SUMMARY
Mice lacking epidermal Langerhans cells (LC) develop exaggerated contact-hypersensitivity (CHS) responses due to the absence of LC during sensitization/initiation. Examination of T cell responses reveals that the absence of LC leads to increased numbers of hapten-specific CD4 and CD8 T cells but does not alter cytokine expression or development of Treg. CHS responses and antigen-specific T cells are increased in mice in which MHC-II is ablated specifically in LC suggesting that direct cognate interaction between LC and CD4 cells is required for LC suppression. LC-derived IL-10 is also required for optimal inhibition of CHS. Both LC-derived IL-10 mediated suppression and full LC activation require LC expression of MHC-II. These data support a model in which cognate interaction of LC with CD4 T cells enables LC to inhibit expansion of antigen-specific responses via elaboration of IL-10.
INTRODUCTION
Langerhans cells (LC) have long been considered to be an archetypal tissue-resident DC. They reside in the epidermis of the skin and acquire local antigen. During inflammation, they migrate to T cell areas in the regional LN and present processed antigen to T cells thereby initiating adaptive immune responses(1). Contact hypersensitivity (CHS) is a mouse model of allergic contact dermatitis which is one of the most common inflammatory skin disorders and is responsible for considerable morbidity(2). CHS is an ideal assay in which to investigate LC biology since unmanipulated mice are immunized through intact skin, the response is well characterized, and alterations in T cell responses can be correlated with biological function(3). The classic model of LC function has been recently tested by the study of CHS in two types of LC-deficient mice(4, 5).
Langerin-DTR mice were generated by the introduction of the primate receptor for diphtheria toxin (DT) into the endogenous murine langerin locus(6, 7). Langerin is expressed by LC as well as a subset of dermal DC(8-10). DC subsets in the lung, thymus, spleen and LN also express Langerin (6, 11, 12). Injection of DT ablates all Langerin expressing DC and results in reduced contact hypersensitization (CHS) responses (5-7). However, many of the early studies showing a role for LC in generating cutaneous inflammatory responses were performed before ablation of dermal DC was appreciated. Interestingly, at time points when Langerin+ dermal DC have partially repopulated the dermis but LC are still absent, CHS is unaffected (8). Thus, the absence of LC does not appear to affect CHS. However, if very low dose hapten is used, CHS appears to be reduced in the absence of LC (13). Importantly, Langerin+ DC found in the dermis are required for optimal CHS responses and fit the pro-inflammatory role originally ascribed to LC.,
Langerin-DTA mice express the active subunit of diphtheria toxin (DTA), not the receptor, as a BAC transgene under control of the human Langerin genomic locus(14). Expression is limited to LC leading to their constitutive ablation while leaving all other cells including Langerin+ dermal DC intact(8). CHS responses in LC-deficient mice were exaggerated due to the absence of LC during the sensitization/priming phase but not the challenge/elicitation phase. Thus, LC actually function to suppress the CHS response. LC-mediated suppression also inhibits rejection of minor-mismatched skin grafts and limits Th1 responses after tick infestation(15, 16).
There have been many investigations into the mechanism of CHS. Mice sensitized with hapten develop CD8+ CTLs that secrete IFNγ and IL-17(17-19). CTLs induce keratinocyte apoptosis and are required for development of CHS(20). However, the control of CTLs is only partially understood. During CHS, CD4 T cells produce both Th1 and Th2 cytokines(21, 22). CD4 T cells are required for optimal CHS and also regulate CHS responses(17, 23, 24). It has been proposed that CD4 T cells suppress CD8 responses via alterations in Th phenotype or development Foxp3+ Treg (17, 25-27). Thus, there are a variety of potential mechanisms through which LC could regulate CHS. Herein we examine LC-mediated suppression of CHS by correlating CHS responses with the development of hapten-specific T cells in mice with absent or defective LC.
MATERIALS AND METHODS
Mice
Langerin-DTA(14), Langerin-Cre(28), I-Aβ-flox(29), and IL-10-flox(30) mice have been previously described. Rag1−/− mice were obtained from Jackson Laboratories (Bar Harbor, ME). All experiments were performed with 6- to 10-week-old age- and sex-matched mice. Mice were housed in micro-isolator cages and fed autoclaved food and acidified water. The University of Minnesota institutional care and use committee approved all mouse protocols.
Antibodies
Antibodies to the following targets were used: muLangerin (929F3, Dendritics, France), CD4, CD8, CD11c, CD11b, CD40, CD44, CD62L, CD69, CD80, CD86, CD90.2, CD103, B7DC, ICOS, ICOS-L, OX40-L, 4-1BB, I-A/E, FoxP3, Fas-L (BioLegend) CD90.1, B7H1, and BrdU (eBioscience). 2-4G2 (anti-FcR) was purified from hybridoma supernatants, as previously described(14).
Contact Hypersensitivity
Mice were sensitized on day 0 by epicutaneous application of 25 μl of 0.5 % DNFB (2,4-dinitro-fluorobenzene, Sigma) in aceton:olive oil (4:1) or vehicle alone onto dry shaved abdominal skin. All mice used in a given experiment were derived from the same mating cages (i.e. siblings) to minimize the possibility of small changes in background affecting CHS. Groups of 4-8 experimental and 3 vehicle control mice were used in all experiments. Lower concentrations of DNFB were used as noted. On day 5, baseline ear thickness was measured with an engineer micrometer followed by challenge with 10 μl of 0.2 % DNFB to both sides of one ear. Ear thickness was measured daily after challenge and data were expressed as the ear size at 24 hr minus the baseline thickness. CHS to FITC used 0.5% FITC diluted in acetone/dibutylpthalate (1:1) for sensitization and challenge.
Flow cytometry
Single-cell suspensions of dermis, LN, and spleen were obtained and stained as previously described(14). Live/dead discrimination was obtained using propidium iodide or ethidium monoazide (Invitrogen, Carlsbad, CA). To evaluate cytokine expression, cells were cultured for 5 hours with phorbol myristic acid and ionomycin, including monensin during the last 2 hours of culture as described(31). The intracellular cytokine staining was performed using BD Bioscience kit (BD Biosciences, San Jose, CA) according to manufacturers directions. For BrdU incorporation, BrdU was continuously administered in drinking water at a concentration of 0.8mg/mL for 4 days before sacrifice. A modified BrdU staining protocol was used for analysis of BrdU and Foxp3. Lymphocytes from the skin-draining inguinal, axillary and brachial lymph nodes were isolated and surface stained, followed by fixation in eBioscience FixPerm for 30 min. Cells were then stained for BrdU following the BrdU Flow Kit manufacturer’s instructions (BD Pharmingen). Detection of CCR4, E-selectin, and P-selectin were performed as described(32, 33). Samples were analyzed on a FACSCalibur or LSR-II flow cytometers (BD Biosciences). Data were analyzed with FlowJo software (Treestar, Ashland, OR).
Immunofluorescence
Eight-μm-thick cryostat sections were made from frozen tissue samples and mounted on poly-L-lysine coated slides. After 10 min of cold acetone fixation, the sections were air-dried and stored in a freezer. Sections were stained and mounted as described(15). Epidermal sheets were prepared by affixing ears to slides using double-sided adhesive tape (3M), followed by incubation in 10 mM EDTA in PBS for 2 h at 37° and physical removal of the dermis, as previously described(28). Images were captured using a microscope (DM5500; Leica) with digital system and LAS AF software (version 1.5.1).
Antigen specific in vitro T-cell restimulation assay
Mice were painted on belly with 25 μl of 0.5 % DNFB diluted in aceton:olive oil. Five days later the painted skin and the draining lymph nodes were harvested and processed. Skin was minced and digested with collagenase and hyaluronidase as described(14). Cells were further purified using Ficoll gradient separation 670g for 30 minand labeled with CFSE (Invitrogen, Carlsbad, CA) according to manufacturers directions and used as responders. For stimulators, Rag1−/− CD90.1 spleens were harvested and haptenated with 5 mM DNBS (2,4-dinitrobenzenesulfonic acid, Sigma) for 30 minutes at 37 °C. Cells were washed and irradiated with cesium-irradiator with 2500 rad. 4×105 responders were co-cultured with 2×105 stimulators in 200 μl complete RPMI in CO2 incubator for 4 days. After culture the cells were harvested and analyzed by flowcytometry. The supernatant cytokine content was determined using Luminex.
Multiplex cytokine analysis
Cell culture supernatants were analyzed for following cytokine: IL-4, IL-5, IL-10, IL-13, IL-17 and IFNγ using Bio-Rad (Hercules, CA) custom made Luminex kit on the basis of manufacturers recommended protocol. TGF-β was assessed using Panomics (Fremont, CA) single-plex kit. The samples were run and analyzed using Bio-Rad Luminex® 200™ system.
Statistics
Statistical comparisons between groups were made with a Student’s two-tailed t test.
RESULTS
Langerin-DTA mice develop exaggerated CHS responses
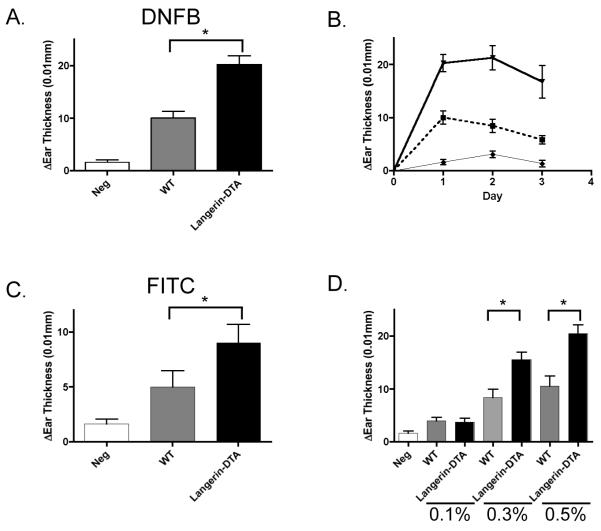
We have previously reported increased CHS responses in Langerin-DTA mice that had been generated and maintained on the FVB background(14). To assess whether this phenotype was related to the background strain of the mice, we backcrossed Langerin-DTA mice a total of 9 generations onto C57BL/6 mice. These mice consistently developed exaggerated CHS responses to DNFB (Figure 1a and b). The degree and timing of the increased ear swelling in Langerin-DTA mice on the C57BL/6 background was similar to that observed on the FVB background. Similar CHS results were also obtained using the hapten FITC (Figure 1c). Finally, we examined CHS responses at different doses of DNFB (Figure 1d). The degree of ear swelling in both wild-type and Langerin-DTA mice decreased with lower concentrations of DNFB. However CHS responses were exaggerated in Langerin-DTA mice at both normal (0.5%) and lower (0.3%) doses. We do not observe decreased CHS in the absence of LC at very low hapten dose (0.1%) as has been reported by others(13). Thus, the increased CHS responses that develop in the absence of LC are not isolated to a single strain, hapten, or dose. All subsequent experiments used C57BL/6 mice and the standard 0.5% DNFB regimen.
Figure 1. Langerin-DTA mice develop exaggerated CHS response.
Langerin-DTA (black bars) and transgene negative littermate control mice (WT, grey bars) were sensitized with 0.5% DNFB on shaved abdomens on day 0. Mice were then challenged with 0.2% DNFB on their ears on day 5. Negative control mice (Neg, white bars) were sensitized with vehicle and challenged with 0.2% DNFB. Langerin-DTA and WT vehicle controls are equivalent and are grouped together. Data represents the change of ear thickness over baseline at 24 hr (*p<0.05). B) As in (A), specific ear swelling is shown over 3 days in Langerin-DTA (solid line), littermate controls (broken line) and vehicle alone controls (thin line). C) CHS responses to 0.5% FITC, as in (A). D) As in (A), mice were sensitized with 0.1%, 0.3% or 0.5% DNFB as indicated and challenged with 0.2% DNFB. Representative experiments from at least 3 repeats are shown. Error bars represent +/−SEM.
Langerin-DTA mice develop increased numbers of hapten-specific T cells
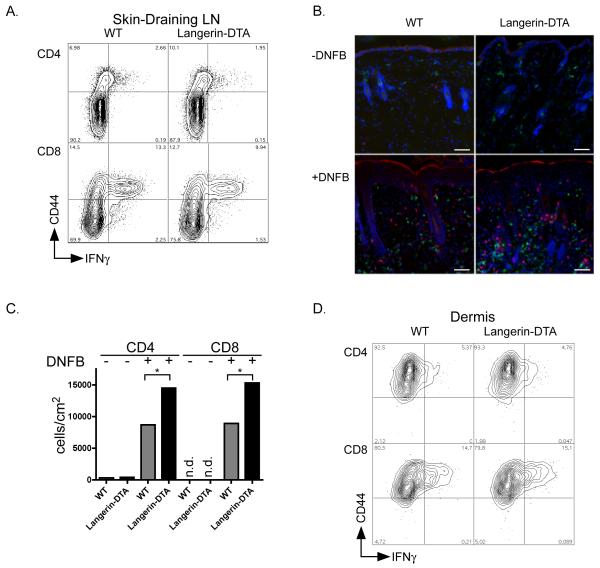
To understand how LC limit the development of CHS responses, we first focused on alterations in T cell populations in secondary lymphoid tissues. Analysis of bulk T cell populations from LN and spleen showed no alteration in numbers of CD4 or CD8 cells. Expression of CD69, CD44, and CD62L were similar in naïve or DNFB-sensitized WT and Langerin-DTA mice (data not shown). Expression of IFNγ was also similar in skin-draining LN (Figure 2a) and spleen (data not shown) harvested from DNFB-sensitized mice. Since DNFB painted mice develop brisk skin inflammation, we next evaluated cells obtained from the dermis. In naïve mice, the number of CD4 cells was similar in both strains and virtually no CD8 cells were present (Figure 2b,c). In DNFB-sensitized mice, there was a dramatic increase in cell number which was augmented in Langerin-DTA. Despite the increase in cell number, the percentage of IFNγ secreting cells was similar in both strains (Figure 2d).
Figure 2. Dermal inflammation is increased in Langerin-DTA but bulk T-cell responses are unaltered.
A) Skin-draining lymph nodes cells from WT (left) and Langerin-DTA (right) mice 5 days after 0.5% DNFB sensitization were stimulated ex vivo with PMA/ionomycin and analyzed for expression of IFNγ by intracellular flow cytometry. Live cells were gated on CD4 (top) or CD8 (bottom). B) DNFB painted (bottom) and unpainted (top) skin from WT (left) and Langerin-DTA mice (right) were examined by immunofluorescence for the presence of CD4 (green) and CD8 (red) cells. Nuclei were highlighted by DAPI staining (blue). Bar represents 100μm C) The number of infiltrating T-cells was determined flow cytometry from collagenase treated DNFB-painted or unpainted skin (as indicated). T cell number/cm2 is shown for Langerin-DTA (black bars) and WT (grey bars) (*p<0.05). D) As in (A), cells were isolated from dermis 5 days after DNFB painting and analyzed for IFNγ expression. Representative experiments from at least 3 repeats are shown.
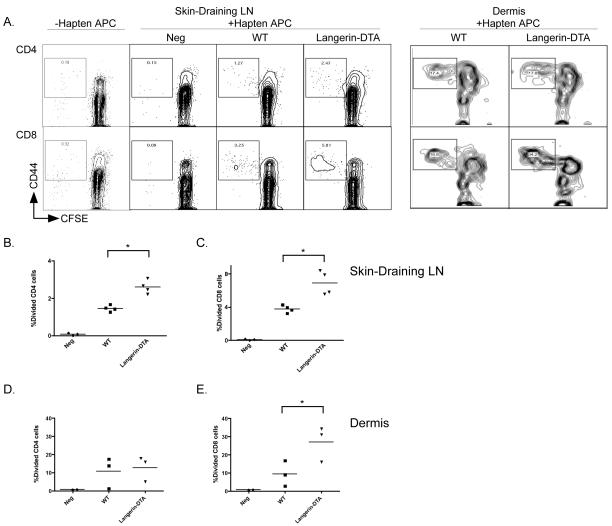
To examine the number of hapten specific T cells in sensitized hosts, we employed a modified DNBS restimulation assay. WT and Langerin-DTA mice were sensitized with DNFB. On day +5 when the CHS challenge is normally performed, cells from skin-draining LN were harvested, CFSE labeled and cultured in vitro with irradiated splenocytes from Thy1.1 Rag1−/− mice that had been haptenated in vitro with DNBS (a water soluble form of DNFB). After 4 days in culture the degree of proliferation was assayed based on dilution of CFSE. We consistently observed an approximately two-fold increase in frequency of divided CD4 and CD8 T cell from Langerin-DTA mice (Figure 3a-c). As expected, divided cells expressed higher levels of CD44 compared with cells from isolated from unsensitized control (Neg) mice which were infrequent and CD44low. The total frequency of divided cells was increased but the number of divisions was similar in both groups indicating the presence of an increased number of hapten-specific CD4 and CD8 cells in Langerin-DTA skin-draining LN. Similar results were obtained using purified CD4 and CD8 cells (data not shown). In the dermis the overall frequency of DNFB-specific cells was higher than in the LN (Figure 3a,d,e). The number of CD8 cells was increased by 2 fold in the absence of LC, but the number of CD4 was unchanged. Interestingly, in both the LN and skin, antigen-specific T cells were increased by approximately two-fold in Langerin-DTA mice which is consistent with the observed two-fold increase in CHS (Figure 1a).
Figure 3. Langerin-DTA mice develop increased hapten-specific T cells.
A) Cells from skin-draining lymph node (left) and dermis (right) from DNFB sensitized WT and Langerin-DTA mice were labeled with CFSE and cultured for 4 days with DNBS-haptenated spleen cells from Thy1.1 congenic Rag−/− mice. Proliferation was assessed by flow cytometry gating on live responders and is shown for CD4 (top) and CD8 (bottom) cells. Cells harvested from unsensitized WT and Langerin-DTA mice gave identical results and are shown as negative (Neg) controls. Cells harvested from sensitized WT and Langerin-DTA mice and stimulated with unhaptenated APC (−Hapten APC) also gave identical results and are pooled. The percentages of proliferating cells from multiple experiments is shown for B) CD4 and C) CD8 cells derived from LN. Percentages of cells from dermis is shown for D) CD4 and E) CD8 cells. Each data point represents an independent experiment with 4-6 pooled mice. *p<0.05
DNFB-Specific T cells in Langerin-DTA and WT mice are phenotypically similar
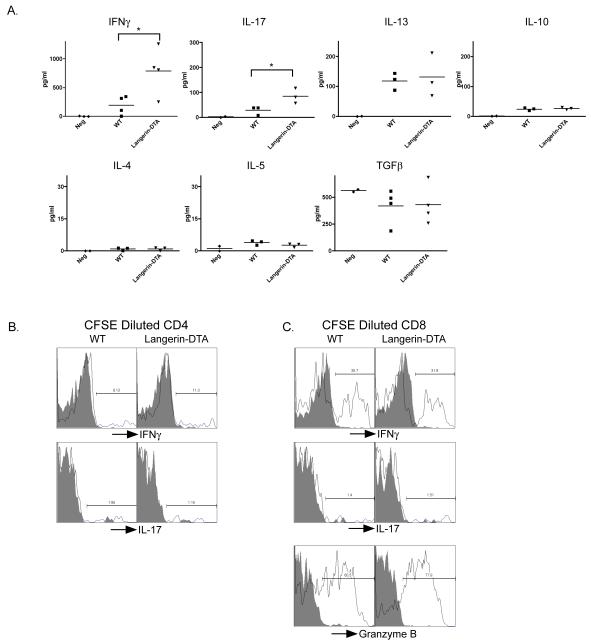
CHS to DNFB has been shown to generate a mixed Th1/Th2 response(23). LC could potentially promote Th2-type responses such that an exaggerated Th1 response would develop in their absence. This hypothesis predicts a decreased production of Th2 cytokines in Langerin-DTA mice. To test this, we examined the cultured supernatants from DNBS stimulated cells using multiplex cytokine analysis. We observed a two-fold increase in amounts of IFNγ and IL-17 in Langerin-DTA cultures (Figure 4a). Importantly, levels of IL-13 and IL-10, which are associated with Th2-type responses, were increased compared to naïve controls but were similar in WT and Langerin-DTA mice. TGFβ1 was not increased compared to negative controls and we did not observe detectable amounts of IL-4 or IL-5. Thus the absence of LC does not alter the production of Th2-type cytokines.
Figure 4. DNFB-specific T-cells in Langerin-DTA and WT mice are phenotypically similar.
A) Cultured supernatants from LN cells stimulated with haptenated APC in figure 3 were analyzed by multiplex cytokine analysis. B) LN cells cultured with haptenated APC for 3-4 days were stimulated with PMA/ionomycin and gated on live, Thy1.2+, CD4+, CFSE diluted cells. Shaded lines represent cells stained with isotype control C) As in (B) but cells are gated on CD8 cells. Representative experiments from at least 3 repeats are shown.
Intracellular cytokine analysis of divided cells, revealed modest numbers of CD4 cells expressing IFNγ and very few expressing IL-17 (Figure 4b). Approximately 30% of CD8 cells expressed IFNγ and very few expressed IL-17 (Figure 4c). This suggests that IFNγ is largely derived from CD8 cells. The percentage of cells expressing IFNγ or IL-17 was similar in WT and Langerin-DTA mice. In addition, the granzyme-B expression was unaltered in Langerin-DTA mice. Thus, although the number of antigen specific cells is increased in the absence of LC, the primarily Th1/Tc1 response to DNFB is unaltered.
Foxp3+ Treg are unaltered in Langerin-DTA mice
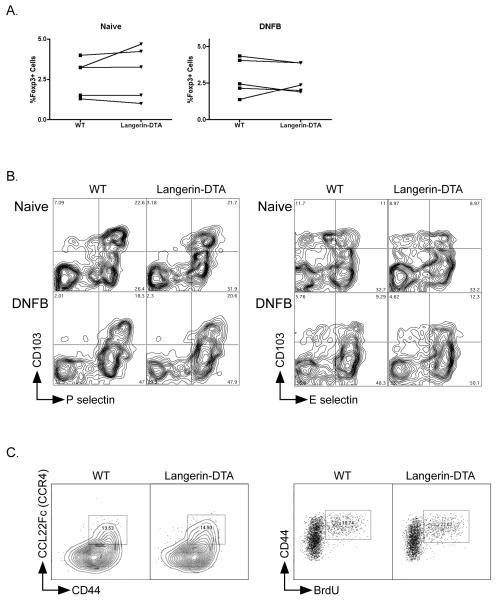
We next sought to determine whether Foxp3 Treg cells participated in LC-mediate suppression of CHS. Bulk analysis of CD4+ cells from naïve and sensitized WT showed equal percentages (Figure 5a) and numbers (data not shown) of Foxp3+ cells in WT and Langerin-DTA mice. Skin homing Treg can be identified in the skin-draining LN based on expression of Foxp3, CD103 and P or E-selectin(32). As with the total Foxp3 population, the percentages of these cells in skin-draining LN was similar in WT and Langerin-DTA mice (Figure 5 b and c).
Figure 5. Foxp3+ Treg unaltered in Langerin-DTA mice.
A) The percentages of Foxp3+ T-cells in LN from naïve (left) and DNFB sensitized (right) mice was analyzed in Langerin-DTA and WT control mice by flow cytometry. Each line represents paired data from littermates within the same experiment. B) LN cells from WT and Langerin-DTA mice were analyzed for alterations in skin homing Treg. Live cells are gated on CD4 and Foxp3. Expression of CD103 and P-selectin (left) and E-selectin(right) is shown. Cells from naïve (top) and 5 days after DNFB sensitization (bottom) are shown. C) Expression of CCR4 on CD4+ CD25+ gated LN cells from WT and Langerin-DTA mice is shown (left). Incorporation of BrdU in CD4+ Foxp3+ gated LN cells after 4 days of continuous administration of BrdU is shown (right). Representative experiments from at least 3 repeats are shown.
The homeostasis of skin-tropic Treg cells depends on their expression of the chemokine receptor CCR4(33). In skin-draining LN, LC produce the CCR4 ligand CCL22, and this may help attract CCR4+ Treg cells, and promote their survival and/or proliferation in vivo. However, Langerin-DTA mice had a normal frequency of CCR4+ Treg cells in the skin-draining LN (Figure 5c, left). Treg cell proliferation as measured by incorporation of 5-bromo-2′-deoxyuridine (BrdU) was equivalent to that seen in wild-type mice (Figure 5c, right) indicating that Treg homeostasis is normal in the absence of LC.
There are several potential mechanisms other than Foxp3 Treg that could participate in LC-mediated regulation. We did not observe an increase in IL-10 or TGFβ producing T cells (Figure 4a) suggesting that Tr1 and Th3 are not affected by the absence of LC. In addition, numbers of other DC subsets (data not shown) as well as surface expression of co-stimulatory molecules were similar in WT and Langerin-DTA mice (Supplemental Figure 1a).
LC-mediated suppression requires LC expression of MHC-II
Since we observed an increase in proliferation of both CD4 and CD8, we next examined whether interaction between LC and CD4 T cells is required for LC-mediated suppression of CHS. We bred mice that express Cre recombinase selectively in epidermal LC (Langerin-Cre) (28) to mice in which the gene I-Aβ had been floxed(29). The floxed allele was created on a H-2b background that lacks the I-E gene, so that Langerin-Cre x I-Aβf/f mice have a LC-selective deficiency of MHC-II. We have previously demonstrated that Langerin-Cre mice ablated floxed genes only in LC with very high efficiency(28). As with Langerin-DTA mice, Langerin+ DC other than LC are not affected(28). Epidermal sheets from Langerin-Cre x I-Aβf/f were stained for CD11c to identify LC and MHC-II (Figure 6a). In WT mice both antibodies co-localized on LC. Langerin-Cre x I-Aβf/f mice, however, have similar numbers of CD11c+ LC but no staining with MHC-II was detectable.
Figure 6. LC-mediated suppression requires LC expression of MHC-II.
A) Epidermal sheets from Langerin-Cre x I-Aβf/f (bottom) and I-Aβf/f littermate controls (WT, top) were stained for CD11c (left) to identify LC and MHC-II (right). Bar represents 100μm B) Langerin-Cre x I-Aβf/f (black bars) and WT (grey bars) were sensitized with 0.5% DNFB and challenged with 0.2% DNFB. Vehicle sensitized WT and Langerin-Cre x I-Aβf/f are similar are shown together (Neg , white bars). Data represents the change of ear thickness over baseline after 24 hr (left, *p<0.05). Specific ear swelling is shown over 3 days in Langerin-Cre x I-Aβf/f (solid line, right), WT (broken line) and vehicle control (thin line). C) CHS on Langerin-Cre I-Aβf/+ (left, black bars), WT (grey bars) and vehicle control (white bars). Specific ear swelling is shown over 3 days in Langerin-Cre I-Aβf/+ (right, solid line), WT(broken line) and vehicle controls (thin line). D) The percentage of CFSE diluted CD4 (left) and CD8 (right) cells from multiple DNBS restimulation experiments. Each data point represents an independent experiment with 4-6 pooled mice. *p<0.05
To examine the functional requirement of MHC-II expression by LC, CHS responses to DNFB in Langerin-Cre x I-Aβf/f mice and littermate controls were examined. CHS responses were consistently increased in Langerin-Cre x I-Aβf/f mice (Figure 6b). Importantly, Langerin-Cre mice not bred onto a homozygous floxed background did not show altered CHS compared to littermate controls (Figure 6c). Thus, the enhanced CHS observed in Langerin-Cre x I-Aβf/f mice was due to the absence of MHC-II on LC and not from expression of the Cre transgene itself.
As was observed for Langerin-DTA mice, the number of DNFB-specific CD4 and CD8 T cells was increased in Langerin-Cre x I-Aβf/f mice (Figure 6d). Thus, the absence of MHC-II on LC recapitulated the phenotype observed in Langerin-DTA mice and suggests that LC-mediated suppression of CHS requires cognate interaction with CD4 T cells.
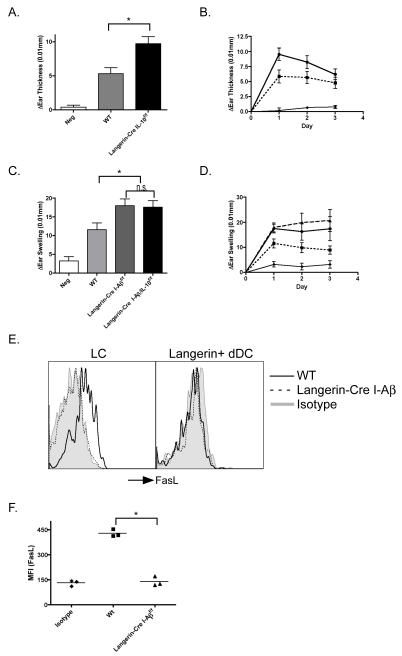
Optimal suppression of CHS requires LC-derived IL-10
IL-10 is known to be secreted by LC and could limit CD4 and CD8 expansion(34-37). To evaluate whether LC-derived IL-10 participates in regulating CHS, we bred Langerin-Cre mice to mice carrying floxed IL-10. Langerin-Cre x IL-10f/f mice develop enhanced CHS compared to littermate controls (Figure 7a and b). Although CHS responses were significantly increased, the enhancement of ear swelling was somewhat less than that observed with Langerin-DTA or Langerin-Cre x I-Aβf/f mice. To evaluate whether LC-derived IL-10 and MHC-II are synergistic, we generated Langerin-Cre I-Aβf/f/IL-10f/f mice. These mice develop CHS responses similar to Langerin-Cre x I-Aβf/f mice (Figure 7c and d), demonstrating that IL-10 mediated suppression and MHC-II-dependent suppression do not act synergistically but are in the same pathway.
Figure 7. Optimal suppression of CHS requires LC-derived IL-10.
A) Langerin-Cre x IL-10f/f (black bars) and IL-10f/f littermate controls (WT, grey bars) were sensitized with 0.5% DNFB or vehicle alone (white bars) and challenged with 0.2% DNFB. Data represents the change of ear thickness over baseline after 24 hr (*p<0.05). B) Specific ear swelling is shown over 3 days in Langerin-Cre x IL-10f/f (solid line),WT (broken line) and vehicle controls (thin line). C) As in (A) Langerin-Cre I-Aβf/f/IL-10f/f (black bar), Langerin-Cre I-Aβf/f (dark grey bar) and WT (grey bar) (*p<0.05). D) As in (B) Langerin-Cre I-Aβf/f/IL-10f/f (solid line), Langerin-Cre I-Aβf/f (long-dashed line), littermate controls (short-dashed line) and vehicle controls (thin line). E) FasL expression was assessed on LC (left) and Langerin+ DC (right) isolated from the lymph node of Langerin-Cre I-Aβf/f (dashed line) and littermate control (solid line) mice (p<0.05 MFI for LC). Shaded area represents isotype control. F) Total LN cells from WT (left) and Langerin-Cre I-Aβf/f mice (right) were incubated in complete RPMI overnight with a 10ug/ml blocking anti-CD40 antibody (dashed line) or isotype control (solid line). Levels of FasL expression on cells gated on LC are shown. Representative experiments from at least 3 repeats are shown.
Ligation of CD40 by CD40L expressed by T cells is required to fully activate/condition DC for optimal elaboration of cytokines(38). Expression of CD40 and co-stimulatory molecules was unchanged on LC from Langerin-Cre x I-Aβf/f mice (Supplemental Figure 1b). However, expression of FasL, was absent on LC from Langerin-Cre I-Aβf/f mice (Figure 7e). Expression of FasL by LC results from CD40 ligation and is a marker of LC activation but does not lead to apoptosis of LC (39). Moreover, LC from WT mice incubated with blocking antibodies to CD40 lose FasL expression (Figure 7f). This is consistent with the absence of FasL on LC from Langerin-Cre I-Aβf/f mice. Since these LC cannot interact CD4 T cells, they do not receive CD40 stimulation from CD40L (CD154) on CD4 T cells and remain unactivated. Interestingly, FasL is not expressed by dermal DC and may be represent another mechanism in addition to LC-derived IL-10 by which LC limit antigen-specific responses.
DISCUSSION
We have demonstrated that LC-mediated suppression of CHS responses requires cognate interaction with CD4 T cells to limit the number of hapten-specific CD4 and CD8 T cells via an IL-10-dependent mechanism. The absence of LC does not alter cytokine expression by hapten-specific T cells or impair the development and homeostasis of Treg.
The fact that Langerin-Cre I-Aβf/f mice recapitulate the exaggerated CHS responses and increased numbers of hapten-specific cells seen in Langerin-DTA mice argues that direct LC-CD4 interaction is an obligate step in LC-mediated regulation. Furthermore, increased CHS in Langerin-Cre IL-10f/f mice demonstrates that optimal LC-mediated suppression requires LC-derived IL-10 and suggests that increased CHS in IL-10−/− mice(40) is at least partially dependent on LC-derived IL-10. IL-10 can directly inhibit T cell function, induce a state of anergy/unresponsiveness, promote Treg development, and decrease APC activation(37). As we did not detect any alterations in Treg development or in DC populations, we favor the hypothesis that LC-derived IL-10 acts directly on effector T cells and propose the following model. LC arrive in the skin-draining LN 3-4 days after hapten application, which is well after skin-resident Langerin+ dDC (and quite likely other dDC) have transported haptenated antigens to the LN and initiated adaptive responses (41, 42). Upon cognate interaction with CD4 cells presumably via CD40-CD40L interaction, LC express IL-10 which inhibits hapten-specific T cells. In the absence of LC-CD4 T cell interaction, LC are not stimulated to secrete IL-10. This results in a failure to suppress expansion of hapten-specific cells thereby leading to the development of exaggerated CHS. The absence of any effect on bulk T cell population in Langerin-DTA mice argues that LC-mediated suppression is antigen-specific and does not affect by-stander cells.
Although we have demonstrated a role for LC-derived IL-10, other regulatory molecules are also likely to help dampen CHS responses. FasL is selectively expressed by LC that have cognate CD4 T cell interatction (Figure 7 and (39)) and could reduce effector responses by directly inducing apoptosis of hapten-specific T cells(43). In vitro LC negatively regulate T cell activation via cell-cell contact (44). However, Fas-FasL interactions appear to be redundant as CHS is unaffected in lpr and gld mice(20), though the absence of Fas-FasL interactions in all cell types may obscure the role of FasL on LC. Since we detect antigen specific T cells based on their ability to proliferate, we cannot use the DNFB-restimulation assay to determine whether cells in Langerin-DTA undergo reduced levels of apoptosis. TGFβ is a potent immunoregulatory cytokine secreted by LC and could suppress CHS responses. We have generated Langerin-Cre TGFβf/f mice but LC do not develop without autocrine/paracrine TGFβ which precludes the use of these mice in functional assays(28). Other LC-specific molecules such 2-3-indolamine-deoxygenase, could also participate(45).
The presence of a population of CD4 T cells that regulate CHS responses has been suggested by increased CHS responses in CD4 depleted mice(21, 22, 24, 46). It has been speculated that these cells represent Foxp3+ Treg and/or Th2 cells that balance the predominantly Th1 CHS response (23, 25). Data for Treg mediated suppression comes from studies in which CHS responses can be suppressed by adoptive transfer of either Foxp3 transduced cells or Treg from orally tolerized mice (26, 27, 47). Transgenic over-expression of RANKL by keratinocytes dramatically increases the number of Treg in skin draining LN(48). Since LC express RANK, the receptor for RANKL, we anticipated that elimination of LC in Langerin-DTA mice would lead to the development of many fewer Treg. However, we observed normal Treg development and homeostasis.
Cytokine analysis of DNFB sensitized mice revealed increased IFNγ and IL-17 in the absence of LC. These were primarily produced by CTL. Increased quantities of these effector cytokines are consistent with increased CHS in LC deficient mice. We observed virtually no expression of IFNγ or IL-17 by CD4 T cells and instead found modest levels of IL-13 and IL-10. A role for Th2 cells in CHS regulation derives from observations that CD4 cells in hapten-treated mice produce primarily IL-4, IL-5, IL-10 and little IFNγ(17). Interestingly, LC-deficient mice immunized by gene gun develop reduced IgG1 and enhanced IgG2a antibody production(49). However, the levels of IL-13 were similar in WT and Langerin-DTA mice indicating that Th2 responses develop normally. We did not detect IL-4 or IL-5 expression as has been seen previously after DNFB sensitization(17) possibly due to differences in genetic background. In addition, CHS responses were increased in Langerin-DTA mice to both DNFB which generates a Th1-type response and to FITC which has been reported to generate Th2-type responses(50). Thus, though Treg and Th2 cells may participate in limiting the extent of CHS responses in intact mice, LC-mediated suppression appears to operate via an alternative mechanism.
Langerin-DTR mice in which LC and other Langerin+ DC are ablated by injection of DT do not develop enhanced CHS even at time points after DT administration, when Langerin+ dermal DC have partially repopulated the dermis but LC are still largely absent (6-8). A potential compensatory response to the constitutively empty epidermal DC niche or expression of DTA cannot explain increased CHS responses in Langerin-DTA mice since Langerin-Cre I-Aβf/f and Langerin-Cre IL-10f/f mice both develop enhances CHS. In addition, heterozygous-flox Langerin-Cre mice have normal CHS responses indicating that genes present on the BAC transgene or expression of Cre itself does not affect CHS. The discrepancy between Langerin-DTA and DTR mice could reflect the still depleted number of Langerin+ dDC at the time of CHS sensitization or that repopulating Langerin+ dDC have properties distinct from those present in the steady-state. Alternatively, since Langerin-DTA, Langerin-Cre I-Aβf/f and Langerin-Cre IL-10f/f mice have defective/absent LC from birth, we cannot exclude that MHC-II/IL-10 dependent LC-mediated suppression may function during ontogeny. Future inducible ablation systems that are more LC-specific should help resolve the acute role of LC in skin immune responses.
In summary, we have described a novel mechanism of immuno-regulation in the skin. LC-mediated suppression may function to drive the resolution of cutaneous immune responses and could also inhibit responses against skin commensal organisms and benign environmental antigens.
Supplementary Material
Supplemental Figure 1. The expression of co-stimulatory molecules is unaffected by the absence of LCs. A) The expression of indicated surface molecules on DC obtained from skin-draining LN isolated from Langerin-DTA (red) and littermate control (blue) was compared by flow cytometry. Live cells were gated on CD11c+, MHC-IIbright and as indicated. LC were gated as Langerin+, CD103−, CD11b+. Shaded area represents isotype control. B) The expression of the indicated surface molecules on LC obtained from skin-draining LN isolated from Langerin-Cre I-Aβf/f (solid line) andWT (dashed) mice was compared by flow cytometry. Shaded area represents isotype control.
ACKNOWLEDGEMENTS
We would like to thank Drs. Kris Hogquist and Matthew Mescher for helpful suggestions and careful reading of the manuscript. We would also like to thank Dr. Kensuke Takada for technical assistance. We thank the University of Minnesota Research Animal Resources staff for outstanding animal care.
Supported by NIH R01-AR056632 to DHK and R01-AR44077 to MJS.
Footnotes
The authors have no conflicting financial interests.
REFERENCES
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117–124. doi: 10.2310/6620.2004.04007. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Esche C, Mamelak A, Freed I, Watanabe H, Sauder DN. Cytokine knockouts in contact hypersensitivity research. Cytokine Growth Factor Rev. 2003;14:381–389. doi: 10.1016/s1359-6101(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 4.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells--changing views on their function in vivo. Immunol Lett. 2006;106:119–125. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 6.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and Function of Langerhans Cells In Vivo Dermal Dendritic Cells Colonize Lymph Node AreasDistinct from Slower Migrating Langerhans Cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valladeau J, Clair-Moninot V, Dezutter-Dambuyant C, Pin JJ, Kissenpfennig A, Mattei MG, Ait-Yahia S, Bates EE, Malissen B, Koch F, Fossiez F, Romani N, Lebecque S, Saeland S. Identification of mouse langerin/CD207 in Langerhans cells and some dendritic cells of lymphoid tissues. J Immunol. 2002;168:782–792. doi: 10.4049/jimmunol.168.2.782. [DOI] [PubMed] [Google Scholar]
- 12.Douillard P, Stoitzner P, Tripp CH, Clair-Moninot V, Ait-Yahia S, McLellan AD, Eggert A, Romani N, Saeland S. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- 13.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Obhrai JS, Oberbarnscheidt M, Zhang N, Mueller DL, Shlomchik WD, Lakkis FG, Shlomchik MJ, Kaplan DH. Langerhans cells are not required for efficient skin graft rejection. J Invest Dermatol. 2008;128:1950–1955. doi: 10.1038/jid.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vesely DL, Fish D, Shlomchik MJ, Kaplan DH, Bockenstedt LK. Langerhans cell deficiency impairs Ixodes scapularis suppression of Th1 responses in mice. Infect Immun. 2009;77:1881–1887. doi: 10.1128/IAI.00030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006;177:6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 20.Kehren J, Desvignes C, Krasteva M, Ducluzeau MT, Assossou O, Horand F, Hahne M, Kagi D, Kaiserlian D, Nicolas JF. Cytotoxicity is mandatory for CD8(+) T cell-mediated contact hypersensitivity. J Exp Med. 1999;189:779–786. doi: 10.1084/jem.189.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bour H, Peyron E, Gaucherand M, Garrigue JL, Desvignes C, Kaiserlian D, Revillard JP, Nicolas JF. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 22.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–4128. [PubMed] [Google Scholar]
- 23.Watanabe H, Unger M, Tuvel B, Wang B, Sauder DN. Contact hypersensitivity: the mechanism of immune responses and T cell balance. J Interferon Cytokine Res. 2002;22:407–412. doi: 10.1089/10799900252952181. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Fujisawa H, Zhuang L, Freed I, Howell BG, Shahid S, Shivji GM, Mak TW, Sauder DN. CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J Immunol. 2000;165:6783–6790. doi: 10.4049/jimmunol.165.12.6783. [DOI] [PubMed] [Google Scholar]
- 25.Gorbachev AV, Fairchild RL. CD4+ T cells regulate CD8+ T cell-mediated cutaneous immune responses by restricting effector T cell development through a Fas ligand-dependent mechanism. J Immunol. 2004;172:2286–2295. doi: 10.4049/jimmunol.172.4.2286. [DOI] [PubMed] [Google Scholar]
- 26.Loser K, Hansen W, Apelt J, Balkow S, Buer J, Beissert S. In vitro-generated regulatory T cells induced by Foxp3-retrovirus infection control murine contact allergy and systemic autoimmunity. Gene Ther. 2005;12:1294–1304. doi: 10.1038/sj.gt.3302567. [DOI] [PubMed] [Google Scholar]
- 27.Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood. 2003;102:3295–3301. doi: 10.1182/blood-2003-03-0727. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGF{beta}1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimoda M, Mmanywa F, Joshi SK, Li T, Miyake K, Pihkala J, Abbas JA, Koni PA. Conditional ablation of MHC-II suggests an indirect role for MHC-II in regulatory CD4 T cell maintenance. J Immunol. 2006;176:6503–6511. doi: 10.4049/jimmunol.176.11.6503. [DOI] [PubMed] [Google Scholar]
- 30.Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, Muller W. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O’Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J Exp Med. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshiki R, Kabashima K, Sugita K, Atarashi K, Shimauchi T, Tokura Y. IL-10-producing Langerhans cells and regulatory T cells are responsible for depressed contact hypersensitivity in grafted skin. J Invest Dermatol. 2009;129:705–713. doi: 10.1038/jid.2008.304. [DOI] [PubMed] [Google Scholar]
- 35.Flacher V, Bouschbacher M, Verronese E, Massacrier C, Sisirak V, Berthier-Vergnes O, de Saint-Vis B, Caux C, Dezutter-Dambuyant C, Lebecque S, Valladeau J. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 36.Morelli AE, Rubin JP, Erdos G, Tkacheva OA, Mathers AR, Zahorchak AF, Thomson AW, Falo LD, Jr., Larregina AT. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J Immunol. 2005;175:7905–7915. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- 37.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 38.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibaki A, Katz SI. Activation through CD40 ligation induces functional Fas ligand expression by Langerhans cells. Eur J Immunol. 2001;31:3006–3015. doi: 10.1002/1521-4141(2001010)31:10<3006::aid-immu3006>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Wang B, Zhuang L, Fujisawa H, Shinder GA, Feliciani C, Shivji GM, Suzuki H, Amerio P, Toto P, Sauder DN. Enhanced epidermal Langerhans cell migration in IL-10 knockout mice. J Immunol. 1999;162:277–283. [PubMed] [Google Scholar]
- 41.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, Burnham K, Saeland S, Handman E, Shortman K. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 42.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 43.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803–13809. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- 45.von Bubnoff D, Bausinger H, Matz H, Koch S, Hacker G, Takikawa O, Bieber T, Hanau D, de la Salle H. Human epidermal langerhans cells express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. J Invest Dermatol. 2004;123:298–304. doi: 10.1111/j.0022-202X.2004.23217.x. [DOI] [PubMed] [Google Scholar]
- 46.Gorbachev AV, Fairchild RL. Regulatory role of CD4+ T cells during the development of contact hypersensitivity responses. Immunol Res. 2001;24:69–77. doi: 10.1385/IR:24:1:69. [DOI] [PubMed] [Google Scholar]
- 47.Ring S, Schafer SC, Mahnke K, Lehr HA, Enk AH. CD4+ CD25+ regulatory T cells suppress contact hypersensitivity reactions by blocking influx of effector T cells into inflamed tissue. Eur J Immunol. 2006;36:2981–2992. doi: 10.1002/eji.200636207. [DOI] [PubMed] [Google Scholar]
- 48.Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372–1379. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 49.Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, Clausen BE, Merad M, Udey MC. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang A, Judge TA, Nickoloff BJ, Turka LA. Suppression of murine allergic contact dermatitis by CTLA4Ig. Tolerance induction of Th2 responses requires additional blockade of CD40-ligand. J Immunol. 1996;157:117–125. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The expression of co-stimulatory molecules is unaffected by the absence of LCs. A) The expression of indicated surface molecules on DC obtained from skin-draining LN isolated from Langerin-DTA (red) and littermate control (blue) was compared by flow cytometry. Live cells were gated on CD11c+, MHC-IIbright and as indicated. LC were gated as Langerin+, CD103−, CD11b+. Shaded area represents isotype control. B) The expression of the indicated surface molecules on LC obtained from skin-draining LN isolated from Langerin-Cre I-Aβf/f (solid line) andWT (dashed) mice was compared by flow cytometry. Shaded area represents isotype control.