Abstract
Summary: The discovery of Wolbachia intracellular bacteria within filarial nematodes, including Onchocerca volvulus, the causative agent of onchocerciasis or “river blindness,” has delivered a paradigm shift in our understanding of the parasite's biology, to where we now know that the bacterial endosymbionts are essential for normal development of larvae and embryos and may support the long-term survival of adult worms. The apparent mutualistic dependency has also offered a novel approach to the treatment of onchocerciasis through the use of antibiotics to eliminate Wolbachia, delivering for the first time a treatment which has significant macrofilaricidal efficacy. Studies with other filarial nematode species have also highlighted a role for Wolbachia in transmission and infection of the mammalian host through a fascinating manipulation of mast cell-mediated vasodilation to enhance infectivity of vector-borne larvae. Wolbachia has also been identified as the principal driver of innate and adaptive Th1 inflammatory immunity, which can either contribute to disease pathogenesis or, with the Wolbachia-mediated recruitment of mast cells, enhance infectivity. The Wolbachia activation of innate inflammation also drives inflammatory adverse events in response to chemotherapy with either diethylcarbamazine (DEC) or ivermectin. In this review we summarize the experimental and field trial data which have uncovered the importance of Wolbachia symbiosis in onchocerciasis.
INTRODUCTION
Most filarial nematodes, including those responsible for onchocerciasis, harbor an intracellular bacterial symbiont, Wolbachia. The presence of intracellular bacteria in Onchocerca volvulus was first reported by Kozek and Marroquin (45). In nematodes, Wolbachia bacteria are obligate mutualistic endosymbionts, in contrast to the case in most arthropod-Wolbachia associations, in which they are generally considered to be reproductive parasites due to their manipulation of host reproduction to enhance transmission (65). In Onchocerca volvulus all individual worms and all life cycle stages, contain the endosymbionts. They are present in all developmental stages and inhabit the lateral chords of adult worms and the reproductive system of females, where they are transmitted transovarially (68). Wolbachia bacteria are an important target for antifilarial therapy (66). Clearance of the endosymbionts by antibiotic treatment causes inhibition of worm development, blocks embryogenesis and fertility, and reduces viability (36). Our understanding of the biological basis of the symbiotic relationship is limited and is based on comparative genomics of Wolbachia and host nematodes. This has suggested that various biochemical pathways which are intact in Wolbachia but absent or incomplete in the nematode, including heme, nucleotide, and enzyme cofactor biosynthesis, are candidates for Wolbachia's contribution to nematode biology (61).
INTRODUCTION TO ONCHOCERCIASIS (RIVER BLINDNESS)
Parasitological and Epidemiological Features
Onchocerciasis is caused by the filarial nematode Onchocerca volvulus, which is transmitted by Simulium sp. black flies, intermediate hosts that require fast-flowing water for their breeding and development; the disease is thus restricted to areas adjacent to river systems. An estimated 37 million people in 34 countries in sub-Saharan Africa and South America are infected with the disease (4). The large adult female worms are contained within fibrous nodules or onchocercomas in subcutaneous or deeper tissues. Males migrate between nodules to inseminate the females, which when fertilized give birth to 1,000 to 3,000 microfilariae per day that migrate into the skin to be transmitted to their black fly vectors. In communities where the infection is highly endemic, disease prevalence increases with age up to the 30- to 40-year age group and is accompanied by an increasing prevalence of troublesome itching and chronic papular onchodermatitis. Older age groups then begin to acquire skin depigmentation, impaired vision, and blindness (69).
Disease Manifestations
The socioeconomic and public health importance of visual impairment, blindness, and the more widespread dermatitis are profound (4). The spectrum of disease manifestations ranges from asymptomatic/paucisymptomatic infection, or generalized onchocerciasis (GEO), to severe pathology presenting as visual impairment and blindness and acute and chronic skin disease. A large body of evidence supports host immunity to the microfilarial stage as the cause of pathology, with a hyporesponsive immunological state present in the majority of asymptomatic infections (35). The type and magnitude of the immune response and consequent clinical manifestations may be influenced by host genetic factors (48, 50).
Onchocerciasis is characterized by cutaneous and ocular pathology that occurs after the invasion and death of microfilariae in the skin and eye, while adult worms are enclosed in nodules (onchocercomas) in the subcutaneous and deeper tissues. Cutaneous pathology with troublesome itching is the most common manifestation in infected people, driving social stigma due to skin appearance (82) and accounting for 60% of the 1 million disability-adjusted life years (DALYs) for onchocerciasis (85). The spectrum of skin pathology manifestations is broad. The more common generalized form presents with subclinical or intermittent dermatitis (acute and chronic papular dermatitis) that may progress to skin hyperpigmentation or depigmentation (leopard skin) and atrophy with loss of elasticity (hanging groin). A less common but severe hyperreactive form (lichenified onchodermatitis or sowda), a feature of onchocerciasis common in certain geographical areas such as Yemen and Sudan, is characterized by pruritic hyperpigmented hyperkeratotic plaques, often asymmetrical and localized, associated with local lymphadenopathy (49).
Visual impairment and blindness represent the most severe pathological outcomes of onchocerciasis, with 500,000 and 270,000 cases estimated, respectively (85). Their incidence has been dramatically reduced in areas where control programs are implemented (59). The occurrence of ocular pathology varies between geographical locations, being more common in savannah areas of West Africa and Central Africa and in Latin America (6), and has been related to various factors, such as localization of nodules in the upper part of the body (60), vector species (3), microfilarial burdens (47), and parasite strain (88), and more recently to a higher Wolbachia load in the more virulent savannah strain (28). The most common ocular pathology involves the cornea, but other structures of the anterior segment and the posterior segment can also be affected. Corneal pathology begins with “fluffy” or “snow-flake” opacities (punctate keratitis), which later coalesce and may become hyperpigmented (sclerosing keratitis). In the anterior chamber dead microfilariae can cause uveitis with formation of sinechiae, cataract, and glaucoma. Posterior segment lesions include atrophy of the retinal-pigment epithelium, choroido-retinal scarring, subretinal fibrosis, and postneuritic optical atrophy (17).
Immune Responses and Pathogenesis of Onchocerciasis in Humans
The host inflammatory response to microfilariae and Wolbachia is thought to be the driver of onchocercal keratitis and dermatitis, while retinal lesions may result from autoimmune processes driven by cross-reaction between retinal and parasite proteins (53). Nematode-derived molecules such as proteases may also be involved (26). Consistent evidence shows that pathology is caused by the immune response to the parasite, in a balance between pro- and anti-inflammatory regulation of immune responses, modulated by host factors (genetic background and prenatal exposure). Studies on human onchocerciasis tend to classify patients into three groups. Patients with generalized onchocerciasis (GEO) represent the vast majority of infected subjects and are characterized by having weak or no skin inflammation despite high parasite burdens, while patients with severe chronic dermatitis (sowda) suffer from severe symptoms and present low microfilaria and adult burdens. A third, small subgroup of people living in areas of endemicity do not acquire detectable patent infection despite exposure to infective vector bites and have been termed “endemic normals” (EN) or “putatively immune” (PI). EN have been studied to shed light on the immune mechanisms involved in their supposed refractoriness to infection; however, their true classification as uninfected is difficult to prove and has been debated (75, 81). The majority of studies investigating the relationship between immune responses and pathogenesis of onchocerciasis compared GEO and sowda patients, correlating the low microfilarial loads and the strong T helper 2 (Th2) response to greater severity of pathological manifestations, as seen in sowda (1, 70). Nevertheless, this comparison could be misleading. Indeed, sowda is relatively rare compared to GEO, it is geographically confined, and it has been correlated with specific genetic polymorphisms (2, 31). Sowda is characterized by strong Th2 cytokine responses, high levels of anti-O. volvulus immunoglobulin G1 (IgG1) and IgG3 (isotypes involved in EN resistance to infection) and IgE, pronounced eosinophilia, increased circulating levels of eosinophil cationic protein, and increased delayed-type hypersensitivity (7, 9, 70, 72). Eosinophils are probably the effector cells involved in microfilarial killing in sowda. Eosinophils from these patients show high chemotactive responsiveness in vitro, and they were the major effector cells of immunity to Onchocerca microfilaria in a mouse model of onchocerciasis (18, 55). Taken together, these characteristics suggest that the mechanisms behind the pathogenesis of sowda and GEO might not lie on a continuum, with sowda being instead the result of a hyperreactive Th2 immune reaction to microfilaria, mirroring tropical pulmonary eosinophilia observed in lymphatic filariasis.
GEO is characterized by weak proliferative responses to filarial antigens, low levels of gamma interferon (IFN-γ), and increasing generalized Th2 responses with increasing severity of pathology, but the correlation between low microfilarial loads and severity of pathology in these patients is not clear-cut (70). IgG4 and IgE are the prominent antibody responses, with IgG4 possibly acting as a blocking isotype (20, 40). Vital nondegenerating microfilariae are not attacked by effector cells (granulocytes and mast cells) except after death either through natural attrition or following microfilaricidal treatment (12, 25, 86). In comparison to EN, who show a mixed Th1/Th2 response, both responses appear to be downregulated in infected people and partially restored after ivermectin (IVM) therapy (15, 27).
The majority of infected patients clearly have mechanisms modulating the host response to prevent immune-mediated damage while allowing high parasite burdens. The fact that both arms (Th1 and Th2) of the immune response are restored after antimicrofilarial treatment shows that the hyporesponsiveness in generalized onchocerciasis is not simply due to a shift from Th1 to Th2 (15). Various mechanisms appear to be involved in the immune downregulation. Production of interleukin-10 (IL-10) has been repeatedly associated with hyporesponsiveness in onchocerciasis (27, 62). The additional neutralization of transforming growth factor β (TFG-β) enhanced but did not completely restore proliferation of peripheral blood mononuclear cells (PBMC) from microfilaridermic patients (15). Antigen-specific regulatory T cells (Tr1), first described in human infectious diseases in onchocerciasis, seem to play a key role in inducing peripheral tolerance by production of IL-10 and TGF-β and expression of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (57). Alternatively activated macrophages have been studied extensively in mouse models of filariasis (35), and recently macrophages with an alternative activation phenotype in onchocercomas have been described (44). Moreover, the antigen-presenting functions of dendritic cells have been found to be impaired by exposure to filarial parasites in vitro (58). This complex immune modulation has been attributed to molecules secreted by the parasite, such as antioxidants, proteases, cytokine homologues (TGF-β), glycoproteins (ES-62), and lipid mediators (prostaglandin E2 [PGE2]) (reviewed in reference 7), and to in utero exposure to the parasite (16). Genetic background has also been implicated in the diverse clinical outcomes after exposure, with polymorphisms of several immune response genes, including those for Fc gamma RIIa (CD32), IL-13, and HLA, associated with chronic and hyperreactive skin lesions (2, 13, 50, 71).
WOLBACHIA AND THE INFLAMMATORY PATHOGENESIS OF ONCHOCERCIASIS
Wolbachia and the Inflammatory Response
Wolbachia and Wolbachia-derived molecules, as demonstrated in another filarial species, Brugia malayi, can be released from worms and come in contact with the host immune system after parasite death or release of excretory/secretory products (5, 8, 43). The characteristics of this interaction can be replicated in vitro by exposing innate immune cells to Wolbachia-containing parasite extracts, which, in contrast to extracts from aposymbiotic species (Acanthocheilonema viteae and Loa loa) or extracts from worms depleted of Wolbachia by antibiotic treatment of the host, mount a potent proinflammatory cytokine response (67, 76). Figure 1 illustrates Wolbachia-induced responses in different immune cell types. Experiments using Wolbachia-containing extracts of O. volvulus in a mouse model of onchocercal keratitis demonstrated that the presence of the bacteria was essential for neutrophil-mediated inflammation, opacity, and corneal haze (Fig. 2) (see “Role of Wolbachia in a Murine Model of Onchocercal Keratitis” below). Neutrophil activation is also observed during adverse reactions following treatment with diethylcarbamazine (DEC) and IVM, which include fever, generalized body pain, pruritus, edema, lymphoadenopathy, and in the case of DEC, which kills microfilaria much more rapidly than IVM, ocular inflammation (10). The occurrence and severity of adverse reactions correlate with microfilarial load (19) and the presence of Wolbachia DNA, proinflammatory cytokines, and neutrophil-derived antibacterial calprotectin levels in the blood after microfilaricidal treatment (43). Neutrophils are an abundant inflammatory component of the nodule tissues and are most numerous adjacent to adult worms. Following doxycycline depletion of Wolbachia, the neutrophil infiltrate of nodules is drastically reduced (8). This depletion of neutrophils does not depend on the presence of dead worms after treatment and suggests that intact viable parasites are a source of Wolbachia inflammatory products released via secretory or excretory processes. The role of Wolbachia in the pathogenesis of river blindness is illustrated in Fig. 2.
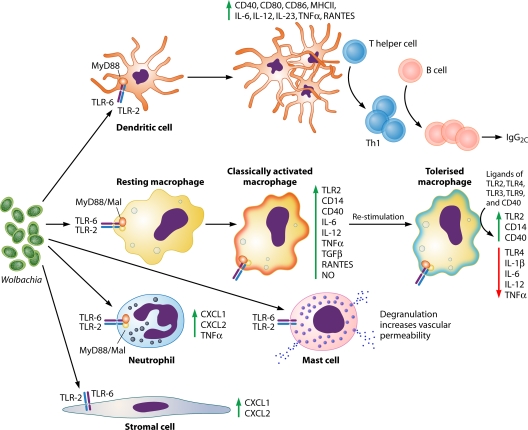
Fig. 1.
Wolbachia-induced responses of specific cell types. Wolbachia-exposed dendritic cells are activated via the TLR2/6-MyD88-Mal pathway, as shown by enhanced expression of surface costimulatory molecules and produce proinflammatory cytokines, inducing a preferential type 1 (Th1) immune response (11, 77). Macrophages stimulated with Wolbachia or Wolbachia-containing but not Wolbachia-depleted filarial extracts enhance their surface expression of costimulatory molecules and produce proinflammatory cytokines and oxidative products. Macrophages can be homo- and heterotolerized by a subsequent stimulation, contributing to the immune downregulation characterizing the majority of filarial infections (29, 67, 76, 77). Neutrophils and corneal stromal cells are also able to interact with Wolbachia via the TLR2-MyD88 pathway, producing CXC chemokines and contributing to the inflammatory response to the parasite (22–24). Mast cells are stimulated by Wolbachia via TLR2 to degranulate and increase vascular permeability to facilitate establishment of infection (63). Abbreviations: TLR, Toll-like receptor; CD, cluster of differentiation; MHCII, major histocompatibility complex class II; IL, interleukin; TNF-α, tumor necrosis factor alpha; RANTES, regulated upon activation, normal T-cell expressed, and secreted; TGF-β, transforming growth factor β; NO, nitric oxide; MyD88, myeloid differentiation primary response gene (88); Mal, MyD88 adaptor-like.
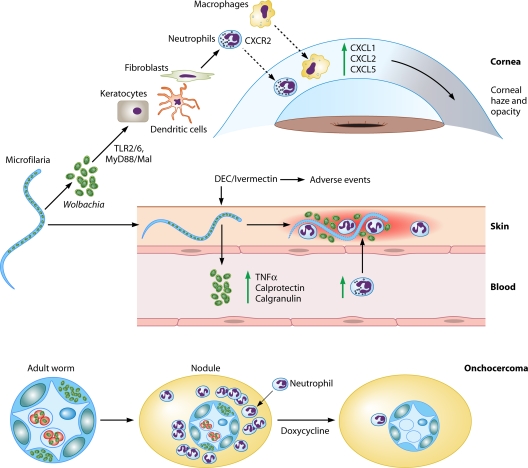
Fig. 2.
Role of Wolbachia in river blindness. Wolbachia release after microfilaria death in the cornea causes corneal edema and opacity by inducing neutrophil and macrophage infiltration and activation in the corneal stroma that are dependent on TLR2-MyD88 activation and production of CXC chemokines. Keratocytes and bone marrow-derived cells in the corneal stroma can initiate this response, which is then perpetuated by inflammatory cells (22–24, 29, 56, 77). When large loads of Wolbachia bacteria are released from microfilariae after microfilaricidal treatment, this induces cutaneous and systemic side effects such as fever, tachycardia, hypotension, lymphadenopathy, and pruritus. In the skin, neutrophils are the first cells to be recruited and activated, inducing dermal inflammation. At a systemic level, adverse events correlate with microfilarial loads and are associated with Wolbachia DNA and whole bacterial levels in blood, proinflammatory cytokines, neutrophilia, and antibacterial peptides (calprotectin and calgranulin) (25, 43, 52, 80). The presence of Wolbachia is associated with neutrophil infiltration in the cornea, skin, and onchocercomas (8, 22, 23). Abbreviations: TLR, Toll-like receptor; MyD88, myeloid differentiation primary response gene (88); TNF-α, tumor necrosis factor alpha; DEC, diethylcarbamazine.
Role of Wolbachia in a Murine Model of Onchocercal Keratitis
Microfilariae invade both the anterior and the posterior segments of the eye (17). In the latter case, they cause uveitis and chorioretinitis, resulting in loss of vision. Due to ethical restrictions on the availability of ocular tissue from human cases of onchocerciasis, tissues from Onchocerca dermatitis have been studied, and these show microfilariae surrounded by neutrophils, eosinophils, or macrophages (12, 25). The likely explanation is that neutrophils surround recently dead and degenerating worms, whereas macrophages and eosinophils migrate to the site at later time points. Our findings in a murine model (23) show that neutrophils surround microfilariae in the cornea within 24 h, and immunogold labeling of the major Wolbachia surface protein shows neutrophils in close proximity to Wolbachia (Fig. 3). Using a similar mouse model of O. volvulus keratitis in which filaria-Wolbachia extracts were injected into the corneal stroma (24, 56), we demonstrated that endosymbiotic Wolbachia bacteria are essential for the pathogenesis of O. volvulus keratitis, as (i) O. volvulus from individuals depleted of Wolbachia by antibiotic treatment does not induce corneal inflammation, (ii) related filarial species containing Wolbachia induce keratitis, in contrast to filarial species lacking Wolbachia, and (iii) isolated Wolbachia bacteria induce neutrophil recruitment to the corneal stroma.
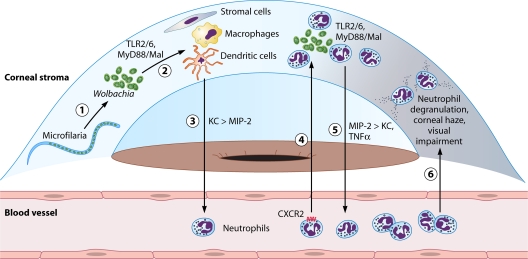
Fig. 3.
Predicted sequence of events in O. volvulus/Wolbachia-induced keratitis. Wolbachia released from dying microfilariae in the corneal stroma (1) activates resident cells, including fibroblasts, dendritic cells, and macrophages (2). These cells produce chemokines MIP-2 and predominantly KC (3). KC induces a CXCR2-dependent neutrophil migration to the corneal stroma, where neutrophils are also activated via TLR2/6 by Wolbachia (4). Neutrophils produce additional chemokines, predominantly MIP-2, inducing further neutrophil migration (5). Neutrophils degrade the corneal matrix, causing corneal haze and visual impairment (6). Abbreviations: TLR, Toll-like receptor; MyD88, myeloid differentiation primary response gene (88); Mal, MyD88 adaptor-like; KC, keratinocyte-derived chemokine; MIP, macrophage-inflammatory protein 2; TNF-α, tumor necrosis factor alpha; DC, dendritic cell.
Wolbachia and Toll-Like Receptors in the Cornea
Toll-like receptors (TLR) are surface and endosomal receptors that are expressed in the cornea and respond to microbial products. TLR2 forms heterodimers with TLR1 or TLR6 to initiate signaling through adaptor molecules to induce nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) translocation to the nucleus and the production of proinflammatory and chemotactic cytokines (42). Our findings using gene knockout mice clearly demonstrate that O. volvulus extracts containing Wolbachia or isolated nematode or insect Wolbachia selectively activates TLR2 and TLR6 and the adaptor molecules myeloid differentiation primary response gene 88 (MyD88) and MyD88 adaptor-like molecule (Mal) (29). Figure 3 shows that corneal inflammation (neutrophil infiltration and increased corneal haze) is entirely dependent on activating TLR2, and the use of chimeric mice also shows that TLR2 expressed on bone marrow-derived cells has an important role (22).
Taken together, these findings indicate that Wolbachia induces TLR2 activation in resident macrophages in the corneal stroma and produces proinflammatory cytokines and CXC chemokines, which mediate neutrophil recruitment from peripheral limbal vessels into the corneal stroma. Neutrophil responses to Wolbachia are also dependent on TLR2/MyD88, which mediate cytokine production by these cells and may contribute to degranulation and secretion of reactive oxygen species and matrix metalloproteinases, resulting in cell death and loss of corneal clarity (22, 24).
In chronically infected, untreated individuals, there is also an ongoing adaptive immune response, due to repeated invasion of microfilariae into the corneal stroma and consistent worm degeneration and release of Wolbachia. Upon infiltration, eosinophils and macrophages combine to cause permanent tissue damage, which manifests as corneal opacification, loss of vision, and blindness. We found TLR2-dependent Wolbachia activation of dendritic cells and T-cell production of IFN-γ but not IL-4 or IL-5 (11). IFN-γ also has an indirect role in enhancing proinflammatory and chemotactic cytokine production and thereby increasing neutrophil recruitment to the corneal stroma (21). Together, these findings demonstrate that TLR2 governs the host response to Wolbachia at several levels, including systemic and corneal responses through activation of innate and adaptive immunity.
Identification of a Wolbachia TLR2/TLR6 Ligand
The TLR2/TLR6 heterodimer is activated by diacylated lipoproteins. Biochemical removal of lipid and protein from native parasite extracts eliminates all inflammatory activity. Two lipoproteins were consistently predicated from database mining as candidate TLR2/TLR6 ligands, Wolbachia peptidoglycan-associated lipoprotein (wBmPAL) and type IV secretion system-VirB6. To examine the response of wBmPAL, corneas of C57BL/6, TLR1−/−, TLR2−/−, and TLR6−/− mice were injected with synthetic diacylated peptides of wBmPAL, and corneal inflammation was examined as before. We found that TLR2−/− and TLR6−/− corneas had significantly impaired neutrophil infiltration, indicating that the interaction between Wolbachia lipoproteins and TLR2/6 heterodimers on resident cells in the corneal stroma induces the early stages of O. volvulus keratitis (77).
Predicted Sequence of Events in O. volvulus/Wolbachia-Induced Keratitis
Taken together, studies using the mouse model of ocular onchocerciasis are consistent with the following sequence of events (Fig. 3): (i) the inflammatory response to Wolbachia is initiated after death and degeneration of microfilariae and release of bacteria into the corneal stroma; (ii) Wolbachia bacteria activate TLR2/6 and MyD88 on resident cells in the cornea, including resident fibroblasts and bone marrow-derived macrophages and dendritic cells; (iii) these cells produce proinflammatory and chemotactic cytokines (21, 22, 29); (iv) neutrophils migrate in a CXCR2-dependent manner through the stromal matrix to the site of microfilarial degradation and release of Wolbachia (22); (v) as neutrophils also express TLR2/6 and MyD88, they ingest Wolbachia and produce proinflammatory and chemotactic cytokines (22), which stimulate further neutrophil infiltration; and (vi) neutrophil degranulation and secretion of cytotoxic products such as nitric oxide, myeloperoxidase, and oxygen radicals have a cytotoxic effect on resident cells in the cornea, including fibroblasts and corneal endothelium, resulting in corneal edema and further loss of corneal clarity.
Alternative Roles for Wolbachia-Mediated Inflammation in Establishment of Infective Larvae
In addition to driving inflammatory components of disease pathogenesis and inflammatory adverse reactions to drugs, the stimulation of inflammation by Wolbachia appears to have been exploited by the rodent filaria Litomosoides sigmodontis to facilitate its infection of the mammalian host (63). Exposure of mice to infective bites from the mite intermediate host results in a chemokine (C-C motif) ligand 17 (CCL17)-dependent infiltration of mast cells into the skin, which degranulate and increase vascular permeability. The recruitment of mast cells and subsequent increase in vascular permeability facilitate the entry of infective third-stage larvae into the host, leading to higher worm burdens in mice deficient in CCL17 than in wild-type controls. Vascular permeability could be blocked by chemical inhibition of mast cell degranulation and was dependent upon the presence of Wolbachia and TLR2. Thus, in this case the parasite appears to exploit the Wolbachia-mediated induction of inflammatory cells to its own advantage to promote its establishment in the mammalian host.
WOLBACHIA AS A TARGET FOR CHEMOTHERAPY
In Vitro and In Vivo Animal Studies
Following the resurgence of Wolbachia in filarial research, an obvious priority was to determine whether targeting the bacteria with antibiotics could provide an alternative approach to the treatment and control of onchocerciasis. Initial studies screened a series of antibiotics against Onchocerca spp. in vitro and in animal models, which showed that tetracyclines and rifamycins were the most potent drug classes to result in adult parasite death (73, 74). Macrofilaricidal activity was first demonstrated in a species infecting cattle, O. ochengi, in which a protracted course of oxytetracycline resulted in the elimination of Wolbachia, killing of adult worms, and resolution of nodules at 9 months posttreatment (46).
Human Field Trials with Antiwolbachial Drugs
Currently the treatment and control of onchocerciasis rely on a single drug, ivermectin. This is used in mass drug administration (MDA) either annually or biannually or for individual treatment every 3 to 6 months. It is effective at reducing microfilarial loads but is only marginally effective against adult worms and so requires sustained delivery for more than 15 to 17 years in order to interrupt transmission (14, 69).
Doxycycline was the first drug used in trials in human onchocerciasis to deplete Wolbachia. First, open trials performed in Ghana administered doxycycline at a daily dose of 100 mg for 6 weeks. This treatment resulted in Wolbachia depletion of more than 90% (38), which in later studies has become an empirical threshold for a reduction in bacteria that will lead to death of the worms (13, 78). After 4 to 6 months, embryogenesis was interrupted (33, 38), and patients showed sustained amicrofilaridermia after a single additional dose of the microfilaricidal drug IVM, in contrast to patients who had received IVM only (32). This study was terminated too early to allow assessment of a macrofilaricidal effect, which is seen only after approximately 2 years following treatment with doxycycline. Such an effect was, however, observed in further double-blind, placebo-controlled, randomized trials in Ghana, after administration of 200 mg/day of doxycycline for 4 or 6 weeks or of 100 mg/day for 5 weeks, whereby the highest dose showed a macrofilaricidal effect of up to 60% (70% if worms that had been newly acquired during the observation period were subtracted), in contrast to the 50% (60% after subtraction of newly acquired worms) seen after the 4-week or the 5-week dose (36, 37). The female worms that were still alive at the time of nodulectomy (extirpation of the onchocercomata) were sterile.
Importantly, both sterilizing and macrofilaricidal effects are due to doxycycline alone, as could be shown in a parallel study from Cameroon (79), where doxycycline monotherapy was administered without subsequent IVM, in contrast to trials in Ghana that had used IVM after 200-mg doxycycline regimens. Doxycycline efficacy without IVM had also been observed in the trial using doxycycline at 100 mg/day for 5 weeks (37).
Based on these trials, doxycycline at 200 mg/day for 6 weeks is recommended for patients in whom the highest possible macrofilaricidal activity is desired and who have moved away from areas with ongoing transmission. In those areas where reinfection will be frequent, single-dose IVM yearly is easier to comply with. However, exceptions should be made if the patient is suffering from severe skin disease, as this will resume a few months after IVM since this drug does not lead to a sustained interruption of embryogenesis. If doxycycline treatment aims at permanently clearing microfilariae (the inducers of pathology) from the skin of a patient, a regimen of 4 weeks of doxycycline at 200 mg/day will be sufficient (30).
There has been some skepticism as to whether the current doxycycline regimens are deliverable via community-directed interventions adopted for IVM-based control strategies. Wanji and colleagues, however, proved the skeptics wrong when they showed that more than 97% of ∼13,000 people from villages where the infection is endemic who started doxycycline completed a 6-week regimen after community-directed explanation and organization of the delivery (community-directed treatment) (84). Current follow-up studies are aimed at assessing the impact of community-directed doxycycline administration (which comprised, on average, about 74% of the eligible population having started the treatment) on community microfilarial load and thus its impact on transmission.
Even if doxycycline should be administered in restricted areas at the health district level, the problem of contraindication for children and pregnant women remains. The best class of existing registered antibiotics would be macrolides, since they can be given during pregnancy and childhood. Therefore, following promising results by others (54) on the ability of azithromycin to deplete Wolbachia, trials on onchocerciasis (and also lymphatic filariasis) were undertaken. Unfortunately, azithromycin did not fulfill its promise and led to neither Wolbachia depletion nor antifilarial effects (34). This is in contrast to the case for rifampin, which had shown almost equivalent efficacy in the L. sigmodontis mouse model regarding Wolbachia depletion and inhibition of larval development (83) and led to considerable depletion of Wolbachia and interruption of embryogenesis when administered for 4 weeks to onchocerciasis patients (64). However, this human trial covered only a small number of patients and is currently being repeated in a placebo-controlled manner, from which more precise data on the extent of Wolbachia copy number reduction and antifilarial effects will be obtained. Importantly, since rifampin has shown synergistic activity with doxycycline, allowing a reduction of treatment time by 50% in the L. sigmodontis mouse model (A. Hoerauf et al., unpublished results), the current trials also involve combination regimens with doxycycline and rifampin using reduced time frames and doses as part of the regimen refinement objective of the Anti-Wolbachia (A-WOL) Consortium program (www.A-WOL.com) (see below).
Future Prospects for Use of Antiwolbachial Treatment in the Control and Elimination of Onchocerciasis
Although human trials of antiwolbachial therapy have delivered superior efficacy compared with existing antionchocercal drugs through permanent sterilization and macrofilaricidal activity against adult O. volvulus, the prolonged treatment regimens (4 to 6 weeks) and contraindications for doxycycline (pregnancy and age <8 years) remain barriers to the widespread use of these approaches in current control strategy scenarios. The desire to determine whether alternative drugs or combinations could deliver regimens more compatible with MDA approaches led to the formation of the A-WOL Consortium. The A-WOL Consortium product portfolio seeks to deliver (i) optimized regimens of existing drugs for rapid deployment in restricted settings now, (ii) the identification of alternative drugs and combinations which are effective in shorter time frames and safe for children and in pregnancy, and ultimately (iii) narrow-spectrum antiwolbachial drugs which hopefully will ensure that the goal of eliminating onchocerciasis can be ultimately achieved.
Regimen refinement trials are aimed at optimizing regimens of existing antiwolbachial drugs in combination and in reduced time frames for delivery in restricted settings where the urgent deployment of alternative treatment might be warranted. This might include areas where there is evidence for suboptimal efficacy and potential resistance to existing drugs may occur (51), areas where coendemicity of Loa loa and the risk of serious adverse events prevent introduction of IVM-based strategies, or where sustained delivery of IVM is compromised. It may also provide a useful tool in program endgame situations where a test-and-treat strategy to identify and treat residual infection could be used if the aim is to achieve elimination of the infection. The macrofilaricidal and permanent sterilization outcomes of antiwolbachial therapy should also drastically reduce the time frames of control programs compared with annual or biannual IVM delivery, which requires at least 15 to 17 years before transmission is interrupted (14).
A comprehensive drug screening strategy has been used against libraries of registered drugs, focused anti-infective drugs, and larger diversity-based synthetic and natural product libraries to identify existing and novel compounds active against Wolbachia, which conform to the A-WOL Consortium target product profile for drugs compatible with MDA control programs. Target discovery approaches have also been advanced to provide a core list of essential genes and enzyme targets in pathways validated as important to the Wolbachia-nematode symbiosis (39, 41, 61, 87).
The targeting of Wolbachia with antibiotics has already delivered a superior efficacy for individual treatment and has achieved the “holy grail” of a safe macrofilaricidal therapy that has so far eluded the field of onchocerciasis chemotherapy. The goal of our ongoing research is to deliver an antiwolbachial therapy that is compatible with MDA programs to sustain and enhance the achievements in onchocerciasis control and deliver the means for elimination of onchocerciasis.
Biographies
 Francesca Tamarozzi graduated in veterinary medicine in Parma (Italy) in 2003 and in medicine in Pavia (Italy) in 2008. She is currently a Ph.D. student in Professor Mark Taylor's laboratory, Molecular and Biochemical Parasitology Group, Liverpool School of Tropical Medicine, funded through the A-WOL Consortium.
Francesca Tamarozzi graduated in veterinary medicine in Parma (Italy) in 2003 and in medicine in Pavia (Italy) in 2008. She is currently a Ph.D. student in Professor Mark Taylor's laboratory, Molecular and Biochemical Parasitology Group, Liverpool School of Tropical Medicine, funded through the A-WOL Consortium.
 Alice Halliday is a Ph.D. student (BBSRC CASE Award with Pfizer) in Professor Mark Taylor's laboratory, Molecular and Biochemical Parasitology Group, Liverpool School of Tropical Medicine.
Alice Halliday is a Ph.D. student (BBSRC CASE Award with Pfizer) in Professor Mark Taylor's laboratory, Molecular and Biochemical Parasitology Group, Liverpool School of Tropical Medicine.
 Katrin Gentil graduated from the Medical School in Göttingen (Germany) in 2004. She worked under Eric Pearlman at Case Western Reserve University (Cleveland, OH) on ocular onchocerciasis before joining the Institute for Medical Microbiology, Immunology and Parasitology at the University Hospital Bonn (Germany).
Katrin Gentil graduated from the Medical School in Göttingen (Germany) in 2004. She worked under Eric Pearlman at Case Western Reserve University (Cleveland, OH) on ocular onchocerciasis before joining the Institute for Medical Microbiology, Immunology and Parasitology at the University Hospital Bonn (Germany).
 Achim Hoerauf is Full Professor (C4) and Director of the Institute for Medical Microbiology, Immunology and Parasitology (IMMIP), University Clinic of Bonn.
Achim Hoerauf is Full Professor (C4) and Director of the Institute for Medical Microbiology, Immunology and Parasitology (IMMIP), University Clinic of Bonn.
 Eric Pearlman is Professor and Director of the Research Department of Ophthalmology and Visual Sciences, Case Western Reserve University, Cleveland, OH.
Eric Pearlman is Professor and Director of the Research Department of Ophthalmology and Visual Sciences, Case Western Reserve University, Cleveland, OH.
 Mark Taylor is Professor of Parasitology and Head of Molecular and Biochemical Parasitology at the Liverpool School of Tropical Medicine and Director of the A-WOL Consortium.
Mark Taylor is Professor of Parasitology and Head of Molecular and Biochemical Parasitology at the Liverpool School of Tropical Medicine and Director of the A-WOL Consortium.
REFERENCES
- 1. Ali M. M., et al. 2003. Immune responses directed against microfilariae correlate with severity of clinical onchodermatitis and treatment history. J. Infect. Dis. 187:714–717 [DOI] [PubMed] [Google Scholar]
- 2. Ali M. M., et al. 2007. Fc gamma RIIa (CD32) polymorphism and onchocercal skin disease: implications for the development of severe reactive onchodermatitis (ROD). Am. J. Trop. Med. Hyg. 77:1074–1078 [PubMed] [Google Scholar]
- 3. Baker R. H., Abdelnur O. M. 1986. Onchocerciasis in Sudan: the distribution of the disease and its vectors. Trop. Med. Parasitol. 37:341–355 [PubMed] [Google Scholar]
- 4. Basanez M. G., et al. 2006. River blindness: a success story under threat? PLoS Med. 3:e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennuru S., et al. 2009. Brugia malayi excreted/secreted proteins at the host/parasite interface: stage- and gender-specific proteomic profiling. PLoS Negl. Trop. Dis. 3:e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boatin B. A., Richards F. O., Jr 2006. Control of onchocerciasis. Adv. Parasitol. 61:349–394 [DOI] [PubMed] [Google Scholar]
- 7. Brattig N. W. 2004. Pathogenesis and host responses in human onchocerciasis: impact of Onchocerca filariae and Wolbachia endobacteria. Microbes Infect. 6:113–128 [DOI] [PubMed] [Google Scholar]
- 8. Brattig N. W., Buttner D. W., Hoerauf A. 2001. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 3:439–446 [DOI] [PubMed] [Google Scholar]
- 9. Burchard G. D., Brattig N. W., Kruppa T. F., Horstmann R. D. 1999. Delayed-type hypersensitivity reactions to Onchocerca volvulus antigens in exposed and non-exposed African individuals. Trans. R. Soc. Trop. Med. Hyg. 93:103–105 [DOI] [PubMed] [Google Scholar]
- 10. Dadzie K. Y., et al. 1987. Ocular findings in a double-blind study of ivermectin versus diethylcarbamazine versus placebo in the treatment of onchocerciasis. Br. J. Ophthalmol. 71:78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daehnel K., et al. 2007. Filaria/Wolbachia activation of dendritic cells and development of Th1-associated responses is dependent on Toll-like receptor 2 in a mouse model of ocular onchocerciasis (river blindness). Parasite Immunol. 29:455–465 [DOI] [PubMed] [Google Scholar]
- 12. Darge K., Lucius R., Monson M. H., Behrendsen J., Buttner D. W. 1991. Immunohistological and electron microscopic studies of microfilariae in skin and lymph nodes from onchocerciasis patients after ivermectin treatment. Trop. Med. Parasitol. 42:361–367 [PubMed] [Google Scholar]
- 13. Debrah A. Y., et al. 2006. Assessment of microfilarial loads in the skin of onchocerciasis patients after treatment with different regimens of doxycycline plus ivermectin. Filaria J. 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diawara L., et al. 2009. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLoS Negl. Trop. Dis. 3:e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doetze A., et al. 2000. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h) 3/T(r) 1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h) 1 to T(h) 2 shift. Int. Immunol. 12:623–630 [DOI] [PubMed] [Google Scholar]
- 16. Elson L. H., et al. 1996. In utero exposure to Onchocerca volvulus: relationship to subsequent infection intensity and cellular immune responsiveness. Infect. Immun. 64:5061–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Enk C. D. 2006. Onchocerciasis—river blindness. Clin. Dermatol. 24:176–180 [DOI] [PubMed] [Google Scholar]
- 18. Folkard S. G., Hogarth P. J., Taylor M. J., Bianco A. E. 1996. Eosinophils are the major effector cells of immunity to microfilariae in a mouse model of onchocerciasis. Parasitology 112:323–329 [DOI] [PubMed] [Google Scholar]
- 19. Francis H., Awadzi K., Ottesen E. A. 1985. The Mazzotti reaction following treatment of onchocerciasis with diethylcarbamazine: clinical severity as a function of infection intensity. Am. J. Trop. Med. Hyg. 34:529–536 [DOI] [PubMed] [Google Scholar]
- 20. Garraud O., Nkenfou C., Bradley J. E., Perler F. B., Nutman T. B. 1995. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen-specific IgE and IgG4 antibodies. J. Immunol. 155:1316–1325 [PubMed] [Google Scholar]
- 21. Gentil K., Pearlman E. 2009. Gamma interferon and interleukin-1 receptor 1 regulate neutrophil recruitment to the corneal stroma in a murine model of Onchocerca volvulus keratitis. Infect. Immun. 77:1606–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gillette-Ferguson I., et al. 2007. Toll-like receptor 2 regulates CXC chemokine production and neutrophil recruitment to the cornea in Onchocerca volvulus/Wolbachia-induced keratitis. Infect. Immun. 75:5908–5915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gillette-Ferguson I., et al. 2004. Wolbachia-induced neutrophil activation in a mouse model of ocular onchocerciasis (river blindness). Infect. Immun. 72:5687–5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gillette-Ferguson I., et al. 2006. Wolbachia- and Onchocerca volvulus-induced keratitis (river blindness) is dependent on myeloid differentiation factor 88. Infect. Immun. 74:2442–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gutierrez-Pena E. J., Knab J., Buttner D. W. 1996. Neutrophil granule proteins: evidence for the participation in the host reaction to skin microfilariae of Onchocerca volvulus after diethylcarbamazine administration. Parasitology 113:403–414 [DOI] [PubMed] [Google Scholar]
- 26. Haffner A., Guilavogui A. Z., Tischendorf F. W., Brattig N. W. 1998. Onchocerca volvulus: microfilariae secrete elastinolytic and males nonelastinolytic matrix-degrading serine and metalloproteases. Exp. Parasitol. 90:26–33 [DOI] [PubMed] [Google Scholar]
- 27. Henry N. L., Law M., Nutman T. B., Klion A. D. 2001. Onchocerciasis in a nonendemic population: clinical and immunologic assessment before treatment and at the time of presumed cure. J. Infect. Dis. 183:512–516 [DOI] [PubMed] [Google Scholar]
- 28. Higazi T. B., et al. 2005. Wolbachia endosymbiont levels in severe and mild strains of Onchocerca volvulus. Mol. Biochem. Parasitol. 141:109–112 [DOI] [PubMed] [Google Scholar]
- 29. Hise A. G., et al. 2007. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J. Immunol. 178:1068–1076 [DOI] [PubMed] [Google Scholar]
- 30. Hoerauf A. 2008. Filariasis: new drugs and new opportunities for lymphatic filariasis and onchocerciasis. Curr. Opin. Infect. Dis. 21:673–681 [DOI] [PubMed] [Google Scholar]
- 31. Hoerauf A., et al. 2002. The variant Arg110Gln of human IL-13 is associated with an immunologically hyper-reactive form of onchocerciasis (sowda). Microbes Infect. 4:37–42 [DOI] [PubMed] [Google Scholar]
- 32. Hoerauf A., Mand S., Adjei O., Fleischer B., Buttner D. W. 2001. Depletion of Wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet 357:1415–1416 [DOI] [PubMed] [Google Scholar]
- 33. Hoerauf A., et al. 2003. Doxycycline in the treatment of human onchocerciasis: kinetics of Wolbachia endobacteria reduction and of inhibition of embryogenesis in female Onchocerca worms. Microbes Infect. 5:261–273 [DOI] [PubMed] [Google Scholar]
- 34. Hoerauf A., et al. 2008. Effects of 6-week azithromycin treatment on the Wolbachia endobacteria of Onchocerca volvulus. Parasitol. Res. 103:279–286 [DOI] [PubMed] [Google Scholar]
- 35. Hoerauf A., Satoguina J., Saeftel M., Specht S. 2005. Immunomodulation by filarial nematodes. Parasite Immunol. 27:417–429 [DOI] [PubMed] [Google Scholar]
- 36. Hoerauf A., et al. 2008. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: a randomized placebo-controlled study. Med. Microbiol. Immunol. 197:295–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoerauf A., et al. 2009. Efficacy of 5-week doxycycline treatment on adult Onchocerca volvulus. Parasitol. Res. 104:437–447 [DOI] [PubMed] [Google Scholar]
- 38. Hoerauf A., et al. 2000. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet 355:1242–1243 [DOI] [PubMed] [Google Scholar]
- 39. Holman A. G., Davis P. J., Foster J. M., Carlow C. K., Kumar S. 2009. Computational prediction of essential genes in an unculturable endosymbiotic bacterium, Wolbachia of Brugia malayi. BMC Microbiol. 9:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hussain R., Poindexter R. W., Ottesen E. A. 1992. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J. Immunol. 148:2731–2737 [PubMed] [Google Scholar]
- 41. Johnston K. L., et al. 2010. Lipoprotein biosynthesis as a target for anti-Wolbachia treatment of filarial nematodes. Parasites Vectors 3:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kawai T., Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373–384 [DOI] [PubMed] [Google Scholar]
- 43. Keiser P. B., et al. 2002. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J. Infect. Dis. 185:805–811 [DOI] [PubMed] [Google Scholar]
- 44. Korten S., Kaifi J. T., Buttner D. W., Hoerauf A. 2010. Transforming growth factor-beta expression by host cells is elicited locally by the filarial nematode Onchocerca volvulus in hyporeactive patients independently from Wolbachia. Microbes Infect. 12:555–564 [DOI] [PubMed] [Google Scholar]
- 45. Kozek W. J., Marroquin H. F. 1977. Intracytoplasmic bacteria in Onchocerca volvulus. Am. J. Trop. Med. Hyg. 26:663–678 [DOI] [PubMed] [Google Scholar]
- 46. Langworthy N. G., et al. 2000. Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc. Biol. Sci. 267:1063–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Little M. P., Basanez M. G., Breitling L. P., Boatin B. A., Alley E. S. 2004. Incidence of blindness during the onchocerciasis control programme in western Africa, 1971–2002. J. Infect. Dis. 189:1932–1941 [DOI] [PubMed] [Google Scholar]
- 48. Meyer C. G., et al. 1994. HLA-D alleles associated with generalized disease, localized disease, and putative immunity in Onchocerca volvulus infection. Proc. Natl. Acad. Sci. U. S. A. 91:7515–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murdoch M. E., et al. 1993. A clinical classification and grading system of the cutaneous changes in onchocerciasis. Br. J. Dermatol. 129:260–269 [DOI] [PubMed] [Google Scholar]
- 50. Murdoch M. E., et al. 1997. HLA-DQ alleles associate with cutaneous features of onchocerciasis. The Kaduna-London-Manchester Collaboration for Research on Onchocerciasis. Hum. Immunol. 55:46–52 [DOI] [PubMed] [Google Scholar]
- 51. Osei-Atweneboana M. Y., Eng J. K., Boakye D. A., Gyapong J. O., Prichard R. K. 2007. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet 369:2021–2029 [DOI] [PubMed] [Google Scholar]
- 52. Pearlman E., et al. 1999. Temporal recruitment of neutrophils and eosinophils to the skin in a murine model for onchocercal dermatitis. Am. J. Trop. Med. Hyg. 61:14–18 [DOI] [PubMed] [Google Scholar]
- 53. Pearlman E., Hall L. R. 2000. Immune mechanisms in Onchocerca volvulus-mediated corneal disease (river blindness). Parasite Immunol. 22:625–631 [DOI] [PubMed] [Google Scholar]
- 54. Rao R. U. 2005. Endosymbiotic Wolbachia of parasitic filarial nematodes as drug targets. Indian J. Med. Res. 122:199–204 [PubMed] [Google Scholar]
- 55. Rubio de Kromer M. T., Medina-De la Garza C. E., Brattig N. W. 1995. Differences in eosinophil and neutrophil chemotactic responses in sowda and generalized form of onchocerciasis. Acta Trop. 60:21–33 [DOI] [PubMed] [Google Scholar]
- 56. Saint Andre A., et al. 2002. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science 295:1892–1895 [DOI] [PubMed] [Google Scholar]
- 57. Satoguina J., et al. 2002. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 4:1291–1300 [DOI] [PubMed] [Google Scholar]
- 58. Semnani R. T., Sabzevari H., Iyer R., Nutman T. B. 2001. Filarial antigens impair the function of human dendritic cells during differentiation. Infect. Immun. 69:5813–5822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shibuya K., Bernard C., Ezzati M., Mathers C. D. 2000. Global burden of onchocerciasis in the year 2000: summary of methods and data sources. Epidemiology and Burden for Disease (EBD), Global Programme on Evidence for Health Policy (GPE), World Health Organization. http://www.who.int/healthinfo/statistics/bod_onchocerciasis.pdf
- 60. Simonsen P. E. 2009. Filariases, p. 1477–1513 In Cook G. C. (ed.), Manson's tropical diseases, 22nd ed Saunders-Elsevier, Philadelphia, PA [Google Scholar]
- 61. Slatko B. E., Taylor M. J., Foster J. M. 2010. The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis 51:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Soboslay P. T., et al. 1999. Regulatory effects of Th1-type (IFN-gamma, IL-12) and Th2-type cytokines (IL-10, IL-13) on parasite-specific cellular responsiveness in Onchocerca volvulus-infected humans and exposed endemic controls. Immunology 97:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Specht S., et al. 2011. CCL17 controls mast cells for the defense against filarial larval entry. J. Immunol. 186:4845–4852 [DOI] [PubMed] [Google Scholar]
- 64. Specht S., et al. 2008. Efficacy of 2- and 4-week rifampicin treatment on the Wolbachia of Onchocerca volvulus. Parasitol. Res. 103:1303–1309 [DOI] [PubMed] [Google Scholar]
- 65. Taylor M. J., Bandi C., Hoerauf A. 2005. Wolbachia bacterial endosymbionts of filarial nematodes. Adv. Parasitol. 60:245–284 [DOI] [PubMed] [Google Scholar]
- 66. Taylor M. J., Bandi C., Hoerauf A. M., Lazdins J. 2000. Wolbachia bacteria of filarial nematodes: a target for control? Parasitol. Today 16:179–180 [DOI] [PubMed] [Google Scholar]
- 67. Taylor M. J., Cross H. F., Bilo K. 2000. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J. Exp. Med. 191:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Taylor M. J., Hoerauf A. 1999. Wolbachia bacteria of filarial nematodes. Parasitol. Today 15:437–442 [DOI] [PubMed] [Google Scholar]
- 69. Taylor M. J., Hoerauf A., Bockarie M. 2010. Lymphatic filariasis and onchocerciasis. Lancet 376:1175–1185 [DOI] [PubMed] [Google Scholar]
- 70. Timmann C., et al. 2003. Cutaneous pathology in onchocerciasis associated with pronounced systemic T-helper 2-type responses to Onchocerca volvulus. Br. J. Dermatol. 149:782–787 [DOI] [PubMed] [Google Scholar]
- 71. Timmann C., et al. 2008. Human genetic resistance to Onchocerca volvulus: evidence for linkage to chromosome 2p from an autosome-wide scan. J. Infect. Dis. 198:427–433 [DOI] [PubMed] [Google Scholar]
- 72. Tischendorf F. W., Brattig N. W., Buttner D. W., Pieper A., Lintzel M. 1996. Serum levels of eosinophil cationic protein, eosinophil-derived neurotoxin and myeloperoxidase in infections with filariae and schistosomes. Acta Trop. 62:171–182 [DOI] [PubMed] [Google Scholar]
- 73. Townson S., et al. 2000. Antibiotics and Wolbachia in filarial nematodes: antifilarial activity of rifampicin, oxytetracycline and chloramphenicol against Onchocerca gutturosa, Onchocerca lienalis and Brugia pahangi. Ann. Trop. Med. Parasitol. 94:801–816 [DOI] [PubMed] [Google Scholar]
- 74. Townson S., Tagboto S., McGarry H. F., Egerton G. L., Taylor M. J. 2006. Onchocerca parasites and Wolbachia endosymbionts: evaluation of a spectrum of antibiotic types for activity against Onchocerca gutturosa in vitro. Filaria J. 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Turaga P. S., et al. 2000. Immunity to onchocerciasis: cells from putatively immune individuals produce enhanced levels of interleukin-5, gamma interferon, and granulocyte-macrophage colony-stimulating factor in response to Onchocerca volvulus larval and male worm antigens. Infect. Immun. 68:1905–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Turner J. D., et al. 2006. Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner. J. Immunol. 177:1240–1249 [DOI] [PubMed] [Google Scholar]
- 77. Turner J. D., et al. 2009. Wolbachia lipoprotein stimulates innate and adaptive immunity through Toll-like receptors 2 and 6 to induce disease manifestations of filariasis. J. Biol. Chem. 284:22364–22378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Turner J. D., et al. 2006. A randomized, double-blind clinical trial of a 3-week course of doxycycline plus albendazole and ivermectin for the treatment of Wuchereria bancrofti infection. Clin. Infect. Dis. 42:1081–1089 [DOI] [PubMed] [Google Scholar]
- 79. Turner J. D., et al. 2010. Macrofilaricidal activity after doxycycline only treatment of Onchocerca volvulus in an area of Loa loa co-endemicity: a randomized controlled trial. PLoS Negl. Trop. Dis. 4:e660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Turner P. F., et al. 1994. Interleukin-6 and tumor necrosis factor in the pathogenesis of adverse reactions after treatment of lymphatic filariasis and onchocerciasis. J. Infect. Dis. 169:1071–1075 [DOI] [PubMed] [Google Scholar]
- 81. Udall D. N. 2007. Recent updates on onchocerciasis: diagnosis and treatment. Clin. Infect. Dis. 44:53–60 [DOI] [PubMed] [Google Scholar]
- 82. Vlassoff C., et al. 2000. Gender and the stigma of onchocercal skin disease in Africa. Soc Sci. Med. 50:1353–1368 [DOI] [PubMed] [Google Scholar]
- 83. Volkmann L., Fischer K., Taylor M., Hoerauf A. 2003. Antibiotic therapy in murine filariasis (Litomosoides sigmodontis): comparative effects of doxycycline and rifampicin on Wolbachia and filarial viability. Trop. Med. Int. Health 8:392–401 [DOI] [PubMed] [Google Scholar]
- 84. Wanji S., et al. 2009. Community-directed delivery of doxycycline for the treatment of onchocerciasis in areas of co-endemicity with loiasis in Cameroon. Parasites Vectors 2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. WHO 1995. Onchocerciasis and its control. Report of a WHO expert committee on onchocerciasis control. World Health Organ. Tech. Rep. Ser. 852:1–104 [PubMed] [Google Scholar]
- 86. Wildenburg G., Korten S., Mainuka P., Buttner D. W. 1998. Ivermectin influence on the mast cell activity in nodules of onchocerciasis patients. Trop. Med. Int. Health 3:918–925 [DOI] [PubMed] [Google Scholar]
- 87. Wu B., et al. 2009. The heme biosynthetic pathway of the obligate Wolbachia endosymbiont of Brugia malayi as a potential anti-filarial drug target. PLoS Negl. Trop. Dis. 3:e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zimmerman P. A., et al. 1992. Onchocerca volvulus DNA probe classification correlates with epidemiologic patterns of blindness. J. Infect. Dis. 165:964–968 [DOI] [PubMed] [Google Scholar]