the entire ROI. Three to six independent measures were taken from several consecutive sections for each animal. All cell or fiber counts were averaged for each brain region per hamster and used for statistical analysis.
Figure 1.
Diagram showing the location of the brain areas selected to quantify VGLUT2. Platesfrom pages 50, 54 and 59 of A Stereotaxic Atlas of the Golden hamster Brain were modified and reflect specific positions in the rostrocaudal plane ((Morin & Wood, 2001)(i.e., distance in millimeters from bregma to the plane section at the skull surface). Abbreviations: LS, lateral septum; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; AH, anterior hypothalamus; LAH, ventro-lateral portion of the AH; MeA, medial amygdala; VLH, ventrolateral hypothalamus.
2.2.5. Statistics
Aggression results (i.e., composite aggression score) from Experiment 1 were compared between AAS- and SO-treated groups Student’s t-test (two-tailed). Immunohistochemical data from Experiment 1 were compared between AAS- and SO-treated animals using Student’s t-test (two-tailed). Aggression and immunohistochemical results from Experiments 2 and 3 were analyzed using two-way ANOVA (treatment × time point) followed by Fisher’s protected least significant test post hoc when applicable (AAS vs SO for each time group). The alpha level for all experiments was set at 0.05.
3. RESULTS
3.1. Experiment 1
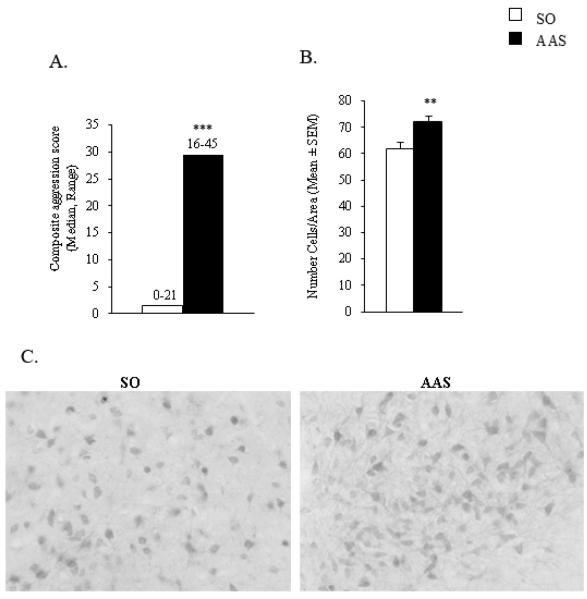
As previously reported in several studies, exposure to AAS throughout adolescence results in a significant increase in offensive aggression as measured using a composite aggressive score [t(12) = 5.3, p < 0.0001 ] (Figure 2A). In fact, AAS-treated animals exhibited a six-fold increase in composite aggression score compared to control animals. Interestingly, examination of VGLUT2-ir in the AH showed that this glutamate marker is expressed in both cell bodies (i.e., mainly bipolar like cells) and fibers. Moreover, neuroanatomical analysis revealed a dense VGLUT2 -ir staining pattern within the ventro-lateral portion of the AH (LAH; region located between the mSON and NC) of AAS-treated animals, compared to controls. Indeed, the number of VGLUT2-ir cells in the LAH of AAS-treated animals was significantly higher in AAS-treated animals compared to controls [t (16) = 2.13, p < 0.01] (Figure 2B and 2C).
Figure 2.
(A) Adolescent AAS treatment increases offensive aggression. Composite aggression scores in SO- (white bar) and AAS- (black bar) treated hamsters. (B) Number of VGLUT2 immunopositive cells in the latero-anterior hypothalamus (LAH) of SO- (white bar) and AAS- (black bar) treated animals. (C) Brightfield photomicrographs at 20× magnification showing VGLUT2-ir cells in the LAH of SO- and AAS-treated animals. **p < 0.01, ***p < 0.005, Student’s t-test (two-tailed).
3.2. Experiment 2
Similar to previous results (Grimes et al., 2007), behavioral data from the current study showed that exposure to AAS throughout adolescence resulted in a time-dependent increase in overall aggression score [F(3,44) = 15.7, p < 0.0001], such that the longer hamsters were exposed to AAS the higher were their aggression levels compared to controls (Figure 3). Closer investigation revealed that the emergence of aggressive behavior occurred after only 2weeks of exposure to AAS (p < 0.05). Notably, following the onset of aggressive behavior, aggression levels continued to rise after 3 weeks (p < 0.0001) and 4 weeks (p < 0.0001) of exposure to AAS. Within AAS group comparison, showed that animals exposed to AAS for 1 week had significantly reduced aggression levels compared to animals treated with AAS for 2, 3 and 4 weeks (p < 0.05 for week 2 and p < 0.0001 for weeks 3 and 4). Moreover, animals exposed to AAS for 2 weeks also exhibited reduced aggression levels when compared to animals treated with AAS for 3 and 4 weeks (p < 0.0001). Lastly, no significant changes in aggression levels were detected between animals exposed to AAS for 3 and 4 weeks.
Figure 3.
Composite aggression scores during the time course of AAS/SO treatment. Comparisons to SO-treated animals. *p < 0.05, ****p < 0.001, two-way ANOVA (treatment × time point) followed by Fisher’s protected least significant post hoc (AAS vs SO for each time group).
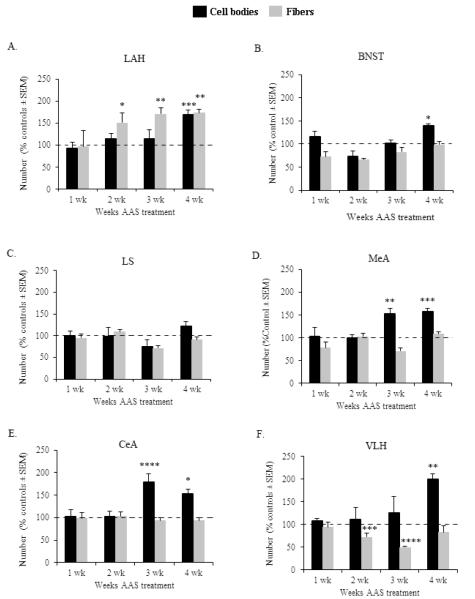
Animals exposed to AAS throughout adolescence exhibited significant alterations in the developmental pattern of VGLUT2-ir within the LAH compared to controls (Figure 4A, Table 1). Specifically, compared to SO-treated controls, AAS-treated animals showed significant changes in the expression pattern of both VGLUT2-ir cell bodies [F(3,44) = 2.946, p < 0.05] and fibers [F(3,44) = 3.776, p < 0.01] throughout the 4 weeks of AAS treatment. When examined in more detail, the results showed that AAS-dependent changes in the number of VGLUT2-ir cell bodies occurred after 4 weeks of treatment. At this time point, a significant increase in the number of LAH-VGLUT2-ir cell bodies was observed compared to control animals (p < 0.005). Interestingly, AAS-induced increase in LAH-VGLUT2-ir fibers occurred following a shorter period of exposure to AAS (i.e., 2 weeks) compared to the effect of AAS on VGLUT2-ir cell bodies. At this time, increases in VGLUT2-ir fibers were significantly higher in AAS-treated animals compared to controls (p < 0.05). Moreover, the number of LAH-VGLUT2-ir fibers remained significantly elevated following 3 (p < 0.01) and 4 (p < 0.01) weeks of AAS administration.
Figure 4.
Graph depicts the time course of AAS-induced neurodevelopmental changes in VGLUT2-immunoreactive cell bodies (black bars) and fibers (grey bars) within the (A) latero-anterior hypothalamus (LAH), (B) bed nucleus of the stria terminalis (BNST), (C) lateral septum (LS), (D) medial amygdala (MeA), (E) central amygdala (CeA) and (F) ventrolateral hypothalamus (VLH) of animals treated with AAS for 1, 2, 3 and 4 weeks. Data are expressed as percentage of vehicle responding, as indicated by the 100% dashed lines in graph. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001, two-way ANOVA (treatment × time point) followed by Fisher’s protected least significant post hoc (AAS vs SO for each time group).
Table 1.
Summary results (i.e., mean and standard error to mean [SEM]] of the time course of AAS-induced developmental effects on VGLUT2-immunoreactive cell bodies and fibers within the latero-anterior hypothalamus (LAH), bed nucleus of stria terminalis (BNST), lateral septum (LS), medial amygdala (MeA), central amygdala (CeA) and ventrolateral hypothalamus.
| Cell bodies | Fibers | ||||
|---|---|---|---|---|---|
| Brain | Time | AAS | SO | AAS | SO |
| Area | Point | Mean | Mean | Mean | Mean |
| LAH | Week 1 | 210.9 ± 28.1 | 226.2 ± 28.1 | 169.2 ± 11.2 | 171.9 ± 17.8 |
| Week2 | 145.7 ± 14.4 | 127.3 ± 43.8 | 158.1 ± 23.1* | 104.8 ± 15.5 | |
| Week 3 | 190.8 ± 32.3 | 166.6 ± 49.9 | 158.7 ± 10.3** | 93 ± 10.7 | |
| Week 4 | 382.01 ± 21.1*** | 225.4 ± 59.9 | 160 ± 7.4** | 92.3 ± 8.6 | |
| BNST | Week 1 | 292.2 ± 22.4 | 231.2 ± 25.3 | 226 ± 33.8 | 312.4 ± 29.8 |
| Week2 | 187.1 ± 37.3 | 254.1 ± 26.7 | 338 ± 12.7 | 444 ± 37.9 | |
| Week 3 | 338.6 ± 24.8 | 332.4 ± 22.1 | 265.6 ± 40.2 | 395.6 ± 7.2 | |
| Week 4 | 330.9 ± 8.3* | 237.3 ± 36.4 | 332.4 ± 23.2 | 336.8 ± 8.1 | |
| LS | Week 1 | 137.9 ± 8.2 | 131.7 ± 10.4 | 220 ± 18.7 | 231.3 ± 12.7 |
| Week2 | 76.2 ± 17.4 | 77.2 ± 20.5 | 271.6 ± 13.9 | 248.8 ± 23.8 | |
| Week 3 | 79.3 ± 23.8 | 117.9 ± 15.3 | 195.6 ± 16.3 | 274 ± 15.1 | |
| Week 4 | 116.7 ± 9.6 | 95.1 ± 7.9 | 244 ± 17.1 | 269.6 ± 15.7 | |
| MeA | Week 1 | 196.9 ± 36.3 | 188.8 ± 14.9 | 206.3 ± 36.8 | 280.9 ± 15.4 |
| Week2 | 244.8 ± 16.2 | 243.6 ± 10.1 | 358.4 ± 22.4 | 348 ± 58.9 | |
| Week 3 | 328.6 ± 24.6** | 214.1 ± 39.3 | 212.4 ± 18.6 | 299.2 ± 37.4 | |
| Week 4 | 314.6 ± 14.1*** | 207.5 ± 37.1 | 302.8 ± 11.9 | 278 ± 40.3 | |
| CeA | Week 1 | 172.4 ± 17.2 | 167.5 ± 36.7 | 229.7 ± 37.6 | 237.7 ± 25.6 |
| Week2 | 73.02 ± 9.6 | 73.4 ± 22.8 | 240.8 ± 23.4 | 233.6 ± 21 | |
| Week 3 | 278.3 ± 28.3**** | 131.9 ± 31.1 | 246 ± 16.5 | 262 ± 48.2 | |
| Week 4 | 255.3 ± 17.8* | 166.6 ± 20.9 | 266.9 ± 15.6 | 285.6 ± 16.2 | |
| VLH | Week 1 | 207.8 ± 10.9 | 193.1 ± 24 | 276.2 ± 32.9 | 294.3 ± 31.5 |
| Week2 | 186.1 ± 42.4 | 167.3 ± 65.5 | 319.6 ± 36.1*** | 444 ± 37.9 | |
| Week 3 | 146.7 ± 41.9 | 117.1 ± 41.3 | 196.4 ± 10.6**** | 395.6 ± 7.2 | |
| Week 4 | 266.8 ± 18.3** | 133.7 ± 16.8 | 277.2 ± 50.3 | 336.8 ± 8.1 | |
Comparisons to SO-treated animals: p < 0.05,
p < 0.01,
p < 0.005,
p < 0.001, two-way ANOVA (treatment × time point) followed by Fisher’s protected least significant post hoc (AAS vs SO for each time group).
Treatment with AAS during adolescence also induced changes in the developmental time course of VGLUT2-ir cell bodies in various brain regions involved in aggression control and sharing reciprocal connections with the AH, including the BNST [F(3,42) = 2.772, p < 0.05], MeA [F(3,43) = 3.015, p < 0.05], CeA [F(3,42) = 3.034, p < 0.05] and VLH [F(3,44) = 3.81, p < 0.01] (Figure 4B, 4D, 4E, 4F and Table 1). Similar to the LAH, augmented number of VGLUT2-ir cell bodies within the BNST and VLH were only observed after 4 weeks of treatment with AAS (BNST: p < 0.05 VLH: p < 0.01) (Figures 4B and 4F). Moreover, compared to controls, increases in VGLUT2-ir cell bodies within the MeA and CeA were detected after a shorter period of AAS exposure (i.e., 3 weeks, MeA: p < 0.01; CeA: p < 0.0001) (Figures 4D and 4E). This elevated number of VGLUT2-ir cell bodies within the MeA and CeA remained significantly elevated following 4 weeks of treatment with AAS (MeA: p < 0.005; CeA: p < 0.05). In contrast, no differences between AAS- and SO-treated animals in the number of VGLUT2-ir cell bodies were observed in the LS throughout the 4 weeks of AAS treatment (p > 0.05 for all comparisons) (Figure 4C). Further analysis showed that opposite to AAS-induced increases in the number of VGLUT2-ir cell bodies within the BNST, MeA and CeA, AAS exposure did not alter the number of VGLUT2-ir fibers within these brain regions (Figures 4B, 4D and 4E). However, significant alterations in the number of VGLUT2-ir fibers were detected in the VLH of adolescent AAS-treated animals (Figure 4F). Specifically, animals administered AAS for 2 and 3 weeks showed significant reductions in the number of VLH-VGLUT2-ir fibers compared to SO-treated controls (2 weeks: p < 0.005; 3 weeks: p < 0.0001). Lastly, AAS- and SO-treated animals had comparable levels in LS-VGLUT2-ir fibers throughout the 4 weeks of treatment.
3.3. Experiment 3
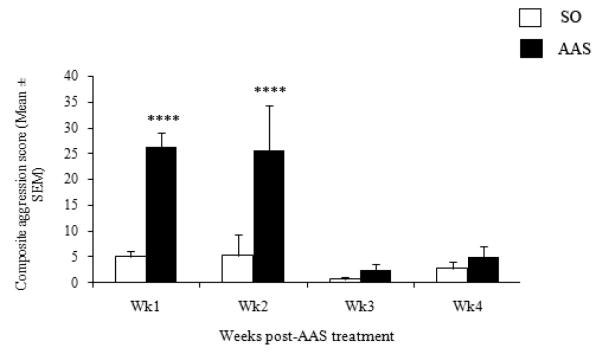
In agreement with previous data (Grimes & Melloni, 2006; Grimes et al., 2006), the current results showed that administration of AAS throughout the majority of adolescence leads to long-term alterations in aggressive behavior [F(3,52) = 6.332, p < 0.001] (Figure 5). Particularly, elevated levels of aggression (i.e., measured using composite aggression score) were observed following 1 and 2 weeks of withdrawal from AAS, compared to vehicle-treated hamsters (1 week: p < 0.00001, 2 weeks: p < 0.0001). Interestingly, animals withdrawn from AAS for three weeks exhibited a rapid reduction in aggression levels (i.e., compared to animals withdrawn from AAS for 2 weeks) that was comparable to controls (p > 0.05). Similar to animals withdrawn from AAS for 3 weeks, hamsters withdrawn from AAS for 4 weeks continued to display low aggression levels that were not significantly different from controls (p > 0.05). Within AAS group comparisons indicated that while animals withdrawn from AAS for 1 and 2 weeks had comparable aggression levels (p > 0.05), both of these groups had significantly elevated aggression levels compared to animals withdrawn from AAS for 3 and 4 weeks (p < 0.0001). Moreover, no differences in aggression levels were detected between animals withdrawn from AAS for 3 and 4 weeks (p > 0.05).
Figure 5.
Composite aggression scores following 1, 2, 3 and 4 weeks withdrawal from AAS treatment. Comparisons to SO-treated animals. ****p < 0.001, two-way ANOVA (treatment × time point) followed by Fisher’s protected least significant post hoc (AAS vs SO for each time group).
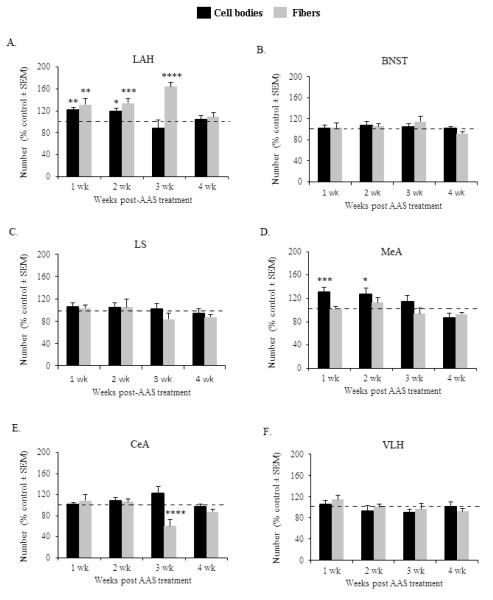
Investigation of immunohistochemical results showed that adolescent AAS exposure produced long-term alterations in the number of LAH-VGLUT2-ir cell bodies [F(3,44) = 3.03, p < 0.05] and fibers [F(3,44) = 2.85, p < 0.05] (Figure 6A, Table 2). Notably, treatment with AAS during adolescence produced long-term increases in the number of VGLUT2-ir cells within the LAH of male Syrian hamsters. Specifically, an elevated number of LAH-VLGUT2-ir cell bodies was detected following 1 (p < 0.01) and 2 weeks (p < 0.05) of withdrawal from AAS compared to controls. However, following 3 and 4 weeks withdrawal from AAS, the number of VLGUT2-ir cell bodies in the LAH returned to baseline levels and was comparable to controls (p > 0.05 for 3 and 4 weeks). Similar to the long term effect of AAS on LAH-VGLUT2-ir cell bodies, adolescent AAS exposure produced significant long-term increases in the number of LAH-VGLUT2-ir fibers. In particular, compared to SO-treated controls, animals exposed to AAS throughout adolescence showed significantly higher number of LAH-VGLUT2-ir fibers following 1 (p < 0.01), 2 (p < 0.005) and 3 (p < 0.0001) weeks of withdrawal from AAS. However, these effects were not permanent, given that the number of LAH-VGLUT2-ir fibers returned to baseline levels after 4 weeks cessation of AAS (p > 0.05).
Figure 6.
Graph depicts the time course of AAS-induced long-term changes in VGLUT2-immunoreactive cell bodies (black bars) and fibers (grey bars) within the (A) latero anterior hypothalamus (LAH), (B) bed nucleus of the stria terminalis (BNST), (C) lateral septum (LS), (D) medial amygdala (MeA), (E) central amygdala (CeA) and (F) ventrolateral hypothalamus (VLH) of animals withdrawn from AAS for 1, 2, 3 and 4 weeks. Data are expressed as percentage of vehicle responding, as indicated by the 100% dashed lines in graph. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001, two-way ANOVA (treatment × time point) followed by Fisher’s protected least significant post hoc (AAS vs SO for each time group).
Table 2.
Summary results (i.e., mean and standard error to mean [SEM]) of the time-course of long-term AAS withdrawal effects on VGLUT2-immunoreactive cell bodies and fibers within the latero-anterior hypothalamus (LAH), bed nucleus of stria terminalis (BNST), lateral septum (LS), medial amygdala (MeA), central amygdala (CeA) and ventrolateral hypothalamus.
| Cell bodies | Fibers | ||||
|---|---|---|---|---|---|
| Brain | Time | AAS | SO | AAS | SO |
| Area | Point | Mean | Mean | Mean | Mean |
| LAH | Week 1 | 419.5 ± 17.4** | 344.4 ± 11.7 | 166.7 ± 14.3** | 128.4 ± 5.9 |
| Week2 | 414.9 ± 8.4* | 334 ± 27.5 | 189.9 ± 13.1*** | 142.1 ± 9.5 | |
| Week 3 | 319.8 ± 39 | 360 ± 43.5 | 177.7 ± 38.9**** | 109.2 ± 4.6 | |
| Week 4 | 287.7 ± 19.6 | 272.8 ± 18.9 | 118.9 ± 6.1 | 108.3 ± 12.5 | |
| BNST | Week 1 | 402.9 ± 23.5 | 384.9 ± 25.9 | 299.7 ± 28.8 | 291.1 ± 21.9 |
| Week2 | 453.1 ± 16.2 | 443.1 ± 35.9 | 337.2 ± 17.5 | 320.4 ± 30.1 | |
| Week 3 | 497.6 ± 20.4 | 456.3 ± 40 | 341.5 ± 29.6 | 297.2 ± 24.4 | |
| Week 4 | 327.2 ± 10.4 | 318.9 ± 23.7 | 327.1 ± 13.5 | 355.1 ± 15.9 | |
| LS | Week 1 | 144.7 ± 8.8 | 135.9 ± 9.4 | 228.9 ± 10.5 | 221.3 ± 7.5 |
| Week2 | 159.7 ± 11.2 | 151.3 ± 3.9 | 206.5 ± 27.4 | 195.6 ± 16.4 | |
| Week 3 | 170.1 ± 15.9 | 166.3 ± 5.1 | 238.5 ± 29.3 | 285 ± 8.5 | |
| Week 4 | 114.03 ± 8.6 | 120.5 ± 12.9 | 226.2 ± 11.7 | 258 ± 13.2 | |
| MeA | Week 1 | 293.4 ± 17.2*** | 225.3 ± 8.2 | 271.1 ± 12.1 | 268.3 ± 20.5 |
| Week2 | 304.5 ± 19.3* | 239.1 ± 26.6 | 345.7 ± 25.3 | 308 ± 27.6 | |
| Week 3 | 307.6 ± 19.9 | 267.1 ± 27.9 | 300 ± 30.9 | 320 ± 9.7 | |
| Week 4 | 234.5 ± 19.3 | 269.1 ± 26.3 | 312.2 ± 10.5 | 336.9 ± 15.5 | |
| CeA | Week 1 | 352.8 ± 10 | 328.2 ± 21.3 | 228.3 ± 23.1 | 209.8 ± 9.3 |
| Week2 | 303.9 ± 17.3 | 278.8 ± 40.1 | 220.8 ± 10.4 | 207.6 ± 12.5 | |
| Week 3 | 383.4 ± 38.3 | 310.9 ± 33 | 156.2 ± 30.1**** | 258.8 ± 30.7 | |
| Week 4 | 279.2 ± 10.8 | 284.3 ± 33 | 282.2 ± 15 | 320.7 ± 10.1 | |
| VLH | Week 1 | 475.6 ± 27.2 | 448.8 ± 12.5 | 316 ± 21.7 | 275.5 ± 19.8 |
| Week2 | 401.3 ± 35.9 | 427.8 ± 36.5 | 341 ± 18.8 | 340.5 ± 58.5 | |
| Week 3 | 415.9 ± 28.7 | 462.2 ± 26.8 | 348.5 ± 39.1 | 360.8 ± 36.1 | |
| Week 4 | 311.5 ± 22.2 | 305.7 ± 25/2 | 322.4 ± 17.2 | 348.9 ± 16.2 | |
Comparisons to SO-treated animals: p < 0.05,
p < 0.01,
p < 0.005,
p < 0.001, two way ANOVA (treatment × time point) followed by Fisher’s protected least significant post hoc (AAS vs SO for each time group).
Of all other brain regions examined, long-term effects in the number of VGLUT2-ir cell bodies were only detected in the MeA [F(3,49) = 4.842, p < 0.01] (p > 0.05 for all other brain areas, (Figure 6, Table 2.). Data indicated that compared to vehicle-treated hamsters the number of VGLUT-ir cell bodies within the MeA was significantly elevated following 1 and 2 weeks of AAS withdrawal (1 week: p < 0.005; 2 weeks: p < 0.05) (Figure 6D). However, the number of VGLUT2-ir cell bodies returned to control levels after 3 weeks cessation of AAS (p > 0.05) and remained comparable to controls after 4 weeks withdrawal (p > 0.05). Further investigation showed that adolescent AAS administration had long-term effects on CeA-VGLUT2-ir fibers [F(3,49) = 3.798, p < 0.01] (Figure 6E). Close inspection revealed that although there were no significant differences in the number of VGLUT2-ir fibers within the CeA following 1 and 2 weeks of AAS withdrawal, the number of VGLUT2-ir fibers was significantly reduced in animals withdrawn from AAS for 3 weeks (p < 0.001). Interestingly, this effect was not lasting, as there were no significant differences between AAS- and SO-treated animals in the number of CeA-VGLUT2-ir fibers following 4 weeks cessation of AAS treatment (p > 0.05). Finally, exposure to AAS throughout adolescence had no lasting effects in the number of VGLUT2-ir fibers in the LS, BNST, MeA or VLH (p > 0.05 for all brain areas) (Figures 6C, 6B, 6D and 6F).
4. DISCUSSION
In a number of previous studies we have shown that administration of an AAS cocktail throughout the developmental period of adolescence produces animals with escalated levels of offensive aggression (Grimes & Melloni, 2006; Grimes, Ricci, & Melloni, 2003; Grimes et al., 2006; Ricci et al., 2007; Ricci, Knyshevski, & Melloni, 2005; Schwartzer, Morrison, Ricci, & Melloni, 2009). In this animal model, exposure to AAS during adolescence changes the normal activity of the glutamatergic system in the LAH, the postulated output nucleus for several brain regions involved in the expression of aggressive behavior (Carrillo et al., 2009; Fischer et al., 2007). To date, however, the time course of neurodevelopmental and long-term effects of adolescent AAS treatment on the glutamatergic system have not been examined. The current results showed that administration of AAS throughout the developmentally sensitive period of adolescence correlates with considerable alterations in the normal development of the glutamatergic system within the aggression neural circuit. Moreover, adolescent AAS-treated animals displayed long-term but not permanent alterations in the glutamatergic system.
Glutamate activity has been implicated in the modulation of aggression in a range of animal models (Brody, DeFeudis, & DeFeudis, 1969; Hrabovszky et al., 2005; Vekovischeva et al., 2004; Vekovischeva, Aitta-aho, Verbitskaya, Sandnabba, & Korpi, 2007). In agreement with the facilitatory role of glutamate in aggression in these animal models and with preliminary results from our laboratory, the current report showed a significant increase in VGLUT2 cells within the LAH of highly aggressive adolescent AAS-treated animals. The finding that in AAS animals there is an increase in glutamate activity within the likely center of aggression control (i.e., the LAH) strengthens the notion that glutamate plays a key role in AAS-induced aggression. Notwithstanding these results glutamate expression was examined after 4 weeks of AAS treatment while aggression levels are known to significantly rise after a shorter period of adolescent AAS exposure (i.e., 2 weeks)(Grimes et al., 2007). Thus, to further explore the temporal relationship between AAS-induced neurodevelopmental effects in the glutamatergic system and aggression, glutamate expression in various brain regions and aggression levels were examined weekly through the time course of AAS treatment (i.e., 4 weeks). As previously reported, the onset of aggression was observed after 2 weeks of AAS exposure (Grimes et al., 2007). After this time point, aggression levels continued to increase showing the highest aggression levels after 3 and 4 weeks of AAS exposure. These data replicate our previous findings and indicate that the emergence of the aggressive phenotype in AAS-treated hamsters occurs after a short period of exposure to AAS. When examining the temporal relationship between the development of aggression and VGLUT2-ir, our results showed that increases in the number of LAH-VGLUT2-ir fibers were first detected in animals exposed to AAS for 2 weeks. Noticeably, these increases were observed at the same time when elevated aggression levels are first detected in AAS-treated animals, suggesting that AAS-induced increases in LAH-glutamate fibers might be necessary for the emergence of the aggressive phenotype. Of particular interest is the overlapping developmental pattern observed between LAH-AVP (Grimes et al., 2007) and LAH-glutamate fibers. In fact, results from a previous study showed that similar to the time course of LAH-glutamate fibers in AAS-treated hamsters, an elevated density of AVP fibers innervating the LAH was first observed after 2 weeks of AAS administration and maximum AVP fiber density occurs after 4 weeks of AAS exposure. High density of AVP fibers innervating the LAH of AAS-treated animals has been shown to be necessary for heightened levels of aggression (Harrison et al., 2000). Notably, it has been previously hypothesized that within the LAH, AVP modulates the aggression output system (i.e., likely glutamate) from the LAH to other aggression areas (Ferris et al., 1997). Moreover, evidence indicates that normal development of glutamate synaptic patterning is dependent on AVP neurotransmission (Chevaleyre, Moos, & Desarmenien, 2002). Given these findings and the similar developmental time course of changes in AVP and glutamate fibers within the LAH it is possible that the LAH serves as a point of convergence for AVP-mediated modulation of the activity of the glutamatergic system. Notwithstanding these results, AAS-induced increases in LAH-VGLUT2-ir cell bodies did not parallel changes in aggression or AVP, as they were only observed after an extended period of AAS exposure (i.e., 4 weeks). Perhaps, within the LAH adolescent AAS administration first stimulates increases in glutamate branching formation and after this process has been completed glutamate production is elevated in glutamate cell bodies.
AAS-mediated neurodevelopmental alterations in the glutamatergic system were also detected within brain regions sharing reciprocal connections with the LAH. However, the VLH was the only brain region examined (i.e., in addition to the LAH) were changes in glutamate activity (i.e., VGLUT2 fibers) coincided (i.e., 2 weeks) with the onset of the aggressive phenotype in AAS-treated animals. Of particular interest was that opposite to the LAH, this area exhibited significant reductions in the number of VGLUT2-ir fibers after 2 weeks of AAS exposure. The number of VGLUT2-ir fibers decreased even more in animals exposed to AAS for 3 weeks and returned to baseline levels after 4 weeks of AAS administration. These data showing that in adolescent AAS-treated animals VGLUT2-ir fibers within the VLH are significantly reduced at the same time point when aggression emerges, suggests that perhaps the VLH does not play a key role in AAS-induced aggression. In agreement with this notion, recent results from a retrograde tracing study showed that the number of glutamate cells within the LAH innervating the VLH is significantly reduced in animals administered AAS throughout adolescence (Carrillo et al., 2009). Behavioral studies indicate that the VLH is involved in the modulation of aggression onset (i.e., latency to first attack or bite). Interestingly, compared to other hamster models of escalated aggression (i.e., cocaine-induced aggression), although AAS-treated animals display comparable levels of aggression intensity, these animals exhibit higher latency to first attack and/or bite. Perhaps, reductions in VGLUT2-fibers within the VLH underlie the higher latency of attack observed in AAS-treated animals compared to other hamster models of aggression. However, it is important to notice that the number of VGLUT2-ir cell bodies was significantly elevated after 4 weeks of exposure to AAS, suggesting that alterations in the number of VGLUT2-ir fibers and cell bodies might represent different neuroplastic events in response AAS administration. Notably, AAS-treated animals also exhibited alterations in VGLUT2-ir within the BNST, MeA and CeA. Nonetheless, within these areas only changes in VGLUT2-ir cell bodies were detected (i.e., no significant alterations detected in VGLUT2-ir fibers). Moreover, the time course of neurodevelopmental increases in VGLUT2-ir cell bodies within the BNST, MeA and CeA did not parallel increases in AVP or in aggression, as they occurred at either 3 (i.e., MeA and CeA) or 4 weeks (BNST) of AAS administration. Lastly, no significant changes in VGLUT2-ir within the LS are consistent with the inhibitory role this region has on aggression (Potegal, Blau, & Glusman, 1981). Overall, these results indicate that in adolescent AAS-treated animals there are significant alterations in the normal development of the glutamatergic system within various brain areas involved in aggression control, suggesting that neurodevelopmental alterations in glutamate neurotransmission might play a role in the emergence of the aggressive phenotype.
Previous studies from our laboratory have reported long-term AAS-induced alterations in aggressive behavior and in various neural systems implicated in the modulation of aggression (i.e., AVP and 5-HT). To determine if adolescent AAS exposure produced long-term changes in the glutamatergic system and whether these changes correlated with alterations in aggression, VGLUT2-ir and aggression levels were examined at various time points up to 4 weeks following cessation of AAS treatment. The behavioral results from the current report replicate the behavioral pattern observed in previous studies and showed that administration of AAS throughout the majority of adolescence leads to long-term but transient alterations in aggressive behavior. Specifically, AAS-treated hamsters exhibited heightened aggression for up to 2 weeks following cessation of AAS exposure. However, after 3 weeks cessation of AAS exposure, animals displayed aggression levels comparable to control animals. Moreover, AAS-treated animals maintained this non-aggressive phenotype through the last week of testing (i.e., 4 weeks).The sustained aggressive phenotype observed during the two week period after cessation of AAS exposure may be due to alterations in neurochemical systems involved in aggression control that are maintained after withdrawal from AAS. In agreement with this notion increases in LAH-VGLUT2-ir fibers and cell bodies were detected in AAS-treated animals after cessation of AAS treatment. Similar to developmental findings, AAS-treated animals exhibited differential changes on the number of VGLUT2-ir fibers and cell bodies, such that increased number of VGLUT2-ir fibers was detected for a longer period of time (i.e., 3 weeks) compared to VGLUT2-ir cell bodies (i.e., 2 weeks). Interestingly, increases in LAH-VGLUT2 expression closely paralleled the time course of AAS withdrawal effects on aggression, where elevated aggression levels lasted up to 2 weeks after cessation from AAS and increased VGLUT-ir was observed after 2 to 3 weeks following AAS withdrawal. Moreover, similar to developmental data, the pattern of AAS-mediated long-term effects on VGLUT2 expression temporally coincided with changes in AVP, such that an elevated number of AVP fibers was detected for 2 weeks after withdrawal from AAS (Grimes et al., 2006). Together with developmental results, these data strengthen the notion that interactions between the AVPergic and glutamatergic systems within the LAH likely play an important role in the modulation of adolescent AAS-induced aggression. Further examination of AAS-mediated effects in VGLUT2-ir during the withdrawal period showed long-term but not permanent alterations in the MeA. Although no changes in the number of VGLUT2-ir fibers were detected within this region, long-term increases in the number of VGLUT2-ir cell bodies were observed following 2 weeks cessation of AAS. Similar to the LAH, the number of VGLUT2-ir cell bodies returned to control levels in animals withdrawn from AAS for 3 weeks. Moreover, the finding that within the CeA a significant reduction in VGLUT2-ir fibers occurred only in animals withdrawn from AAS for 3 weeks was of particular interest and puzzling given that no changes in CeA-VGLUT2-ir fibers or cell bodies were detected prior to or following this 3 week time point. Interestingly, recent findings indicate that although aggression levels return to baseline after 3 weeks withdrawal, adolescent AAS-treated animals exhibit other behavioral long-term alterations (i.e., increased anxiety) which are only detected after 3 weeks cessation of AAS treatment (unpublished results). Given that in various animal models the CeA has been implicated in the modulation of anxiety behavior (Kalin, Shelton, & Davidson, 2004; Liebsch et al., 1995; Pandey, Roy, & Zhang, 2003) it is possible that alterations in the glutamatergic system within the CeA regulate this anxiogenic behavioral response exhibited by adolescent AAS-treated animals. Aside from speculation, these data underscores the role of the glutamatergic system in AAS-induced aggression and highlights the role of the MeA and LAH as critical regions where AAS-induced long-term neuroplastic events occur.
Overall, the results from the current report showed that in AAS-treated animals the time course of changes in glutamate development and withdrawal effects was region specific, suggesting functional heterogeneity of glutamate activity dependent on brain region. This is not surprising given that glutamate neurotransmission is regionally segregated and that neuroplastic events in the glutamatergic system are strongly dependent on local tonic environment (Bar-Peled et al., 1997; Chen, Trombley, & van den Pol, 1995; Furuta, Rothstein, & Martin, 1997; Meeker et al., 1993). Moreover, from a behavioral standpoint, these differential glutamate-dependent effects on brain region indicate that perhaps glutamate development plays a role in the expression of various aspects of the aggressive phenotype. The LS, BNST, MeA, CeA and VLH modulate different critical aspects of the aggressive act and alterations in the normal function of either one of these regions can alter the aggressive response. For example, the MeA, CeA and BNST relay hormonal and chemosensory cues, thus playing a critical role in interpretation of environmental and social events and the consequent behavioral response (Amaral et al., 2003; Bamshad, Karom, Pallier, & Albers, 1997b; Shaikh, Brutus, Siegel, & Siegel, 1986; Vinkers et al., 2010). Disruption of the normal function of these regions through generalized pharmacologic blockade or electrolytic lesions inhibits flank marking behavior, a critical component of social communication in Syrian hamsters (Bamshad, Karom, Pallier, & Albers, 1997a; Petrulis & Johnston, 1999). Moreover, in the Syrian hamster the VLH appears to modulate the initiation of the aggressive act (e.g., latency to attack or bite). For instance, disruption of VLH function through blockade of AVP V1a receptor subtype significantly increases the latency to first bite during an aggressive encounter (Delville, Mansour, & Ferris, 1996a, 1996b). In contrast to the stimulatory role these regions have on aggression, stimulation of the LS inhibits the aggressive response, which is consistent with results showing no developmental or long-term changes in VGLUT2 expression within this region (Potegal et al., 1981). Notwithstanding the critical role these regions play in the modulation of different aspects of the aggressive phenotype, the LAH appears to be a key brain area that not only integrates aggressive-related information from other aggression brain regions but also controls aggression intensity.
In conclusion, adolescent AAS-treated Syrian hamsters display significant changes in the normal development of the glutamatergic system within various aggression brain areas. However, these changes were considerably heterogeneous and dependent on brain area. Notably, the time course of alterations in the development of the glutamatergic system, particularly within the LAH, correlated with the emergence of the heightened aggressive phenotype observed in these animals. In addition, animals withdrawn from AAS exhibited long-term but not permanent increases in glutamate immunoreactivity within the LAH, CeA and MeA. Of particular interest, were the findings showing that AAS-induced increases in LAH glutamate closely parallel changes in LAH-AVP, supporting the notion that close interactions between glutamate and AVP might be critical for the modulation of AAS-induced aggression. Our findings here are novel and significant such that these studies are the first to show developmental and long-term but transient effects of AAS on glutamate within brain areas part of the neural circuit modulating aggression.
ACKNOWLEDGMENTS
R.H.M. extends special thanks to J.J. Schwartzer for critical insight and editorial comments incorporated in the construction of this manuscript. This publication was made possible by Grant (RO1) DA10547 from NIDA to R.H.M and (DDIG) 0909854from NSF to R.H.M and M.C. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA or NSF.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
REFERENCES
- Amaral DG, Bauman MD, Capitanio JP, Lavenex P, Mason WA, Mauldin-Jourdain ML, Mendoza SP. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41(4):517–522. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Karom M, Pallier P, Albers HE. Role of the central amygdala in social communication in Syrian hamsters (Mesocricetus auratus) Brain Research. 1997a;744(1):15–22. doi: 10.1016/s0006-8993(96)01061-x. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Karom M, Pallier P, Albers HE. Role of the central amygdala in social communication in Syrian hamsters (Mesocricetus auratus) Brain Research. 1997b;744(1):15–22. doi: 10.1016/s0006-8993(96)01061-x. [DOI] [PubMed] [Google Scholar]
- Bar-Peled O, Ben-Hur H, Biegon A, Groner Y, Dewhurst S, Furuta A, Rothstein JD. Distribution of glutamate transporter subtypes during human brain development. Journal of Neurochemistry. 1997;69(6):2571–2580. doi: 10.1046/j.1471-4159.1997.69062571.x. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Vaughn MG, Delisi M, Wright JP. Anabolic-androgenic steroid use and involvement in violent behavior in a nationally representative sample of young adult males in the United States. American Journal of Public Health. 2008;98(12):2185–2187. doi: 10.2105/AJPH.2008.137018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB. Glutamate: a major neuroendocrine excitatory signal mediating steroid effects on gonadotropin secretion. Journal of Steroid Biochemistry and Molecular Biology. 1995;53(1-6):325–329. doi: 10.1016/0960-0760(95)00070-g. [DOI] [PubMed] [Google Scholar]
- Brody JF, DeFeudis PA, DeFeudis FV. Effects of micro-injections of L-glutamate into the hypothalamus on attack and flight behaviour in cats. Nature. 1969;224(5226):1330. doi: 10.1038/2241330a0. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Melloni RH., Jr. Adolescent anabolic androgenic steroids reorganize the glutamatergic neural circuitry in the hypothalamus. Brain Research. 2009;1249:118–127. doi: 10.1016/j.brainres.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. GABA receptors precede glutamate receptors in hypothalamic development; differential regulation by astrocytes. Journal of Neurophysiology. 1995;74(4):1473–1484. doi: 10.1152/jn.1995.74.4.1473. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Moos FC, Desarmenien MG. Interplay between presynaptic and postsynaptic activities is required for dendritic plasticity and synaptogenesis in the supraoptic nucleus. Journal of Neuroscience. 2002;22(1):265–273. doi: 10.1523/JNEUROSCI.22-01-00265.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs JM, Jr., Jurkovic GJ, Frady RL. Salivary testosterone and cortisol among late adolescent male offenders. Journal of Abnormal Child Psychology. 1991;19(4):469–478. doi: 10.1007/BF00919089. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain, Behavior and Evolution. 2000;55(2):53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiology and Behavior. 1996a;59(4-5):813–816. doi: 10.1016/0031-9384(95)02166-3. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiology and Behavior. 1996b;60(1):25–29. doi: 10.1016/0031-9384(95)02246-5. [DOI] [PubMed] [Google Scholar]
- Eyigor O, Centers A, Jennes L. Distribution of ionotropic glutamate receptor subunit mRNAs in the rat hypothalamus. Journal of Comparative Neurology. 2001;434(1):101–124. doi: 10.1002/cne.1167. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y. Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology. 1994;19(5-7):593–601. doi: 10.1016/0306-4530(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr., Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. Journal of Neuroscience. 1997;17(11):4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JW, Susman EJ, Chinchilli VM, Kunselman SJ, D’Arcangelo MR, Schwab J, Demers LM, Liben LS, Lookingbill G, Kulin HE. Estrogen or testosterone increases self-reported aggressive behaviors in hypogonadal adolescents. Journal of Clinical Endocrinology and Metabolism. 1997;82(8):2433–2438. doi: 10.1210/jcem.82.8.4165. [DOI] [PubMed] [Google Scholar]
- Fischer SG, Ricci LA, Melloni RH., Jr. Repeated anabolic/androgenic steroid exposure during adolescence alters phosphate-activated glutaminase and glutamate receptor 1 (GluR1) subunit immunoreactivity in Hamster brain: correlation with offensive aggression. Behavioural Brain Research. 2007;180(1):77–85. doi: 10.1016/j.bbr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floody OR, Pfaff DW. Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. Journal of Comparative and Physiological Psychology. 1977;91(3):443–464. doi: 10.1037/h0077341. [DOI] [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. Journal of Neuroscience. 1997;17(21):8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes JM, Melloni RH., Jr. Prolonged alterations in the serotonin neural system following the cessation of adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behavioral Neuroscience. 2006;120(6):1242–1251. doi: 10.1037/0735-7044.120.6.1242. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH., Jr. Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Hormones and Behavior. 2003;44(3):271–280. doi: 10.1016/s0018-506x(03)00138-7. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH., Jr. Plasticity in anterior hypothalamic vasopressin correlates with aggression during anabolic-androgenic steroid withdrawal in hamsters. Behavioral Neuroscience. 2006;120(1):115–124. doi: 10.1037/0735-7044.120.1.115. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH., Jr. Alterations in anterior hypothalamic vasopressin, but not serotonin, correlate with the temporal onset of aggressive behavior during adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behavioral Neuroscience. 2007;121(5):941–948. doi: 10.1037/0735-7044.121.5.941. [DOI] [PubMed] [Google Scholar]
- Harrison RJ, Connor DF, Nowak C, Nash K, Melloni RH., Jr. Chronic anabolic-androgenic steroid treatment during adolescence increases anterior hypothalamic vasopressin and aggression in intact hamsters. Psychoneuroendocrinology. 2000;25(4):317–338. doi: 10.1016/s0306-4530(99)00057-8. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Halasz J, Meelis W, Kruk MR, Liposits Z, Haller J. Neurochemical characterization of hypothalamic neurons involved in attack behavior: glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience. 2005;133(3):657–666. doi: 10.1016/j.neuroscience.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neuroscience Research. 2002;42(4):243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Lerwill CJ, Makings P. The agonistic behavior of golden hamster. Animal Behaviour. 1971;19 [Google Scholar]
- Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, Holsboer F, Montkowski A. Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regulatory Peptides. 1995;59(2):229–239. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- Mattsson A, Schalling D, Olweus D, Low H, Svensson J. Plasma testosterone, aggressive behavior, and personality dimensions in young male delinquents. Journal of the American Academy of Child Psychiatry. 1980;19(3):476–490. doi: 10.1016/s0002-7138(09)61065-7. [DOI] [PubMed] [Google Scholar]
- Meeker RB, Greenwood RS, Hayward JN. Glutamate receptors in the rat hypothalamus and pituitary. Endocrinology. 1994;134(2):621–629. doi: 10.1210/endo.134.2.7905409. [DOI] [PubMed] [Google Scholar]
- Meeker RB, Swanson DJ, Greenwood RS, Hayward JN. Quantitative mapping of glutamate presynaptic terminals in the supraoptic nucleus and surrounding hypothalamus. Brain Research. 1993;600(1):112–122. doi: 10.1016/0006-8993(93)90408-f. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr., Connor DF, Hang PT, Harrison RJ, Ferris CF. Anabolic-androgenic steroid exposure during adolescence and aggressive behavior in golden hamsters. Physiology and Behavior. 1997;61(3):359–364. doi: 10.1016/s0031-9384(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr., Ricci LA. Adolescent exposure to anabolic/androgenic steroids and the neurobiology of offensive aggression: A hypothalamic neural model based on findings in pubertal Syrian hamsters. Hormones and Behavior. 2009 doi: 10.1016/j.yhbeh.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Morin RH, Wood RI. A Sterotaxic Atlas of the Golden Hamster Brain. Academic Press; San Diego: 2001. [Google Scholar]
- NIDACapsules 2007 http://www.nida.nih.gov/NIDACapsules/NCIndex.html.
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcoholism, Clinical and Experimental Research. 2003;27(3):396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sidney, Australia: 1997. [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behavioral Neuroscience. 1999;113(2):345–357. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Archives of General Psychiatry. 1994;51(5):375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Potegal M, Blau A, Glusman M. Effects of anteroventral septal lesions on intraspecific aggression in male hamsters. Physiology and Behavior. 1981;26(3):407–412. doi: 10.1016/0031-9384(81)90167-0. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Grimes JM, Melloni RH., Jr. Lasting changes in neuronal activation patterns in select forebrain regions of aggressive, adolescent anabolic/androgenic steroid-treated hamsters. Behavioural Brain Research. 2007;176(2):344–352. doi: 10.1016/j.bbr.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci LA, Knyshevski I, Melloni RH., Jr. Serotonin type 3 receptors stimulate offensive aggression in Syrian hamsters. Behavioural Brain Research. 2005;156(1):19–29. doi: 10.1016/j.bbr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: relationship to aggressive, hyperactive, and internalizing behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33(8):1174–1184. doi: 10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Morrison RL, Ricci LA, Melloni RH., Jr. Paliperidone suppresses the development of the aggressive phenotype in a developmentally sensitive animal model of escalated aggression. Psychopharmacology. 2009;203(4):653–663. doi: 10.1007/s00213-008-1412-4. [DOI] [PubMed] [Google Scholar]
- Shaikh MB, Brutus M, Siegel HE, Siegel A. Regulation of feline aggression by the bed nucleus of stria terminalis. Brain Research Bulletin. 1986;16(2):179–182. doi: 10.1016/0361-9230(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Su TP, Pagliaro M, Schmidt PJ, Pickar D, Wolkowitz O, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269(21):2760–2764. [PubMed] [Google Scholar]
- van den Pol AN. Glutamate and aspartate immunoreactivity in hypothalamic presynaptic axons. Journal of Neuroscience. 1991;11(7):2087–2101. doi: 10.1523/JNEUROSCI.11-07-02087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Hermans-Borgmeyer I, Hofer M, Ghosh P, Heinemann S. Ionotropic glutamate-receptor gene expression in hypothalamus: localization of AMPA, kainate, and NMDA receptor RNA with in situ hybridization. Journal of Comparative Neurology. 1994;343(3):428–444. doi: 10.1002/cne.903430307. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Trombley PQ. Glutamate neurons in hypothalamus regulate excitatory transmission. Journal of Neuroscience. 1993;13(7):2829–2836. doi: 10.1523/JNEUROSCI.13-07-02829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekovischeva OY, Aitta-Aho T, Echenko O, Kankaanpaa A, Seppala T, Honkanen A, Sprengel R, Korpi ER. Reduced aggression in AMPA-type glutamate receptor GluR-A subunit-deficient mice. Genes Brain Behav. 2004;3(5):253–265. doi: 10.1111/j.1601-1848.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- Vekovischeva OY, Aitta-aho T, Verbitskaya E, Sandnabba K, Korpi ER. Acute effects of AMPA-type glutamate receptor antagonists on intermale social behavior in two mouse lines bidirectionally selected for offensive aggression. Pharmacology, Biochemistry and Behavior. 2007;87(2):241–249. doi: 10.1016/j.pbb.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Bijlsma EY, Houtepen LC, Westphal KG, Veening JG, Groenink L, Olivier B. Medial amygdala lesions differentially influence stress responsivity and sensorimotor gating in rats. Physiology and Behavior. 2010;99(3):395–401. doi: 10.1016/j.physbeh.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Yesalis CE, Kennedy NJ, Kopstein AN, Bahrke MS. Anabolic-androgenic steroid use in the United States. JAMA. 1993;270(10):1217–1221. [PubMed] [Google Scholar]