Abstract
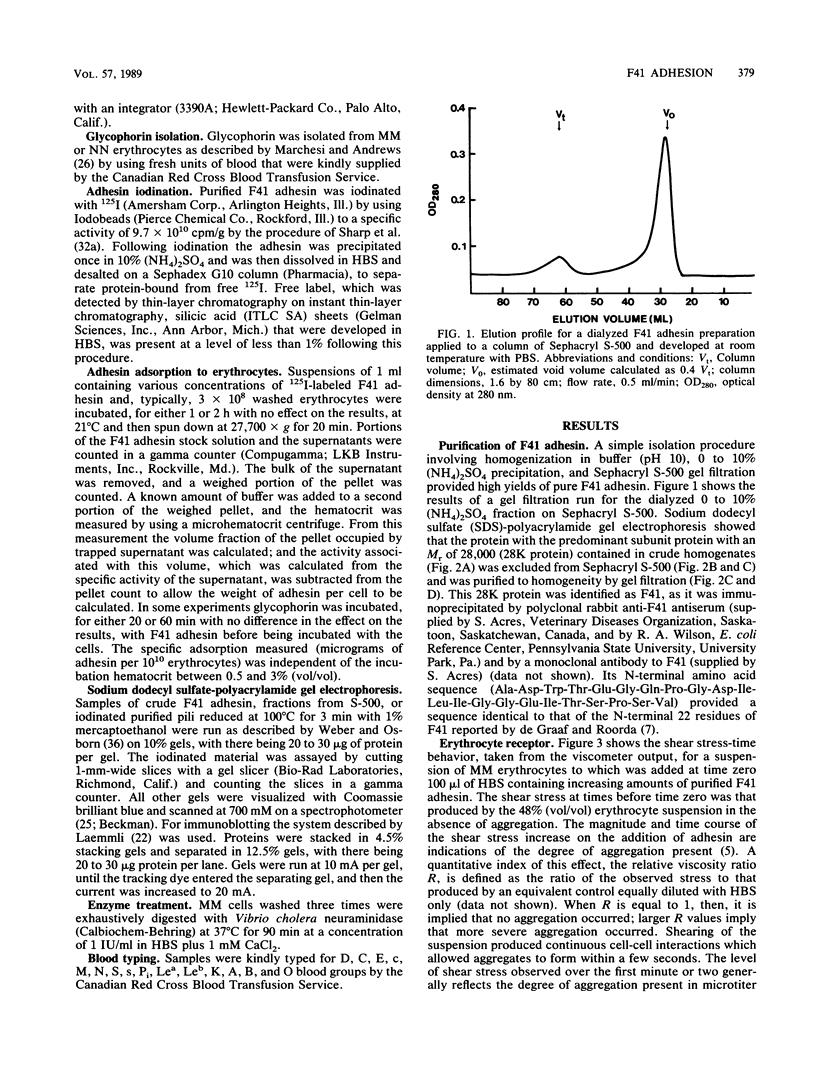
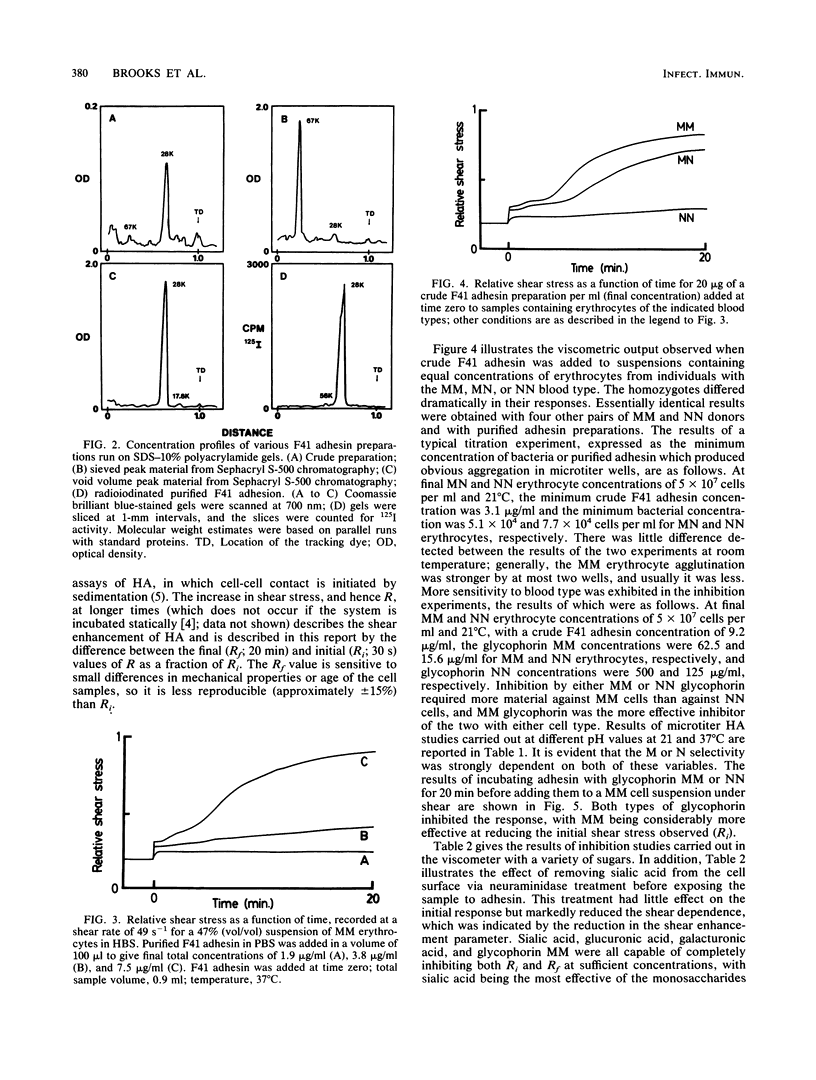
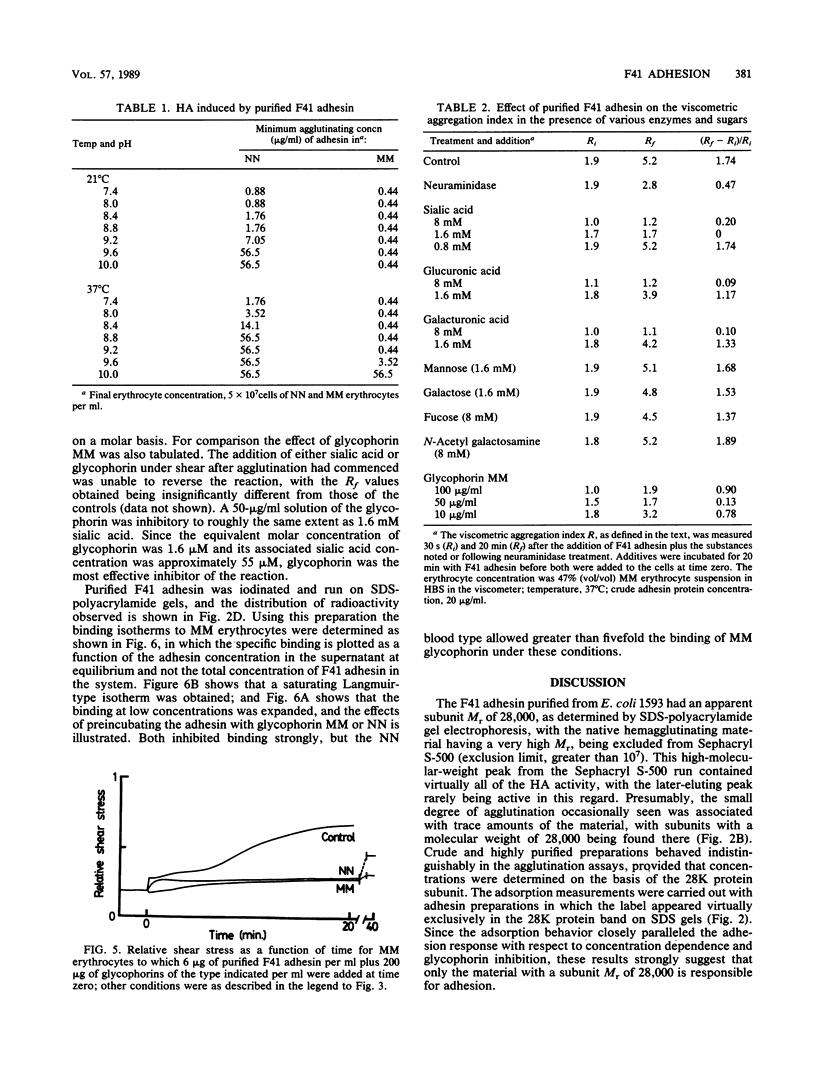
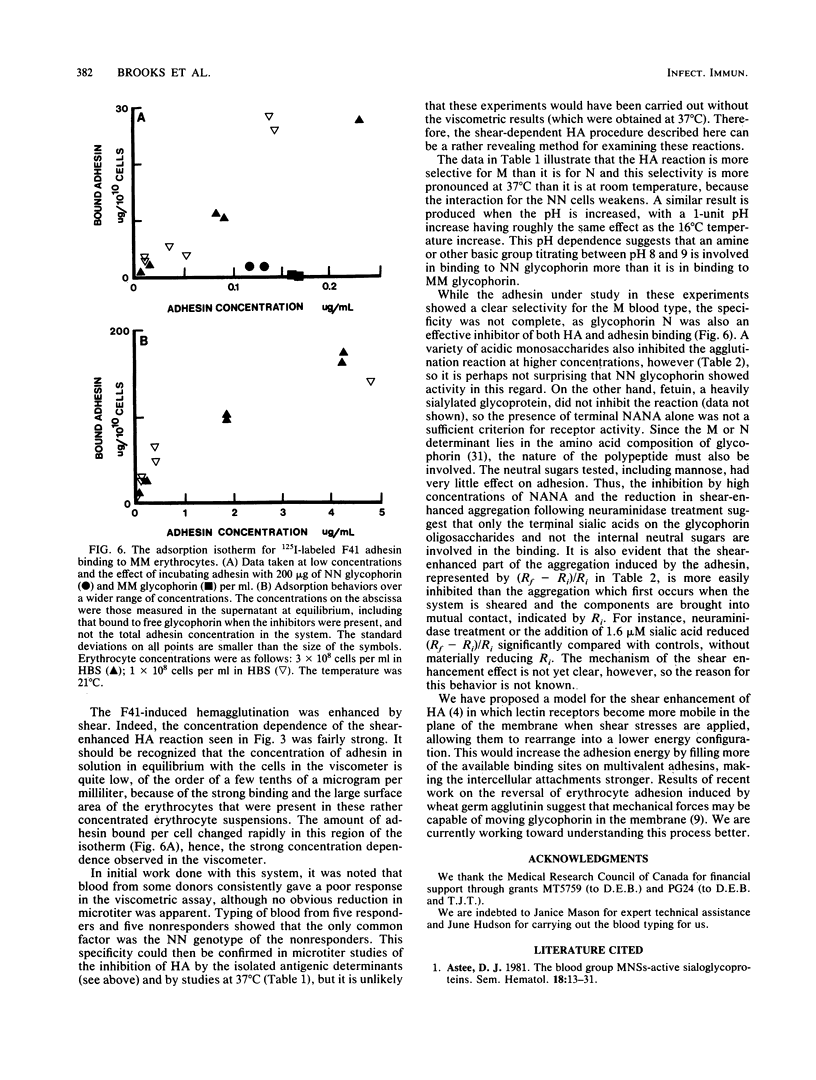
An adhesin from Escherichia coli F41 with an apparent subunit molecular weight of 28,000 daltons was isolated by using (NH4)2SO4 precipitation at pH 10 and Sephacryl S-500 gel filtration. The hemagglutination (HA) properties of the native high-molecular-weight adhesin were studied by using a viscometric assay, which provided a quantitative index of the degree of agglutination present as a function of time at a known rate of shear. Shear was found to enhance the degree of agglutination over a 20-min period. A strong, shear-enhanced HA was observed for all donors with the MM or MN blood type studied, but those with the NN blood type showed very little HA. In the microtiter HA assay, the selectivity of the adhesin for MM over NN erythrocytes was found to be dependent on pH and temperature. At 21 degrees C and pH 7.4, there was little difference in HA between the two blood types, but NN cells were progressively more weakly agglutinated than MM cells as the pH or the temperature was increased. Glycophorin A, which bears the M or N determinant, was isolated from individuals with the MM and NN blood types and was shown to be an effective inhibitor of the reaction, with the MM type being the more effective in both microtiter and viscometric assays. Acidic monosaccharides, particularly sialic acid, were also effective inhibitors of HA, although they were less potent on a molar basis than glycophorin. The adsorption isotherm of 125I-labeled adhesin was measured, and the binding was shown to be strongly inhibited by MM glycophorin and somewhat less strongly by NN glycophorin. Collectively, these data strongly suggest that glycophorin AM is a receptor for the F41 adhesin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartus H., Actor P., Snipes E., Sedlock D., Zajac I. Indications that the erythrocyte receptor involved in enterotoxigenic Escherichia coli attachment is a sialoglycoconjugate. J Clin Microbiol. 1985 Jun;21(6):951–954. doi: 10.1128/jcm.21.6.951-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A. F41 antigen as a virulence factor in the infant mouse model of Escherichia coli diarrhoea. J Gen Microbiol. 1985 Nov;131(11):3037–3045. doi: 10.1099/00221287-131-11-3037. [DOI] [PubMed] [Google Scholar]
- Brooks D. E., Trust T. J. Enhancement of bacterial adhesion by shear forces: characterization of the haemagglutination induced by Aeromonas salmonicida strain 438. J Gen Microbiol. 1983 Dec;129(12):3661–3669. doi: 10.1099/00221287-129-12-3661. [DOI] [PubMed] [Google Scholar]
- Brooks D. E., Trust T. J. Interactions of erythrocytes with bacteria under shear. Ann N Y Acad Sci. 1983;416:319–331. doi: 10.1111/j.1749-6632.1983.tb35196.x. [DOI] [PubMed] [Google Scholar]
- Duchet-Suchaux M. Protective antigens against enterotoxigenic Escherichia coli O101:K99,F41 in the infant mouse diarrhea model. Infect Immun. 1988 May;56(5):1364–1370. doi: 10.1128/iai.56.5.1364-1370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Leung A. Adhesivity and rigidity of erythrocyte membrane in relation to wheat germ agglutinin binding. J Cell Biol. 1984 Apr;98(4):1201–1208. doi: 10.1083/jcb.98.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig R. G., Brooks D. E. Enhanced concanavalin A agglutination of trypsinised erythrocytes is due to a specific class of aggregation. Biochim Biophys Acta. 1981 Mar 6;641(2):410–415. doi: 10.1016/0005-2736(81)90497-1. [DOI] [PubMed] [Google Scholar]
- Greig R. G., Brooks D. E. Shear-induced concanavalin A agglutination of human erythrocytes. Nature. 1979 Dec 13;282(5740):738–739. doi: 10.1038/282738a0. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Veldkamp J., Jansen W. H. Improved minca medium for the detection of K99 antigen in calf enterotoxigenic strains of Escherichia coli. Infect Immun. 1977 Feb;15(2):676–678. doi: 10.1128/iai.15.2.676-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., Venema J., Leeven R., van Pelt-Heerschap H., de Graaf F. K. Inhibition of adhesive activity of K88 fibrillae by peptides derived from the K88 adhesin. J Bacteriol. 1987 Feb;169(2):735–741. doi: 10.1128/jb.169.2.735-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen M., Ehnholm C., Väisänen-Rhen V., Korhonen T., Pipkorn R., Kalkkinen N., Gahmberg C. G. Identification of the major human sialoglycoprotein from red cells, glycophorin AM, as the receptor for Escherichia coli IH 11165 and characterization of the receptor site. Eur J Biochem. 1985 Feb 15;147(1):47–52. doi: 10.1111/j.1432-1033.1985.tb08716.x. [DOI] [PubMed] [Google Scholar]
- Källenius G., Svenson S. B., Möllby R., Korhonen T., Winberg J., Cedergren B., Helin I., Hultberg H. Carbohydrate receptor structures recognized by uropathogenic E. coli. Scand J Infect Dis Suppl. 1982;33:52–60. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadstrom T. Colonization factor antigen on enterotoxigenic Escherichia coli is a sialic-specific lectin. Lancet. 1982 Jul 31;2(8292):280–280. doi: 10.1016/s0140-6736(82)90368-3. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Maurer L., Orndorff P. E. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J Bacteriol. 1987 Feb;169(2):640–645. doi: 10.1128/jb.169.2.640-645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. A., Thorns C. J., Sojka W. J. Evidence for two adhesive antigens on the K99 reference strain Escherichia coli B41. J Gen Microbiol. 1980 May;118(1):107–113. doi: 10.1099/00221287-118-1-107. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Thorns C. J., Wells G. A., Scott A. C., Sojka W. J. The production of F41 fimbriae by piglet strains of enterotoxigenic Escherichia coli that lack K88, K99 and 987P fimbriae. J Gen Microbiol. 1983 Sep;129(9):2753–2759. doi: 10.1099/00221287-129-9-2753. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Thorns C., Scott A. C., Sojka W. J., Wells G. A. Adhesion in vitro and in vivo associated with an adhesive antigen (F41) produced by a K99 mutant of the reference strain Escherichia coli B41. Infect Immun. 1982 Jun;36(3):1146–1153. doi: 10.1128/iai.36.3.1146-1153.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska R., Koerner T. A., Jr, Armitage I. M., Furthmayr H. Chemical and carbon-13 nuclear magnetic resonance studies of the blood group M and N active sialoglycopeptides from human glycophorin A. J Biol Chem. 1981 Jun 10;256(11):5781–5791. [PubMed] [Google Scholar]
- Runnels P. L., Moseley S. L., Moon H. W. F41 pili as protective antigens of enterotoxigenic Escherichia coli that produce F41, K99, or both pilus antigens. Infect Immun. 1987 Mar;55(3):555–558. doi: 10.1128/iai.55.3.555-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp K. A., Yalpani M., Howard S. J., Brooks D. E. Synthesis and application of a poly(ethylene glycol)-antibody affinity ligand for cell separations in aqueous polymer two-phase systems. Anal Biochem. 1986 Apr;154(1):110–117. doi: 10.1016/0003-2697(86)90503-8. [DOI] [PubMed] [Google Scholar]
- Smit H., Gaastra W., Kamerling J. P., Vliegenthart J. F., de Graaf F. K. Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia coli K99 fimbrial adhesin. Infect Immun. 1984 Nov;46(2):578–584. doi: 10.1128/iai.46.2.578-584.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Hultberg H., Källenius G., Korhonen T. K., Möllby R., Winberg J. P-fimbriae of pyelonephritogenic Escherichia coli: identification and chemical characterization of receptors. Infection. 1983 Jan-Feb;11(1):61–67. doi: 10.1007/BF01651362. [DOI] [PubMed] [Google Scholar]
- Väisänen V., Korhonen T. K., Jokinen M., Gahmberg C. G., Ehnholm C. Blood group M specific haemagglutinin in pyelonephritogenic Escherichia coli. Lancet. 1982 May 22;1(8282):1192–1192. doi: 10.1016/s0140-6736(82)92264-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Roorda I. Production, purification, and characterization of the fimbrial adhesive antigen F41 isolated from calf enteropathogenic Escherichia coli strain B41M. Infect Immun. 1982 May;36(2):751–758. doi: 10.1128/iai.36.2.751-758.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



