Abstract
Objective
To test the association between self-reported unfair treatment and objective and self-reported sleep characteristics in African American and Caucasian adults.
Design
Cross-sectional study of 97 African American and 113 Caucasian middle-aged adults.
Main Outcome Measures
Participants completed: a) two night in-home, polysomnography (PSG) sleep study, b) sleep diaries and actigraph assessments across nine days and nights, and c) self-report measures of sleep quality in the past month, and daytime sleepiness in the past two weeks.
Results
Greater unfair treatment was associated with reports of poorer self-reported sleep quality and greater daytime sleepiness, shorter sleep duration and lower sleep efficiency as measured by actigraphy and PSG, and a smaller proportion of rapid eye movement (REM) sleep. Racial/ethnic differences were few. Exploratory analyses showed that nightly worry partially mediated the associations of unfair treatment with sleep quality, daytime sleepiness, sleep efficiency (actigraphy), and proportion of REM sleep.
Conclusion
Perceptions of unfair treatment are associated with sleep disturbances in both African American and Caucasian adults. Future studies are needed to identify the pathways that account for the association between unfair treatment and sleep.
Keywords: unfair treatment, discrimination, sleep disturbance, worry, race/ethnicity
Chronic stress is a significant correlate of sleep disturbance (Akerstedt, 2006; De Lange et al., 2009; Hall et al., 2008; Hall et al., 2009). Characterized by persistent or recurring adverse situations or events, chronic stressors, such as occupational and financial strain, poor relationship quality, and caregiving stress, are associated with poorer self-reported sleep quality, and poorer objective measures of sleep duration, continuity, and architecture (Brummett et al., 2006; De Lange et al., 2009; Hall et al., 2008; Hall et al., 2009; Healey et al., 1981; Lee, Lee, Rankin, Weiss, & Alkon, 2007; Rowe, McCrae, Campbell, Benito, & Cheng, 2008; Sadeh, Keinan, & Daon, 2004; Vahtera et al., 2007).
Racial/ethnic discrimination, a chronic stressor common to minority groups (Contrada et al., 2001), may also be associated with poor sleep. Steffen and Bowden (2006) found that Hispanics who reported unfair treatment due to racial/ethnic discrimination reported poorer sleep quality in the past month. Thomas, Bardwell, Ancoli-Israel, and Dimsdale (2006) found that middle-aged African American and Caucasian adults who reported unfair treatment due to racial/ethnic discrimination had less slow wave sleep (i.e., stages 3–4 sleep) as measured by laboratory-based polysomnography (PSG) for one night than those who did not. Unfair treatment due to racial/ethnic discrimination was not associated with sleep duration, continuity (i.e., efficiency or wakefulness after sleep onset, WASO), or rapid eye movement (REM) sleep. African Americans had less slow wave sleep than Caucasians, with the difference mediated by reports of racial/ethnic discrimination.
The mixed findings on racial/ethnic discrimination and sleep from these prior studies (Steffen & Bowden, 2006; Thomas et al., 2006) raise the question of whether the exclusive focus on racial/ethnic discrimination should be expanded to assess unfair treatment overall, which can arise from multiple sources including racial/ethnic, gender, or age discrimination. In addition, a longer period of sleep assessment in the environment where persons typically sleep may yield a more reliable estimate of sleep and associations with unfair treatment may be stronger. Thus, the primary aim of the current study is to determine whether reports of unfair treatment are associated with sleep measured by two nights of in-home PSG and nine nights of actigraphy in African American and Caucasian middle-aged participants, a sample similar to the Thomas et al. (2006) sample. We hypothesize that unfair treatment overall will be associated with poorer subjective sleep quality, shorter sleep duration, less efficient sleep, and less stages 3–4 (slow wave) sleep, all indicative of sleep disturbance.
Relative to Caucasians, African Americans report poorer sleep quality and greater sleepiness (Durrence & Lichstein, 2006; Friedman et al., 2006), have shorter sleep and less continuous sleep as measured by actigraphy and PSG (Durrence & Lichstein, 2006; Hall et al., 2009; Jean-Louis, Kripke, Ancoli-Israel, Klauber, & Sepulveda, 2000; Mezick et al., 2008; Redline et al., 2004), and have less slow wave sleep (Durrence & Lichstein, 2006; Hall et al., 2009; Mezick et al., 2008). Associations between unfair treatment and sleep may differ by race/ethnicity as African Americans are exposed to greater levels of racial/ethnic discrimination and report more unfair treatment overall than Caucasians in adult samples (Krieger, Smith, Naishadham, D., Hartman, Barbeau, 2005; Williams, Yu, Jackson, & Anderson, 1997). At the same time, recent studies have documented an inverse association between unfair treatment and health among both Caucasians and African Americans (Pascoe & Smart-Richman, 2009). Thus, a second aim of the current study is to evaluate whether there are racial/ethnic differences in the association between unfair treatment and sleep among African Americans and Caucasians. We do not have directional hypotheses because of the absence of racial/ethnic differences observed in a recent meta-analysis on the association of unfair treatment and health (Pascoe & Smart-Richman, 2009).
An exploratory aim is to investigate one potential pathway through which unfair treatment may be associated with sleep. One plausible mechanism suggested, albeit not tested by Steffen and Bowden (2006), is that experiences of unfair treatment give rise to perseverative thoughts and rumination that, in turn, impact sleep. Indeed, previous studies have demonstrated a relationship between stress-related intrusive thoughts or worry and self-report and objective measures of sleep (Brosschot et al., 2006; Hall et al., 1997; Hall et al., 2000; Hall et al., 2007). Further, there is some experimental evidence that stress and stress-related intrusive thoughts are associated with sustained increases in physiological arousal during sleep and may contribute to wakefulness during the night (e.g., Hall et al., 2004). Although the current study protocol did not have measures of rumination or intrusive thoughts, either general or specific to unfair treatment, participants did report on the sleep diary whether they had worried last night. Thus, we explored whether a general measure of nightly worry partially accounted for the association between unfair treatment and self-reported sleep quality and objective sleep measures.
Method
Research Participants
Participants in the current study, SleepSCORE, were recruited as part of a larger study, Heart Strategies Concentrating on Risk Evaluation (HeartSCORE). HeartSCORE is a single center, prospective community-based cohort study investigating the mechanisms for racial/ethnic disparities in cardiovascular disease (CVD) risk. A subset of individuals who had moderate/high Framingham risk scores were offered a standard life-style intervention designed to provide health education information and behavioral counseling to improve diet, exercise, smoking, and stress levels. Eligibility criteria for HeartSCORE included age 45 to 75, residence in the greater Pittsburgh, Pennsylvania metropolitan area, absence of comorbid conditions expected to limit life expectancy, and the ability to undergo baseline and annual follow-up visits. Data collection included measures of demographics, medical history, physiological data, physical activity, and psychosocial factors.
The overall purpose of SleepSCORE was to evaluate the associations between cardiovascular risk and sleep characteristics in a community sample with no known sleep disorders, (i.e., not currently being treated for sleep apnea using continuous positive airway pressure therapy or nightly use of medications for sleep disturbance), or heart disease, stroke, or diabetes. SleepSCORE enrolled 224 participants from HeartSCORE: 97 (43.3%) African Americans and 123 (54.9%) Caucasians, and 4 (1.8%) Asian Americans; mean age 60.0 years (SD = 7.2) at study entry; 50% women; 62.5% (n = 140) married; and 50% (n = 114) with a 4-year college degree or greater. The current analyses are based on 217 of the 224 SleepSCORE participants, as 3 were missing unfair treatment data and the 4 Asian American participants in the sample were removed due to the small number in this racial/ethnic group. Exclusion criteria for SleepSCORE included: current pregnancy, use of continuous positive airway pressure treatment for sleep-disordered breathing, medication for sleep problems on a regular basis, nighttime work schedule, medication for diabetes, and prior diagnosis of stroke, myocardial infarction, or interventional cardiology procedures. Both HeartSCORE and SleepSCORE studies were approved by the University of Pittsburgh Biomedical Institutional Review Board and all participants provided written informed consent. Participants were compensated $200 for study completion.
Overview
Participants were recruited during HeartSCORE assessment visits by study personnel. Potential participants interested in the study were provided with detailed information and then screened for study eligibility criteria. Participants were scheduled for the sleep study within approximately 3 months of providing consent. The study protocol lasted 10 days. Three methods were used to collect sleep data: PSG, actigraphy, and self-report. On nights 1 and 2 of the study, in-home PSG was conducted to obtain nighttime sleep measurements. Across the two nights of PSG with the 217 participants (i.e., 434 nights of PSG assessment), we asked participants who had inadequate in-home PSG data due to equipment or user errors to repeat a night of sleep measures. This was done in 32 cases (7.6%), for 30 (13.8%) participants. After the repeated assessments, one participant had no usable data due to faulty operation and another had only one night of usable data due to unreliable signaling. Thus, the final sample for PSG analyses included 216 participants with 431 nights of PSG data.
Across all 10 days and 9 nights of the study, participants wore an actigraph and completed a morning and evening diary to assess objective and subjective sleep quality, respectively. Self-report measures of unfair treatment, global sleep quality, and daytime sleepiness were collected on day 2.
Demographic and Clinical Variables
Several demographic and clinical variables were assessed. Age, sex, and race were determined by self-report. Highest education obtained was assessed using a six-level ordinal scale: high school or less, some college/no degree, vocational/technical school/associate (2-year) degree, 4-year degree, Master’s degree, and professional degree. Annual income was assessed using a five-level ordinal scale: <$10,000, $10,000 –< $20,000, $20,000 – < $40,000, $40,000 – < $80,000, and > $80,000. Values for education and income were standardized and then averaged for each participant to create a composite SES variable. Resting blood pressure was taken in the laboratory and blood pressure-related medication use was assessed during in-home PSG studies. Resting blood pressure > 140/90, self-reported history of hypertension, and current blood pressure medication (i.e., ACE inhibitors, angiotensin II blockers, beta blockers, calcium channel blockers, alpha I blockers, alpha II agonists, and diuretics) were assessed to create a dichotomous (yes/no) “Hypertensive and/or Related Medication Use.” Body mass index (BMI) was assessed in the HeartSCORE study protocol and was calculated as weight in kilograms divided by height in meters squared.
Measures
Sleep Parameters
Actigraphy
The Actiwatch-16 (Respironics, Bend, OR), a watch-like activity monitor worn on the non-dominant wrist, records physical movements to provide behavioral data used to infer sleep/wake patterns. Data were stored in 1-minute epochs and validated Minimitter Action 5.0 software algorithms (Philips Respironics, Inc.) were used to estimate sleep parameters, which were averaged across the nine consecutive nights of data collection. Actigraphy was used to assess sleep duration (actual sleep time while in bed, excluding periods of wakefulness during the night) and continuity as measured by efficiency (percentage of time in bed spent sleeping). The sleep efficiency variable was log transformed due to skewness.
PSG
Study participants were monitored with a Compumedics Siesta monitor (Charlotte, North Carolina) for 2 consecutive nights of in-home PSG recording, concurrent with actigraphy and sleep diary. The PSG montage included bilateral central and occipital electroencephalogram channels, bilateral electrooculograms, bipolar submentalis electromyograms, and one channel of electrocardiogram recording. On the first night of PSG, participants were monitored for sleep-disordered breathing using nasal pressure, inductance plethysmography, and fingertip oximetry. High-frequency filter settings were 100 Hz for electroencephalogram and electrooculograms, and 100 Hz for electromyograms. Low frequency filter settings were 0.3 Hz for electroencephalogram and 10 Hz for electromyogram. Trained PSG technologists scored sleep records using standard sleep stage scoring criteria for each 20-s epoch (Rechtschaffen & Kales, 1986). These records were collected and scored prior to the publication of the new American Academy of Sleep Medicine guidelines (Iber, Ancoli-Israel, Chesson, & Quan, 2007). The American Academy of Sleep Medicine Task Force (1999) definitions were used to identify apneas and hypopneas; oximetry readings were used to quantify average and minimum oxygen saturation levels.
PSG was used to measure sleep duration (actual sleep time, excluding periods of wakefulness during the night), continuity as measured by efficiency (percentage of time in bed spent sleeping) and wakefulness after sleep onset (WASO; total number of minutes scored as awake following sleep onset), architecture (percentage (REM and non-REM sleep time spent in stages 3–4), and disordered breathing expressed as the apnea/hypopnea index (AHI; number of apneas and hypopneas per hour of sleep). Values from the 2 nights of the study were averaged for each of these variables, with the exception of AHI, which was measured only on the first night. Due to skewed distributions, some PSG variables were transformed using either a log (sleep efficiency, WASO, and AHI) or square root (percentage for Stages 3–4 sleep) transformation.
Self-Report
Two forms of self-report were used: daily diary and retrospective measures. The 10-day diary was used to capture sleep and wake times used in conjunction with actigraphy, and perceptions of sleep. Every morning, participants rated the previous night’s sleep quality and how rested they felt on wakening using a 0 to 5 Likert-type scale; 0 represented “very poor” sleep quality and feeling “not at all rested,” and 5 represented “very good” sleep quality and feeling “extremely rested.” Responses to these two items were averaged across the 9 study nights. Average sleep quality and average rested ratings were correlated at r = .76, (217), p = < .0001; therefore, the two measures were averaged together to create a composite variable of diary sleep quality.
The Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) is a standardized measure of subjective sleep quality over the previous month. Eighteen individual items on the PSQI are grouped to create seven component scores (e.g., subjective sleep quality and sleep duration). These subscores are summed to generate a global score between 0 and 21, with a higher score indicating worse sleep quality. The Epworth Sleepiness Scale (ESS; John, 1991) is an 8-item measure of the likelihood of falling asleep in specific situations, with higher scores indicating greater daytime sleepiness. The PSQI and ESS scores were treated as continuous variables.
Unfair Treatment
The 9-item Detroit Area Study Everyday Unfair Treatment Scale was used to assess unfair treatment (Williams et al., 1997). Respondents were asked about the frequency of their experiences with various forms of interpersonal unfair treatment in their daily lives without reference to specific reasons for the unfair treatment (e.g., race or ethnicity). Representative items range from relatively minor and subtle negative events, such as receiving poorer service compared with others in restaurants or stores, to more blatant and extreme negative events, such as being threatened or harassed. Participants were not given a specific timeframe for recall of these events and the measure was administered once. The frequency of each type of mistreatment was assessed using a 4-point scale (0 = never, 1 = rarely, 2 = sometimes, 3 = often). A 10th item, “People ignore you or act as if you are not there?” was included and the total scores range from 0–30, with a higher score indicating more experiences of unfair treatment. Other papers have included this 10th item (e.g., Lewis et al., 2006; Troxel, Matthews, Bromberger, & Sutton-Tyrrell, 2003). The 9-item measure has been previously validated in a sample of African American adults (Taylor, Kamarck, & Shiffman, 2004) and the Cronbach’s alpha in that paper was .80. In the present sample, the alpha was .85, and .86 and .83 in Caucasians and African Americans, respectively.
Nightly Worry
Every morning, participants were asked to indicate the severity of a number of symptoms including worries they may have experienced last night. A Likert-type scale was used with a range of 0–4, 0 represented “not at all,” and 4 represented “extremely,” and this score was averaged across the nine nights of the study.
Other Psychosocial Characteristics
To determine whether associations between unfair treatment and sleep were independent of personality characteristics, we assessed anger, anxiety, hostility, and depressive symptoms, using the 10-item Trait Anger and 10-item Trait Anxiety subscales from the Spielberger Trait Anger and Trait Anxiety Inventories (Spielberger, 1970), the 27-item Cook-Medley Hostility Scale (Barefoot, Dodge, Peterson, Dahlstrom, & Williams, 1989), and the 20-item Center for Epidemiological Studies Depression scale (CES-D; scored without the sleep item to prevent spurious associations with sleep; Radloff, 1977), respectively. These measures were administered as part of the HeartSCORE protocol prior to the start of the SleepSCORE protocol. The Trait Anger, Trait Anxiety, and CES-D variables were square root transformed due to skewness.
Statistical Analysis
Descriptive statistics for continuous (mean, SD or %, SD; as appropriate) and categorical (n, %) variables were assessed. To determine whether there were racial/ethnic differences in the demographic, clinical, psychosocial, and sleep variables, unadjusted models using t-tests, chi-squares, and Pearson correlations were assessed.
To test the primary study hypothesis that unfair treatment was associated with sleep parameters, multiple linear regression analyses were conducted with age, BMI, gender, hypertensive status, and composite SES included as covariates in all models. Race/ethnicity was included as a covariate in models where it was not entered as a moderator variable. To test whether associations between unfair treatment and sleep variables were independent of anger, anxiety, hostility, and depressive symptoms, subsequent models including the covariates listed above were analyzed controlling for each of these negative affect variables separately. To test whether the association of unfair treatment with the sleep parameters would vary by race/ethnicity, an Unfair Treatment X Race/Ethnicity interaction term was created where the unfair treatment was standardized. The individual predictor (unfair treatment) and moderator (i.e., race/ethnicity) variables were also included in these models. For significant interaction terms, simple effects analyses were conducted to determine which slopes were significant.
Mediational analyses tested whether nightly worry attenuated the association between unfair treatment and sleep indices. If a main effect of unfair treatment on a given sleep variable was established, then we assessed whether unfair treatment was associated with the mediator variable, nightly worry, and finally whether the association between the unfair treatment and the sleep variable was either eliminated or attenuated when both the mediator and the predictor variables were entered into the model simultaneously. Once these criteria were met, the statistical significance of the indirect association of unfair treatment with the given sleep variable via nightly worry was evaluated using the Sobel method (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002). All analyses were conducted using Statistical Analysis Software, vs. 9.2 (Cary, NC) and a p-value ≤ .05 was used to determine significance.
Results
Racial/Ethnic Differences in Psychosocial, Demographic, and Clinical Variables
As shown in Table 1, African Americans reported slightly but significantly higher levels of unfair treatment than Caucasians, and tended to report more hostile attitudes, p < .08. The mean unfair treatment scores were similar to those obtained in other samples of African Americans and Caucasians (e.g., Beatty & Matthews, 2009; Lewis, Aiello, Leurgans, Kelly, & Barnes, 2009; Lewis, Barnes, Bienias, Lackland, Evans, Mendes de Leon, 2009). There were no significant racial/ethnic differences in reports of nightly worry, Trait Anger, Trait Anxiety, and depressive symptoms. Caucasians had significantly higher composite SES scores than African Americans, as African Americans reported fewer years of education and lower annual incomes. A significantly higher number of African Americans were classified as hypertensive and had higher BMI levels relative to Caucasians.
Table 1.
Demographic, Clinical, and Psychosocial Characteristics of the Full Sample and Stratified by Race/Ethnicity
| Measure | Full Sample | African Americans | Caucasians |
|---|---|---|---|
| Total, n (%) | 217 | 96(44.2) | 121(55.8) |
| Women, n (%) | 108(49.8) | 59(54.6) | 49(45.4) |
| Age, mean (SD) | 59.9(7.1) | 59.4(7.3) | 60.3(7.0) |
| Body Mass Index, mean (SD) | 29.6 (5.0) | 31.1(5.4)** | 28.3(4.4) |
| Hypertensive and/or Related Medication Use, n (%) | 100(46.1) | 60(60.0)** | 40(40.0) |
| Socioeconomic Status Variables, n (%) | |||
| Years education, n (%)** | |||
| High School Diploma or Less | 36(16.6) | 21(21.9) | 15(12.4) |
| Some college/No degree | 38(17.5) | 22(22.9) | 16(13.2) |
| Vocational/Technical school/Associate, 2-year College Degree | 35(16.1) | 19(19.8) | 16(13.2) |
| 4-year College Degree | 44(20.3) | 20(20.8) | 24(19.8) |
| Master’s Degree | 39(18.0) | 7(7.3) | 32(26.4) |
| Professional Degree | 25(11.5) | 7(7.3) | 18(14.9) |
| Annual Income+, n (%)** | |||
| <$10,000 | 15(7.4) | 12(13.2) | 3(2.7) |
| $10,000 – < $20,000 | 17(8.4) | 13(14.3) | 4(3.6) |
| $20,000 – < $40,000 | 62(30.5) | 32(35.2) | 30(27.0) |
| $40,000 – < $80,000 | 74(36.4) | 30(33.0) | 44(39.3) |
| ≥ $80,000 | 35(17.2) | 4(4.4) | 31(28.0) |
| Psychosocial Factors, mean (SD) | |||
| Negative Emotion Variables | - | - | - |
| Trait Anger | 5.4(4.0) | 5.0(4.4) | 5.7(3.7) |
| Hostility | 1.5(1.3) | 1.3(1.3) | 1.6(1.3) |
| Depressive Symptoms++ | 12.1(10.5) | 11.9(10.4) | 12.3(10.7) |
| Trait Anxiety | 6.0(5.0) | 5.7(4.7) | 6.3(5.2) |
| Mediator Variable | - | - | - |
| Diary Nightly Worry | .37(.45) | .33(.42) | .40(.46) |
| Predictor Variable | - | - | - |
| Unfair Treatment | 7.4(4.5) | 8.4(4.7)* | 6.7(4.3) |
Note.
p ≤ .001,
p ≤ .05.
Annual income was not available for 14 participants.
The depressive symptoms variable is the Center for Epidemiological Studies Depression scale minus the sleep item.
Racial/Ethnic Differences in Sleep Variables
Consistent with our earlier report on a subsample of SleepSCORE (Mezick et al., 2008), there were significant racial/ethnic differences in several sleep variables (see Table 2). Relative to Caucasians, African Americans slept for a shorter period of time and had lower sleep efficiency by both actigraphy and PSG. There were no racial/ethnic differences for REM or AHI. African Americans had less slow wave sleep and reported poorer sleep quality in the past month, relative to Caucasians. There were no racial/ethnic differences in nightly sleep quality or daytime sleepiness in the past two weeks.
Table 2.
Self-Report and Objective Characteristics of Sleep in the Full Sample and Stratified by Race/Ethnicity
| Measure | Full Sample (N = 217) | African Americans (n = 96) | Caucasians (n = 121) |
|---|---|---|---|
| M(SD) | M(SD) | M(SD) | |
| Self-Reported Sleep Quality Variables | |||
| Diary Sleep Quality | 2.3(.54) | 2.3(.55) | 2.3(.53) |
| PSQI Sleep Quality | 6.4(3.4) | 7.0(3.5)* | 5.9(3.2) |
| Epworth Sleepiness | 8.2(3.9) | 8.2(4.5) | 8.1(3.4) |
| Objective Sleep Variables | |||
| Actigraphy | |||
| Duration, hr | 5.8(.88) | 5.4(.82)** | 6.1(.84) |
| Efficiency, % | 80.5(8.0) | 77.9(8.9)** | 82.6(6.4) |
| Polysomnography | - | - | - |
| Duration, hr | 6.1(1.0) | 5.8(1.1)* | 6.2(.93) |
| Efficiency, % | 76.8(11.3) | 74.2(12.6)* | 78.8(9.7) |
| Wakefulness After Sleep Onset, min | 81.0(52.0) | 87.9(59.7) | 75.5(44.0) |
| Sleep Architecture (%) | |||
| Stage 3–4 Sleep | 5.3(6.4) | 3.5(4.9)** | 6.7(7.0) |
| Rapid Eye Movement Sleep | 22.4(5.0) | 22.1(4.9) | 22.7(5.1) |
| Apnea-Hypopnea Index | 13.3(15.1) | 13.6(16.6) | 13.1(13.9) |
Note.
p ≤ .001,
p ≤ .05. Reported mean(SD) and %(SD) values for the actigraphy measure of sleep efficiency and the polysomnography measures of sleep efficiency, wakefulness after sleep onset, sleep stages 3– 4, and apnea-hypopnea are based on the non-transformed variables.
Associations among Unfair Treatment, Negative Emotion, and Nightly Worry Variables
Unfair treatment was significantly correlated with Trait Anger, r(215) = .29, p < .0001, Trait Anxiety, r(215) = .33, p < .0001, and Hostility, r(214) = .25, p = .0002, but not with depressive symptoms (excluding the sleep item), r(216) = .04, p =.55. Unfair treatment was significantly correlated with nightly worry, r(217) = .18, p = .006. Higher Trait Anger, r(215) = .26, p < .0001, Trait Anxiety, r(215) = .38, p < .0001, and Hostility, r(214) = .20, p = .003 scores, but not depressive symptoms (excluding the sleep item), r(216) = .11, p =.11, were correlated with nightly worry.
Associations between Unfair Treatment and Sleep Variables
Results of models of the association between unfair treatment and the self-report and objective sleep variables are shown in Table 3. Models are presented unadjusted and adjusted for standard covariates and negative emotions. In models unadjusted and adjusted for standard covariates, greater unfair treatment was associated with poorer self-reported sleep quality in the past month using the PSQI and greater daytime sleepiness in the past two weeks; shorter sleep duration and lower sleep efficiency as measured by actigraphy and PSG; and a smaller proportion of REM sleep. The findings for REM sleep remained unchanged when selective serotonin reuptake inhibitor (SSRI) use, a potential suppressor of REM sleep (Rasch, Pommer, Diekelmann, & Born, 2008), was included as a covariate. Unfair treatment was not associated with nightly sleep quality using the nightly diary measure, WASO, proportion of slow wave sleep, or AHI.
Table 3.
Association of Unfair Treatment with Sleep in Linear Regression Models, Unadjusted, Adjusted for Covariates+, and Adjusted for Covariates + and Negative Emotions in the Full Sample.
| Unfair Treatment (Predictor) | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for Covariates+ | Trait Anger+ | Hostility+ | Trait Anxiety+ | ||
| B(SE) | B(SE) | ΔR2 | B(SE) | B(SE) | B(SE) | |
| Outcome Measure | ||||||
| Self-Report Diary | ||||||
| Nightly Sleep Quality | −.01(.01) | −.01(.01) | .01 | −.01(.01) | −.01(.01) | .01(.01) |
| PSQI Sleep Quality | .17(.05)*** | .14(.05)** | .04 | .09(.05)* | .10(.05)** | .07(.05) |
| Epworth Sleepiness | .16(.06)** | .17(.06)** | .03 | .14(.06)** | .14(.06)** | .11(.06) |
| Actigraphy | ||||||
| Duration, hr | −.05(.01)*** | −.04(.01)*** | .05 | −.03(.01)** | −.04(.01)** | −.04(.01)** |
| Efficiency, % ++ | .01(.01)** | .01(.01)** | .02 | .01(.01) | .01(.01)* | .01(.01) |
| Polysomnography | ||||||
| Duration, hr | −.04(.01)** | −.03(.01)** | .02 | −.03(.02) | −.03(.02)* | −.03(.02)* |
| Efficiency, % ++ | .02(.01)** | .01(.01)** | .02 | .01(.01)* | .01(.01)** | .01(.01) |
| Wakefulness After Sleep Onset, min ++ | .01(.01) | .01(.01) | .01 | .01(.01) | .01(.01) | .01(.01) |
| Stage 3–4 Sleep ++ | −.03(.02) | −.01(.02) | .01 | −.01(.02) | −.01(.02) | −.01(.02) |
| Rapid Eye Movement | −.26(.07)*** | −.27(.08)*** | .05 | −.24(.08)** | −.26(.08)*** | −.24(.08)** |
| Apnea-Hypopnea Index ++ | .02(.01) | .01(.01) | .01 | .01(.01) | .02(.01) | .02(.01) |
Note.
p ≤ .001,
p ≤ .05,
p ≤ .10.
Covariates included in these models were age, sex, body mass index, composite socioeconomic status (score based on participants’ education and annual income), hypertensive and/or related medication use, and race/ethnicity.
Reported B coefficients are based on transformed variables.
Race/ethnicity coded as 0 for African Americans and + 1for Caucasians.
None of the significant associations became nonsignificant with the inclusion of depressive symptoms in the model (data not shown). In models adjusting for Trait Anger, Hostility, and Trait Anxiety sequentially, most associations between unfair treatment and sleep outcomes remained significant or approached significance. The exceptions were the associations between unfair treatment and sleep efficiency as measured by actigraphy, p = .24, and sleep duration as measured by PSG, p = .12, after adjusting for Trait Anger. Of note, Trait Anger was not an independent predictor of these two outcomes, p = .12, and p = .32, respectively, in analyses that did not include unfair treatment.
As shown in Table 3, in models adjusting for the standard covariates and Trait Anxiety, unfair treatment no longer predicted self-reported sleep quality using the PSQI, p = .16, daytime sleepiness, p = .13, and sleep efficiency as measured by actigraphy, p = .28, and PSG, p = .14. Of note, Trait anxiety independently predicted only the two self-reported sleep outcomes; self-reported sleep quality using the PSQI, B = .87, SE = .20, p <.0001 and self-reported daytime sleepiness, B = .69, SE = .24, p = .005.
Racial/Ethnic Differences in the Associations between Unfair Treatment and Sleep
Out of 13 possible interactions, the Race/Ethnicity X Unfair Treatment term was significant only for WASO as measured by PSG, p < .02. Simple effects analyses indicated that greater unfair treatment was associated with greater WASO among Caucasians, B = .13, SE = .05, p = .009, but not among African Americans, B = −.06, SE = .05, p = .31.
Nightly Worry as a Potential Mediator of the Association between Unfair Treatment and Sleep
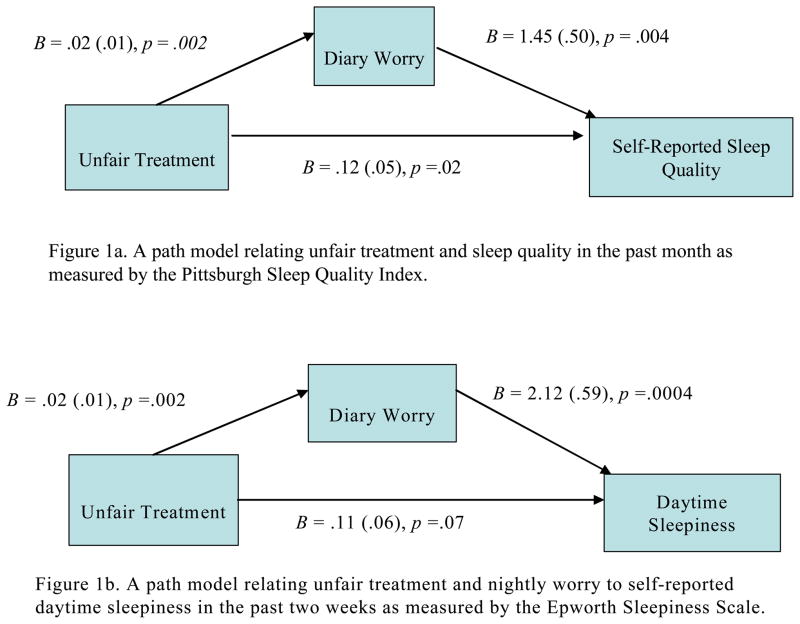
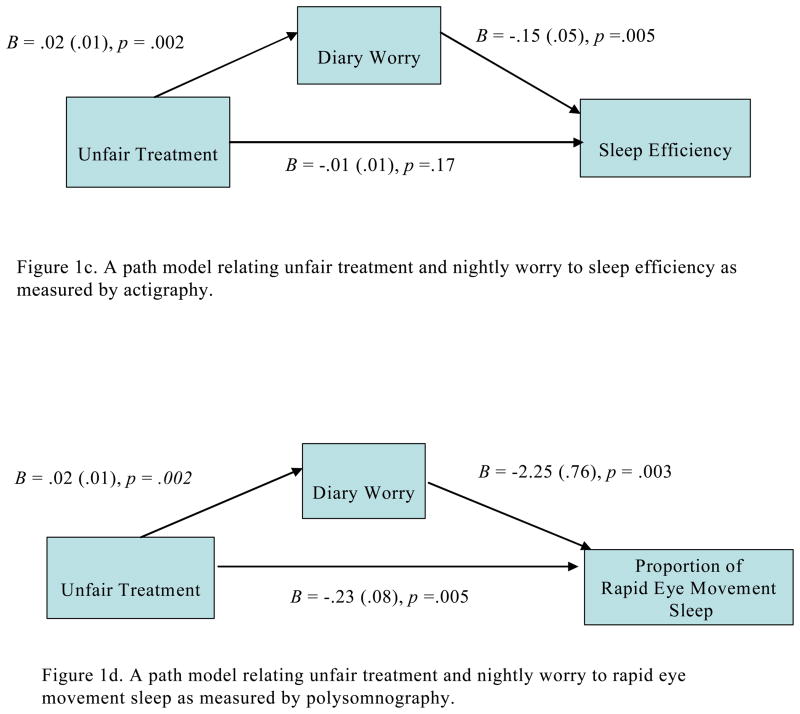
As shown in Figures 1a–1d, nightly worry partially mediated the associations between greater unfair treatment and four measures: poorer sleep quality in the past month using the PSQI, z = 2.13, SE = .01, p = .03, greater daytime sleepiness in the past two weeks, z = 2.36, SE = .02, p = .02, lower sleep efficiency, z = 2.12, SE = .001, p = .03, as measured by actigraphy, and a lower proportion of REM sleep, z = −2.15, SE = .02, p = .03. Of note, SSRI use did not change the meditational findings for REM sleep. Nightly worry did not alter the associations between unfair treatment and sleep duration as measured by actigraphy and PSG, or sleep efficiency as measured by PSG.
Figure 1.
Figures 1a–d. Mediational models testing the association between unfair treatment and sleep quality as measured by the PSQI, daytime sleepiness as measured by the Epworth, sleep efficiency as measured by actigraphy, and rapid eye movement as partially accounted for by nightly worry reported using the diary in the full sample. In all models, age, sex, race/ethnicity, BMI, hypertensive status and/or related medication use and composite SES were covariates.
Discussion
The current study sought to investigate the association between unfair treatment and sleep across self-report and objective measures, specifically, questionnaire and diary measures, and actigraphy and PSG. In line with our primary hypothesis, we found that greater unfair treatment was associated with poorer self-reported sleep quality in the past month1, greater daytime sleepiness in the last two weeks, and shorter sleep duration (PSG and actigraphy), poorer sleep efficiency (PSG and actigraphy), and a smaller proportion of REM sleep during the in-home monitoring period. Our findings extend the current literature (i.e., Steffen & Bowden, 2006; Thomas et al., 2006) by providing a more comprehensive examination of the association between unfair treatment defined broadly and a number of sleep characteristics concurrently and repeatedly assessed.
We also determined that the association between unfair treatment and sleep is not a function of depressive symptoms and hostility. Trait Anger and Trait Anxiety did render nonsignificant several associations with unfair treatment. Trait Anger only predicted self-reported sleep in the past month and was not a significant predictor without unfair treatment in the model, whereas Trait Anxiety predicted the self-report measures in models adjusted or unadjusted for unfair treatment. This suggests that the variance overlapping unfair treatment, anger, and anxiety predicts these sleep measures.
We evaluated whether the association between unfair treatment and self-reported sleep quality and objective sleep outcomes differed by race/ethnicity. There was only one difference by race/ethnicity: greater unfair treatment was associated with greater WASO among Caucasians. This pattern is similar to that reported in a meta-analysis of the relationship of unfair treatment and health, i.e., that few racial/ethnic differences were observed (Pascoe & Smart-Richman, 2009). Thus, unfair treatment appears to have a negative effect on sleep regardless of race/ethnicity.
An exploratory aim was to investigate whether general nightly worry (measured by a daily diary) may be a possible pathway through which unfair treatment is associated with sleep. We found that higher levels of worry partially accounted for the associations between greater unfair treatment and self-reported poorer sleep quality in the past month and daytime sleepiness in the past two weeks, a lower proportion of REM sleep, and poorer sleep efficiency as measured by actigraphy. The fact that nightly worry did not alter the association with poor sleep efficiency measured by PSG may be due to different nights of the study, PSG was assessed across two of those nights, with the presumably better reliability of the actigraphy measures. Note that the primary hypotheses for the main effects of unfair treatment on sleep efficiency were similar across the PSG and actigraphy measures.
Albeit preliminary, our findings point to important future research that could elucidate ruminative processes as a pathway in the association between unfair treatment and sleep. Rather than using a single item about general nightly worry, future research should use a multi-item assessment of worry that includes reasons for the worry, such as distressing interpersonal interactions, finances, or work-related issues. Also, assessing the temporal nature of these events would be helpful in identifying whether daily variations in experiences of unfair treatment and self-reported worry, and sleep quality track across the week or whether one precedes the other. Further, a state measure of worry such as a daily diary measure combined with the use of a trait measure of worry would tease out individual differences in ongoing worry that perhaps may contribute to more frequent reports of unfair treatment. Of note, the current analyses suggest that nightly worry may overlap with anxiety, and that future studies should attempt to disentangle the two. Ultimately, longitudinal studies would be instrumental in disentangling the temporal nature of these associations, which is particularly important given the overlap between key constructs of this research, namely negative emotions, self-reported unfair treatment, and worry (Paradies, 2006; Verkuil, Brosschot, Putman, & Thayer, 2009).
This study had some limitations. First, the study design was cross-sectional, although these data were collected over multiple days. Thus, causation cannot be inferred from our findings. Second, the sample was not representative of the general population as it was drawn from a community-based study of CVD risk in the Pittsburgh, PA area. Third, as already discussed approaches to sleep measurement; the association between the two measures of efficiency was moderate, r(214) = .34, p < .001. It is also the case that while nightly worry and actigraphy were assessed all 9 nights of the study, PSG was assessed across two of those nights, with the presumably better reliability of the actigraphy measures. Note that the primary hypotheses for the main effects of unfair treatment on sleep efficiency were similar across the PSG and actigraphy measures.
Albeit preliminary, our findings point to important future research that could elucidate ruminative processes as a pathway in the association between unfair treatment and sleep. Rather than using a single item about general nightly worry, future research should use a multi-item assessment of worry that includes reasons for the worry, such as distressing interpersonal interactions, finances, or work-related issues. Also, assessing the temporal nature of these events would be helpful in identifying whether daily variations in experiences of unfair treatment and self-reported worry, and sleep quality track across the week or whether one precedes the other. Further, a state measure of worry such as a daily diary measure combined with the use of a trait measure of worry would tease out individual differences in ongoing worry that perhaps may contribute to more frequent reports of unfair treatment. Of note, the current analyses suggest that nightly worry may overlap with anxiety, and that future studies should attempt to disentangle the two. Ultimately, longitudinal studies would be instrumental in disentangling the temporal nature of these associations, which is particularly important given the overlap between key constructs of this research, namely trait anxiety, self-reported unfair treatment, and worry.
This study had some limitations. First, the study design was cross-sectional, although these data were collected over multiple days. Thus, causation cannot be inferred from our findings. Second, the sample was not representative of the general population as it was drawn from a community-based study of CVD risk in the Pittsburgh, PA area. Third, as already discussed, our daily diary measure of worry was not specific to interpersonal interactions or unfair treatment, but assessed overall worry and the rate of worry was rather low in the group. Thus, caution should be exercised in interpreting the mediational findings.
The current findings are noteworthy in the context of a literature that demonstrates an association between unfair treatment and risk factors for CVD, including ambulatory blood pressure (Beatty & Matthews, 2009; Brondolo et al., 2008; Hill, Kobayashi, & Hughes, 2008; Steffen, McNeilly, Anderson, & Sherwood, 2006), inflammatory markers (Friedman, Williams, Singer, & Ryff, 2009), and risky health behaviors (for review see Williams, Neighbors, & Jackson, 2003; Paradies, 2006) such as poor eating habits and limited physical activity. Sleep is a critical component of health maintenance. Poor sleep has been associated with weight gain, learning and memory problems, negative mood, and may be a risk factor for CVD (Buysse, 2004; Centers for Disease Control and Prevention, 2007; Dinger, 1995; Howard et al., 2004; Stickgold, 2006). Taken together, the confluence of perceived unfair treatment as a chronic stressor and poor sleep and the interplay between the two may have critical roles in long-term health problems.
Acknowledgments
This research was supported by grants from the National Heart, Lung and Blood Institute; 076379 and 007560.
Footnotes
We published previously that unfair treatment was not associated with PSQI scores in a subsample of these participants (Buysse et al. 2008). In that study, we used p < .01 because of a large number of analyses and had different covariates because of the different purpose of that paper.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea
Contributor Information
Danielle L. Beatty, Department of Psychiatry, University of Pittsburgh
Martica H. Hall, Department of Psychiatry, University of Pittsburgh
Thomas A. Kamarck, Department of Psychology, University of Pittsburgh
Daniel J. Buysse, Department of Psychiatry, University of Pittsburgh
Jane F. Owens, Department of Psychiatry, University of Pittsburgh
Steven E. Reis, Department of Psychiatry, University of Pittsburgh
Elizabeth J. Mezick, Department of Psychology, University of Pittsburgh
Patrick J. Strollo, Department of Psychiatry, University of Pittsburgh
Karen A. Matthews, Department of Psychiatry, University of Pittsburgh
References
- Akerstedt T. Psychosocial stress and impaired sleep. Scandinavian Journal of Work and Environmental Health. 2006;32(6):493–501. [PubMed] [Google Scholar]
- Barefoot KC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB. The Cook-Medley Hostility Scale: Item content and ability to predict survival. Psychosomatic Medicine. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Beatty DL, Matthews KA. Unfair treatment and trait anger in relation to nighttime blood pressure in African American and White adolescents. Psychosomatic Medicine. 2009;71:813–820. doi: 10.1097/PSY.0b013e3181b3b6f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondolo E, Rieppi R, Erickson SA, Bagiella E, Shapiro PA, McKinley P, et al. Hostility, interpersonal interactions, and ambulatory blood pressure. Psychosomatic Medicine. 2003;65:1003–1011. doi: 10.1097/01.psy.0000097329.53585.a1. [DOI] [PubMed] [Google Scholar]
- Brondolo E, Libby DJ, Denton E, Thompson S, Beatty DL, Schwartz J, et al. Racism and ambulatory blood pressure in a community sample. Psychosomatic Medicine. 2008;70:49–56. doi: 10.1097/PSY.0b013e31815ff3bd. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Siegler IC, Vitaliano PP, Ballard EL, Gwyther LP, et al. Associations among perceptions of social support, negative affect, and quality of sleep in caregivers and noncaregivers. Health Psychology. 2006;25:220–225. doi: 10.1037/0278-6133.25.2.220. [DOI] [PubMed] [Google Scholar]
- Buysse DJ. Insomnia, depression and aging. Assessing sleep and mood interactions in older adults. Geriatrics. 2004;59:47–51. [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, Reis SE, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. Journal of Clinical Sleep Medicine. 2008;4:563–571. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Disease Prevention. Sleep and sleep disorders: A public health challenge. 2009 Retrieved from http://www.cdc.gov/sleep/index.htm.
- Contrada RJ, Ashmore RD, Gary ML, Coups E, Egeth JD, Sewell A, et al. Measures of Ethnicity-Related Stress: Psychometric properties, ethnic group differences, and associations with well-being. Journal of Applied Social Psychology. 2001;31:1775–1820. [Google Scholar]
- De Lange AH, Kompier MAJ, Taris TW, Geurts SAE, Beckers DGJ, Houtman ILD, et al. A hard day’s night: a longitudinal study on the relationships among job demands and job control, sleep quality and fatigue. Journal of Sleep Research. 2009;18:374–383. doi: 10.1111/j.1365-2869.2009.00735.x. [DOI] [PubMed] [Google Scholar]
- Dinges DF. An overview of sleepiness and accidents. Journal of Sleep Research. 1995;4:4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Durrence HH, Lichstein KL. The sleep of African Americans: A comparative review. Behavioral Sleep Medicine. 2006;4:29–44. doi: 10.1207/s15402010bsm0401_3. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Williams DR, Singer BH, Ryff CD. Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: The MIDUS study. Brain, Behavior, and Immunity. 2009;23:684–692. doi: 10.1016/j.bbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M, Bliznikas D, Klein M, Duggal P, Somenek M, Joseph NJ. Comparison of the incidences of obstructive sleep apnea-hypopnea syndrome in African Americans versus Caucasian Americans. Otolaryngology–Head and Neck Surgery. 2006;134:545–550. doi: 10.1016/j.otohns.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Hall M, Buysse DJ, Dew MA, Prigerson HG, Kupfer DJ, Reynolds CF. Intrusive thoughts and avoidance behaviors are associated with sleep disturbances in bereavement-related depression. Depression and Anxiety. 1997;6:106–112. [PubMed] [Google Scholar]
- Hall M, Buysse DJ, Nofzinger EA, Reynolds CF, Thompson W, Mazumdar S, et al. Financial strain is a significant correlate of sleep continuity disturbances in late-life. Biological Psychology. 2008;77:217–222. doi: 10.1016/j.biopsycho.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CF, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosomatic Medicine. 2000;62:227–230. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- Hall MH, Matthews KA, Kravitz HM, Gold EB, Buysse DJ, Bromberger JT, et al. Race and financial strain are independent correlates of sleep in midlife women: The Swansleep study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- Hall M, Thayer JF, Germain A, Moul D, Vasko R, Puhl M, et al. Psychological stress is associated with heightened physiological arousal during Nrem sleep in primary insomnia. Behavioral Sleep Medicine. 2007;5:178–193. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- Hall M, Vasko R, Buysse D, Ombao H, Chen Q, Cashmere JD, et al. Acute stress and heart rate variability in sleep. Psychosomatic Medicine. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, Soldatos CR. Onset of insomnia: Role of life-stress events. Psychosomatic Medicine. 1982;43:439–451. doi: 10.1097/00006842-198110000-00007. [DOI] [PubMed] [Google Scholar]
- Howard ME, Desai AV, Grunstein RR, Hukins C, Armstrong JG, Joffe D, et al. Sleepiness, sleep-disordered breathing, and accident risk factors in commercial vehicle drivers. American Journal of Respiratory and Critical Care Medicine. 2004;170:1014–1021. doi: 10.1164/rccm.200312-1782OC. [DOI] [PubMed] [Google Scholar]
- Hill LK, Kobayashi I, Hughes JW. Perceived racism and ambulatory blood pressure in African American college students. Journal of Black Psychology. 2007;33:404–421. [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biological Psychiatry. 2000;47:921–927. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science and Medicine. 2005;61:1576–1596. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee KA, Rankin SH, Weiss SJ, Alkon A. Sleep disturbance, fatigue, and stress among Chinese-American parents with ICU hospitalized infants. Issues in Mental Health Nursing. 2007;28:593–605. doi: 10.1080/01612840701354505. [DOI] [PubMed] [Google Scholar]
- Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, et al. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: The SWAN heart study. Psychosomatic Medicine. 2006;68:362–368. doi: 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets VA. Comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, Strollo PJ, Buysse DJ, Kamarck TW, et al. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosomatic Medicine. 2008;70:410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies Y. A systematic review of empirical research on self-reported racism and health. International Journal of Epidemiology. 2006;35:888–901. doi: 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- Pascoe EA, Smart-Richman L. Perceived discrimination and health: A meta-analytic review. Psychological Bulletin. 2009;135:531–554. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rasch B, Pommer J, Diekelmann S, Born J. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nature Neuroscience. 2008;12:396–397. doi: 10.1038/nn.2206. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. (NIH Publication No. 204) Washington, DC: Government Printing Office; 1986. [Google Scholar]
- Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex ethnicity, and sleep-disordered breathing on sleep architecture. Archives of Internal Medicine. 2004;164:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- Rowe MA, McCrae CS, Campbell JM, Benito AP, Cheng J. Sleep pattern differences between older adult dementia caregivers and older adult noncaregivers using objective and subjective measures. Journal of Clinical Sleep Medicine. 2008;15(4):362–369. [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Keinan G, Daon K. Effects of stress on sleep: The moderating role of coping style. Health Psychology. 2004;23:542–545. doi: 10.1037/0278-6133.23.5.542. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Steffen PR, McNeilly M, Anderson N, Sherwood A. Effects of perceived racism and anger inhibition on ambulatory blood pressure in African Americans. Psychosomatic Medicine. 2003;65:746–750. doi: 10.1097/01.psy.0000079380.95903.78. [DOI] [PubMed] [Google Scholar]
- Steffen PR, Bowden M. Sleep disturbance mediates the relationship between perceived racism and depressive symptoms. Ethnicity and Disease. 2006;16:16–21. [PubMed] [Google Scholar]
- Stickgold R. A memory boost while you sleep. Nature. 2006;444(30):559–560. doi: 10.1038/nature05309. [DOI] [PubMed] [Google Scholar]
- The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- Thomas KS, Bardwell WA, Ancoli-Israel S, Dimsdale JE. The toll of ethnic discrimination on sleep architecture and fatigue. Health Psychology. 2006;25:635–642. doi: 10.1037/0278-6133.25.5.635. [DOI] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychology. 2003;22:300–309. doi: 10.1037/0278-6133.22.3.300. [DOI] [PubMed] [Google Scholar]
- Vahtera J, Kivimäki M, Hublin C, Korkeila K, Suominen S, Paunio T, et al. Liability to anxiety and severe life events as predictors of new-onset sleep disturbances. Sleep. 2007;30:1537–1546. doi: 10.1093/sleep/30.11.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuil B, Brosschot JF, Putman P, Thayer JF. Interacting effects of worry and anxiety on attentional disengagement from threat. Behaviour Research and Therapy. 2009;47:146–152. doi: 10.1016/j.brat.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: Findings from community studies. American Journal of Public Health. 2003;93:200–208. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: Socioeconomic status, stress and discrimination. American Journal of Health Psychology. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]