Abstract
Calcitonin gene-related peptide (CGRP) is a multifunctional neuropeptide implicated in inflammatory diseases involving trigeminal ganglion nerve activation. Within trigeminal ganglia, satellite glia and Schwann cells are found in close association with neuronal cell bodies and fibers, respectively, and are known to express functional CGRP receptors. The goal of this study was to use array analysis to provide a more comprehensive understanding of CGRP regulation of inflammatory proteins and genes in trigeminal glia. Primary trigeminal ganglia cultures enriched for glia were treated with 500 nM CGRP for 8 or 24 h. CGRP caused a >3-fold increase in the level of 19 cytokines 8 h after CGRP treatment and the levels of each of these cytokines remained significantly elevated over basal unstimulated levels at 24 h. While mRNA levels of many genes involved in mitogen-activated protein (MAP) kinase signaling were increased 8 h after CGRP treatment, the number of responsive genes was greatly increased at 24 h. Specifically, CGRP was shown to temporally regulate expression of multiple MAP kinases as well as numerous MAP kinase-responsive genes including transcription factors, scaffold/anchoring proteins, and cell cycle proteins. Thus, our data provide evidence of an emerging role of CGRP as an important modulator of trigeminal ganglion glia by stimulating cytokine release as well as inducing expression of a diverse array of proteins involved in MAP kinase signaling.
Keywords: CGRP, Trigeminal ganglion, Satellite glial cells, Arrays
Calcitonin gene-related peptide (CGRP), which is expressed by peripheral sensory neurons of the trigeminal ganglia, is implicated in the underlying pathology of migraine, rhinosinusitis, and temporomandibular joint disorder (TMD) [3,4,24]. In response to activation of trigeminal nociceptors, CGRP facilitates neurogenic inflammation in peripheral tissues by functioning as a potent vasodilator and causing mast cell degranulation [17]. Stimulated CGRP release in the CNS contributes to pain, central sensitization, and allodynia by mediating excitation of second order neurons and glial cells [12,24]. In addition, CGRP released within trigeminal ganglia could function in an autocrine manner to stimulate its own synthesis as well as function in a parcrine manner to stimulate glia activity [27,32]. Within trigeminal ganglia, neuronal cell bodies are ensheathed by several satellite glial cells and together are thought to form distinct functional units [27]. Another glial cell found in trigeminal ganglia is the Schwann cell, which is located in close proximity to nerve fibers. A primary role of trigeminal glia is to regulate the microenvironment around the neuronal cell body and fibers under normal and pathological conditions [11]. Hence, these glial cells function to control the excitability state of trigeminal neurons. Since both satellite glia and Schwann cells are known to express CGRP receptors [16,18,32], CGRP regulation of glia activity is likely to have important implications in inflammatory diseases involving excitation of trigeminal nerves.
The CGRP receptor is a heterodimer formed between G protein-coupled receptor known as calcitonin receptor-like receptor (CLR) and another transmembrane protein named receptor activity-modifying protein 1 (RAMP1). RAMP1 is required for facilitating CLR expression at the cell surface and is critical to receptor function for it defines the relative potency of ligands for the receptor [19]. Activation of CGRP receptors is most commonly reported to couple to stimulation of adenylate cyclase and increases in intracellular cAMP levels in multiple cell types [25,31]. However, CGRP-responsive signaling proteins in satellite glia and Schwann cells that have not been thoroughly investigated.
We have recently reported that CGRP can stimulate expression of inducible nitric oxide synthase (iNOS) and release of nitric oxide (NO), as well as differentially regulate the expression of multiple cytokines in cultured trigeminal ganglion glial cells [18,27,28]. The CGRP-mediated increase in iNOS expression was shown to involve activation of mitogen-activated protein (MAP) kinases [28]. Importantly, cytokines and MAP kinase signaling pathways are known to play important roles in regulating inflammatory responses within tissues. The goal of this study was to use microarray analysis to more thoroughly investigate time-dependent changes in cytokine expression as well as changes in expression of genes involved in MAP kinase signaling in cultured trigeminal ganglia glia in response to CGRP.
All animal experimental procedures were conducted in accordance with institutional and National Institutes of Health guidelines. Pregnant female Sprague–Dawley rats (Charles River, Wilmington, MA) were housed in plastic cages on a 12 h light/dark cycle with unrestricted access to food and water. Every effort was made to minimize animal suffering and reduce the number of animals used.
Primary cultures of trigeminal ganglia enriched in glia were established based on previously published protocols [5,9,10,18]. Briefly, trigeminal ganglia were isolated from 3- to 4-day-old neonatal rats and dissociated in L15 media containing Dispase II, and RQ1 DNase. After centrifugation, cell pellets were resuspended in L15 media and respun to pellet neuronal cells while the resultant supernatant respun to concentrate glia. The resulting glial cell pellet was resuspended in L15 media supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA), 50 mM glucose, 250 µMascorbic acid, 8 µM glutathione, 2 mM glutamine, and 10 ng/mL mouse 2.5 S nerve growth factor (Alomone Laboratories, Jerusalem, Israel), as well as penicillin (100 units/mL), streptomycin (100 µg/mL), and amphotericin B (2.5 µg/mL). For array studies, glia were plated at a density of 2 ganglia per well in poly-d-lysine coated 24-well tissue culture plates and at a density of half a ganglion on 11 mm glass coverslips coated with poly-d-lysine for immunocytochemistry studies. Glia cultures were incubated at 37 °C at ambient CO2 and culture medium was changed after 24 h and every other day thereafter as needed.
Immunocytochemical studies were conducted using untreated 2-day-old primary glia cultures as previously described [18,28]. Fixed and permeabilized cells were incubated in PBS containing 5% donkey serum and then with a rabbit polyclonal antibody directed against RAMP1 (1:200 in PBS; ProteinTech Group, Inc., Chicago, IL), and immunoreactive proteins detected following incubation with Alexa Fluor 594 (diluted 1:500 in PBS; Invitrogen). As a control, some cultures were incubated with only anti-rabbit secondary antibodies. All images were taken at 400× magnification on a Zeiss Imager.Z1 Microscope with an Apotome using AxioVision Version 4.7.2.0 software (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Cell counts were performed over 10 random fields by two independent researchers blinded to the experimental design and values reported as percentage of RAMP1 positive glia to total number of counted glia.
Primary glia-enriched cultures of trigeminal ganglia maintained for 2–5 days were left untreated (control) or treated with 500 nM CGRP (in sterile water; American Peptides, Sunnyvale, CA) in L15 complete media for 8 or 24 h. After incubation, media was removed and replaced with PBS (pH 7.4) for 1 h at 37 °C. Media or cells were collected from 4-well per condition and pooled for antibody or mRNA arrays. Each experimental condition was repeated in at least 3 independent experiments. As a control, cell viability was determined in untreated and CGRP-treated cultures using the CellTiter96 assay (Promega, Madison, WI).
Levels of 19 cytokines were determined in control or CGRP-treated glia cultures using the RayBio Rat Cytokine Antibody Array 1.1 per manufacturer’s instructions (RayBiotech, Norcross, GA). Briefly, cytokine membranes were blocked for 30 min and 1 mL of media was added to each array and incubated overnight at 4 °C. Following washing and addition of biotin-conjugated primary antibodies, membranes were incubated overnight at 4 °C. Membranes were then incubated in streptavadin-conjugated peroxidase for 1 h and exposed to a peroxidase substrate for 5 min prior to developing on X-ray film. Densiometric analysis was performed on a Kodak ImageStation 4000 M (Eastman Kodak Company, Rochester, NY) with background subtraction from spot edges. Spot data was normalized to a positive control spot on each array.
RNA was isolated from pellets of untreated glia or cells treated with 500 nM CGRP using the MagMax-96 for Microarrays Total RNA Isolation Kit according to manufacturer’s instructions (Applied Biosystems, Foster City, CA). The RT2 First Strand Kit (SABiosciences, Frederick, MD) was used to create cDNA from isolated RNA (1 µg). Resultant cDNA was used to perform qPCR on RT2 MAP Kinase Signaling Pathway PCR Arrays (SABiosciences) with SABiosciences RT2 qPCR Master Mix according to manufacturer’s instructions. Plates were read on a Stratagene Mx3000p qPCR machine (Stratagene, La Jolla, CA) with 1 cycle of 10 min at 95 °C followed by 45 cycles of 15 s at 95 °C and 1 min at 60 °C. SYBER Green fluorescence was monitored at the annealing step of each cycle and analyzed with MX Pro software (Stratagene).
Statistics were performed with a non-parametric Mann–Whitney U-test. Differences were considered statistically significant at p < 0.05. All statistical tests were performed using SPSS Statistical Software, Release 16 (Chicago, IL).
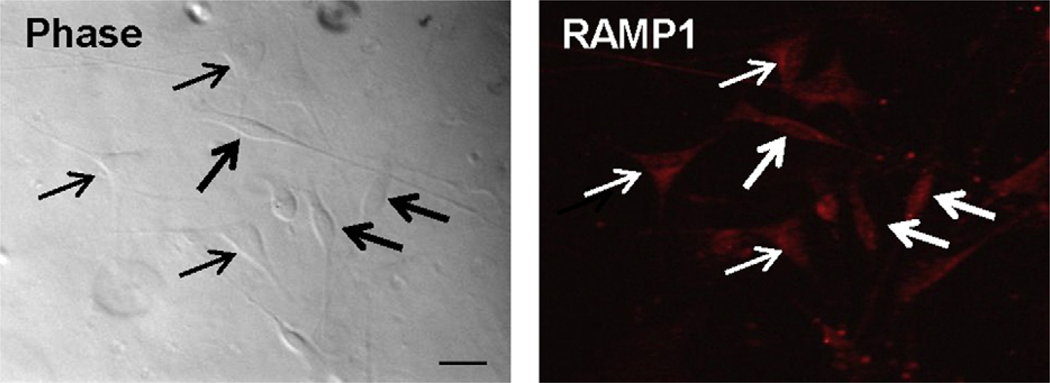
To determine the effect of CGRP on protein and mRNA expression in trigeminal glia, cultures of trigeminal ganglia enriched for glia were established. As viewed with phase microscopy, cultures were comprised of mostly glia (>98%), which consisted of satellite glia (two processes extending from elongated cell body) and Schwann cells (multiple processes extending from triangular shaped cell body) (Fig. 1). Under our culture conditions, the percentage of glia cells expressing RAMP1 was 95.5 ± 1.8% (193/203 glia cells). Based on cell counts, the number of RAMP1 positive Schwann cells (51.8 ± 5.7%; 105/203 total glia) were greater than that observed for satellite glia (43.1 ± 5.5%; 88/203).
Fig. 1.
RAMP1 expression in trigeminal ganglion glia. Cultures enriched in glia were immunostained for the presence of the CGRP receptor protein RAMP1 and imaged at 400× magnification using phase contrast microscopy (phase, left panel) as well as fluorescent microscopy (RAMP1). Thick arrows indicate satellite glial cells while thin arrows indicate Schwann cells. Scale bar equals 25 µm.
To investigate the temporal effect of CGRP on release of multiple cytokines from trigeminal glia, media collected from cultured glia was analyzed using RayBio Rat Cytokine Antibody Arrays. Somewhat surprisingly, treatment with 500 nM CGRP caused a significant (>3-fold) increase in the amount of all 19 cytokines detected in the media after 8 or 24 h when compared to unstimulated control levels (Table 1). For some stimulatory cytokines, such as CINC-3, fractalkine, GM-CSF, IL-1α, leptin, and MIP-3α, the amount of protein detected 8 h after addition of CGRP was greater than levels at 24 h, which remained significantly higher than control levels. The inhibitory cytokine TIMP-1 followed a similar temporal pattern. In contrast, levels of other stimulatory cytokines, including β-NGF, CTNF, IL-1β, IL-6, LIX, TNF-α, and VEGF, as well as the inhibitory cytokines IL-10 and IL-4, were greatest 24 h after CGRP treatment. Levels of the stimulatory cytokines CINC-2, IFN-γ, and MCP-1, which were significantly elevated over control levels 8 h after CGRP stimulation, remained at similar levels at 24 h. Importantly, CGRP treatment at 500 nM for 8 or 24 h was not associated with glial toxicity when compared to control as determined by the CellTiter96 assay (>98% viable at 24 h; n = 6).
Table 1.
Average fold change in release of cytokines from glia in response to CGRP (500 nM) for 8 or 24 h when compared to unstimulated control levels. CGRP stimulation caused a >3-fold increase in all the cytokines over control (p < 0.05). Grey boxes indicate cytokines thought to have an anti-inflammatory role.
| RayBio rat cytokine antibody array |
8 h | 24 h | ||
|---|---|---|---|---|
| Fold change | ±SEM | Fold change | ±SEM | |
| CINC-3 | 40.77 | 8.82 | 25.29 | 2.60 |
| Fractalkine | 77.11 | 5.11 | 15.74 | 2.47 |
| GM-CSF | 30.51 | 2.87 | 5.17 | 0.10 |
| IL-1α | 32.08 | 9.50 | 12.47 | 1.71 |
| Leptin | 8.5 | 2.28 | 4.69 | 0.48 |
| MIP-3a | 9.58 | 0.77 | 3.99 | 0.88 |
| TIMP-1 | 13.56 | 0.77 | 10.79 | 2.49 |
| β-NGF | 7.67 | 0.31 | 21.49 | 2.62 |
| CNTF | 9.08 | 0.34 | 26.51 | 0.35 |
| IL-1β | 12.78 | 0.27 | 17.22 | 0.14 |
| IL-6 | 10.67 | 0.47 | 22.77 | 0.98 |
| LIX | 7.49 | 0.45 | 22.88 | 0.83 |
| TNF-α | 10.47 | 0.06 | 16.74 | 0.88 |
| VEGF | 6.72 | 0.23 | 33.66 | 4.08 |
| IL-10 | 10.04 | 0.33 | 26.91 | 0.72 |
| IL-4 | 15.45 | 0.60 | 22.68 | 0.07 |
| CINC-2 | 15.28 | 1.99 | 15.88 | 1.31 |
| IFN-γ | 20.92 | 2.21 | 19.67 | 1.31 |
| MCP-1 | 8.33 | 0.12 | 8.02 | 0.12 |
Rat MAP Kinase Signaling Pathway arrays (SABiosciences) were used to study changes in mRNA levels of MAP kinase signaling proteins in cultured glia cells in response to CGRP. Treatment of glia with 500 nM CGRP resulted in a significant 3-fold or higher increase in expression of multiple MAP kinases and transcription factors in trigeminal glia at 8 and 24 h when compared to unstimulated cells (Table 2). While the level of only one MAP kinase was greater at 8 h than 24 h, levels of six kinases were higher at 24 h. In addition, three kinases were not stimulated at 8 h but were significantly increased at 24 h. One kinase was not increased at either time point. An even more diverse temporal pattern of expression was observed for transcription factor genes. The mRNA levels of six transcription factors were greatest 8 h after CGRP treatment. Although the levels of three other factors were increased at 8 h, a higher level of expression was seen at 24 h. In contrast, the levels of two factors were only significantly elevated at 8 h while expression of seven factors was only significantly increased at 24 h. In addition, CGRP was found to differentially regulate the expression of many other genes involved in MAP kinase signaling including the kinases MKKK, MKK, and MEKK1 interacting proteins (Supplemental Table). Similarly, mRNA levels for proteins involved in the regulation of Raf as well as scaffold/anchoring and cell cycle regulation were significantly elevated over control levels. Taken together, these data provide evidence that CGRP differentially regulates a vast array of genes and proteins involved in cytokine release and MAP kinase signaling in trigeminal glia.
Table 2.
Average fold change in mRNA levels in glia in response to CGRP (500 nM) for 8 or 24 h when compared to unstimulated control levels. Means are reported for genes whose levels were significantly increased >3-fold over control (p < 0.05); (–), not statistical different than control.
| RT2 profiler PCR array—MAP kinase signaling genes | 8 h | 24 h | |||
|---|---|---|---|---|---|
| Fold change | ±SEM | Fold change | ±SEM | ||
| MAPK | |||||
| Mapk3 (ERK1) | Mitogen-activated protein kinase 3 | 11.02 | 4.09 | 3.18 | 1.76 |
| Mapk7 (ERK7) | Mitogen-activated protein kinase 7 | 3.61 | 1.04 | 4.21 | 0.01 |
| Mapk9 (JNK2) | Mitogen-activated protein kinase 9 | 3.28 | 0.78 | 6.93 | 0.1 |
| Mapk10 (JNK3) | Mitogen-activated protein kinase 10 | 9.86 | 3.72 | 77.87 | 32.33 |
| Mapk12 (p38gMAPK) | Mitogen-activated protein kinase 12 | 3.54 | 1.13 | 10.5 | 1.34 |
| Mapk13 | Mitogen-activated protein kinase 13 | 4.35 | 1.26 | 6.16 | 1.66 |
| Mapk14 (p38 MAPK) | Mitogen-activated protein kinase 14 | 4.67 | 1.66 | 11.26 | 2.33 |
| LOC689314 (Mapk11) | Similar to mitogen-activated protein kinase 11 | – | – | 9.53 | 0.72 |
| Mapk1 (ERK2) | Mitogen-activated protein kinase 1 | – | – | 5.07 | 0.49 |
| Mapk8 (JNK1) | Mitogen-activated protein kinase 8 | – | – | 3.66 | 0.48 |
| Mapk6 (ERK3) | Mitogen-activated protein kinase 6 | – | – | – | – |
| Activated transcription factors | |||||
| Jun | Jun oncogene | 5.00 | 1.71 | 2.99 | 0.48 |
| Kcnn1 | Potassium small conductance calcium-activated channel, subfamily N-1 | 9.27 | 3.58 | 6.03 | 0.48 |
| MGC112775 (Mknk1) | Similar to map kinase interacting kinase | 13.57 | 5.12 | 4.43 | 1.69 |
| Myc | Myelocytomatosis viral oncogene homolog (avian) | 8.59 | 3.3 | 4.12 | 0.95 |
| Nfatc4 (NFAT3) | Nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 4 | 5.25 | 1.87 | 3.07 | 0.41 |
| Tp53 (p53) | Tumor protein p53 | 11.98 | 4.31 | 4.9 | 0.45 |
| Atf2 (CREB-2) | Activating transcription factor 2 | 4.06 | 1.37 | 6.31 | 1.85 |
| CREB1 | cAMP-responsive element binding protein 1 | 3.98 | 1.15 | 39.21 | 5.92 |
| Max | Max protein | 3.66 | 1.14 | 5.85 | 1.21 |
| CREBbp (CBP) | CREB binding protein | 3.95 | 1.41 | – | – |
| Egfr | Epidermal growth factor receptor | 3.14 | 1.05 | – | – |
| Ets1 | V-ets erythroblastosis virus E26 oncogene homolog 1 (avian) | – | – | 3.56 | 0.13 |
| Ets2 | V-ets erythroblastosis virus E26 oncogene homolog 2 (avian) | – | – | 4.54 | 1.16 |
| Kcnh8 (Elk1) | Potassium voltage-gated channel, subfamily H (eag-related), member 8 | – | – | 8.39 | 2.34 |
| Mapkapk2 | MAP kinase-activated protein kinase 2 | – | – | 3.6 | 0.21 |
| MGC116327 (Mapkapk5) | Similar to MK-5 type 2 | – | – | 5.02 | 2.16 |
| RGD1563119 (Mef2c) | Similar to MADS box transcription enhancer factor 2, polypeptide C | – | – | 4.16 | 0.93 |
| Smad4 (Madh4/DPC4) | MAD homolog 4 (Drosophila) | – | – | 3.16 | 0.38 |
In this study, we found that CGRP significantly increased the release of many cytokines as well as mRNA levels of proteins involved in MAP kinase signaling pathways in cultured trigeminal ganglion glia in a temporal manner. Within trigeminal ganglia, two types of glia have been identified corresponding to satellite glia and Schwann cells [11,22]. These cells function to control the microenvironment of neurons by regulating the levels of extracellular ions as well as cytokines, chemokines, and other inflammatory mediators found in proximity to cell bodies and nerve fibers. In agreement with previous studies, we showed that both satellite glia and Schwann cells express the CGRP receptor subunit protein RAMP1 [16,18], which is required for the formation of functional CGRP receptors. Thus, it is likely that the stimulatory effects of CGRP observed in our study are being mediated through activation of CGRP receptors present on satellite and Schwann cells.
Results of this study demonstrate that 500 nM CGRP causes a prolonged stimulation of cytokine release from cultured trigeminal glia. In fact, under our culture conditions, treatment with 500 nM CGRP significantly (>3-fold) increased release of all 19 cytokines 8 and 24 h post-treatment. This finding differs somewhat from our previous results in which treatment with 100 nM CGRP caused an increase in only six of the 19 cytokines after 24 h. Another difference between the two studies was that the overall magnitude of changes caused by 100 nM CGRP (range 2–4-fold) was much less than that seen with 500 nM CGRP (many >10-fold). The dissimilarity in the response to CGRP is most likely due to the difference in CGRP concentrations since the method used to establish the glia-enriched cultures was the same in both studies. While the total level of CGRP in trigeminal ganglia is not known, current literature suggests that CGRP content in tissues and exudates can be detected from low nanomolar to micromolar concentrations in normal or pathological conditions, respectively [1,23]. In our studies, it appears that the duration and magnitude of the stimulatory effects of CGRP on cytokine release from trigeminal glia is concentration dependent. We can only speculate that elevated levels of multiple stimulatory cytokines within the media, as seen in this study, would be expected to cause sensitization and possibly even activation of trigeminal neurons [6,8,15]. Thus, release of CGRP in the ganglia in response to nerve activation would create an inflammatory loop by stimulating prolonged release of stimulatory cytokines from satellite glia known to cause sensitization or activation of neurons in a concentration dependent manner. In this way, a CGRP-cytokine loop would lead to peripheral sensitization of trigeminal neurons, a central event that contributes to the underlying pathology of migraine, allergic rhinitis, and TMJ disorders. Similarly, CGRP activation of Schwann cells would release cytokines such as TNF-α, IL-1β and IL-6 that are known to cause sensitization of nociceptive neurons [30]. However, CGRP was also shown to cause a prolonged increased release of the inhibitory cytokines TIMP-1, IL-10, and IL-4 that would likely function in a compensatory manner to restore normal cytokine levels in the extracellular environment around ganglion neurons. Taken together, our findings support the concept that CGRP functions to initiate a complex regulatory loop involving differential expression of multiple cytokines that are likely to play an important role in diseases involving trigeminal ganglia.
Given the large body of evidence that supports a direct role of MAP kinase signaling pathways in the increased expression and release of cytokines from multiple cell types [7,14,29], it is likely that the prolonged elevation of cytokine release from glia is mediated at least in part by MAP kinase signaling pathways. Importantly, MAP kinase pathways connect activation of cell surface receptors to key regulatory events implicated in the initiation and maintenance of inflammatory and nociceptive responses in sensory ganglion neurons and glia [13,20,21]. In this study, we found that CGRP significantly increased the mRNA levels for numerous genes involved in MAP kinase signaling including isoforms of the MAP kinases ERK, JNK, and p38. This finding is in agreement with previous published results in which CGRP stimulation of iNOS gene expression in trigeminal glia was shown to involve activation of these MAP kinases [28]. Our results also are in agreement with other reports that CGRP activation of its receptor couples to increases in cAMP levels and subsequently in levels of the transcription factor, cAMP-responsive element binding protein 1 or CREB1 [2,26]. Taken together, data from our study provide evidence that CGRP is likely to modulate the excitability state of trigeminal glia by regulating expression of amultitude of diverse genes that includes MAP kinase-responsive transcription factors, scaffolding/anchoring proteins, and cell cycle proteins.
In conclusion, results from our study provide evidence of an emerging role of CGRP as an important modulator of trigeminal ganglion glia by stimulating cytokine release as well as increased expression of numerous proteins involved in MAP kinase signaling.
Supplementary Material
Acknowledgment
This research was funded by Merck & Co., Inc.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neulet.2010.01.074.
References
- 1.Alstergren P, Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Co-variation of neuropeptide Y, calcitonin gene-related peptide, substance P and neurokinin A in joint fluid from patients with temporomandibular joint arthritis. Arch. Oral Biol. 1995;40:127–135. doi: 10.1016/0003-9969(94)00141-w. [DOI] [PubMed] [Google Scholar]
- 2.Anderson LE, Seybold VS. Calcitonin gene-related peptide regulates gene transcription in primary afferent neurons. J. Neurochem. 2004;91:1417–1429. doi: 10.1111/j.1471-4159.2004.02833.x. [DOI] [PubMed] [Google Scholar]
- 3.Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Neuropeptides in the arthritic TMJ and symptoms and signs from the stomatognathic system with special considerations to rheumatoid arthritis. J. Orofac. Pain. 1995;9:215–225. [PubMed] [Google Scholar]
- 4.Baraniuk J. Neurogenic mechanisms in rhinosinusitis. Curr. Allergy Asthma Rep. 2001;1:252–261. doi: 10.1007/s11882-001-0016-4. [DOI] [PubMed] [Google Scholar]
- 5.Bowen E, Schmidt T, Firm C, Russo A, Durham P. Tumor necrosis factor-α stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J. Neurochem. 2006;96:65–77. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol. Pain. 2009;5:43. doi: 10.1186/1744-8069-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem. Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damodaram S, Thalakoti S, Freeman SE, Garrett FG, Durham PL. Tonabersat inhibits trigeminal ganglion neuronal-satellite glial cell signaling. Headache. 2009;49:5–20. doi: 10.1111/j.1526-4610.2008.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durham P, Russo A. Regulation of calcitonin gene-related peptide secretion by a serotonergic antimigraine drug. J. Neurosci. 1999;19:3423–3429. doi: 10.1523/JNEUROSCI.19-09-03423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durham P, Russo A. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J. Neurosci. 2003;23:807–815. doi: 10.1523/JNEUROSCI.23-03-00807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res. Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Hargreaves R. New migraine and pain research. Headache. 2007;47:S26–S43. doi: 10.1111/j.1526-4610.2006.00675.x. [DOI] [PubMed] [Google Scholar]
- 13.Ji R. Mitogen-activated protein kinases as potential targets for pain killers. Curr. Opin. Investig. Drugs. 2004;5:71–75. [PubMed] [Google Scholar]
- 14.Ji R, Suter M. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AA, Diogenes A, Jeske NA, Henry MA, Akopian A, Hargreaves KM. Tumor necrosis factor alpha enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience. 2008;155:503–509. doi: 10.1016/j.neuroscience.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 16.Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J. Comp. Neurol. 2008;507:1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- 17.Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr. Pain Headache Rep. 2009;13:237–240. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Vause CV, Durham PL. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallee J, Salvatore C, LeBourdelles B, Oliver K, Longmore J, Koblan K, Kane S. Receptor activity-modifying protein 1 determines the species selectivity of non-peptide CGRP receptor antagonists. J. Biol. Chem. 2002;277:14294–14298. doi: 10.1074/jbc.M109661200. [DOI] [PubMed] [Google Scholar]
- 20.Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K. Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur. J. Neurosci. 2004;20:2881–2895. doi: 10.1111/j.1460-9568.2004.03754.x. [DOI] [PubMed] [Google Scholar]
- 21.Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J. Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pannese E. The satellite cells of the sensory ganglia. Adv. Anat. Embryol. Cell Biol. 1981;65:1–111. doi: 10.1007/978-3-642-67750-2. [DOI] [PubMed] [Google Scholar]
- 23.Petersson G, Malm L, Ekman R, Hakanson R. Capsaicin evokes secretion of nasal fluid and depletes substance P and calcitonin gene-related peptide from the nasal mucosa in the rat. Br. J. Pharmacol. 1989;98:930–936. doi: 10.1111/j.1476-5381.1989.tb14623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietrobon D. Migraine: new molecular mechanisms. Neuroscientist. 2005;11:373–386. doi: 10.1177/1073858405275554. [DOI] [PubMed] [Google Scholar]
- 25.Poyner D, Sexton P, Marshall I, Smith D, Quirion R, Born W, Muff R, Fischer J, Foord S. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 26.Simonetti M, Giniatullin R, Fabbretti E. Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons. J. Biol. Chem. 2008;283:18743–18752. doi: 10.1074/jbc.M800296200. [DOI] [PubMed] [Google Scholar]
- 27.Thalakoti S, Patil V, Damodaram S, Vause C, Langford L, Freeman S, Durham P. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vause CV, Durham PL. CGRP stimulation of iNOS and NO release from trigeminal ganglion glial cells involves mitogen-activated protein kinase pathways. J. Neurochem. 2009;110:811–821. doi: 10.1111/j.1471-4159.2009.06154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Fu S, Wang Y, Yu P, Hu J, Gu W, Xu XM, Lu P. Interleukin-1 beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol. Cell. Neurosci. 2007;36:343–354. doi: 10.1016/j.mcn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Watkins L, Milligan E, Maier S. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv. Exp. Med. Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 31.Wimalawansa S. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr. Rev. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Winborn C, Marquez de Prado B, Russo A. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J. Neurosci. 2007;27:2693–2703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.