Abstract
We have shown previously in normal subjects that a sensory measure, the Urge-to-Cough rating, increases at concentrations of inhaled capsaicin that are lower than those necessary to elicit reflex cough. This finding suggests that the Urge-to-Cough may represent an index of the cough response. Research on cough in the human has most often employed challenge with inhaled capsaicin to induce reflex cough. Current measures of cough sensitivity in the human provide no information regarding the intensity of cough. The influence of codeine on cough perceptual sensitivity and the relationship to cough intensity with capsaicin-induced cough in normal subjects has not been evaluated. This study determined the effect of codeine on capsaicin-induced cough perceptual sensitivity and motor response in normal subjects in a double-blind, placebo-controlled, crossover study. This approach investigated the relevance of cough sensitivity, intensity, and sensory modalities in the assessment of cough suppression in humans.
This study consisted of three experimental trials: administration of placebo, 30 mg codeine and 60 mg codeine. The study was double-blinded. The order of the three trials was randomized. Respiratory motor pattern was recorded with EMGs from the rectus abdominis, lateral abdominal muscles and eighth intercostal space. The subjects leaned into a fume hood to inspire deeply for 2 s once through a mouthpiece connected to the nebulizer. A modified Borg scale was used to estimate their Urge-to-Cough. The experimental trial consisted of eight test solutions of 0–200 μM capsaicin. Each solution was presented three times in a randomized block order for a total of 24 presentations. The lowest capsaicin concentration to elicit a cough was determined. The lowest capsaicin concentration to elicit an Urge-to-Cough greater than zero was identified. The Urge-to-Cough sensitivity was determined from the log–log slope.
For placebo, the Urge-to-Cough was zero with inhalation of the vehicle and no coughs were observed. The threshold capsaicin concentration for subjects to report an Urge-to-Cough was 15.6 μM (±2.6 SEM). The capsaicin concentration threshold for eliciting a cough was significantly greater, 39.3 μM (±5.6 SEM). As the capsaicin concentration increased, the magnitude estimation of the Urge to-Cough increased. The slope of the log–log relationship for the Urge-to-Cough was 0.94 (±0.07 SEM). As the capsaicin concentration increased, the number and intensity of the coughs increased. The administration of 30 and 60 mg codeine had no significant effect on the threshold capsaicin concentration for the Urge-to-Cough. There was also no significant codeine effect on the slope of the log–log Urge-to-Cough relationship. Thirty and sixty milligram codeine had no significant effect on the relationship between the capsaicin concentration and the number and intensity of the coughs.
The results of this study demonstrate that the threshold for a subject to perceive an Urge-to-Cough was less than the capsaicin concentration that elicits the cough motor response. There was a direct relationship between the sensory intensity (magnitude estimation of the Urge-to-Cough) and the cough number and intensity. Thus, as the sense of an Urge-to-Cough increased the cough motor response increased. Neither the 30 nor 60 mg codeine affected the perceptual or motor sensitivity to capsaicin-induced cough. These results showed that the initial threshold for responding to capsaicin-induced cough is the perception of an Urge-to-Cough, followed by a motor cough response if the capsaicin is increased above the perceptual threshold. As the capsaicin concentration increases, both the perceptual need to cough and the cough motor response increase. The response of subjects to inhalation of capsaicin consisted of both a sensory component leading to perception of an Urge-to-Cough and motor cough behavior.
Keywords: Perception, Magnitude estimation, Respiratory muscle, Cough intensity
1. Introduction
We have previously shown that adding capsaicin to the breathing line results in a sensation, which we termed the Urge-to-Cough [1]. We also showed that this Urge-to-Cough increases with increasing capsaicin concentration. It is also known that there are correlations between the Urge-to-Cough, the number of coughs and the capsaicin concentration [1,2]. That initial study also reported that the Urge-to-Cough occurred at a capsaicin concentration less than the capsaicin concentration eliciting the motor cough [1]. This suggests that the cough cognitive sensory process precedes the cough motor event. In the present study, we hypothesized that if stimulation of receptors that initiate irritant reflex cough project to cognitive cortical centers, then the perception of a need to cough is the initial sensory process and will elicit the sensation of an Urge-to-Cough before a motor cough event is produced.
It is also known that as capsaicin concentration increases, there is an increase in the number of coughs counted [3–8]. This cough number response to capsaicin concentration is one measure of cough sensitivity [9]. This increase in cough number suggests that as the stimulus that elicits cough increases there is an increase in the intensity of the cough. This also suggests that there is a relationship between the cough stimulus (such as capsaicin), the Urge-to-Cough and the intensity of the cough. It is, however, very difficult to define cough intensity using cough sounds or cough number. Cough intensity has been reported to be measured by the expiratory airflow and the expiratory muscle EMG activity generated during coughing [10–14]. In fact the area under the integrated EMG curve is an expiratory muscle measure of cough intensity [10]. It seemed reasonable that the best measures of cough intensity were the parameters related to the cough mechanical output. The mechanical output of a cough comes from the airflow pattern [15]. The cough motor pattern is characterized by lung inflation (the lung volume priming phase), closure of the glottis with the compression phase and a rapid expulsive phase followed by a sustained plateau of airflow [15]. However, the airflow expulsive phase of the cough is often interrupted by reclosure of the glottis [2,6,7,15,16]. This results in more than one expulsive event for an individual priming inflation. What that means is that for a single inflation, the lung is compressed to generate the subglottic pressure for the cough expulsion. Then the lung is recompressed with interruption of the expiratory airflow by closure of the glottis resulting in reacceleration of the airflow with the subsequent expulsive event when the glottis opens a second time. The number of the reaccelerated expulsive events for each inflation, the airflow pattern and the expiratory muscle EMG activity was hypothesized to measure cough intensity. It was further hypothesized that there should be a correlation between the magnitude of the stimulus, the magnitude of the Urge-to-Cough and the intensity of the cough.
Previous capsaicin studies have asked the subjects to provide a perceptual measure of their cough using the doubling dose paradigm [9]. In these types of studies, to control for subject anticipation of the capsaicin presentation, vehicle aerosol has been presented randomly between increasing capsaicin concentrations [9]. A limitation of this type of protocol is that when the presence of capsaicin is sensed the subject knows that the capsaicin concentration will be an increase from the previous concentration. This can predispose the subject to anticipate the increasing concentration and increase their perceptual estimate of the capsaicin concentration accordingly. Thus, the increase in their magnitude estimation as the trial number increases has the potential of an artifactual increase in magnitude estimation of capsaicin. The presentation protocol for determining the perceptual measure of the Urge-to-Cough must control for anticipation of capsaicin concentration. In our previous study on the Urge-to-Cough, we used a randomized block order for the range of capsaicin concentrations [1]. The order for the multiple capsaicin concentrations was randomized to form a presentation block. The capsaicin concentration was then rerandomized for the second and third presentation blocks. Each capsaicin concentration was thus presented three times and each block contained only one presentation of an individual capsaicin concentration. The magnitude estimation response was a function of the individual concentration presentation because the subject did not know if the capsaicin concentration they received was greater or less than the previous aerosol. However, it was unknown if multiple capsaicin presentations, especially the higher concentrations, would produce tachyphylaxis. This was tested in the present study by determining the perceptual and motor response for each presentation block.
The final aspect of this study was to examine the effect of an antitussive agent, codeine, on the Urge-to-Cough and cough intensity [1]. We used a double-blind placebo controlled protocol to test 60 and 30 mg of codeine and no-codeine. Each experimental trial was separated by a week to allow the subject to clear the codeine. It hypothesized that codeine would inhibit the Urge-to-Cough and the intensity of the cough.
2. Methods
2.1. Study design
This study consisted of three experimental trials. The three experimental trials consisted of oral administration of placebo, 30 mg codeine and 60 mg codeine. The vehicle and codeine doses were administered in single color-coded capsules. The capsules (lactose, 30 mg codeine, and 60 mg codeine) were prepared by the University of Florida, Clinical Research Center (CRC). The CRC determined the color code and blinded the investigators to the color code until all the subjects were tested in all three treatment sessions and the data analyzed. Thus, the study was double-blinded. The order of the three treatment trials was randomized. Each experimental trial (placebo, 30 mg codeine and 60 mg codeine) was performed in the morning. Each experimental trial was separated by a week with the trials occurring for each individual subject at the same time and day of the week. Thus, each subject was tested over a 3 week experimental time period.
2.2. Experimental preparation
The subject was instrumented for the measurement of respiratory pattern during spontaneous breathing and capsaicin-induced cough. The airway mechanical events were measured using a custom-designed mouthpiece with integral pneumotachograph. A dental impression was made of the subjects upper teeth and a plastic retainer was constructed. A plastic penumotachograph was cemented to the palatal surface of the retainer. The pneumotachograph was connected to a differential pressure transducer by flexible tubing. This device allowed the subject to close their mouth around the nebulizer mouthpiece and cough while measuring mouth airflow velocity. This device did not allow the measurement of airflow volume because all the mouth airflow did not pass through the penumotachograph. The device did permit unobstructed breathing, aerosol inhalation, cough and measurement of airflow pattern. The pressure transducer was connected to a computer processing system (ADInstruments, Inc.). Respiratory motor pattern was recorded with respiratory muscle EMG. Active EMG recording electrodes (Delsys, Inc.) with integral amplifiers and band pass filters were placed over the rectus abdominis, lateral abdominal wall and the eighth intercostals space. The signals were amplified, band pass filtered (100–1000 Hz) and recorded on a computer processing system (PowerLab, ADInstruments, Inc.). The computer signals were analyzed to determine inspiratory breath phase, expiratory breath phase, inspiratory cough phase, cough compression phase, expiratory cough phase, cough phase mechanical timing, cough phase muscle intensity, cough phase muscle timing and integrated outcome parameters. After the subject was instrumented, the subject was seated comfortably in a chair in front of an airflow fume hood. The subjects leaned into the airflow fume hood to breathe on the experimental apparatus. The airflow fume hood prevented exposure of the subject to the nebulized solution except through inspiration via the mouthpiece. The subject inspired deeply once for 2 s through a mouthpiece connected to the nebulizer (KoKo Dosimeter). The outflow neublizer gas was passed through an isopropyl alcohol solution to remove capsaicin from the vented gas.
2.3. Experimental protocol
Informed consent was obtained from each subject upon arrival to the laboratory. Simple standard instructions were given to each subject. The modified Borg scale [1] was used to allow subjects to estimate their Urge-to-Cough. The Urge-to-Cough scale was placed in front of the subject. During the experimental trials, the subject was given a verbal cue to lean into the airflow fume hood, place the mouthpiece into their mouth and take a single deep inspiration of the test nebulized air. The subject made an estimate immediately after completion of the single inspiration using the modified Borg category scale of the Urge-to-Cough. The subject pointed at the scale number, which was recorded by the experimenter. The experimenter and subjects were blinded to the capsaicin concentration. Each test inspiration was separated by an interval of 2 min during which the subject was breathing away from the airflow fume hood. The experimental trial consisted of eight test solutions: 0, 5, 10, 25, 50, 100, 150 and 200 μM capsaicin in 80% physiological saline, 10% Tween 20, and 10% ethanol. Each test solution was presented three times in a randomized block order for a total of 24 test solution presentations. The number of cough-like expiratory events was counted for each test solution trial by the experimenter during the experiment and recorded on a data sheet in ink. Coughs were counted for 30 s after each dose. When the aerosol challenge paradigm was completed, the subject was seated in a lounge chair and watched a videotaped movie for a post-treatment observation period of 3–4 h. Subjects were tested every 30 min with the Stanford Sleepiness Scale (SSS). The subjects were released from the laboratory only when they had scores on the SSS indicating slight or no sedation and normal alertness. The capsaicin test began 90 min after administration of the codeine or placebo.
2.4. Randomized block design
There were eight test solutions. A single presentation of each of these eight different test solutions, a total of eight test presentations, was called a block. The entire protocol required three presentations of each test solution, which was a presentation of three blocks. This made a total of 24 test solution presentations. An order effect occurs if the total 24 test presentations are not block randomized. To prevent this order effect, a randomized block order was used. The presentation order of the eight individual test solutions was randomized for each block generating three randomly ordered blocks with only one presentation of each test solution in each block. The three random blocks were then presented to obtain the total 24 test presentations with each test solution presented three times.
2.5. Data analysis
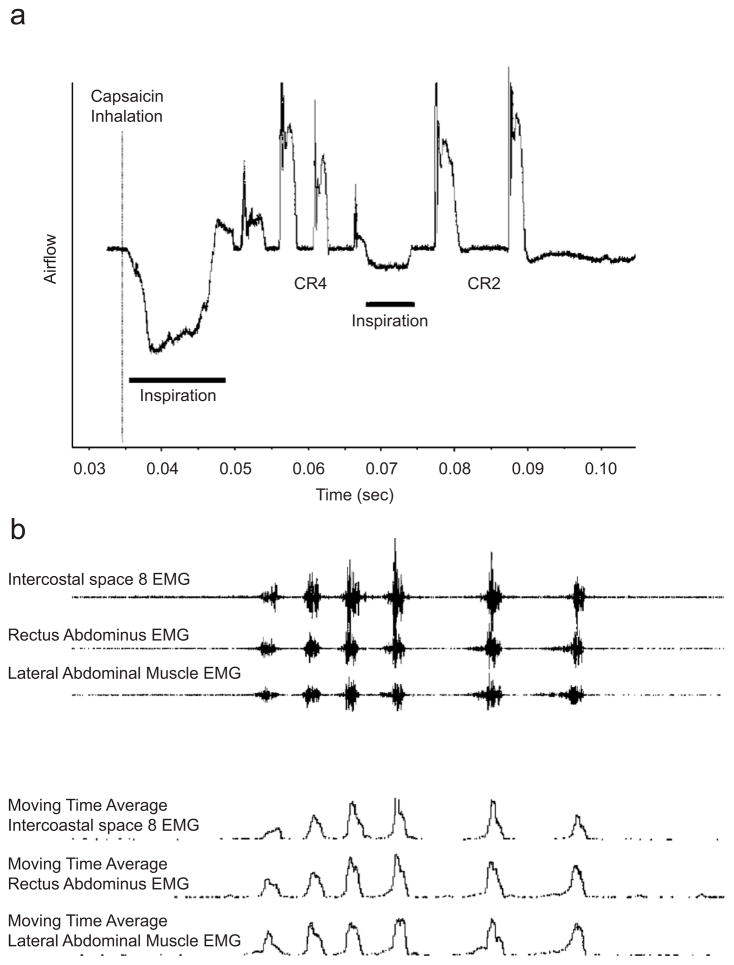
A cough was defined as a priming inspiration followed by one or more expulsive events. Throughout a single expiratory phase as lung volume decreased following that priming inspiration one or more expulsive events occurred. Repeated interruption of the expiratory phase was defined as reacceleration of airflow and we termed this cough as cough reaccelerated (CR) subscripted with the number of expulsive events (Fig. 1a). Cough airflow was measured from the expulsive event airflow. The expulsive event intensity measured from the airflow pattern was defined as the integrated area under the plateau phase (Fig. 2). The post-peak plateau integrated area (PPPIA) was calculated from the point at which the rapid transient airflow peak ended and that the airflow plateau had a rapid decline (Fig. 2). A line was drawn between these two points and the area under the plateau phase was calculated. The total CR intensity for each priming inspiration was the sum of the expulsive events PPPIA. A second measure of cough intensity was determined by calculating the area under the moving time average of the EMG (Fig. 1b) for each expulsive phase. Again a cough was defined as the priming inspiration and one of more expulsive events (CR). The muscle EMG measure of CR intensity was the sum of the expulsive event areas under the moving time average of the EMG.
Fig. 1.
Airflow and expiratory muscle EMG pattern in response to a capsaicin-elicited cough. (a) The airflow pattern of the capsaicin-elicited cough. The dose of capsaicin was 100 μM inhaled for about 2 s. The cough response was associated with two inspirations and six expulsive events. The first inspiration had four expulsive events before the subsequent second inspiration. The second inspiration was followed by two expulsive events. The cough reacceleration, CR, definition of the cough resulted in a CR4 for the first cough response and CR2 for the second cough response. (b) The corresponding EMG activity for the airflow pattern in (a). The top three traces are the raw EMG signal for intercostal space 8, rectus abdominis and lateral abdominal wall surface recordings. The bottom three traces are the moving time average (50 ms time constant) of the full wave rectified of the 3 EMG recordings.
Fig. 2.

Dependent cough variables as measured from the airflow pattern of a cough, CR1. The vertical lines on the airflow trace mark the time points for phase delineation. The hatched area under the expiratory airflow indicates the area of the plateau phase.
The number of coughs recorded on paper during the experiment was the auditory observation of cough-like expulsive events. The lowest capsaicin concentration that elicited a cough-like expulsive event was determined as the motor cough threshold. The number of coughs counted by listening to the subject and the motor cough threshold for each of the three randomized blocks was determined individually and averaged across the three presentations of each capsaicin concentration. The Urge-to-Cough magnitude estimation score was determined for each individual block and averaged across blocks for each test solution. The average Urge-to-Cough magnitude estimation was plotted against the corresponding capsaicin concentration on a log–log scale. The slope of the log–log relationship was determined by linear regression analysis. The Urge-to-Cough sensitivity was reported as the log–log slope. The capsaicin concentration that elicited an Urge-to-Cough magnitude estimation greater than zero was determined as the Urge-to-Cough threshold. The results were compared using a paired-t-test, repeated measures ANOVA and post-hoc between group analyses performed with Student–Newman–Keuls method for paired multiple comparisons as appropriate. The significance criterion was set at p<0.05.
3. Results
After administration of the vehicle solution, the subjects reported their Urge-to-Cough as zero. No coughs were recorded with inhalation of vehicle. There is no significant difference between capsaicin concentration presentation blocks 1, 2 and 3 for the Urge-to-Cough, airflow PPPIA, muscle EMG and cough number.
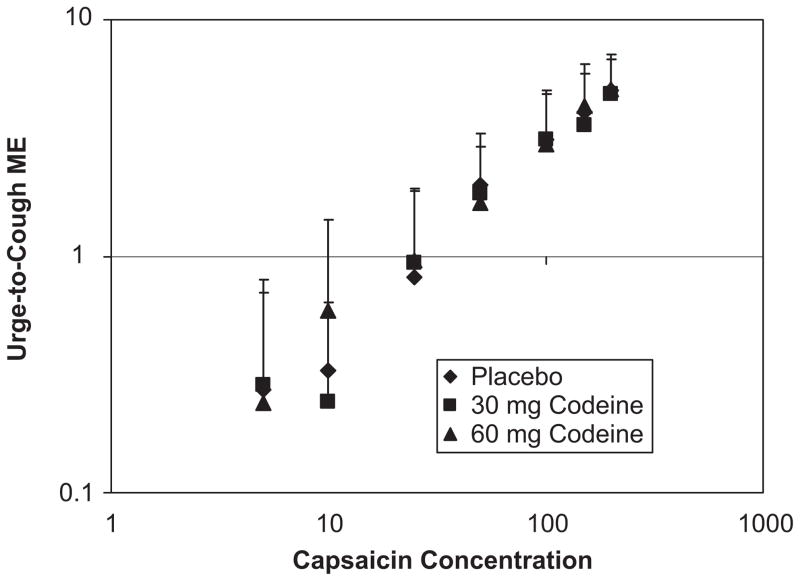
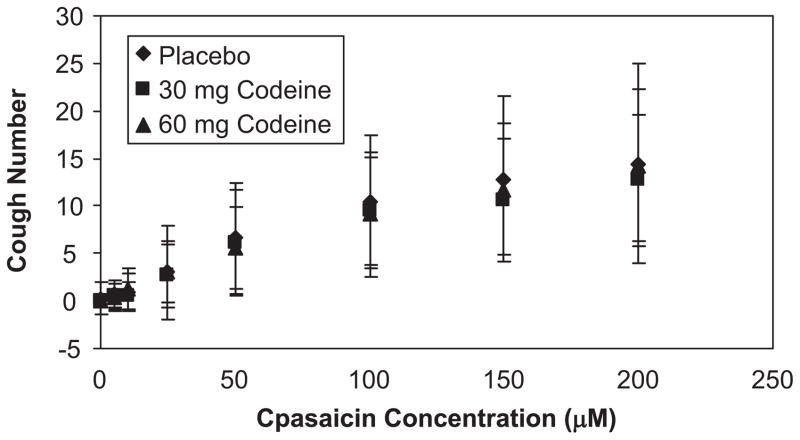
As a capsaicin concentration increased the magnitude estimation of the Urge-to-Cough increased. There was a linear log–log relationship (Fig. 3) between the magnitude estimation of the Urge-to-Cough and capsaicin concentration, mean slope = 0.94 (±0.07 SEM). There was also a significant increase in the auditory counted cough number as capsaicin concentration increased (Fig. 4). The auditory counted cough number was the number of expulsive events for each test solution. When cough was counted by measuring the cough bouts, CR for each priming inspiration, both the number of CR increased with capsaicin concentration and the number of expulsive events for each CR increased. The most frequent cough bout CR was a CR2. At higher capsaicin concentrations the number of expulsive events increased from CR1 to as much as CR7.
Fig. 3.
Relationship between the Urge-to-Cough magnitude estimation and the capsaicin concentration. The group means and standard deviations of the magnitude estimations are plotted against the corresponding capsaicin concentrations on a log–log scale. The diamond symbols represent the mean magnitude estimation for placebo treatment, the square symbols represent the mean magnitude estimation for 30 mg codeine treatment and the triangle symbols represent the mean magnitude estimation for 60 mg codeine treatment. There were no significant treatment effects.
Fig. 4.
Relationship between the total cough number with auditory counting and the capsaicin concentration. The group means and standard deviations of the cough numbers are plotted against the corresponding capsaicin concentrations on a linear scale. The diamond symbols represent the mean cough numbers for placebo treatment, the square symbols represent the mean cough numbers for 30 mg codeine treatment and the triangle symbols represent the mean cough numbers for 60 mg codeine treatment. There were no significant treatment effects.
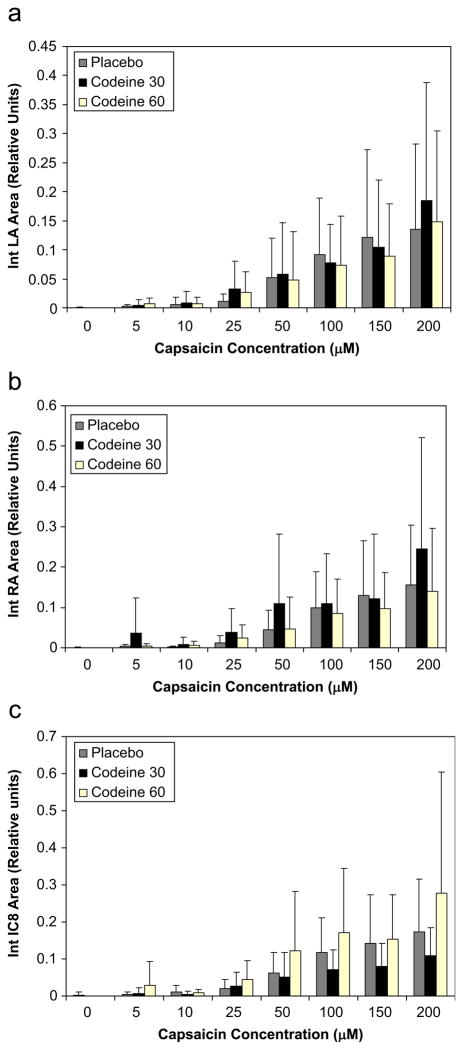
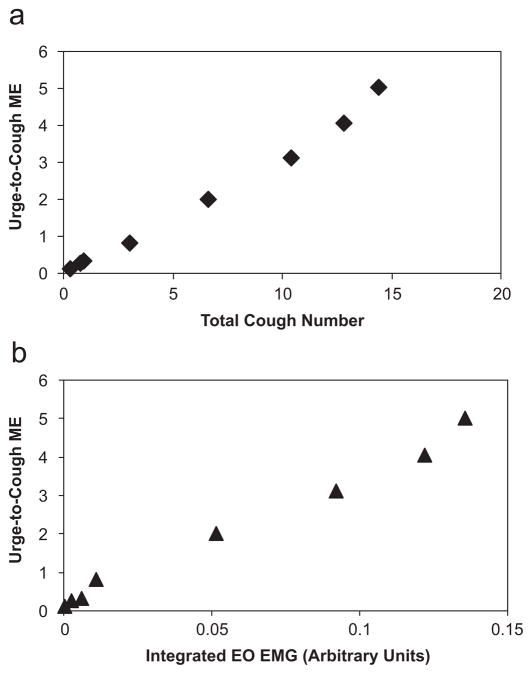
As capsaicin concentration increased, the sum of the PPPIA increased. The summed integrated area of the moving time average for each muscle system recorded also increased significantly with increasing capsaicin concentration (Fig. 5). There was a near-linear relationship between the Urge-to-Cough magnitude estimation and the auditory counted cough number (Fig. 6a). There was a near-linear relationship between the Urge-to-Cough magnitude estimation and integrated moving time average of the muscle EMG (Fig. 6b) and the PPPIA. Administration of either 30 mg codeine or 60 mg codeine did not significantly alter the relationship between capsaicin concentration and Urge-to-Cough (Fig. 3), auditory counted cough number (Fig. 4), integrated area of muscle EMG (Fig. 5) and PPPIA.
Fig. 5.
Relationship between the summed integrated EMG area and the capsaicin concentration. The integrated areas of each expulsive event were summed for all the expulsive events at each capsaicin concentration. Panel (a) is the relationship for intercostals space 8 measurements, (b) is for rectus abdominis measurements and (c) is for lateral abdominal wall measurements. The group means and standard deviations of the summed integrated areas are plotted against the corresponding capsaicin concentration. The gray bars represent the mean summed integrated area for placebo treatment, the black bars represent the mean summed integrated area for 30 mg codeine treatment and the open bars represent the mean summed integrated area for 60 mg codeine treatment. There were no significant treatment effects.
Fig. 6.
(a) The relationship between the group mean Urge-to-Cough magnitude estimation and total cough number. As the capsaicin concentration increased, the Urge-to-Cough magnitude estimation and corresponding total number of coughs increased. (b) The relationship between the group mean Urge-to-Cough magnitude estimation and summed integrated area of the lateral abdominal EMG activity. As the capsaicin concentration increased, the Urge-to-Cough magnitude estimation and corresponding summed integrated EMG increased. Only the lateral abdominal EMG response is presented, however, an identical relationship was found for intercostals space 8 and rectus abdominis EMG activity.
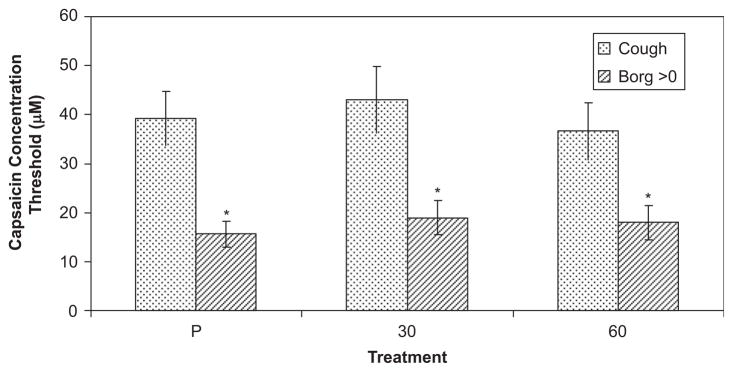
The threshold capsaicin concentration (Fig. 7) for subjects to report an Urge-to-Cough was a mean of 15.6 μM (±2.6 SEM). The capsaicin concentration threshold for eliciting a cough was significantly greater, 39.3 μM (±5.6 SEM). Codeine also had no significant effect on the capsaicin concentration threshold for eliciting either the Urge-to-Cough or the motor cough event.
Fig. 7.
Threshold for eliciting a motor cough and perceptual sense of an Urge-to-Cough. The group mean and standard deviation for the minimum capsaicin concentration to elicit a motor cough is represented by the stippled bars. The group mean and standard deviation for the minimum capsaicin concentration to elicit a Borg perceptual score greater than zero for the Urge-to-Cough is represented by the cross hatched bars. The cough motor perceptual Urge-to-Cough responses for placebo, 30 mg codeine and 60 mg codeine treatments are indicated. There were no significant treatment effects.
4. Discussion
The first issue addressed by this study was the definition of a cough. When subjects inhale for a cough, after the initial compression phase, they usually had more than one expulsive event following the inhalation. The most common pattern was an inhalation followed by two expulsive events, each preceded by a compression phase. We defined this pattern as CR. We defined the total individual cough pattern as an inspiration and the number of expulsive events that follow that inspiration. In the example of the first cough bout with four expulsive events and the second cough bout with two expulsive events following an inspiration (Fig. 1a), we symbolized that CR with a subscript for the number of expulsive events. In this example (Fig. 1a), the first cough would be referred to as a CR4 and the second cough bout as a CR2. The number of expulsive events that follow the inflation increased as the concentration of capsaicin increased. The incidence of expulsive events for each cough bout also increased such that there was a greater incidence of CR3, CR4 and CR5 as the concentration of capsaicin increased. The number of expulsive events is one measure of the intensity of a cough [6,7,9].
Another measure of cough intensity is the mechanical effects of the cough [11,14,15]. We measured the mechanical output in two different ways. One measure was from the cough airflow pattern. The expulsive airflow event has a rapid acceleration phase followed by a sustained expiratory airflow plateau phase [15]. The duration of the plateau phase and the mean airflow rate of the plateau phase is a function of the airflow velocity that is forcing the air out as a result of the cough. The area under the plateau phase is a measure of the total airflow sustained over time. The area under the plateau phase was directly related to the EMG generated by the abdominal muscles [10,12]. The abdominal EMG was the second measure of cough intensity [10,12]. We measured EMG from rectus abdominis, lateral abdominal muscles and lower thoracic wall muscles. We observed that the EMG preceded the expiratory flow. The integrated EMG increased with increased sustained air-flow. The airflow plateau phase correlated with the EMG integrated area for the expulsive events. The summed EMG generated for the series of expulsions for each cough priming inspiration is a measure of the intensity of the individual cough intensity. As the number of expulsive events increased (CR2–CR7) the summed EMG increased. There was a direct relationship between both airflow and EMG measures of cough intensity and capsaicin concentration. While auditory cough counting score, and the number of expulsive events increased with for each cough priming inspiration and the counted number of “coughs” increased with increasing capsaicin concentration [6–9], unfortunately the increase in the number of counted coughs does not provide a measure of the intensity of the cough. Thus, the combination of the measurement of airflow pattern and EMG activity allows determination of the expulsive event intensity, and the sum of the expulsion intensities for each priming inspiration provides a measure of the cough intensity. Using these measures, we found an increase in individual CR intensity as the capsaicin concentration increased.
This study used a random block presentation of capsaicin concentrations [1]. Each capsaicin concentration was present three times. The perceptual (Urge-to-Cough) and cough intensity response to capsaicin concentration were not significantly different between blocks suggesting that tachyphylaxis did not occur with repeated capsaicin presentations when separating the presentations by at least 2 min. For the Urge-to-Cough, there was a direct relationship between the capsaicin concentration and the magnitude estimation of the Urge-to-Cough. Neither the absolute measures of the Urge-to-Cough nor the log–log slope were altered by the administration of either 30 or 60 mg codeine. This suggests that codeine did not suppress the perception of irritant-elicited cough. As capsaicin concentration increased there was an increase in the integrated area of the expulsive events, however, there was no effect of codeine on cough numbers, airflow measures of cough intensity and EMG measures of cough intensity. Thus, codeine did not alter the response of normal subjects to irritant-elicited cough.
The Urge-to-Cough threshold was measured as the concentration of capsaicin that elicited a greater than zero Borg score magnitude estimation of an Urge-to-Cough [1]. The threshold concentration of capsaicin that elicited a cough or expulsive event was determined as the threshold for the motor cough response. The concentration of capsaicin that elicited a magnitude estimation greater than zero was significantly less than the concentration of capsaicin required to elicit an expulsive event. There was no effect of codeine on the threshold to elicit either the perceptual threshold Urge-to-Cough or the motor cough threshold. These results demonstrate the subject can reliably estimate their Urge-to-Cough; the Urge-to-Cough is a measure of the perceptual sensation of a need to cough; and the sensation of an Urge-to-Cough occurs at a concentration of capsaicin less than the capsaicin concentration required to elicit the motor action of a cough. These results also demonstrate that there is a positive correlation between the Urge-to-Cough, the cough intensity and the number of coughs. In addition, the Urge-to-Cough threshold shows that the sensation of an impending cough precedes the production of a cough. The slope of the Urge-to-Cough and capsaicin log–log relationship is a measure of perceptual cough sensitivity [1] and was approximately 1 showing neither sensory compression nor amplification. The magnitude estimation slope and threshold do not change with codeine and codeine administration does not change the number of capsaicin elicited cough numbers or the intensity of capsaicin-elicited coughs. Stimulation of sensory receptors initiates the perceptual events that reflect cough afferent information projection to cognitive cortical centers [2]. This cognitive activation results in the perception of the need to cough. The afferent projection to the cognitive centers is the initial sensory event mediating the Urge-to-Cough. The cognitive sensory event is then followed by the motor process, which is the final output of a cough. The relationship between cough intensity and the Urge-to-Cough and the fact that perception of the need to cough occurs before the motor cough allows the subject to behaviorally modify cough before the motor cough action occurs. For example, if a subject feels the Urge-to-Cough and the urge is not of a magnitude that is mandatory for eliciting a cough, the subject can suppress that cough. The ability to suppress cough or to alter cough motor pattern is thus a function of sensory processes, which involve cognition of the cough. The results of this study suggest that capsaicin-elicited cough is a sensory-motor process which has a threshold for eliciting the sensory component of the cough reflex; once that threshold is exceeded the subject is able to perceive a need to cough (Urge-to-Cough); the perception of the need to cough precedes the motor cough event.
This study used measures of intensity of cough, which are based on the assumption that the intensity of the cough is directly proportional to the cough mechanical events [10–13]. Thus, the airflow and muscle motor patterning are the parameters, which reflect the mechanical processes producing the cough-related high velocity airflows. There is a direct relationship between the area under the airflow plateau phase of the expulsive event and the integrated EMG suggesting that expiratory muscle activity is generating the forces for the airflow. The muscle motor output is a combination of multiple muscle systems producing compression of both the abdomen and the thorax [12]. Active expiratory muscle activity was observed in all muscles recorded including rectus abdominis, the lateral abdominal muscles and muscle activity recorded from the surface of the thoracic wall. This supports previous reports [10–12] that the cough motor pattern is a result of coordinated contraction of multiple muscle systems that compress the abdomen and thorax, compressing the lungs and producing a positive pressure within the alveolar space. This positive pressure increases against the closed glottis during the compression phase and then the pressure provides the driving force for high velocity airflow when the glottis is rapidly opened. The sheer force of the airflow is maintained during the airflow plateau phase. The plateau phase of the airflow is due to the expiratory muscles sustaining their contraction and maintaining the driving pressure for airflow at a level that is a function of the intensity of the expulsive event. After a period of time and with declining lung volume, the airflow-related sheer force of the expulsive event decreases, possibly sensed by respiratory mechanoreceptors. The expulsive event is terminated by closure of the glottis, interrupting the expiratory airflow. The subglottal pressure is then increased behind the closed glottis. The glottis reopens and airflow is rapidly reaccelerated for the second expulsive event. The actual peak airflow is less because the lung volume is less yet the reacceleration processes regenerates the sheer producing airflow. This reacceleration is repeated as a function of the intensity of the cough. The priming event for this multiple expulsive event cough motor pattern is the inspiration. Thus, as the capsaicin concentration increased, the number of reaccelerated expulsive events for a single priming inspiration increased. The CR number (CR2, CR3, CR4, etc.) was indicative of the cough intensity and was why the number of “coughs” counted by listening to the subject increased. The greater the intensity of the irritant stimulation the more the cough motor system reaccelerated the airflow. The most common pattern observed in our subjects was an inspiration followed by two expulsive events or a CR2.
In summary, these results demonstrate that there is a sensory process, which initiates a cognitive awareness of the need to cough; this cognitive awareness then can modulate the brainstem motor cough pattern. As capsaicin concentration increased the intensity of the cough increased as measured by output from the respiratory muscles and airflow. There was a direct correlation between the magnitude of the perceptual Urge-to-Cough and the magnitude of the cough motor output. One specific application of the perceptual measure of cough is using the cognitive awareness of an Urge-to-Cough to have a subject signal an impending motor cough. Cough artifacts are a major problem in therapeutic treatments such as radiation therapy. It is critical to maintain target orientation during targeted radiation therapy treatments. When the patient coughs, the body motion can shift the target tissue location. The perceptual awareness of an impending motor cough could allow the patient and clinician to compensate for the cough before the cough related body motion occurs.
Acknowledgments
This research was supported by a grant from the Schering-Plough Research Institute, Kenilworth, New Jersey. The authors would also like to acknowledge the assistance of R. Kimberly Kelly, Sarah Alexander-Miller, Erin Robertson and Patrick Shahan.
References
- 1.Davenport PW, Sapienza CM, Bolser DC. Psychophysical assessment of the Urge-to-Cough. Eur Respir Rev. 2002;12:249–53. [Google Scholar]
- 2.Widdicombe J, Fontana G. Cough: what’s in a name? Eur Respir J. 2006 July;:2810–5. doi: 10.1183/09031936.06.00096905. [DOI] [PubMed] [Google Scholar]
- 3.Choudry NB, Fuller RW. Sensitivity of the cough reflex in patients with chronic cough. Eur Respir J. 1992;5:296–300. [PubMed] [Google Scholar]
- 4.Choudry NB, Fuller RW, Pride NB. Sensitivity of the human cough reflex: Effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am Rev Respir Dis. 1989;140:137–41. doi: 10.1164/ajrccm/140.1.137. [DOI] [PubMed] [Google Scholar]
- 5.Fuller RW, Karlsson J-A, Choudry NB, Pride NB. Effect of inhaled and systemic opiates on responses to inhaled capsaicin in humans. J Appl Physiol. 1988;65:1125–30. doi: 10.1152/jappl.1988.65.3.1125. [DOI] [PubMed] [Google Scholar]
- 6.Hsu JY, Stine RA, Logan-Sinclair RB, Wordsell M, Busst CM, Chung KF. Coughing frequency in patients with persistent cough: assessment using a 24 h ambulatory recorder. Eur Respir J. 1994;7:1246–53. doi: 10.1183/09031936.94.07071246. [DOI] [PubMed] [Google Scholar]
- 7.Langlands J. The dynamics of cough in health and in chronic bronchitis. Thorax. 1967;22:88–96. doi: 10.1136/thx.22.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morice AH, Higgins KS, Yeo WW. Adaptation of the cough reflex with different types of stimulation. Eur Respir J. 1992;5:841–7. [PubMed] [Google Scholar]
- 9.Dicpinigaitis PV, Rauf K. The influence of gender on cough reflex sensitivity. Chest. 1998;113:1319–21. doi: 10.1378/chest.113.5.1319. [DOI] [PubMed] [Google Scholar]
- 10.Cox ID, Wallis PJW, Apps MCP, Hughes DTD, Empey DW, Osman RCA, et al. An electromyographic method of objectively assessing cough intensity and use of the method to assess effects of codeine on dose-response curve to citric acid. Br J Clin Pharmacol. 1984;18:377–82. doi: 10.1111/j.1365-2125.1984.tb02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana GA, Lavorini F. Cough motor mechanisms. Respir Physiol Neurobiol. 2006;152:266–81. doi: 10.1016/j.resp.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Fontana GA, Pantaleo T, Lavorini F, Boddi V, Panuccio P. A noninvasive electromyographic study on threshold and intensity of cough in humans. Eur Respir J. 1997;10:983–9. doi: 10.1183/09031936.97.10050983. [DOI] [PubMed] [Google Scholar]
- 13.Fontana GA, Pantaleo T, Lavorini F, Mutolo D, Polli G, Pistolesi M. Coughing in laryngectomized patients. Am J Respir Crit Care Med. 1999;160:1578–84. doi: 10.1164/ajrccm.160.5.9901093. [DOI] [PubMed] [Google Scholar]
- 14.Fontana GA, Pantaleo T, Lavorini F, Benvenuti F, Gangemi S. Defective motor control of coughing in Parkinson’s disease. Am J Respir Crit Care Med. 1998;158:458–64. doi: 10.1164/ajrccm.158.2.9705094. [DOI] [PubMed] [Google Scholar]
- 15.Leith DE, Butler JP, Sneddon SL, Brain JD. Handbook of physiology: the respiratory system, V. III: mechanics of breathing. Part I. Bethesda: American Physiological Society; 1986. Cough; pp. 315–36. [Google Scholar]
- 16.Young S, Abdul-Sattar N, Caric D. Glottic closure and high flows are not essential for productive cough. Bull Eur Physiopathol Respir. 1987;23(Suppl):11s–7s. [PubMed] [Google Scholar]